Abstract
The use of animals in research and education dates back to the period when humans started to look for ways to prevent and cure ailments. Most of present day's drug discoveries were possible because of the use of animals in research. The dilemma to continue animal experiments in education and research continues with varied and confusing guidelines. However, the animal use and their handling vary in each laboratory and educational institution. It has been reported that the animals are being subjected to painful procedures in education and training unnecessarily. The extensive use of animals in toxicity studies and testing dermatological preparations has raised concerns about the ways animals are sacrificed for these “irrelevant experiments”. On the other side of the coin are scientists who advocate the relevant and judicious use of animals in research so that new discoveries can continue. In this review, we discuss the evolution of the use of animals in education and research and how these have been affected in recent times owing to concerns from animal lovers and government regulations. A number of computer simulation and other models have been recommended for use as alternatives to use of animals for pharmacology education. In this review we also discuss some of these alternatives.
KEY WORDS: Alternatives, animal experiments, pharmacology education, regulations
Introduction
The mission of medicine is to eliminate suffering to maintain a good health, which may prolong the life. Drugs, an important tool in healthcare, are introduced in therapeutics after experimental evaluation. Since the beginning of humanity, the nature of human mind has led man to exploit his environment for his own requirements. Amidst endless efforts to expand his knowledge about living organisms, himself included, he began using animals for experimentation. Animal experiments, for long, have been an integral part of the pharmacology education at medical colleges in India.[1] Thousands of animals are used annually in educational institutes despite efforts by concerned teachers and activists to reduce this number. Many medical schools in India and other countries have either introduced alternatives to these experiments or are deliberating on this contentious issue.[2,3]
There is a belief that just as medicine cannot be taught or learnt without exposure to wards and clinics, pharmacology cannot be taught without experimentation in animals. However, with changing trends in teaching methods and practices, it is increasingly felt that animals should not be sacrificed just to acquire skills and techniques of experimentation. These experiments are expensive, time consuming and tedious.[3,4] Also, the availability of animals is becoming sparse. Guidelines by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), University Grants Commission (UGC) and the Medical Council of India (MCI), suggest 3 Rs i.e., replacement, refinement and reduction in animal experiments, with the fourth R added, that is their rehabilitation, as an added measure for their care.[5,6,7] In this changing scenario, development of alternatives are the need of the day. The use of live animal experiments is decreasing in many medical colleges across India. These are gradually being replaced by certain alternatives that are available at relatively low cost and with proven educational efficacy. In this review, we discuss the evolution of the use of animals in education and research and how these have been affected in recent times owing to concerns from animal lovers and government regulations. We also discuss certain viable alternatives to animal experiments.
What is animal experimentation?
Animal experimentation is the term used to explain the use of animals in experimentation in education, training and research. The terms animal testing, animal experimentation, animal research, in vivo testing and vivisection are often used interchangeably although they carry different meanings. “Vivisection”, a term preferred by those who oppose the use of animals in research, means cutting into or dissecting a living animal. Researchers prefer to use the term ‘animal experimentation’.[8,9]
Number and types of animals used in experimentation
About 50-100 million animals ranging from zebra fish to non-human primates are used for experimentation every year. While experiments of vertebrates are regulated in most countries, those on invertebrates are not, and hence their accurate usage statistics are lacking.[10] The total number of animals used in the USA in 2010 was almost 1.37 million. It is important to note that these statistics do not include rats, mice (which make up about 90% of research animals), birds and fish, as these animals are not covered by the Animal Welfare Act in the USA. Most procedures have been carried out on mice and rats (96%). Other animals used commonly include guinea pigs (19%), rabbits (18%) and hamsters (13%).[11] In the UK, over three million animals were used in 2011, which chiefly included mice (71%), fish (15%), rats (7%) and birds (4%).[10,12,13] Similar statistics for extensive use of animals in European Union are also available. Nearly 200,000 fish and 20,000 amphibians were used in the UK in 2004, predominantly zebra fish, Danio rerio and the African clawed frog, Xenopus laevis. Over 20,000 rabbits were used for animal testing in the UK in 2004 for eye irritancy tests (Draize test). The biological effects in the eye are easier to visualize because of lesser tear flow and absence of eye pigment in these animals. Rabbits are also frequently used for production of polyclonal antibodies.[12] While most of the above-mentioned animals are used in India too, the use of frogs in India needs special permission from the respective State Chief Wildlife warden, since the frogs are labeled as endangered species. Frogs are included under Schedule IV of the Wildlife Protection Act 1972 of India as well as under the red list of International Union for the Conservation of Nature and Natural Resources.[6,14] Larger animals like dogs, cats and non-human primates together account for less than 1% of the animals used in research every year. They are commonly used as models for human diseases in cardiology, endocrinology, bone and joint studies. Cats are most commonly used in neurological research.[9] As per CPCSEA guidelines amendment in 2006, a separate permission from CPCSEA is required to carry out any experiments on these large animals. However, the Institutional Animal Ethics Committee (IAEC) of the respective establishments is empowered to permit experiments on small animals.[6,15]
The number of animal used in teaching varies from 1% to 10% of total animals used.[16] Most of the animals used are small rodents.[17] These are mostly used for fundamental biological research and breeding purposes.[17] Most animals are used in only one procedure and are less commonly reused. These laboratory animals are obtained from various sources in different countries. The sources also vary based on species required. These experimental animals are mostly obtained through breeders. However, at some places these animals might be caught from the wild areas or from auctions.[18] In India, animals have been mostly provided by small time traders, until a few years ago, after the CPCSEA guidelines were modified and affected, which bans the procurement of animals from unauthorized sources.
