Abstract
Objectives:
The objective of this study is to develop an experimental model of hyperlipidemia and insulin resistance (IR), markers of coronary heart disease (CHD) using high fat and high sugar (HFHS) diet and to evaluate the efficacy of the model using atorvastatin, a known antihyperlipidemic drug, pioglitazone, a known insulin sensitizer, and Tinospora cordifolia (Tc), an antidiabetic plant.
Materials and Methods:
Following Institutional Animal Ethics Committee permission, the study was conducted in male Wistar rats (200-270 g). The model was developed using a high fat (vanaspati ghee: coconut oil, 3:1) oral diet along with 25% fructose (high sugar) added in drinking water over a period of 6 weeks. Atorvastatin (2.1 mg/kg/day), pioglitazone (2.7 mg/kg/day) and Tc (200 mg/kg/day) were administered 3 weeks after initiation of HFHS diet and continued for another 3 weeks. Parameters assessed were weight, lipid profile, fasting blood glucose, insulin, and gastric emptying. Serum malondialdehyde (MDA) and catalase were assessed as markers of oxidative stress.
Results:
Administration of HFHS diet demonstrated a significant increase in blood glucose, insulin, total and low density lipoprotein cholesterol and triglycerides with a decrease in high density lipoprotein cholesterol. Treatment with test drugs decreased blood sugar, insulin, lipid parameters, increased gastric emptying rate, decreased MDA levels, and catalase activity when compared to HFHS diet group, confirming the efficacy of the model. Atherogenic index of all the test drugs (0.48, 0.57, and 0.53) was significantly lower as compared to HFHS diet group (1.107).
Conclusion:
This study confirms the development of a diet based cost-effective and time efficient experimental model, which can be used to study two important markers of cardiovascular disease that is, hyperlipidemia and IR and to explore the efficacy of new molecules in CHD.
KEY WORDS: Coronary heart disease, high fat, high sugar, hyperlipidemia, insulin resistance, Tinospora cordifolia
Introduction
Hyperlipidemia has been ranked as one of the greatest risk factors contributing to the prevalence and severity of coronary heart diseases (CHDs).[1] CHD, stroke, atherosclerosis and hyperlipidemia are the primary cause of death.[2] Hyperlipidemia is characterized by elevated serum total cholesterol (TC), low density lipoprotein (LDL), and very low density lipoprotein (VLDL) and decreased high density lipoprotein (HDL) levels. Among these, hypercholesterolemia and hypertriglyceridemia are closely related to ischemic heart disease.[3]
Hyperlipidemia is a common predicament in society due to change of lifestyle and food practice. Besides medication, diet also plays an important role in the management of lipid and lipoprotein concentrations in blood. Previous studies have shown that the uncontrolled consumption of high fat diet also leads to insulin resistance (IR) because the saturated fatty acids (SFA) interfere with the action of insulin.[4,5] High fat diet leads to IR, which increases the chances of developing diabetes mellitus and its associated complications such as diabetic nephropathy, retinopathy, neuropathy, gastroparesis, oxidative stress, etc. Oxidative stress also occurs as a result of decreased antioxidant defenses and has been related to the pathogenesis of atherosclerosis, and diabetes mellitus.[6]
Developing societies worldwide are shifting away from an agrarian existence to the current environment of high energy consumption, minimal physical activity and a lifestyle that include stress and anxiety which are some of the several factors implicated in the development of CHD and diabetes. The fundamental aspect in the etiology of these disorders is IR, which is linked to a wide array of other complications including hyperlipidemia. Thus, it is of paramount importance to establish an animal model, to have a better understanding of the pathological process involved in IR and hyperlipidemia, which would further aid in developing new therapeutic drugs which would modulate both the conditions.
Several high fat diet animal models are available, including hereditary ob/ob mice and SD or Wistar rat models developed by either injecting low-dose dexamethasone into the abdominal cavity or feeding food rich in fructose and sucrose leading to the development of insulin resistant conditions.[7,8] All these models have looked upon in ameliorating hypercholesterolemia and hypertriglyceridemia however failed to assess the hyperglycemic and hyperinsulinemia, which are also the important aspect of both IR and hyperlipidemia.
