Abstract
Aim:
Epilepsy is a chronic neurological disorder with complex pathophysiology. Several evidences suggest a role of oxidative stress and mitochondrial dysfunction in pathophysiology of epilepsy. Hesperidin (Hesp) acts as a powerful anti-oxidant agent against superoxide, singlet oxygen, and hydroxyl radicals. Thus, this study was undertaken to evaluate the possible neuroprotective mechanism of Hesp against pentylenetetrazole (PTZ)-induced convulsions in mice.
Materials and Methods:
Sixty males Laca mice (20-25 g) were randomly divided into 10 treatment groups (n = 6). Seven days pretreatment of Hesp (100, 200 mg/kg, p.o.) was carried out before PTZ (80 mg/kg, intraperitoneal [i.p.]) challenge, whereas diazepam (DZP) (0.2, 0.5 mg/kg) and gabapentin (Gbp) (10, 20 mg/kg) were administered i.p. 30 min before PTZ administration, that is, on 7th day. Following PTZ challenge, severity of convulsions (onset of jerks, myoclonic seizures, extensor phase and death), brain anti-oxidant enzyme levels and mitochondrial complex enzymes activities were estimated.
Results:
Single i.p. PTZ (80 mg/kg) challenge demonstrated severe convulsions, oxidative damage (raised lipid peroxidation [LPO], nitrite concentration as well as depleted reduced glutathione, superoxide dismutase and catalase levels), and depletion of mitochondrial enzyme Complex (I, II, IV) activities. Hesp (200 mg/kg), DZP (0.5 mg/kg) and Gbp (20 mg/kg) pretreatments attenuated PTZ induced behavioral, biochemical and mitochondrial alterations. However, administration of Hesp (100 mg/kg) in combination with DZP (0.2 mg/kg) or Gbp (10 mg/kg) potentiated their neuroprotective effect, which was significant as compared to their effects in PTZ treated animals.
Conclusion:
Hesp possesses potent anticonvulsant activity which might be mediated through modulation of gamma-amino butyric acid/benzodiazepine receptor action.
KEY WORDS: Mitochondrial dysfunction, myoclonic jerks, oxidative stress, seizure
Introduction
Epilepsy is a complex neurological condition associated with significant alteration in psychological, emotional and educational parameters affecting approximately 1% of the population worldwide. A deficiency in the gamma-amino butyric acid (GABA) concentration or over-excitation of glutamate may result in many pathological alterations in central nervous system (CNS) that can further implicate in epilepsy.[1] Oxidative stress has been recognized as a common pathway to explain neuronal damage in most of the neurodegenerative conditions. Reactive oxygen species (ROS) arising from molecular oxygen originate by the activation of excitatory amino acids and further release glutamate not only causing long lasting seizure formation, but leading to neuronal death. Generation of free radicals also contributes to seizure generation directly by inactivating glutamine synthase and glutamate decarboxylase; thereby, causing an abnormal build-up of excitatory (glutamate) and inhibitory GABA neurotransmitters.[2,3,4]
Flavonoids, well-known for their antioxidant activity which is attributed to the aromatic hydroxyl groups in their moiety, are widely used as dietary supplements. Hesperidin (Hesp), a bioflavonoid, richly found in oranges and lemons, has been reported to possess significant neuroprotective, anti-inflammatory and analgesic, antibacterial, antifungal, antiviral, anti-hypercholesterolemic, and anticancer properties.[2,3,4] Further, in in-vitro studies Hesp has been reported to possess anticonvulsant activity.[5] Taken together, Hesp significantly contributes to the intracellular antioxidant defense system and act as a powerful anti-oxidant agent against superoxide, singlet oxygen and hydroxyl radicals. Various reports suggest that the use of antioxidants can be a potential neuroprotective approach in arresting the seizure genesis either by ameliorating the excitotoxicity or neurotoxicity.[2,4,5]
Over the last decade, newer antiepileptic drugs that have been approved for use by the Food and Drug Administration in the treatment of epilepsy. While most new antiepileptic drugs are approved as second line agents for the treatment of refractory seizures, topiramate, oxcarbazepine and lamotrigine are also approved for monotherapy in certain situations. Regardless of the available antiepileptic drugs, about one-third of the population suffers from un-preventable neurological changes induced by epileptic seizure and also exhibits some accompanied side-effects and chronic toxicity. It was found that chronic administration of GABA enhancing drugs can lead to reduced GABAergic function, alterations in GABAA receptors as well as tolerance and dependence, especially to sedatives and effects of anticonvulsants like benzodiazepines (BDZ). Recently, it has been reported that some naturally occurring flavonoids possess a selective and relatively mild affinity for the central BDZ binding site in the GABAA receptors. Several synthesized flavone derivatives with added electronegative groups have been reported to enhance affinities for the BDZ binding site.[6,7,8]
Therefore, this study was designed to find out the neuroprotective potential of Hesp against pentylenetetrazole (PTZ)-induced convulsions in mice. Further, efforts have been made to explore possible interaction of Hesp with standard anticonvulsants like diazepam (DZP) and gabapentin (Gbp) (ligands for GABA-BDZ receptor) against PTZ-induced convulsions in mice.