Contribution of animal experimentation to therapeutic discoveries
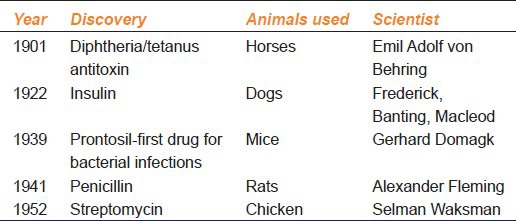
The greatest drug discoveries in the 19th and 20th centuries were possible due to the use of animals. Over the last century, every Nobel Prize for medical research has been dependent on animal research. The first Nobel prize in 1901 in medicine was for serum therapy and research involving use of horses. The latest 2012 Nobel laureates in physiology or medicine also worked on animals.[19] There is a strong relationship between rapid progress in experiments on animals and progress in clinical medicine [Table 1]. In the 1880s, Behring used horses for production of diphtheria antitoxin and the development of a vaccine against diphtheria and tetanus leading to the first Noble prize in physiology or medicine in 1901. Insulin was first isolated from dogs in 1922 and it revolutionized the treatment of diabetes. In the 1970s, antibiotic treatment and vaccines for leprosy were developed using armadillos. Domagk introduced antibacterial activity of prontosil in 1939 by experiments on chicken.[18]
Table 1.
Salient drug discoveries that involved use of animals
Primary uses of animals in experimentation
Education
Until some years ago, it was a common sight to see a hapless frog jumping under a pithing needle. It is difficult to see the rationale of such experimental adventures in making of a “good skilled doctor”. In 1987, Jennifer Graham from California sued her high school for insisting that dissection was the only method they recognized for learning anatomy. Several students objected openly against animal dissection exercises thereafter. Following these incidents, several states of the USA have passed “choice-in-dissection” laws, which provide a choice to the student to use alternatives to dissection. Several medical schools in the USA, like the Mayo, Harvard, Columbia and Yale now, have no live-animal laboratories. Romania has the highest use of animals in teaching among European countries.[20] Similarly, in India, the guidelines of UGC state similar option for students in 2011 to phase out animal experiments in life sciences. However, debates and confusions prevail on this issue, in the absence of clear-cut guidelines.[6]
In India, animals are used in pharmacology for the following:
Undergraduate teaching to demonstrate effects of various drugs although this has been phased out in most institutes. Typical experiments included effect of drugs on rabbit intestine, rabbit eye, central nervous system, reproductive system, as also the frog heart and frog rectus. The latter have however declined owing to the ban on use of frogs.[3,21,22] Although no data is available from India, most medical colleges have begun using or in transition phase to implement alternatives to animal experiments in undergraduate courses.[1,3,16]
Postgraduate teaching to demonstrate the effects of various drugs, to determine the nature of an unknown drug for bioassay, screening methods and to learn skills e.g. administering drugs. The syllabus of M.D. pharmacology has animal experiments as one of the major components. There has been a concern about continuing the animal experiments in the postgraduate courses, but the rules and guidelines are confusing and unclear.[23]
Research
Animals have been used and are still permitted for screening for drugs, in bioassay and for preclinical testing including general and specific toxicity studies. This preclinical safety and efficacy data is needed for submission to drug regulatory authorities before the permission for further studies in humans are granted.[24,25]
A larger number and a greater variety of animals are used in pure research than in applied research. This usually involves studies on embryogenesis, developmental biology, behavior and breeding in Fruit flies, nematodes, mice and rats.[25,26] Applied research that aims to answer specific questions is usually carried out in the pharmaceutical industry or by universities. Animal models of disease, discovered or generated by pure research programs, are used for applied research. Examples include use of transgenic animals, animal models of naturally occurring diseases, induced animal models of human diseases, cosmetic testing and toxicity studies. A few of these are discussed below:
i. Cosmetic testing
Cosmetic testing on animals is particularly controversial and involves general toxicity, eye and skin irritancy, phototoxicity and mutagenicity.[27,28] The famed cosmetics giant L’Oréal, had said it would respect the ban and “no longer sell in Europe any finished product with an ingredient that was tested on animals”.[9] Cosmetics testing is banned in many countries, including the Netherlands, Belgium and the UK. The European Union (EU) approved a near-total ban on the sale of animal-tested cosmetics from 2009. However, companies were allowed the sale of animal-tested products if the tests were conducted elsewhere. As of 11 March 2013, cosmetics tested on animals can no longer be sold in Europe, even if the testing was done outside Europe.[28] Since then major companies have completely stopped testing of cosmetics in animals. Necessity being the mother of invention, superior, cheaper and more effective non-animal methods have been developed for this purpose.
ii. Toxicology testing
Preclinical toxicology tests use one million animals every year in Europe, which are about 10% of all procedures. For each chemical test, approximately 5000 animals are used.[28,29] The most stringent tests are reserved for drugs and food. A number of tests are performed, lasting less than a month to years to test general toxicity, eye and skin irritancy, mutagenicity, carcinogenicity and teratogenicity.[25,29] These toxicity tests provide critical information for assessing hazard and risk potential. The utility of toxicity tests is however debated, since many animal toxicity tests do not accurately reflect toxicity in humans, with false positive results being a particular problem.[27,30] Alternatively, false negative tests, as in the case of thalidomide toxicity in rodents are also observed. Poor reproducibility of Draize test in rabbits, poor extrapolation of safety of acetyl salicylic acid, citalopram and recombinant antibodies from animals to humans are other examples that validate this fact. To add to this, withdrawal and ban of a number of drugs in recent years including rofecoxib, which was proven safe in animal testing highlight these legitimate concerns about extrapolation of animal test results to human beings.[31] Data from acute tests may meet classification and labeling regulations, but may be of limited value for hazard and risk assessment. Also, high doses of chemicals are used in a small number of laboratory animals to predict the effects of low doses in large number of humans.[26] Hence, opinion is divided on how to use this data in a meaningful manner.
Rodents (rats, mice) and non-rodents (rabbits) are usually used in these toxicity studies.[30,32] International variation in testing requirements can result in duplication in toxicity testing. For example, within the European Union, the local lymph node assay (LLNA) is the preferred method for assessing skin sensitization potential, whereas guinea pig assays are still preferred in other regions of the world e.g., China.[33] Considering the above facts, it is suggested that International uniform protocols be implemented so as to reduce the number of animals used, if not totally ban the toxicity tests.