Saturated fatty acids and trans-fatty acids (TFA) are known to induce IR. Indian vanaspati contains high levels of trans-fatty acid. It contains >20% TFA and >60% of SFA. Coconut oil also contains a high proportion of saturated fatty acids. In certain parts of India, transfats from hydrogenated vegetable oil in the form of vanaspati and coconut oil are consumed in greater quantity than in the United States. The consumption of fructose too along with high-fat in the form of bakery foods, sweets, fruit juices, etc. is well-known in India and worldwide. High fructose consumption increases the likelihood of weight gain and reduces circulating leptin concentration leading to IR associated with hyperinsuliemia, hypertriglyceridemia and hyperglycemia.[9,10]
In this study, we thus aimed to develop an experimental model of hyperlipidemia and IR using high fat and high sugar (HFHS) diet consisting of Indian vanaspati and coconut oil with fructose in the form of sugar. We also planned to confirm the efficacy of the model using two standard drugs, atorvastatin, a known antihyperlipidemic drug and pioglitazone, a known insulin sensitizer. We also wished to confirm the efficacy of Tinospora cordifolia (Tc), an antidiabetic plant from Indian traditional systems of medicine that has been claimed to exhibit both these properties.
Materials and Methods
Test Drug
Standardized aqueous extract of stem Tc was procured from natural remedies, Bangalore. The authentication report and certificate of analysis is available on file.
Standard Drug
Pioglitazone and atorvastatin were procured in the pure form from Glenmark Pharmaceuticals, Mumbai, India.
Experimental Animals
Wistar rats of either sex aged 12 weeks (180-300 g) were used in the study. The animals were housed in groups of 6 animals each, for 1 week, in a 12:12 h light and dark cycle, in temperature and humidity controlled room. The animals were given free access to food and water. After the 1 week adaptation period, the animals were used for the study.
High Fat and High Sugar Diet
High fat was prepared by mixing Indian vanaspati ghee and coconut oil in the ratio of 3:1 (v/v). It was given to the rats at a dose of 3 ml/kg body weight per day. High sugar diet consisted of 25% fructose which was orally fed to the rats.
Experimental Design
The hyperlipidemia and insulin resistant model was developed using the HFHS diet. 3 ml of fat emulsion along with a high sugar diet consisting of 25% fructose was fed to the rats orally for 24 h for 6 weeks. Animals were weighed, recorded, numbered and randomly divided into five groups of six animals each. All the animals were taken care of under ethical consideration and the experimental protocol was duly approved by Institutional Ethic Committee (TNMC/IAEC approval dated 6.12.2010).
Group 1: Animals received standard pellet diet and purified water for 24 h over a period of 6 weeks and was considered as normal control (NC) group.
Group 2: Animals received standard pellet diet, 25% fructose water in bottles for 24 h and 3 ml high fat diet each day over a period of 6 weeks and was considered as HFHS diet control (DC) group.
Group 3: Animals received standard pellet diet, 25% fructose in bottles for 24 h and 3 ml high fat diet each day over a period of 6 weeks. After 3 weeks of study, atorvastatin at a dose of 2.1 mg/kg/day was administered after 3 weeks of feeding HFHS diet, and was continued until remaining 3 weeks and was considered as standard control 1 group.
Group 4: Animals received standard pellet diet, 25% fructose in bottles for 24 h and 3 ml high fat diet each day over a period of 6 weeks. After 3 weeks of study, pioglitazone at a dose of 2.7 mg/kg/day was administered after 3 weeks of feeding HFHS diet, and was continued until remaining 3 weeks and was considered as standard control 2 group.
Group 5: Animals received standard pellet diet, 25% fructose in bottles for 24 h and 3 ml high fat diet each day over a period of 6 weeks. After 3 weeks of study aqueous extract of Tc drug (200 mg/kg/day) was administered after 3 weeks of feeding HFHS diet, and was continued until 3 weeks and considered as test control group.
At the end of the experiment, all the animals were taken group wise and blood was collected using the retro-orbital technique on day 0, 21, and 42. The animals were weighed at the start of the experiment and then every week thereafter.
Blood Biochemical Analysis
Blood was collected by retro-orbital puncture from the ether-anesthetized rats and subjected to centrifugation to obtain the serum. Blood glucose levels and insulin levels were evaluated to assess hyperglycemic condition. Serum insulin levels were measured using rat insulin enzyme-linked immunosorbent assay kit (Mercodia, Sweden). IR was assessed by the homeostasis model assessment (HOMA), a mathematical model describing the degree of IR from fasting plasma glucose and insulin. HOMA-estimated IR was calculated by multiplying fasting plasma insulin (mU/L) with fasting plasma glucose (mmol/L) divided by 22.5.
