Abstract
Levetiracetam is well-tolerated and commonly used as a broad spectrum antiepileptic in both partial and generalized seizures. Few cases of levetiracetam-induced psychosis in children are reported in the literature. The present case of levetiracetam-induced acute psychosis highlights the adverse effect of this drug and also emphasizes the need for close monitoring of children on levetiracetam.
KEY WORDS: Antiepileptic drug, children, levetiracetam, psychosis
Introduction
Levetiracetam is an antiepileptic drug, which has a safe and proven efficacy in complex partial seizures, generalized tonic clonic seizures and myoclonic seizures. It is widely used as add-on therapy in patients with epileptic disorders.[1] Though generally well-tolerated, it may cause some mild adverse reactions.[1] However, psychosis associated with its use in children has rarely been reported.[2,3] We report a case of an 11-year-old girl who developed levetiracetam-induced psychosis.
Case Report
An 11-year-old girl was brought to the outpatient department with the complaints of restlessness and “talking to herself” for 1 day. She described hallucinations of either seeing a ghost or of snakes crawling all over her body. She had a history of generalized tonic convulsions 8 days ago for which she was started on tablet levetiracetam by a private practitioner. The convulsion had lasted for 2 min and she recovered after the convulsion. She had been was advised half tablet (500 mg) levetiracetam twice a day (20 mg/kg/day). However, her mother misunderstood the dose and started with one tablet twice a day (40 mg/kg/day). There was no history of fever, vomiting, headache, abdominal pain, or urinary complaints. There was no history of other drug ingestion, contact with tuberculosis or head injury, or history of psychiatric illness in past or in other family members. There was no preceding stressful event at home or in school. Developmentally, the child was normal. She was studying in 5th grade had good academic performance. Birth and family history were insignificant.
On examination, the child was afebrile and her vitals were normal. There was no icterus, pallor or rash. Central nervous system examination revealed a restless child with uninhibited behavior. She spoke excessively, but speech was not slurred. No other abnormalities were detected on systemic examination. Laboratory investigations, e.g., complete blood count, liver function, renal function tests, urine analysis and serum electrolytes, thyroid function tests and antinuclear antibody test were normal. Magnetic resonance imaging of brain and electroencephalography were also normal. Measurement of drug levels in blood facility is not available in our institution hence drug levels were not measured. Levetiracetam was stopped and oral olanzapine was started. The patient recovered from her psychotic symptoms within 72 h and was discharged. Olanzapine was stopped 10 days after the discharge. This type A class of adverse drug reaction was evaluated for causality and was found to have a probable/likely causal relationship with levetiracetam as per Naranjo algorithm.[4]
Discussion
Levetiracetam, a piracetam analog, is a water-soluble pyrrolidone derivative ((S)-alpha-ethyl-2-oxo-pyrrolidine acetamide) with a novel chemical structure and unique mechanism of action. It exerts its antiepileptic effects by specifically binding to synaptic vesicle protein 2A (a 90-kDa-membrane protein), inhibiting calcium release from intra-neuronal stores, opposing the activity of negative modulators of gamma-amino butyric acid- and glycine-gated currents and inhibiting excessive synchronized activity between neurons.[5,6] It also blocks zinc and beta-carbolines from interrupting chloride influx in the GABA and glycine receptors.[5] And inhibits N-type calcium channels.[6] It is absorbed through gastrointestinal tract with high oral bioavailability and is excreted unchanged through the kidneys. It is not associated with clinically significant pharmacokinetic interactions with other drugs, including other antiepileptic drugs.[6]
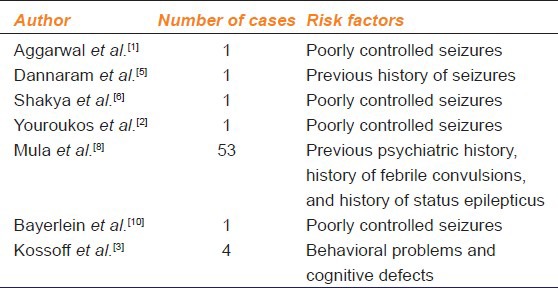