Ethics and animal use
The debate surrounding animal use in experiments and teaching started way back in the 17th century. The animal protection movement was started in 18th century by a group of people known as abolitionists in England. Another worldwide initiative was started in 1975 by Societies for Protection and Care of Animals (SPCA) who opposed all forms of animal research.[34]
Since years, some researchers have favored animal experimentation and emphasized that such experiments were necessary for the advancement of scientific knowledge. Claude Bernard is known as the “prince of vivisection” and the father of physiology. His wife, Marie Françoise Martin, established the first anti-vivisection society in France in 1883. She wrote “the science of life is a superb and dazzlingly lighted hall which may be reached only by passing through a long and ghastly kitchen”. Arguing that “experiments on animals are entirely conclusive for the toxicology and hygiene of man, the effects of these substances are the same on man as on animals, save for differences in degree,” Bernard established animal experimentation as a part of the standard scientific method.[35]
The main concern for animals in experimentation is physical and mental stress and pain. A “painful procedure” in an animal study is defined as one that would “reasonably be expected to cause more than slight or momentary pain or distress in a human being to which that procedure was applied.” In the USA (2006) millions of animals were used in procedures that caused more than momentary pain or pain/distress, while 84,000 were used in studies that would cause pain or distress that would not be relieved by anesthesia.[36] In the UK, research projects are classified as mild, moderate, and substantial in terms of the suffering caused to animals. Animals that are anesthetized and killed without recovering consciousness are categorized as “unclassified”.[37]
Notwithstanding the various regulations, unethical treatment of animals is being reported worldwide. Some of these ‘so cruel’ episodes attracted worldwide attention. iBritches, a macaque monkey, was used for an experiment to test sensory substitution devices for blind people in University of California, Riverside. The monkeys had their eyes sewn shut and hence attracted a lot of ctiticism in 1985. The laboratory was raided by Animal Liberation Front and animals were rescued.[38] The first instance of lab technicians being fined for animal cruelty in the United Kingdom happened in 1997, when employees were ordered to pay £250 by People for the Ethical Treatment of Animals (PETA) for mistreating dogs.[38] Similarly, PETA fined a contract research organization after filming their facility in 2004-05. However, these unethical treatments to animals continued. In 2006, a trial of a monoclonal antibody in primates triggered a disastrous immune reaction and widespread organ failure in the six trial animals. This happening in London generated a lot of media attention.[39]
On the other hand, animal activists were using extreme measures to stop animal use. There have been threats to researchers from animal rights activists. A bomb was placed under the car of a ophthalmologist experimenting on cats and rhesus monkeys.[35] Following this and similar incidents, the US government passed the Animal Enterprise Terrorism Act. The government in U.K. followed by adding the offense of “Intimidation of persons connected with animal research organisation” to the Serious Organised Crime and Police Act 2005.[9]
It is pertinent here to remember that ethics, whether involving humans or animals carries varying connotations to different people. So how do we judge the ethical issues in animal experimentation? Whenever you consider ethical issues in animal experiments, critically analyze the following:
Is the animal the best experimental system for the hypothesis to be tested?
Is the problem under review worth solving?
Can pain and discomfort be minimised for the animal?
Regulations for use of animals for experiments and research
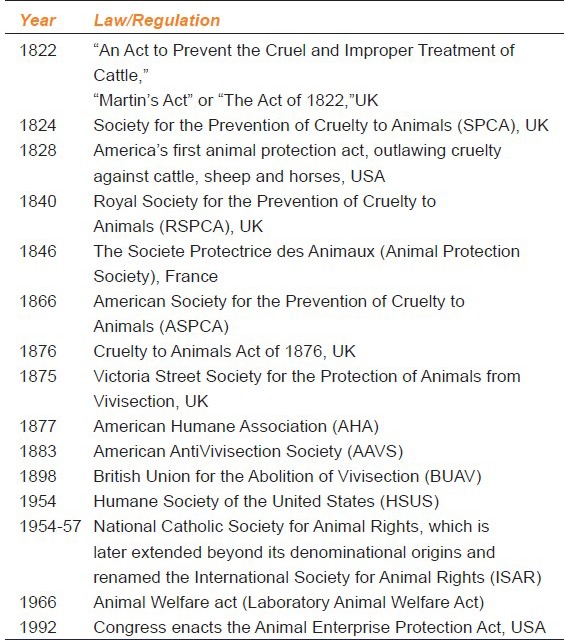
The first animal protection law to regulate animal testing was in enacted in 1822 in the British parliament, followed by the Cruelty to Animals Act (1876). The legislation was supported by Charles Darwin, who wrote way back in 1871, “You ask about my opinion on vivisection. I quite agree that it is justifiable for real investigations on physiology; but not for mere damnable and detestable curiosity. It is a subject which makes me sick with horror, so I will not say another word about it, else I shall not sleep tonight”. This led to formation of American Society for the Prevention of Cruelty to Animals (ASPCA) in 1860s, followed by the American Antivivisection Society (AAVS) in 1883 [Table 2]. Under this law, “any procedure can be performed on an animal if it is successfully proven that it is scientifically justified, as specified under the provisions of the Animal Welfare Act and the guide for the care and use of laboratory animals, published by the National Academy of Sciences”. The institution's veterinarian or institutional animal care or institute animal ethics committee (IAEC), for care and use of animals advise the researchers for the use of animals in their respective research projects. These committees have the responsibility to ensure that alternatives, including non-animal alternatives, have been discussed, the experiments are not unnecessarily duplicative and appropriate pain relief is given unless it would interfere with the research.[40,41]
Table 2.