Total serum cholesterol, triglycerides (TG), LDL cholesterol, VLDL cholesterol, HDL cholesterol, and atherogenic index (AI) were assessed to evaluate the development of dyslipidemia in the model.
Atherogenic index was calculated by using the formula of Schulpis and Karikas[11] Percentage of protection offered by the study drugs was calculated based on Dhandapani.[12]

Measurement of Oxidative Stress
Serum MDA levels and catalase activity from serum were evaluated to assess the oxidative stress developed in the model. Catalase activity was measured by the method of Aebi.[13] MDA levels were assessed using thiobarbituric acid reactive substances method.[14]
Measurement of Gastric Emptying Rate
At the end of the study period, all the animals were kept fasting for 24 h. After 24 h, all the animals were given a known quantity (10 g) of feed. After 20 min of their feeding, all the animals were sacrificed and their stomach was excised and the weight of the stomach and the weight of the feed in the stomach were weighed and the rate of gastric emptying was determined.
Histopathology
Heart and aorta samples were taken and immersed in 10% formalin solution. The fixed specimens were then trimmed, washed. and dehydrated in ascending grades of alcohol. Specimens were then cleared in xylol, embedded in paraffin, sectioned at 4-6 microns thickness and stained with hemtoxylin and eosin stain for examination described by Carleton.[15]
Statistical Analysis
Data obtained were subjected to computerized Graph pad version 3.06. GraphPad Software, Inc. CA, USA. One-way analysis of variance followed by post-hoc tests. The chosen level of significance was P < 0.05.
Results
Effect on Body Weight
An increase in body weight was observed at the end of 6 weeks that is, day 42 in the HFHS diet group as compared with the NC group. Treatment with both atorvastatin and Tc significantly reduced the increase in body weight as compared to the HFHS control group. However, pioglitazone treated group exhibited an increase in body weight.
Effect on Fasting Blood Sugar and Insulin Levels
High fat and high sugar DC group showed an increase in the fasting blood glucose with a significant increase at the end of 42 days as compared to the NC group indicating development of hyperglycemic state. All the three drugs showed a decrease in blood sugar levels proving their antidiabetic action. Similarly, insulin levels were also significantly increased in HFHS diet group on day 21 followed by continuous increase up to day 42. All the three test drugs demonstrated a significant decrease in insulin levels with maximum effect seen with Tc [Figure 1]. The degree of IR as calculated by HOMA was also higher in HFHS diet group. Feeding with all the three drugs significantly reduced the HOMA values [Table 1].
Figure 1.
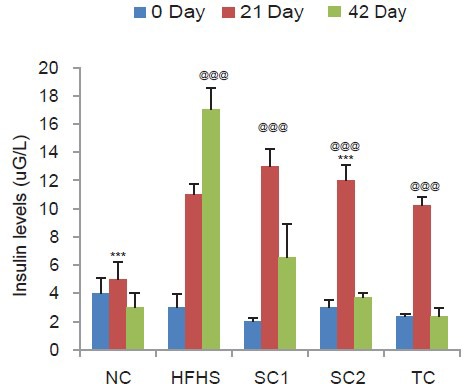
Effect of HFHS diet, atorvastatin, pioglitazone, and Tinospora cordifolia on insulin levels. Values are expressed as mean ± standard deviation; (n = 6). @@P < 0.01, @@@P < 0.001 as compared to normal control group, ***P < 0.001 as compared to HFHS diet control (ANOVA followed by Tukey's test). NC = Normal control group, HFHSC = High fat high sugar diet control group, Standard control 1 = HFHS + atorvastatin (2.1 mg/kg/day), Standard control 2 = HFHS + pioglitazone (2.7 mg/kg/day), Test control = HFHS + Tinospora cordifolia (200 mg/kg/day)
Table 1.