Materials and Methods
Animals
Male Laca mice (20-25 g) bred in central animal house facility were used. The animals were housed under standard laboratory conditions maintained under alternate 12 h light and dark cycle, and had free access to food and water. Animals were acclimatized to laboratory conditions before the experiment. All the experiments were carried out between 900 and 1500 h. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) (IAEC/170-175/UIPS) and was conducted according to the National Science Academy Guidelines for the use and care of animals.
Drug and Treatment Schedule
Pentylenetetrazole (Sigma, USA), DZP (Sigma, USA), Gbp (Lupin Ltd., Pune, India) and Hesp (Sigma, USA) were used, in this study. Doses of these drugs were selected based on the previous studies.[9,10,11] Hesp was suspended in 0.5% w/v sodium carboxymethylcellulose (CMC) and administered orally for 7 days before PTZ challenge. DZP and Gbp were dissolved in normal saline and administered as single intraperitoneal (i.p.) injections 30 min before PTZ administration on 7th day. The experimental protocol comprised of the following groups (n = 6):
Group 1 (Naive): Healthy animals (without any treatment)
Group 2 (PTZ): Vehicle (0.5% w/v Sod. CMC) + PTZ on 7th day
Groups 3,4 (DZP [0.2] and DZP [0.5]): DZP (0.2 and 0.5 mg/kg, i.p.) + PTZ on 7th day
Groups 5,6 (Gbp[10] and Gbp [20]): Gbp (10 and 20 mg/kg, i.p.) + PTZ on 7th day
Groups 7,8 (Hesp[100] and Hesp [200]): Hesp (100 and 200 mg/kg, p.o.) for 7 days + PTZ on 7th day
Group 9 (Hesp[100] + Dzp [0.2]): Hesp (100 mg/kg, p.o.) for 7 days + DZP (0.2 mg/kg, i.p.) on 7th day + PTZ on 7th day
Group 10 (Hesp[100] + Gbp [10]): Hesp (100 mg/kg, p.o.) for 7 days + Gbp (10 mg/kg, i.p.) on 7th day + PTZ on 7th day.
Hesperidin has been reported to possess neuroprotective effect at a 50 and 100 mg/kg oral dose in several neurological conditions such as cerebral ischemia, Huntington's disease, and Parkinson's disease.[9] Accordingly, we selected the doses 100 and 200 mg/kg. DZP has been reported to be neuroprotective at 0.5-2 mg/kg (i.p.) in PTZ induced seizures.[10] Hence, the sub therapeutic dose (0.2 and 0.5 mg/kg) was selected in order to observe any modulation in the neuroprotective effect when administered in combination with Hesp. Gbp has been reported to demonstrate neuroprotective effect in PTZ-induced epilepsy at 50-200 mg/kg dose. It has also been reported to display neuroprotective effect at 10 mg/kg dose in memory impairment conditions.[11] According to the previous study reports, we have selected the above-mentioned doses in our study. Before the start of the protocol, based upon the previous literatures as mentioned, three different doses of Hesp, DZP and Gbp were selected. When we obtained our results, we found that the doses of DZP (0.2 mg/kg) and Gbp (10 mg/kg) did not produce any significant protection against PTZ induced convulsion. However, at their respective higher doses, that is, DZP (0.5) and Gbp (20) significantly ameliorated the PTZ-induced convulsions. Hence, the DZP (0.2 mg/kg) and Gbp (10 mg/kg) doses were considered to be therapeutic. Further, these sub-therapeutic dosed were combined with the lower dose of Hesp (100) in order to observe any possible potentiation in their effects. The control group (PTZ treatment) was compared with the healthy animals (naive animals who have not been treated by any drug) in order to confirm the seizure genesis in control group, which was significantly different from the naive group.