Laws and Regulation for use of experimental animals Worldwide
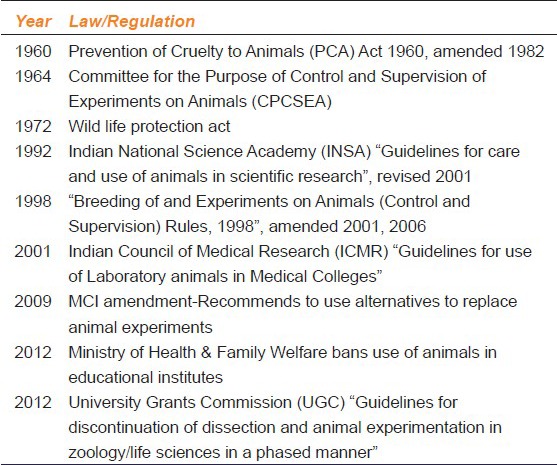
Back home in India, The prevention of cruelty to animals (PCA) Act 1960 was amended in 1982.[42] [Table 3]. For this purpose, the Government has formulated “Breeding of and experiments on animals (Control and Supervision) Rules, 1998” as amended during 2001 and 2006, to regulate the experimentation on animals. The CPCSEA provides guidelines for performing experiments on animals and maintenance of animal house.[5,43] The registration of animal house is mandatory with CPCSEA and is to be renewed every 3 years. There are now some preconditions for renewal by CPCSEA.[5] Besides the rules and procedures laid down by the CPCSEA, the Indian National Science academy (INSA) and Indian Council of Medical Research have also formulated certain guidelines for care and use of animals in scientific research as well as in medical colleges.[44,45]
Table 3.
Laws and regulations for use of experimental animals in India
In view of this, non-animal alternatives are being developed wherever necessary. An apparent 40% decrease in animal use and a concurrent increase in use of tissue culture and biotechnology indicate that scientifically valid, non-animal techniques are implementable. The MCI amendment in 2009 asked all medical colleges to use alternatives to animal experiments in the undergraduate medical course.[7] The proposed new curriculum of MCI “Vision-2015” for undergraduates might make it mandatory to have simulation labs for alternatives.[46] Over the last decade, significant developments in the area have been reported internationally and a few countries have constituted specific agencies for promoting the development and acceptance of these alternatives.
Alternatives to animal experimentation
William Russell and Rex Burch in “The principles of humane experimental technique” proposed that if animals were to be used in experiments, every effort should be made to replace them with non-sentient alternatives. This document published as early as in 1959 also emphasized that researchers need to reduce, replace and refine the use of animals so as to minimize the pain and distress to animals. These “3 Rs” of animal research, were paid little attention initially. However, they have influenced new legislations aimed at controlling the use of experimental animals and are included in the Animal (Scientific) Procedures Act in the United Kingdom and several similar regulations, including the CPCSEA guidelines.[34,47] The 3 Rs also found acceptance among those who opposed use of animals for experimentation, thereby rationalizing the debate to some extent.
Alternatives or substitutes to animal experimentation is defined as anything from absolute to partial replacement of live animals in biomedical research and experimentation.[4] Several alternative methods to replace animal experiments have been accepted worldwide. While the idea seems interesting and feasible, realizing, testing and validating an alternative method from idea to approval can be tedious and time consuming. In India, few software programs which replace animal experiments are available in pharmacology teaching since 1990. The upgraded versions of the earlier software programs are available but no innovative comprehensive software has been introduced so far.[3]
The various steps in designing valid alternatives are as follows:
Step 1: Defining an alternative: The word “alternative” is used to describe any change in an animal test that achieves one or more of the “three Rs” i.e., replaces animals, reduces the number of animals or refines a procedure to alleviate or minimize potential animal pain
Step 2: Developing the alternative: New cell and tissue tests, computer models and other innovative methods can be used to replace existing animal tests. These alternatives are more cost-effective and more reliable than traditional animal experimentation
Step 3: Validating the methods: The developed method is scientifically “validated,” in multiple laboratories to see if its results reliably predict outcomes in humans
Step 4: Acceptance in scientific community: After validation, acceptance of the alternative is necessary for its success.[48,49] The opinions of government regulators strongly influence the extent to which private companies use available alternatives. The various national and international bodies for validation and approval of in vitro methodologies include Organisation for Economic Co-operation and Development (OECD), European Centre for the Validation of Alternative Test Methods (ECVAM), the Interagency Coordinating Committee for the Validation of Alternative Methods in the US (ICCVAM), Japanese Center for the Validation of Alternative Methods (JaCVAM), KorCVAM in Korea and BraCVAM in Brazil.[49,50] The International Cooperation on Alternative Test Methods (ICATM) was created in 2009 as a collaboration between validation bodies from Europe, USA, Canada, Japan and Korea. Approximately 50 alternative methods and testing strategies have been developed, validated and/or accepted by international regulatory authorities.[51] It is interesting to know how the following principle of 3 Rs have been used to develop alternatives:
Replacement: Replacement methods can be absolute replacements i.e., techniques which do not involve animals at any point, such as in silico computer modeling, in vitro methodologies (e.g. tissue engineering) or relative replacements, which avoid or replace the use of ‘protected’ animals. Examples include, established animal cell lines, animal cells, tissues and organs collected from animals sacrificed by a humane technique, abattoir material, invertebrates, such as Drosophila and nematode worms, larval forms of amphibians and fish (until the stage where they become capable of independent feeding) and bacteria, fungi etc[24,30]
Reduction: Methods which minimize animal use and enable researchers to obtain comparable levels of information from fewer animals or to obtain more information from the same number of animals. Examples include, improved experimental design and statistical analysis, modern imaging techniques, avoiding repetitions of tests to prove established hypothesis and sharing data and resources[24,25]
Refinement: It refers to decreased invasiveness, improved instrumentation, improved control of pain which minimize actual or potential pain, suffering and distress.[13,24,34] Examples include, non-invasive techniques; using appropriate anesthetic and analgesic regimes for pain relief.
The 4th R i.e., Rehabilitation of the animals after their use, is also emphasized.
Alternatives are needed at all stages i.e., initial, interim and final in vivo testing. A few examples of these alternatives are:
i. In vitro techniques
The different in vitro techniques include:
Organ cultures
Tissue slices
Primary cell cultures
Established cell lines
Stem cells.