Effect of HFHS diet, atorvastatin, pioglitazone and Tinospora cordifolia on HOMA-insulin resistance
Effect on Lipid Profile
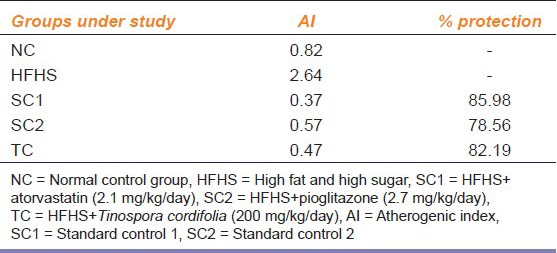
Feeding of HFHS caused a significant increase in serum levels of TC, LDL, VLDL and TG on day 21 followed by continuous increase up to day 42 as compared to a normal diet fed rats indicating development of hyperlipidemic and insulin resistant conditions. Treatment with all the study drugs significantly after day 21 inhibited the increase in the serum levels of total, LDL, VLDL cholesterol and TG, which were induced by the HFHS diet. However, HDL cholesterol level was significantly increased by the treatment in comparison to the HFHS control group Figure 2. The AI and the percent protection offered by the drugs were calculated and are represented in Table 2.
Figure 2.
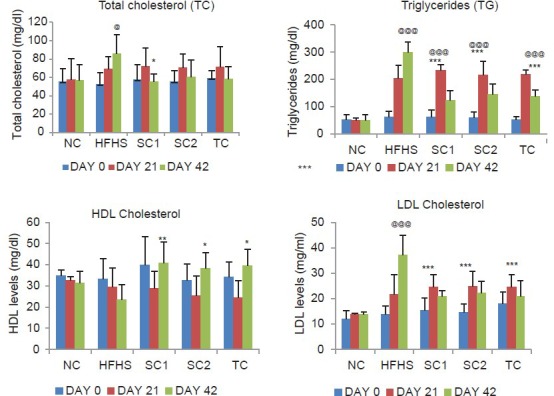
Effect of HFHS diet, atorvastatin, pioglitazone, and Tinospora cordifolia on lipid profile. Values are expressed as mean ± standard deviation; (n = 6). @P < 0.05; @@@P < 0.001 as compared to NC group; *P < 0.05; **P < 0.01; ***P < 0.001 as compared to HFHS diet control. NC = Normal control group, HFHSC = High fat high sugar diet control group, Standard control 1 = HFHS + atorvastatin (2.1 mg/kg/day), Standard control 2 = HFHS + pioglitazone (2.7 mg/kg/day); Test control = HFHS + Tinospora cordifolia (200 mg/kg/day) (ANOVA followed by Tukey's test)
Table 2.
AI and percentage of protection in different groups
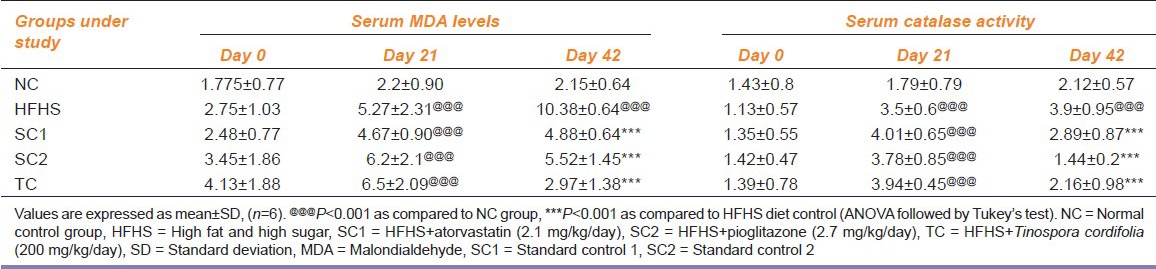
Effect on Oxidative Stress
An increase in MDA levels and catalase activity was observed in the HFHS DC group as compared to NC group indicating oxidative stress. This increase was further decreased by treatment with all the test drugs highlighting their antioxidant properties. The results are represented in Table 3.
Table 3.
Effect of HFHS diet, atorvastatin, pioglitazone and Tinospora cordifolia on oxidative stress
Effect on Gastric Emptying Rate
As seen in Figure 3 the gastric emptying rate was observed to be more in NC group as compared to the HFHS DC group. Following drug administration, the rate of gastric emptying increased as compared to the HFHS DC group with a significant increase seen in case of Tc.
Figure 3.