Pentylenetetrazole-Induced Seizures
Single dose of PTZ (80 mg/kg, i.p.) was administered to induce clonic convulsions in mice.[11] Animals were observed for a period of 30 min post-PTZ administration in the plexiglass chamber (40 × 30 × 20 × 20). Various behavioral parameters such as latency for onset of myoclonic jerks, duration of clonic convulsions, tonic extensor, and mortality were observed. Generalized clonus was described as the involvement of all four limbs and tail, rearing, wild running and jumping, sudden loss of upright posture and autonomic signs such as hyper salivation and defecation.
Immediately after behavioral quantifications, animals were sacrificed by decapitation; brains were rinsed in isotonic saline, weighed and equally divided into two halves. One-half was used for the biochemical estimations (LPO, nitrite, glutathione [GSH], catalase, superoxide dismutase [SOD] and protein) and the other half was used for mitochondrial complex enzymes estimations (Complex-I [NADH dehydrogenase], Complex-II [succinate dehydrogenase] and Complex-IV [cytochrome oxidase]). LPO refers to the oxidative degradation of lipids. It is the process in which free radicals “steal” electrons from the lipids in cell membranes, resulting in cell damage. The magnitude of brain LPO correlates with the extent of neuronal degeneration. Superoxide anion radical, produced during mitochondrial respiration is involved in the generation of several potentially damaging ROS including peroxynitrite. Increased production of superoxide and its derivatives can induce injury by diverse mechanisms including initiation of LPO, inactivation of enzymes, damage to DNA, and protein sulfhydryl oxidation. In particular, in the presence of nitric oxide (NO), O2- and NO rapidly and spontaneously react to form the potent oxidant peroxynitrite (ONOO−), which is capable of nitrating tyrosine contributing to the neuropathological process. In this sense, superoxide radicals have been identified as important mediators of oxidative injury. Endogenous and exogenous nitrites represent sources of NO, which functions in the nervous system as a second messenger. Increased NO is responsible for the hyperexcitation and degeneration of neurons. Catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is an important enzyme in protecting the neuron from oxidative damage by ROS. GSH is an important neuromodulator that directly affects the activity of brain cells. GSH in its reduced form (GSH) is the most important free radical scavenging compound in the mammalian nervous system that prevents membrane LPO. Increased oxidative stress is always associated with mitochondrial dysfunction. Mainly mitochondrial Complex I, II, and IV plays a vital role in the genesis and progression of oxidative damage in epilepsy.
Biochemical Estimations
A 10% (w/v) tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4). The post nuclear fraction for enzyme assay was obtained by centrifugation of the homogenate at 10,000 × g for 20 min at 4°C and used for biochemical estimations.
Measurement of Lipid Peroxidation
The quantitative measurement of LPO in brain was performed according to the method of Wills.[12] The amount of malondialdehyde (MDA), a measure of LPO was measured by reaction with thiobarbituric acid at 532 nm using Perkin Elmer lambda 20 spectrophotometer (Norwalk, CT, USA). The values were calculated using molar extinction coefficient of chromophore (1.56 × 105/M/cm) and expressed as nanomoles of MDA per milligram of protein.
Estimation of Nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of NO, was determined with a colorimetric assay with Greiss reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide and 2.5% phosphoric acid) as described by Green et al.[13] Equal volumes of supernatant and Greiss reagent were mixed and the mixture was incubated for 10 min at room temperature. The absorbance was recorded at 540 nm with Perkin Elmer lambda 20 spectrophotometer (Norwalk, CT, USA). The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and was expressed as micromole per liter.
Estimation of Reduced Glutathione
Reduced GSH in brain was estimated according to the method described by Ellman and its group.[14] A volume of 1 ml supernatant was precipitated with 1 ml of 4% sulfosalicylic acid and cold digested at 4°C for 1 h. The sample was centrifuged at 1200 rpm for 15 min at 4°C. To 1 ml of this supernatant, 2.7 ml of 0.1 M phosphate buffer (pH 8) and 0.2 ml of 5,5-dithiobis 2-nitrobenzoic acid was added. The yellow color developed was read immediately at 412 nm using Perkin Elmer lambda 20 ultraviolet-visible spectrophotometer (Norwalk, CT, USA). Results were calculated using molar extinction coefficient of chromophore (1.36 × l04/M/cm) and were expressed as micromole GSH per milligram protein.
Catalase Estimation
Catalase activity was assayed by the method of Luck, wherein breakdown of hydrogen peroxides (H2O2) is measured at 240 nm.[15] Briefly, assay mixture consisted of 3 ml of H2O2 phosphate buffer and 0.05 ml of supernatant of tissue homogenate (10%), and change in absorbance was recorded at 240 nm. The results were expressed as micromole H2O2 decomposed per milligram of protein/minute.