Cell culture is a promising alternative to animal use. Cultured cells have been developed to create monoclonal antibodies and cell lines have been extensively used in cancer research as well.[32] Hepatocytes are commonly used for evaluation of toxic potential of a compound as also the metabolism studies using cytochromeP450 from hepatocytes. It can predict drug interactions much before a drug is marketed. Various liver-based in vitro model systems include liver tissue slices, isolated microsomes, perfused liver and immortalized cell lines. These techniques can be efficiently used to screen highly toxic compounds at an early stage. Many in vitro techniques have evolved, but the concept is new and awaits validation and standardization.[52]
Human skin cells have been cultured to produce a model of human skin like EpiDerm and EpiSkin. Synthetic replacement using a protein membrane to simulate a skin barrier is approved as a partial replacement. The Organization for Economic Cooperation and Development (OECD) has approved several tissue culture methods which measure the rate of chemical absorption by the skin.[16,29,53] The OECD has also approved 3T3 neutral red uptake (NRU) phototoxicity test and detects the viability of 3T3 cells after phototoxicity. Chitosan films, a substitute for animal and human epidermal sheets are used for in vitro permeation of polar and non-polar drugs. Such films are capable of simulating the flux of drugs like 5-fluorouracil (5-FU) and indomethacin (INDO) across rat, rabbit and human cadaver epidermal sheets.[24] Ames test which can detect 80-90% of all carcinogenic chemicals is used primarily as a screening system, also has a potential as an alternative, after accurate validation. Another example is the use of fungi for studies of the metabolism of drugs. It has been seen that selected group of fungi have the ability to metabolize a wide variety of drugs. Cunninghamella elegans, used for testing anti-coagulants, diuretics, anticonvulsants and hemorheologic agents, also holds promise as a suitable alternative.[24]
Limulus ameobocyte lysate assay and human whole blood pyrogen test have replaced rabbit pyrogen tests. This has saved approximately one million rabbits annually.[29] Replacement of LD50 with acute toxic class test, fixed-dose procedure or up-and-down method can reduce animal use. Similarly, neuroblastoma cell, glioma cells are replacing teratogenic tests. In vitro micronucleus tests are widely used for genotoxocity and photogenotoxicity assays. Bovine corneal opacity and permeability tests have also gained wide acceptance. The reduced LLNA for skin allergy testing makes it possible to reduce animal use by up to 75% compared with traditional guinea pig and mouse tests. Most of these in vitro techniques have been validated.[54]
ii. In Silico (Computer based)
The in silico methods include models of diabetes, asthma and drug absorption. Potential new drugs identified using these techniques require verification in animal and human tests before licensing. Quantitative structure-activity relationship (QSAR) models are also used. These are mechanistic models that aim to predict sensitization from mechanistic knowledge and empirical models that are aimed at predicting from a statistical perspective. Several QSAR systems are available, such as TOPKAT, DEREK, TOPS-MODE, Multi-CASE, TIMES-SS, TOPKAT, ToxCast and DEREK. These are easier to use as compared to wet laboratory processes.[16,25,29,32]
Several “virtual humans” have been constructed by creating mathematical models of known human reactions. A few examples include computer models to model human metabolism, to study plaque buildup and cardiovascular risk and to evaluate toxicity of drugs. For example, the protease inhibitors for patients with HIV were designed by computer and tested in human tissue cultures and computer models, bypassing animal tests due to the urgent need for a treatment. A new cardiovascular drug was developed and approved in 1997 based on data from a virtual heart as animal data were inconclusive.[26] The day is not far off when E-cell which resembles a hypothetical cell is likely to revolutionize the drug research.[53]
Development of alternatives to animal experiments in teaching seems less tedious. These alternatives can be substituted by demonstrations using computer-simulated learning programs. Exercises in the form of graphs, tables obtained from various animal experiments can be used to teach students. They will analyze and interpret these applying different methods, formulae and statistics. After the discussions the learner can draw conclusions and correlate them clinically. Analyzing the results of any experiment or drug trial and drawing conclusions is a good learning experience for students for developing clinical judgment skills. The various alternatives used are mannequins, videos, observational and field studies, materials from slaughter house and fisheries, supervised clinical experience etc.[1,3,32] Exact simulation of real animal experiments on a computer is not easy because the biological responses are very complex. Hence, the results obtained with these simulated models may not be very accurate. It is pertinent to remember however, that the aim of the software is to teach the students about salient facts that have practical utility in their future role as decision makers in patient care.