The usual effective dose is between 20 and 60 mg/kg/day. One can start at 20 mg/kg in two doses and increase every 1-2 weeks till 60 mg/kg/day. Though generally well-tolerated, it may cause some adverse reactions such as asthenia, ataxia, diplopia, dizziness, dysarthria, fatigue, headache, light-headedness, nystagmus, paresthesias, somnolence, and tremors. These are usually either dose related or transient. Behavioral effects including agitation, anxiety, depression, emotional lability, hallucinations, and psychosis also observed.[2,5,6] Psychiatric side-effects are seen in up to 13.3% in adults and 37.6% in pediatric patients. Of these, severe symptoms such as depression, agitation, or hostility, and psychotic behavior are observed in 0.7% of patients.[7] Factors like young age, history of febrile convulsions, status epilepticus, previous history of seizure, poorly controlled seizures, previous psychiatric history or cognitive problems, sensory deprivation and rapid increase in the dose of levetiracetam can increase risk of adverse reactions.[1,2,5,8] Lamotrigine co-therapy has been shown to have a protective effect against psychosis.[8] Although, there is evidence that the drug may trigger behavioral disorders, there are reports that it may reduce hyperactivity, impulsivity, mood instability and aggression in autistic children.[9] Levetiracetam-induced psychosis normally occurs about 1 week after the start of treatment. However, it has also been reported after long term treatment.[10] Table 1 documents some reported cases of levetiracetam-induced psychosis. Most of the patients had certain predisposing risk factors. In the present case, young age and high dose of levetiracetam at the onset were the two risk factors predisposing to psychosis.
Table 1.
Reports of levetiracetam-induced psychosis and associated rise factors in literature
Hence, the authors suggest that children prescribed this drug should be monitored particularly with regard to psychiatric adverse effects. Titration of medication over a period of days or weeks rather than administration of full dose since the beginning may be practiced minimizes these adverse effects.
Acknowledgment
We would like to thank the Dean for permitting us to publish this manuscript.
Footnotes
Source of Support: Nil
Conflict Interest: No
References
- 1.Aggarwal A, Sharma DD, Sharma RC, Kumar R. Probable psychosis associated with levetiracetam: A case report. J Neuropsychiatry Clin Neurosci. 2011;23:E19–20. doi: 10.1176/jnp.23.3.jnpe19. [DOI] [PubMed] [Google Scholar]
- 2.Youroukos S, Lazopoulou D, Michelakou D, Karagianni J. Acute psychosis associated with levetiracetam. Epileptic Disord. 2003;5:117–9. [PubMed] [Google Scholar]
- 3.Kossoff EH, Bergey GK, Freeman JM, Vining EP. Levetiracetam psychosis in children with epilepsy. Epilepsia. 2001;42:1611–3. doi: 10.1046/j.1528-1157.2001.32101.x. [DOI] [PubMed] [Google Scholar]
- 4.Zaki SA. Adverse drug reaction and causality assessment scales. Lung India. 2011;28:152–3. doi: 10.4103/0970-2113.80343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannaram S, Borra D, Pulluri M, Jindal P, Sharma A. Levetiracetam-induced acute psychotic episode. Innov Clin Neurosci. 2012;9:10–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Shakya DR, Dutta A, Gautam R. Hallucination in a seizure patient using levetiracetam: A case report. Case Rep Med 2012. 2012 doi: 10.1155/2012/706243. 706243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delanty N, Jones J, Tonner F. Adjunctive levetiracetam in children, adolescents, and adults with primary generalized seizures: Open-label, noncomparative, multicenter, long-term follow-up study. Epilepsia. 2012;53:111–9. doi: 10.1111/j.1528-1167.2011.03300.x. [DOI] [PubMed] [Google Scholar]
- 8.Mula M, Trimble MR, Yuen A, Liu RS, Sander JW. Psychiatric adverse events during levetiracetam therapy. Neurology. 2003;61:704–6. doi: 10.1212/01.wnl.0000078031.32904.0d. [DOI] [PubMed] [Google Scholar]
- 9.Rugino TA, Samsock TC. Levetiracetam in autistic children: An open-label study. J Dev Behav Pediatr. 2002;23:225–30. doi: 10.1097/00004703-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bayerlein K, Frieling H, Beyer B, Kornhuber J, Bleich S. Drug-induced psychosis after long-term treatment with levetiracetam. Can J Psychiatry. 2004;49:868. [PubMed] [Google Scholar]