Superoxide Dismutase Activity
Superoxide dismutase activity was accessed according to the method as described by Kono, wherein the reduction of nitroblue tetrazolium (NBT) was inhibited by the SOD and measured at 560 nm using spectrophotometer.[16] Briefly, the reaction was initiated by the addition of the hydroxylamine hydrochloride to the mixture containing NBT and sample. The results were expressed as unit/milligram protein, where 1 unit of enzyme is defined as the amount of enzyme inhibiting the rate of reaction by 100%.
Protein estimation
The protein was measured by biuret method using bovine serum albumin (BSA) as standard.[17]
Mitochondrial Complex Enzymes Estimation
Isolation of rat brain mitochondria
Second group of animals were used for mitochondrial isolation as described in the method of Berman and Hastings.[18] The brain regions were homogenized in isolated buffer. Homogenate was centrifuged at 13,000 g for 5 min at 4°C. Pellet was resuspended in isolation buffer with ethylene glycol tetra acetic acid (EGTA) and spun again at 13,000 g at 4°C for 5 min. The resulting supernatant was transferred to new tubes and topped off with isolation buffer with EGTA and again spun at 13,000 × g at 4°C for 10 min. Pellet containing pure mitochondria was resuspended in isolation buffer without EGTA.
NADH dehydrogenase activity
NADH dehydrogenase activity was measured spectrophotometrically by the method of King and Howard.[19] The method involves catalytic oxidation of NADH to NAD+ with subsequent reduction of cytochrome C. The reaction mixture contained 0.2 M glycylglycine buffer (pH 8.5), 6 mM NADH in 2 mM glycylglycine buffer and 10.5 mM cytochrome C. The reaction was initiated by addition of requisite amount of solubilized mitochondrial sample and followed absorbance change at 550 nm for 2 min.
Succinate dehydrogenase activity
Succinate dehydrogenase (SDH) was measured spectrophotometrically according to King.[20] The method involves oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The reaction mixture contained 0.2 M phosphate buffer (pH 7.8), 1% BSA, 0.6 M succinic acid, and 0.03 M potassium ferricyanide. The reaction was initiated by the addition of mitochondrial sample and absorbance change was followed at 420 nm for 2 min.
Cytochrome oxidase assay
Cytochrome oxidase activity was assayed in brain mitochondria according to the method of Sottocasa et al.[21] The assay mixture contained 0.3 mM reduced cytochrome C in 75 mM phosphate buffer. The reaction was started by the addition of solubilized mitochondrial sample and absorbance change was recorded at 550 nm for 2 min.
Statistical Analysis
Results were expressed as mean ± standard error of the mean. Data were analyzed by one-way analysis of variance followed by Tukey's test. P < 0.05 was considered to be statistically significant.
Results
Effect of Hesperidin and its Combinations with Diazepam and Gabapentin Against Pentylenetetrazole-Induced Convulsions in Mice
Pentylenetetrazole (80 mg/kg, i.p.) challenge significantly produced severe convulsions characterized by quick onset of jerks, severe straub tail, myoclonic seizure, extensor phase and mortality as compared to naive animals. However, single i.p. injections of DZP (0.5 mg/kg) and Gbp (20 mg/kg), 30 min before PTZ challenge increased seizure threshold (delayed latency for onset of jerks, myoclonic seizures, and tonus with complete protection against mortality) which was significant as compared to PTZ treated animals. However, sub-convulsive doses of DZP (0.2 mg/kg, i.p.) and Gbp (10 mg/kg, i.p.) administered 30 min before PTZ administration increased the seizure threshold non-significantly as compared to PTZ treated animals. Further, pretreatment with Hesp (200 mg/kg, p.o.) for 7 days significantly increased latency for onset of clonic and tonic phases of convulsion as compared to PTZ treated animals, whereas increase in seizure latency observed with Hesp (100 mg/kg, p.o.) treatment was found to be non-significant. However, unlike combination of lower doses of Hesp and Gbp, pretreatment of Hesp (100 mg/kg, p.o.) in combination with DZP (0.2 mg/kg, i.p.) was found to potentiate their protective effect against seizures which was significant as compared to their effect per se in PTZ treated animals [Figure 1].
Figure 1.