Computer-based alternatives are being used in many countries. In India, two models are currently available: Expharm and Xcology. These are available as free modules and as advanced paid versions.[55,56,57] Both have been well tested and used for years. Computer-based alternatives were used to some extent by all countries. We evaluated these software programs and found that the alternatives are implementable and decrease the cost and time spent on animal experiments. The students appreciated the alternatives and found these more useful to understand the mechanism of action of drugs. Similarly, the response of faculty in India to training on alternatives was very encouraging.[1,3] The digital frog and more recently award-winning virtual frog is available where a student can dissect a frog layer by layer.[4] Surveys show that major barriers to the introduction of alternatives include “resources not available in local languages,” “difficulty finding resources,” and “lack of money.” Major factors that would persuade academic staff to introduce alternatives were: “published evidence of effectiveness,” “colleague's recommendation,” and “students’ objections”.[3,58]
While we ponder on these alternatives and the apparent difficulty in their implementation, it is encouraging to know that similar initiatives have begun worldwide. The reported replacement of animal labs with computer-based alternatives is highest in Spain (73%), whereas in France and Italy, they are low. Poland, Czech Republic and Romania reported a relatively high level of use of computer-based alternatives. Italy (64%) and Holland (67%) make the greatest use of free-of-charge computer-based resources. Countries like Spain (27%), Germany (41%) and Holland (22%) make the greatest use of in-house developed computer-based resources.[58]
iii. Invertebrate animals
Invertebrates can be used to replace the more commonly used laboratory animals. The most used invertebrate species are Drosophila melanogaster, a fruit fly and Caenorhabditis elegans, a nematode worm. Drosophila melonogaster is a classic model used for detecting mutagenicity, teratogenicity and reproductive toxicity. The body of C. elegans is completely transparent. Further, the precise lineage of all the organism's cells can be studied. These organisms have short life cycle and can be studied in large numbers, a distinct advantage over the vertebrates. In spite of some obvious drawbacks like the lack of an adaptive immune system which is a deterrent for their use in certain types of research such as vaccine development, these organisms do have a potential as alternatives to use of conventional animals. Similarly, fruit flies can be useful to identify novel virulence factors or pharmacologically active compounds.[30]
iv. Rapid developing vertebrates
A recent vertebrate model, the Zebra fish has proven to a very good model for toxicity testing. Studies on various chemicals have shown that Zebra fish have shown that 90% of these produced specific tissue, organ and behavioral toxicity. These have been used and validated in large scale high throughput screens for various psychotropic drugs.[59] Orthologues of PS1 and PS2 identified in zebra fish (psen1 and psen2) help in research on pathophysiology and pharmacotherapy of Alzheimer's disease. Their use has increased in the past two decades and a population-based atlas of the zebra fish brain has recently been developed.[60] Zebra fishes develop rapidly, are small, inexpensive to maintain, in large numbers. Chemical administration can be done directly to fish water or by microinjection of small amounts of chemicals. The morphological and molecular basis of tissue and organ development are, in general, either identical or similar to other vertebrates including man. Drug metabolism can also be studied in hydra, a eumetazoan diploblastic organism belonging to the phylum Cnidaria.[60]
v. Microdosing
The failure rate of drugs in phase I clinical trials is 40% and the process is expensive and time consuming. Microdosing studies enable potential new drugs to be tested safely in humans using ultrasensitivity of accelerator mass spectrometry. The time taken is 4-6 months and this method is relatively cost effective. It provides excellent information about human metabolism.[24] While in vivo (phase 0 microdosing with high sensitivity mass spectroscopy) and in silico (using established human biological data) technologies are increasingly being used, in vitro human approaches are rarely employed.
Howerver, it is also worthwile to consider the viewpoints that animal experiments provide an insight into the intricacies of drug action. The pharmacology of the drug unravelled during the preclinical phase of drug development is a prerequisite to understand the potential efficacy and safety of a new drug. Experts also opine that animal experiments are something which very much “belong” to pharmacology; and can serve as an important tool for evaluation of skills, particularly of postgraduate students. On the other hand, experts also opine that while animal experiments may be a necessity for graduates of pharmacy or biosciences, their role is debatable for medical graduates as the objectives can be met with alternative evaluation methods.[61] The debate therfore continues, until suitable alternatives are developed, validated, accepted and implemented.
Conclusion
It seems likely that these alternative methods and models will eventually replace intact animal models in pharmacology education, either partially or completely. The stakeholders need to provide timely information and also upgrade undergraduate and postgraduate curriculum in pharmacology, taking these facts into consideration. Regulatory bodies like the MCI, the CPCSEA, UGC, INSA, and the ICMR should finalize uniform guidelines for all educational institutions keeping in view the expected changes in curriculum viv-a-vis animal experimentation. They must not only initiate and encourage the projects for the development of alternatives in education but also ensure their implementation, by providing the requisite infrastructure in all institutions. In doing so, a broad consensus must be developed for removing or reducing the animal experiments from curriculum. As technology advances, these alternatives, especially in vitro and in silico techniques would receive wide acceptance by researchers and regulatory bodies, as well.
Whether the in vivo effect observed in animals can be compromised, and if the extrapolation should be entirely dependent on in vitro or in silico data, can be a matter of debate. While a combination of newer in vivo and in vitro techniques do provide viable and cost-effective alternatives to certain pharmacodynamic and pharmacokinetic evaluations of drugs, animal testing is yet required for repeat dose toxicity, carcinogenicity of drugs and certain behavioral studies. Hence, it appears that animals are not entirely dispensable, especially in research, which reiterates the need to practice the principles of 4Rs in animal experimentation and intensify our efforts in developing and validating suitable alternatives to their use.
Footnotes
Source of Support: Nil
Conflict Interest: No
References
- 1.Badyal DK, Bala S, Kathuria P. Student evaluation of teaching and assessment methods in pharmacology. Indian J Pharmacol. 2010;42:86–8. doi: 10.4103/0253-7613.64502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu CS, Latha K, Thirunavukkarasu J, Tharani CB. Virtual experimental pharmacology an alternative or not? A global assessment by pharmacology faculties and MBBS students? Rec Res Sci Tech. 2011;3:25–9. [Google Scholar]
- 3.Badyal DK, Modgill V, Kaur J. Computer simulation models are implementable as replacements for animal experiments. Altern Lab Anim. 2009;37:191–5. doi: 10.1177/026119290903700208. [DOI] [PubMed] [Google Scholar]
- 4.Dewhurst DG, Kojic ZZ. Replacing animal use in physiology and pharmacology teaching in selected universities in eastern Europe-charting a way forward. Altern Lab Anim. 2011;39:15–22. doi: 10.1177/026119291103900114. [DOI] [PubMed] [Google Scholar]
- 5.Committee for the purpose of control and supervision of experiments on animals (CPCSEA) [Last accessed on 2013 May 25]. Available from: http://moef.nic.in/modules/divisions/cpcsea .
- 6.University Grants Commission; 2011. [Last accessed on 2013 May 25]. Guidelines for discontinuation of dissection and animal experimentation in zoology/life sciences in a phased manner. Available from: http://www.gujaratuniversity.org.in/web/NWD/NewsEvents/8999_UGC%20Guidelines%20for%20Animal%20Dissection%20and%20Experimentation.pdf . [Google Scholar]
- 7.Medical council of India, New Delhi, amendment notification of 8 July 2009 to the Minimal standard requirements for medical colleges with 150 admissions annually, regulations. 1999. [Last accessed on 2013 May 25]. Available from: http://www.mciindia.org/helpdesk/how_to_start/STANDARD%20FOR%20150.pdf .
- 8.Bertoloni MD. Early modern experimentation on live animals. J Hist Biol. 2013;46:199–226. doi: 10.1007/s10739-012-9327-7. [DOI] [PubMed] [Google Scholar]
- 9.Animal testing. Wikipedia. [Last accessed on 2013 May 25]. Available from: http://en.wikipedia.org/wiki/Animal_testing .