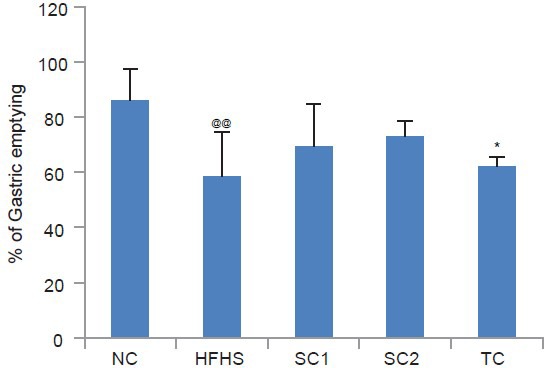
Effect of HFHS diet, atorvastatin, pioglitazone and Tinospora cordifolia on gastric emptying
Histopathology of Abdominal Aorta
Histopathological examination of aorta sections of NC rats showed no histological changes that is., normal tunica media, tunica intima, and tunica adventitia as seen in Figure 4a. In case of HFHS diet fed rats focal and vacuolated cells were seen in tunica initima and hemorrhage was also observed in tunica media [Figure 4b]. Fibro fatty tissues were also seen in tunica intima. Animals in the standard groups and test group showed normal tunica initima with less hemorrhage [Figure 4c–e].
Figure 4.
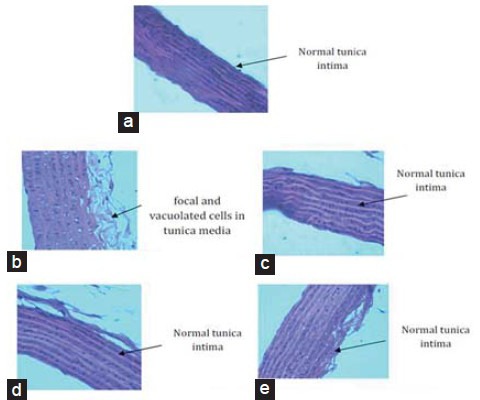
Histopathologiacal changes in abdominal aorta. (a) Normal control, (b) HFHS diet control group, (c) Standard control 1 = HFHS + atorvastatin (2.1 mg/kg/day), (d) Standard control 2 = HFHS + pioglitazone (2.7 mg/kg/day), (e) Test control = HFHS + Tinospora cordifolia (200 mg/kg/day). NC = Normal control group, HFHSC = High fat high sugar diet control group
Histopathology of Heart
Histopathological examination of heart of NC animals [Figure 5a] depicted normal myocardial tissue, whereas HFHS diet group [Figure 5b] showed hemorrhage in the myocardial tissue. This hemorrhage was seen to be decreased in the drug treated groups [Figure 5c–e].
Figure 5.
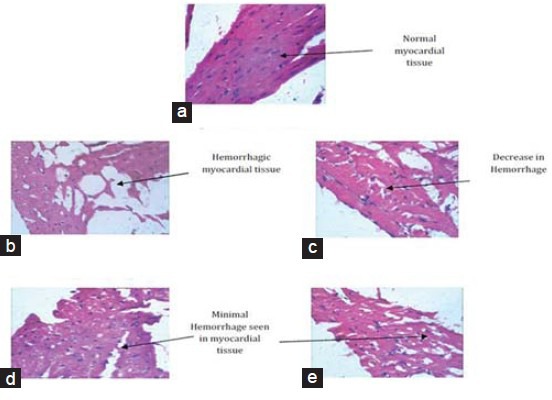
Histopathological changes in heart. (a) Normal control, (b) HFHS diet control group, (c) Standard control 1 = HFHS + atorvastatin (2.1 mg/kg/day), (d) Standard control 2 = HFHS + pioglitazone (2.7 mg/kg/day), (e) Test control = HFHS + Tinospora cordifolia (200 mg/kg/day). NC = Normal control group, HFHSC = High fat high sugar diet control group
Discussion
Coronary heart disease is the leading cause of death in developed countries. This alarming statistic is partly attributable to lifestyle, and partly due to the genetic factors that make humans highly susceptible to atherosclerotic vascular disease. The principal metabolic causes of atherosclerosis include hyperlipidemia, hypertension, obesity, IR, and diabetes mellitus.