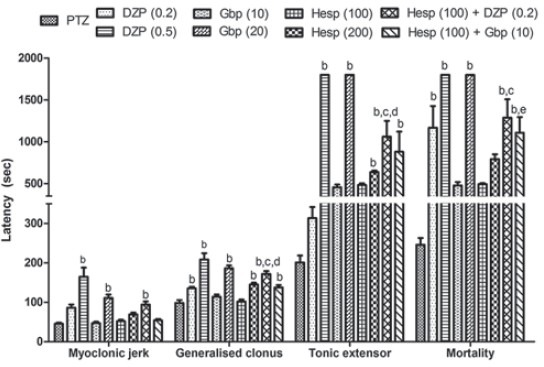
Effect of hesperidin (Hesp) and its combinations with diazepam and gabapentin against pentylenetetrazole (PZZ) induced convulsions in mice. Data expressed as mean ± standard error of the mean. Analysis of variance followed by Tukey's test. bp < 0.05 as compared to PTZ group, cp < 0.05 as compared to Hesp (100), dp < 0.05 as compared to diazepam (0.2), and ep < 0.05 as compared to gabapentin (10)
Effect of Hesperidin and its Combinations with Diazepam and Gabapentin on Brain Lipid Peroxidation and Nitrite Concentration in Pentylenetetrazole Treated Mice
Pentylenetetrazole (80 mg/kg, i.p.) challenge resulted in significant oxidative damage (raised LPO and nitrite concentration) as compared to naive group, whereas Hesp (200 mg/kg, p.o.), DZP (0.5 mg/kg, i.p.) and Gbp (20 mg/kg, i.p.) pretreatment significantly attenuated LPO and nitrite concentration as compared to PTZ (control) group. However, pretreatment of Hesp (100 mg/kg, p.o.) in combination with DZP (0.2 mg/kg, i.p.) or Gbp (10 mg/kg, i.p.) attenuated LPO as well as nitrite concentration which was significant as compared their effect per se in PTZ treated animals [Figure 2a and b].
Figure 2.
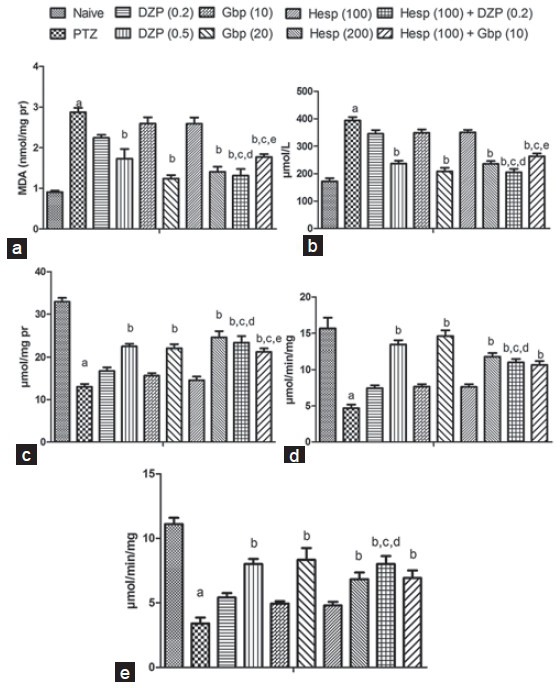
Effect of hesperidin (Hesp) and its combinations with diazepam (DZP) and gabapentin (Gdp) on lipid peroxidation (a), nitrite (b), glutathione (c), superoxide dismutase (d) and catalase (e) levels in brain of pentylenetetrazole (PTZ) challenged mice. Values are expressed as mean ± standard error of the mean. Analysis of variance followed by Tukey test. ap < 0.05 as compared to naive, bp < 0.05 as compared to PTZ, cp < 0.05 as compared to Hesp (100), dp < 0.05 as compared to DZp (0.2), ep < 0.05 as compared to Gbp (10)
Effect of Hesperidin and its Combinations with Diazepam and Gabapentin on Glutathione, Superoxide Dismutase and Catalase Levels in Pentylenetetrazole Treated Mice
Pentylenetetrazole (80 mg/kg, i.p.) challenge caused significant depletion in brain SOD, GSH and catalase levels as compared to the naive group. However, DZP (0.5 mg/kg, i.p.), Gbp (20 mg/kg, i.p.) and Hesp (200 mg/kg, p.o.) pretreatment showed significant protection against depletion in brain SOD, GSH and catalase levels in PTZ treated animals. Further, pretreatment of Hesp (100 mg/kg, p.o.) in combination with DZP (0.2 mg/kg, i.p.) or Gbp (10 mg/kg, i.p.) restored all the three antioxidant enzymes significantly as compared to PTZ (control) group. However, pretreatment of Hesp (100 mg/kg, p.o.) in combination with DZP (0.2 mg/kg, i.p.) was able to restore GSH, SOD and catalase levels significantly as compared to their effect per se in PTZ treated animals whereas combination of Hesp (100 mg/kg, p.o.) with Gbp (10 mg/kg, i.p.) pretreatment was able to restore only GSH levels significantly as compared to their effect per se in PTZ treated animals [Figure 2c–e].