- 10.Nuffield council of bioethics. [Last accessed on 2013 May 25]. Available from: http://www.nuffieldbioethics.org/animal-research/animal-research-what-animals-are-used-research .
- 11.Annual report animal usage by fiscal year, United States department of agriculture, animal and plant inspection service. 2011. [Last accessed on 2013 May 25]. Available from: http://speakingofresearch.files.wordpress.com/2008/03/2010_animals_used_in_research.pdf .
- 12.Statistics of scientific procedures on living animals. Home office, Great Britain. 2012. [Last accessed on 2013 May 25]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/115853/spanimals11.pdf .
- 13.Liebsch M, Grune B, Seiler A, Butzke D, Oelgeschla¨ger M, Pirow R, et al. Alternatives to animal testing: current status and future perspectives. Arch Toxicol. 2011;85:841–58. doi: 10.1007/s00204-011-0718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IUCN the red list of threatened species. [Last accessed on 2013 May 30]. Available from: http://www.iucnredlist.org/search .
- 15.The recommendations of the sub-committee on rehabilitation of animals after experimentation set up by CPCSEA. 2006. [Last accessed on 2013 May 30]. Available from: http://envfor.nic.in/divisions/awd/Rehabiliaion_Guidelines.pdf .
- 16.Mohammad AA, Mohammed Z, Karukayil JM. Alternatives to animals in education, research and risk assessment: An overview with special reference to Indian context. ALTEX. 2013;2:5–19. [Google Scholar]
- 17.Dewhurst D, Hemmi A. A survey of animal use and alternatives in higher education in Europe. University of Edinburgh, UK. ALTEX. 2012;1:1–14. [Google Scholar]
- 18.Animals in scientific procedures, UK Parliament/Chapter 1: Introduction. [Last accessed on 2013 May 30]. Available from: http://www.publications.parliament.uk/pa/ld200102/ldselect/ldanimal/150/15004.htm .
- 19.All noble prizes in physiology or medicine. [Last accessed on 2013 May 30]. Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates .
- 20.Balcombe J. 1st ed. Washington: Humane Society Press; 2000. [Last accessed on 2013 May 30]. The use of animals in higher education: Problems, alternatives and recommendations. Available from: http://www.humanesociety.org/assets/pdfs/parents_educators/the_use_of_animals_in_higher_ed.pdf . [Google Scholar]
- 21.Regulations on graduate medical education, 1997 (amended upto 2010). Medical Council of India. [Last accessed on 2013 May 30]. Available from: http://www.mciindia.org/Rules-and-Regulation/GME_REGULATIONS.pdf .
- 22.Badyal D. 1st ed. New Delhi: Jaypee Publishers; 2008. Practical manual of Pharmacology; pp. 73–93. [Google Scholar]
- 23.Postgraduate medical education regulations, 2000 (amended upto 2012). Medical Council of India. [Last accessed on 2013 May 30]. Available from: http://www.mciindia.org/RulesandRegulations/PGMedicalEducationRegulations2000.aspx .
- 24.Arora T, Mehta AK, Joshi V, Mehta KD, Rathor N, Mediratta PK, et al. Substitute of animals in drug research: an approach towards fulfillment of 4R's. Indian J Pharm Sci. 2011;73:1–6. doi: 10.4103/0250-474X.89750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandebriel RJ, van Loveren H. Non-animal sensitization testing: State-of-the-art. Cri Rev Toxicol. 2010;40:389–404. doi: 10.3109/10408440903524262. [DOI] [PubMed] [Google Scholar]
- 26.Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I. Where is the evidence that animal research benefits humans? BMJ. 2004;328:514–7. doi: 10.1136/bmj.328.7438.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosmetics and animal testing: A historic victory. Peta UK. [Last accessed on 2013 May 30]. Available from: http://www.peta.org.uk/features/new-cosmetics-law/default.asp .
- 28.More than a cosmetic change. News feature. Nature. 2012;438:144–6. doi: 10.1038/438144a. [DOI] [PubMed] [Google Scholar]
- 29.Creton S, Dewhurst IC, Earl LK, Gehen SC, Guest RL, Hotchkiss JA, et al. Acute toxicity testing of chemicals: Opportunities to avoid redundant testing and use alternative approaches. Cri Rev Toxicol. 2010;40:50–83. doi: 10.3109/10408440903401511. [DOI] [PubMed] [Google Scholar]
- 30.Wilson-Sanders SE. Models for biomedical research, testing and education. ILAR J. 2011;52:126–52. doi: 10.1093/ilar.52.2.126. [DOI] [PubMed] [Google Scholar]
- 31.Animal testing is bad science. Point/counterpoint. [Last accessed on 2013 May 30]. Available from: http://www.peta.org/issues/animals-used-for-experimentation/animal-testing-bad-science.aspx .
- 32.Mardas D, Mohammad AA, Bas B, Francesca C, Pierre C, Rodger C, et al. A framework program for the teaching of alternative methods (replacement, reduction, refinement) to animal experimentation. Altex. 20;28:341–52. doi: 10.14573/altex.2011.4.341. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Liu J, Wang F, Xu G, Hou J, Wan X, et al. Improvement of local lymph node assay for cosmetics safety evaluation [Article in Chinese] Wei Sheng Yan Jiu. 2009;38:585–9. [PubMed] [Google Scholar]
- 34.Richmond J. Refinement, reduction, and replacement of animal use for regulatory testing: future improvements and implementation within the regulatory framework. ILAR J. 2002;43:S63–8. doi: 10.1093/ilar.43.suppl_1.s63. [DOI] [PubMed] [Google Scholar]
- 35.Kurosawa TM. Alternatives to animal experimentation v.s. animal school [Article in Chinese] Yakugaku Zasshi. 2008;128:741–6. doi: 10.1248/yakushi.128.741. [DOI] [PubMed] [Google Scholar]
- 36.Report on Enforcement of the Animal Welfare Act. U.S. Department of Agriculture. [Last accessed 2013 May 30]. Available from: http://www.aphis.usda.gov/animal_welfare/downloads/awreports/awreport2005.pdf. 2005 Report on Enforcement of the Animal Welfare Act” (PDF)
- 37.Smith JA, Jennings M. Categorising the severity of scientific procedures on animals. The Boyd Group and the RSPCA, July 2004 RSPCA. Research Animals Department [Google Scholar]
- 38.People for the ethical treatment of animals. [Last accessed on 2013 May 30]. Available from: http://en.wikipedia.org/wiki/People_for_the_Ethical_Treatment_of_Animals .