Most of the Asian countries are undergoing a rapid nutrition transition. From a healthy, traditional, high-fiber, low-fat, low-calorie diet, a shift is being made toward consumption of junk food, refined carbohydrates, high total fat, and red meats, along with low intakes of fiber. Although junk food such as chocolates, French fries, Potato chips cookies, etc., plays an important role in developing the said conditions, not all of our population eat this type of food. On the other side though, most of our Indian population consumes food which is made in oil and/or ghee. Fructose is a simple sugar which is present in fruits and honey and is responsible for their sweet taste. Fructose content is high in a variety of toppings and sauces, ready-made fruit juices, fresh and dried fruits which is consumed by a majority of the population. All these faulty dietary habits and a sedentary lifestyle have contributed to the development of several disorders including hyperlipidemia and IR which further lead to development of CHD. Hence, in this study to develop new drugs which might ameliorate both these conditions, we developed an experimental model using HFHS. High fat diet was prepared by a combination of vanaspati ghee and coconut oil. Coconut oil is also consumed by majority of population to prepare food as same as vanaspati ghee which lead to development of hyperlipidemia. For high sugar diet, 25% of fructose solution was given to the rats. Fructose consumption is reported to induce IR, impaired glucose tolerance, hyperinsulinemia, hypertriglyceridemia, and hypertension in animal models.[16] This model of HFHS diet created hyperlipidemic and insulin resistant condition which mimicked the clinical pathogenesis seen in humans by consumption of faulty diets. The advantage of this model was that it can be easily developed within a shorter period of time. It is also cost effective as compared to other animal models of IR like nicotinamide-induced and genetically developed models.
Hyperlipidemia is associated with heart diseases. The lowering of the levels of harmful lipids to satisfactory values has been confirmed by several experimental animals and interventional studies indicating lowered morbidity and mortality in CHDs. In our study, administration of HFHS diet for 6 weeks, in rats demonstrated a significant increase in serum lipids, such as total, LDL, VLDL cholesterol, and TG along with a decrease in HDL cholesterol. An increase in fasting glucose levels, insulin and HOMA-IR values was also observed. All these conditions represented hyperglycemia, hyperinsulinemia, hypertriglyceridemia mimicking development of hyperlipidemia and IR.
Prolonged consumption of HFHS diet enhances lipid peroxidation, disturbs antioxidant defense mechanism and also leads to delayed gastric emptying where in the food remains in the stomach beyond the length of the time required for healthy digestion. An increase in serum lipid peroxidation evaluated in terms of malondialdehyde (MDA) levels was observed along with increase in antioxidant (catalase) activity demonstrating development of oxidative stress in the model. Oxidative stress has also been suggested as an underlying cause for CHD.[17] The rate of gastric emptying was also observed to be lower in the HFHS diet group as compared to NC group The presence of fat in the small intestine slows gastric emptying, stimulates the release of many gastrointestinal hormones, and suppresses appetite and energy intake as a result of the digestion of fats into free fatty acids; the effects of free fatty acids are, in turn, dependent on their chain length. All these observations in our study confirmed the successful development of a model of hyperlipidemia and IR.
Further the efficacy of the model in our study was assessed using atorvastatin, pioglitazone and Tinospora cordifolia. Atorvastatin is a standard antihyperlipidemic drug, which is used for the treatment of dyslipidemia and the prevention of cardiovasular disease. Pioglitazone is a known insulin sensitizer which improves glycemic control in type 2 diabetes patients by improving insulin sensitivity. Tc is a well-known antidiabetic plant of Indian medicinal system. Aqueous extracts of Tc stem have shown hypoglycemic activity and its aqueous extract reversed hyperglycemia, hyperinsulinemia, hypertriglyceridemia, IR, and elevated levels of hepatic total lipids, cholesterol, TG and free fatty acids in fructose fed rats.[18,19] Based on its reported activities we used it to assess the efficacy of our HFHS diet model.
Treatment with both atorvasatin and pioglitazone exhibited significant changes in the lipid parameters with decreased levels of TC, LDL and VLDL cholesterol and TG and increase in HDL cholesterol. Tc also demonstrated a similar effect as compared to atorvastatin and pioglitazone with a lowering of total, LDL, and VLDL cholesterol and an increase in HDL cholesterol. These results indicate a significant improvement in the AI. AI indicates the deposition of foam cells or plaque or fatty infiltration or lipids in heart, coronaries, aorta, liver and kidneys. The higher the AI, the higher is the risk of above organs for oxidative damage. AI also correlates with the size of the pro- and antiatherogenic lipoprotein particles and is known to predict a cardiovascular risk.[20] A higher AI value as observed in the HFHS DC group is an indication of cardiovascular risk. This risk was observed to be lower in animals treated with either atorvastatin, pioglitazone or Tc. The percentage protection exhibited by all the three drugs also confirmed the role of these drugs in ameliorating the hyperlipidemic and insulin resistant conditions developed in the model, with maximal benefit seen with atorvastatin followed by Tc.