Effect of Hesperidin and its Combinations with Diazepam and Gabapentin on Mitochondrial Complex Enzymes Activities in Pentylenetetrazole Treated Mice
Systemic PTZ (80 mg/kg) treatment significantly impaired mitochondrial Complex enzymes (I, II and IV) activities as compared to PTZ (control) group. Pretreatment of DZP (0.5 mg/kg, i.p.), Gbp (20 mg/kg, i.p.) and Hesp (200 mg/kg, p.o.) significantly restored mitochondrial Complex enzyme (I, II, and IV) activities as compared to PTZ (control) group, whereas they were found to be non-significant at their lower doses. However, pretreatment of Hesp (100 mg/kg, p.o.) in combination with DZP (0.2 mg/kg, i.p.) restored mitochondrial Complex enzyme (I, II and IV) activities which was significant as compared to their effects per se in PTZ treated animals, whereas pretreatment of Hesp (100 mg/kg, p.o.) in combination with Gbp (10 mg/kg, i.p.) was found to restore the mitochondrial Complex (I and IV) only as compared to the control [Figure 3].
Figure 3.
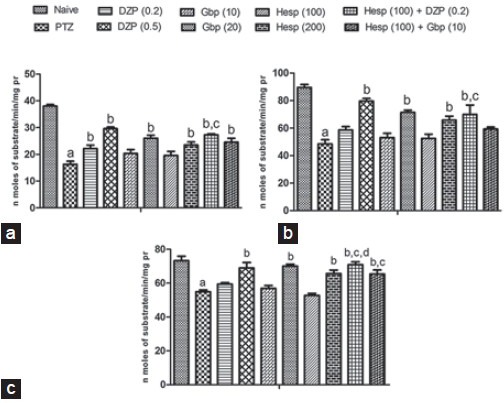
Effect of hesperidin (Hesp) and its combinations with diazepam and gabapentin on mitochondrial Complex I (a), II (b), and IV (c) in brain of pentylenetetrazole (PTZ) challenged mice. Data expressed as mean ± standard error of the mean. Analysis of variance followed by Tukey's test. ap < 0.05 as compared to naive group, bp < 0.05 as compared to PTZ, cp < 0.05 as compared to Hesp (100), dp < 0.05 as compared to diazepam (0.2)
Discussion
It is a well-established fact that impairment of endogenous antioxidants plays a major role in the genesis as well as precipitation of seizure. Role of oxidative stress in CNS has been well demonstrated in several experimental models of epilepsy such as the amygdala kindling model, kainic acid model, PTZ kindling model and acute PTZ-induced seizures. Following PTZ administration, free radicals are produced immediately,[10,11] among which hydroxyl-free radicals (OH.) are considered to be most reactive and hazardous.[11] The demonstration of early seizure induced hydroxyl-free radicals formation establishes the role of oxygen free radicals in the pathophysiology of epilepsy. Thus antioxidants attenuate the oxidative burden as well as seizure generation; hence, play a putative role as adjuncts with low doses of conventional antiepileptic drugs, as reduction of doses of anti-epileptic drugs can decrease the adverse potential, pharmacoresistance, and act through multi-targeted approach. But still, studies have been focusing on elucidating whether prolonged seizure activity in animals results in increased ROS production and whether oxidative injury plays a crucial fundamental role contributing to seizure-induced brain damage.[10,11]
About one-third of the population suffers from un-preventable neurological changes induced by epileptic seizure and also exhibits some accompanied side-effects and chronic toxicity. It was found that chronic administration of GABA enhancing drugs can lead to reduced GABAergic function, alterations in GABAA receptors as well as tolerance and dependence, especially to sedatives and effects of anticonvulsants like BDZ. Recently, it has been reported that some naturally occurring flavonoids (antioxidants) possess a selective and relatively mild affinity for the central BDZ binding site in the GABAA receptors. Several synthesized flavone derivatives have been reported to enhance affinities for the BDZ binding site.[2,3,4,5] Further, antioxidants attenuate the oxidative burden as well as seizure generation. Besides reducing oxidative stress, most of the antioxidants have been reported to have other actions such as anti-inflammatory, anti-microglial, histone de acetylase inhibitory, antiapoptotic activities also. However, as adjuncts with low doses of conventional antiepileptic drugs are expected to target these pathways as well as reduction of doses of anti-epileptic drugs which subsequently result in decrease the adverse potential, pharmacoresistance and toxicity. Thus, addition of anti-oxidants with conventional anti-epileptic drugs can lead to multi targeted approach (reduction of dose, reduced toxicity, reduced drug resistance, and improved therapeutic effects).