- 39.BBC news; [Last accessed on 2013 May 30]. Two drug trial men critically ill. Available from: http://news.bbc.co.uk/2/hi/uk_news/england/london/4808836.stm . [Google Scholar]
- 40.Animal rights [Wikipedia] [Last accessed on 2013 May 30]. Available from: http://en.wikipedia.org/wiki/Animal_rights Animal rights .
- 41.A chronology of animal protection laws. [Last accessed on 2013 May 30]. Available from: http://www.animalsandethics.org/chronology.html .
- 42.The prevention of cruelty to animals act, as amended by Central Act 26 of 1982. [Last accessed on 2013 May 30]. Available from: http://www.moef.nic.in/legis/awbi/awbi01.html .
- 43.Ministry of social justice and empowerment notification, New Delhi, 1998. The Breeding of and Experiments on Animals (Control and Supervision) Rules. [Last accessed on 2013 May 30]. Available from: http://envfor.nic.in/legis/awbi/awbi10.pdf .
- 44.Guidelines for care and use of animals in scientific research. Indian National science academy. First edition, revised edition. 1992, 2000. [Last accessed on 2013 May 30]. Available from: http://icmr.nic.in/bioethics/INSA_Guidelines.pdf .
- 45.Guidelines for use of laboratory animals in medical colleges. Indian council of medical research, New Delhi. 2001. [Last accessed on 2013 May 30]. Available from: http://icmr.nic.in/bioethics/Guidelines_medicalcollege.pdf .
- 46.Vision 2015, Medical Council of India. [Last accessed on 2013 May 30]. Available from: http://www.mciindia.org/tools/announcement/MCI_booklet.pdf .
- 47.Guhad F. Introduction to the 3 Rs (refinement, reduction and replacement) Contemp Top Lab Anim Sci. 2005;4:58–9. [PubMed] [Google Scholar]
- 48.Validation, regulatory acceptance and international harmonization. [Last accessed on 2013 May 30]. Available from: http://alttox.org/ttrc/validation-ra .
- 49.Balls M, Amcoff P, Bremer S, Casati S, Coecke S, Clothier R, et al. The principles of weight of evidence validation of test methods and test strategies. Altern Lab Anim. 2005;34:603–20. doi: 10.1177/026119290603400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wind M, Blakey D, Kojima H, Kreysa J, Stokes W. The international cooperation on alternative test methods (ICATM) ALTEX. 2010;27(Spec Issue):207–10. [Google Scholar]
- 51.Table of validated and accepted alternative methods (updated 2013) [Last accessed 2013 on May 30]. Available from: http://alttox.org/ttrc/validation-ra/validated-ra-methods.html .
- 52.Takahashi K, Ishikawa N, Sadamoto Y, Sasamoto H, Ohta S, Shiozawa A, et al. E-Cell 2: Multi-platform E-Cell simulation system. Bioinformatics. 2003;19:1727–9. doi: 10.1093/bioinformatics/btg221. [DOI] [PubMed] [Google Scholar]
- 53.Ispra, Italy: European centre for the validation of alternative test methods (ECVAM); 2006. [Last accessed on 2013 May 30]. Statement on the scientific validity of the in vitro micronucleus assay as an alternative to the in vitro chromosomal aberration assay for genotoxicity testing. Available from: http://ihcp.jrc.ec.europa.eu/our_labs/eurl-ecvam/scientific-advice-stakeholders-networks/publication/ESAC25_statement_MNT_20061128_C.pdf . [Google Scholar]
- 54.Teaching and learning resources, IndPharnet. Network of Indian pharmacologists. [Last accessed on 2013 May 30]. Available from: http://www.indphar.org/xcology.html .
- 55.CD on X-Cology Pharmacy. [Last accessed on 2013 May 30]. Available from: Http://www.bookshelf.co.in/p/7392/cd-on-x-cology-pharmacy-content-aothors-crpatil-drbodhankar-dr-bhise-nirali-publications .
- 56.Simulated animal experiments in pharmacology. ExPharm pro. [Last accessed on 2013 May 30]. Available from: Http://www.expharmpro.com .
- 57.PETA sponsors emantras’ virtual frog dissection software for schools and colleges. 2012. [Last accessed on 2013 May 30]. Available from: Http://www.petaindia.com/features/Educators-Get-Free-Virtual-Dissection-Software.aspx .
- 58.Stewart AM, Kalueff AV. The Developing utility of zebrafish models for cognitive enhancers. Curr Neuropharmacol. 2012;10:263–71. doi: 10.2174/157015912803217323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahmood F, Mozere M, Zdebik AA, Stanescu HC, Tobin J, Beales PL, et al. Generation and validation of a zebrafish model of EAST (epilepsy, ataxia, sensorineural deafness and tubulopathy) syndrome. Dis Model Mech. 2013;6:652–60. doi: 10.1242/dmm.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segner H, Caroll K, Fenske M, Janssen CR, Maack G, Pascoe D, et al. Identification of endocrine disrupting effects in aquatic vertebrates and in vertebrates: Report from Europen IDEA project. Ecotoxicol Environ Saf. 2003;54:302–14. doi: 10.1016/s0147-6513(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 61.Dikshit RK. Animal experiments: Confusion, contradiction, and controversy. Indian J Pharmacol. 2012;44:661–2. doi: 10.4103/0253-7613.103232. [DOI] [PMC free article] [PubMed] [Google Scholar]