A significant decrease in blood sugar and insulin levels was also observed with the 3 study drugs, thus positively affecting the HOMA-IR values. This decrease in the HOMA-IR values suggest that treatment with atorvastatin, pioglitazone and Tc ameliorate the IR caused by the HFHS diet. The effect seen with Tc was greater than that with atorvastatin and pioglitazone.
Abdominal aorta and heart was sent for histopathological analysis to evaluate the cardiovascular changes. The HFHS DC group showed histopathological changes representing structural alterations of the aortic wall indicating development of atherosclerotic changes. These changes were found to be modulated by all the three study drugs used in our study. Similarly, the myocardial tissue was found to be damaged in the HFHS diet group and this damage was modulated by treatment with the study drugs.
In addition, all the study drugs demonstrated a faster rate of gastric emptying as compared to the HFHS diet group indicating their role in reducing gastroparesis, that is, delayed gastric emptying. These drugs also decreased MDA levels and activity of antioxidant enzyme (Catalase) as compared to the HFHS DC group modulating the oxidative stress developed in the model. All these results indicate reversal of insulin resistant conditions, confirming the efficacy of our model.
Conclusion
This study confirms the development of a diet based cost effective and time efficient experimental model which can be used to study two important markers of cardiovascular disease that is, hyperlipidemia and IR. This model can be further developed to explore new therapeutic moieties to ameliorate cardiovascular heart disease.
Acknowledgment
We thank Ms. Rucha Mhatre and Ms. Neha Morya for their technical assistance.
Footnotes
Source of Support: Nil
Conflict Interest: No
References
- 1.Neil HA, Mant D, Jones L, Morgan B, Mann JI. Lipid screening: Is it enough to measure total cholesterol concentration? BMJ. 1990;301:584–7. doi: 10.1136/bmj.301.6752.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GD, Song F, Sheldon TA. Cholesterol lowering and mortality: The importance of considering initial level of risk. BMJ. 1993;306:1367–73. doi: 10.1136/bmj.306.6889.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson R, Beaglehole R. Evidence-based management of dyslipidaemia. Lancet. 1995;346:1440–2. doi: 10.1016/s0140-6736(95)92466-3. [DOI] [PubMed] [Google Scholar]
- 4.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–9. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Kim da S, Kang S. Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur J Nutr. 2011;50:107–18. doi: 10.1007/s00394-010-0120-0. [DOI] [PubMed] [Google Scholar]
- 6.Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid Med Cell Longev. 2009;2:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: An overview. Indian J Med Res. 2007;125:451–72. [PubMed] [Google Scholar]
- 8.Ai J, Wang N, Yang M, Du ZM, Zhang YC, Yang BF. Development of Wistar rat model of insulin resistance. World J Gastroenterol. 2005;11:3675–9. doi: 10.3748/wjg.v11.i24.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–22. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 11.Schulpis K, Karikas GA. Serum cholesterol and triglyceride distribution in 7767 school-aged Greek children. Pediatrics. 1998;101:861–4. doi: 10.1542/peds.101.5.861. [DOI] [PubMed] [Google Scholar]
- 12.Dhandapani R. Hypolipidemic activity of Eclipta prostrata (L.) L. leaf extract in atherogenic diet induced hyperlipidemic rats. Indian J Exp Biol. 2007;45:617–9. [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Carleton H. 4th ed. New York, USA, Toronto, London: Oxford University Press; 1979. Histological Techniques; p. 267. [Google Scholar]
- 16.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfram R, Oguogho A, Palumbo B, Sinzinger H. Enhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8-epi-PGF (2alpha) Eur J Heart Fail. 2005;7:167–72. doi: 10.1016/j.ejheart.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Reddy SS, Ramatholisamma P, Karuna R, Saralakumari D. Preventive effect of Tinospora cordifolia against high-fructose diet-induced insulin resistance and oxidative stress in male Wistar rats. Food Chem Toxicol. 2009;47:2224–9. doi: 10.1016/j.fct.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SS, Ramatholisamma P, Ramesh B, Baskar R, Saralakumari D. Beneficiary effect of Tinospora cordifolia against high-fructose diet induced abnormalities in carbohydrate and lipid metabolism in Wistar rats. Horm Metab Res. 2009;41:741–6. doi: 10.1055/s-0029-1220922. [DOI] [PubMed] [Google Scholar]
- 20.Frohlich J, Dobiásová M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49:1873–80. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]