In this study, 7 days pretreatment with Hesp (200 mg/kg) increased the seizure threshold and attenuated the severity of PTZ induced convulsions suggesting its neuroprotective potential. Similarly, single i.p. administration of DZP (0.5 mg/kg) and Gbp (20 mg/kg) prolonged the latency for onset of jerks, straub tail, myoclonic seizure and extensor phase and showed complete protection against mortality in PTZ treated animals. These findings suggest that Hesp possesses a possible neuroprotective profile comparable to DZP and several other antioxidants. Further, pretreatment of Hesp (100 mg/kg) in combination with DZP (0.2 mg/kg) or Gbp (10 mg/kg) prolonged the latency for clonic convulsions and interestingly promoted 50% protection against mortality. The above findings implicate that Hesp potentiates the neuroprotective effect of DZP against PTZ induced convulsions.
Pentylenetetrazole has also been reported to trigger the activation of membrane phospholipases, proteases and nucleases, which cause degradation of membrane phospholipids, proteolysis of cytoskeleton proteins and protein phosphorylation.[10,11,22] In consistent with the previous studies, this study demonstrated that acute PTZ-administration leads to an increase in LPO in mice brain. It is well-known that convulsions were followed by an increase in LPO in brain tissue[23] leading to the formation of ROS such as superoxide, hydroxyl radical and hydrogen peroxide (H2O2) radicals. In agreement with these above findings, the current study demonstrated significant alteration in SOD, GSH and catalase activity in the PTZ group, demonstrating an alteration in oxidative stress parameters following PTZ administration. However, Hesp (200 mg/kg), DZP (0.5 mg/kg) and Gbp (20 mg/kg) treatment restored the cellular antioxidant defenses. Moreover, the combination of sub-therapeutic doses of Hesp with DZP or Gbp have abated the LPO and nitrite levels as well as significantly improved the antioxidant enzyme profile as compared their effect per se showing potent anti-oxidant activity. It may also be plausible that Hesp through its free radical scavenging property, especially against superoxide, singlet oxygen and hydroxyl radicals, attenuated the PTZ-induced oxidative damage. Recently, it has been reported that Hesp supports neuronal growth and survival in vitro through activation of MAP kinase and phosphatidylinositol 3-kinase signaling pathway.[6,8,22] However, the exact mechanism of Hesp in restoration of antioxidant defense system is yet to be established.
Mitochondria have critical cellular functions that influence neuronal excitability including ATP production, fatty acid oxidation, excitotoxicity, control of apoptosis and necrosis, neurotransmitter biosynthesis, and regulation of cytosolic Ca2+ homeostasis. Mitochondria are the primary sites of ROS production and are uniquely vulnerable to oxidative damage that may play a critical role in controlling neuronal excitability.[24] It is plausible that prolonged seizures result in sufficient superoxide production to overwhelm the endogenous mitochondrial antioxidant defenses by a cascade of events initiated by increased neuronal firing, excessive glutamate release, N-methyl-D-aspartate receptor activation, cytosolic and mitochondrial calcium influx, and increased ATP consumption. Increasing evidences suggest that mitochondrial dysfunction linked with oxidative damage plays a crucial role in neurodegenerative pathologies and therefore mitochondrial scavenging of ROS can be a promising therapeutic approach in PTZ induced convulsions.[6,9] In this study, PTZ significantly depleted the mitochondrial Complexes I, II and IV as compared to naive. It has also been reported that a synergy exists between Hesp and GABAA binding ligands. Further, it has been suggested that flavonoids may be used with advantage in combination with BDZ as they enhanced the modulatory action of DZP at the recombinant GABAA receptors.[25] Consistent with these reports, in this study pretreatment of Hesp (200 mg/kg), DZP (0.5 mg/kg) and Gbp (20 mg/kg) significantly restored the depleted mitochondrial Complexes I, II, and IV activity demonstrating their protective effect. Further, treatment of Hesp (100 mg/kg) in combinations with subtherapeutic doses of DZP (0.2 mg/kg) or Gbp (10 mg/kg) significantly restored the depleted mitochondrial Complexes I, II, and IV activity demonstrating potentiation in their neuroprotective effect against PTZ-induced convulsion in mice.
Taken together, results suggest that neuroprotective effects of Hesp against PTZ-induced convulsions may be possibly related to its antioxidant properties and the synergy with the ligands at the GABAA/BDZ receptors. However, our hypothesis that anticonvulsant activity might be mediated through modulation of GABA/BDZ receptor action would need additional experiments such as role of Hesp on GABA and glutamate levels. Thus, further studies are now being planned to establish the role of Glutamate and GABA levels.
Acknowledgment
The authors are gratefully acknowledge the financial support of UGC (University Grants Commission), New Delhi to carry out the research work.
Footnotes
Source of Support: Authors gratefully acknowledge the financial support of UGC (University Grants Commission), New Delhi to carry out the research work
Conflict Interest: No
References
- 1.de Oliveira PA, Lino FL, Cappelari SE, da Silva Brum LF, Picada JN, Pereira P. Effects of gamma-decanolactone on seizures induced by PTZ-kindling in mice. Exp Brain Res. 2008;187:161–6. doi: 10.1007/s00221-008-1295-y. [DOI] [PubMed] [Google Scholar]
- 2.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303:19–24. doi: 10.1016/s0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 3.Cho J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res. 2006;29:699–706. doi: 10.1007/BF02968255. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SL, Shih PH, Yen GC. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012;60:877–85. doi: 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- 5.Dimpfel W. Different anticonvulsive effects of hesperidin and its aglycone hesperetin on electrical activity in the rat hippocampus in-vitro. J Pharm Pharmacol. 2006;58:375–9. doi: 10.1211/jpp.58.3.0012. [DOI] [PubMed] [Google Scholar]
- 6.Nones J, E Spohr TC, Gomes FC. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem Res. 2011;36:1776–84. doi: 10.1007/s11064-011-0493-3. [DOI] [PubMed] [Google Scholar]
- 7.Rocha L. Subchronic treatment with antiepileptic drugs modifies pentylenetetrazol-induced seizures in mice: Its correlation with benzodiazepine receptor binding. Neuropsychiatr Dis Treat. 2008;4:619–25. doi: 10.2147/ndt.s2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández SP, Wasowski C, Paladini AC, Marder M. Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur J Pharmacol. 2005;512:189–98. doi: 10.1016/j.ejphar.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Gaur V, Aggarwal A, Kumar A. Possible nitric oxide mechanism in the protective effect of hesperidin against ischemic reperfusion cerebral injury in rats. Indian J Exp Biol. 2011;49:609–18. [PubMed] [Google Scholar]
- 10.Akula KK, Dhir A, Kulkarni SK. Effect of various antiepileptic drugs in a pentylenetetrazol-induced seizure model in mice. Methods Find Exp Clin Pharmacol. 2009;31:423–32. doi: 10.1358/mf.2009.31.7.1393610. [DOI] [PubMed] [Google Scholar]
- 11.Celikyurt IK, Mutlu O, Ulak G, Akar FY, Erden F. Gabapentin, A GABA analogue, enhances cognitive performance in mice. Neurosci Lett. 2011;492:124–8. doi: 10.1016/j.neulet.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 12.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Luck H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1971. pp. 885–93. [Google Scholar]
- 16.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 17.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 18.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: Implications for Parkinson's disease. J Neurochem. 1999;73:1127–37. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 19.King TE, Howard RL. Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol. 1967;10:275–84. [Google Scholar]
- 20.King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol. 1967;10:322–31. [Google Scholar]
- 21.Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967;32:415–38. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, et al. Role of oxidative stress in epileptic seizures. Neurochem Int. 2011;59:122–37. doi: 10.1016/j.neuint.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eraković V, Zupan G, Varljen J, Simonić A. Pentylenetetrazol-induced seizures and kindling: Changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem Int. 2003;42:173–8. doi: 10.1016/s0197-0186(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 24.Naziroğlu M, Kutluhan S, Uğuz AC, Celik O, Bal R, Butterworth PJ. Topiramate and vitamin e modulate the electroencephalographic records, brain microsomal and blood antioxidant redox system in pentylentetrazol-induced seizure of rats. J Membr Biol. 2009;229:131–40. doi: 10.1007/s00232-009-9177-1. [DOI] [PubMed] [Google Scholar]
- 25.Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, et al. Overview - Flavonoids: A new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22:419–25. doi: 10.1023/a:1027303609517. [DOI] [PubMed] [Google Scholar]


