Abstract
Background:
Hydatid disease (HD) is an ancient disease and even was known to Hippocrates. This disease involves all human parts and most common affected organs are liver and lungs. Incidence of unusual site is about 8-10%. The clinical picture depends upon the involved organs, its effects on adjacent structures, complications due to secondary infection, rupture, and anaphylaxis caused by hydatid cysts.
Aim:
The aim of this study was to find out incidence of unusual location of hydatid cyst in the human body.
Materials and Methods:
A retrospective study of HD was carried in a medical college between July 2007 and June 2012. A total 79 cases of HD were treated during this period. Information on clinical presentation and management were reviewed, and results presented as summary statistics.
Results:
Sixty one cases were of liver HD, and 11 were with hydatid lung disease. Fifty cases were with right lobe involvement, and rest 11 were with both lobe involvement. Out of 11 lung hydatid only one case was with bilateral lung involvement. Only eight cases of HD of uncommon locations and presentations were encountered during this period. First case presented with left hypochondriac mass as splenic HD, second with pelvic HD along with obstructive uropathy, third with non-functioning right kidney with bilateral psoas muscles HD, fourth with HD involving mesentery, fifth with pelvic pain due to right ovary HD, sixth with simultaneous involvement of the liver and right subdiaphragmatic region, seventh with HD of right inguinal region, and eighth with hydatid cyst of the left kidney. Even though, there was no mortality found in these patients, there was high morbidity.
Conclusion:
We conclude that Echinococcus granulosus can affect any organ in the body from head to toe, and a high suspicion of this disease is justified in endemic regions. Moreover, medical treatment should be given in the pre-operative period as well as in the post-operative period for 4-6 weeks.
Keywords: Hydatid disease, Ovary, Psoas muscle, Renal, Spleen, Unusual locations
Introduction
Hydatid disease (HD) is endemic in the Middle East Africa, South America, New Zealand, Australia, Turkey, and Southern Europe, but foci are common in almost every part of the world including India where the highest prevalence is reported in Andhra Pradesh, Tamil Nadu, and Jammu and Kashmir. Infestation by Echinococcus granulosus in humans most commonly occurs in the liver (55-70%) followed by the lung (18-35%); the two organs can be affected simultaneously in about 5-13% of cases. Incidence of unusual sites is about 8-10%. Incidence of HD involving the spleen, kidney, peritoneal cavity, skin and muscles is about 2% each and incidence of the heart, brain, vertebral column, ovaries, pancreas, gallbladder, thyroid gland, breast, and bones involvement is about 1% each.[1] The clinical picture depends upon the involved organs, its effects on adjacent structures, complications due to secondary infection, rupture and anaphylaxis caused by hydatid cyst. This benign disease can cause substantial morbidity and mortality. The aim of this study is to highlight the fact that this disease should be suspected in cystic lesions involving any organ in the body, especially in endemic areas like India. Even though, hydatid cysts can affect any organ, the disease is uncommon in the organs cited here.
Materials and Methods
A retrospective study was carried out in our medical college during July 2007 to June 2012. A total 79 cases of HD were treated during this period. Information of clinical presentation and management was reviewed. As a retrospective study, approval to use the data was obtained from the relevant authorities, but no formal ethical clearance was sought.
Clinical features, examination, blood counts (eosinophilia), serological test (indirect hemagglutination [IHA] test), ultrasound, and computed tomography (CT) confirmed the diagnosis. All patients were given albendazole in the pre-operative period and then were operated to minimize the chances of post-operative recurrence. Pain was most important presenting symptom. In two cases, presenting symptoms were due to compression on adjacent organs.
Results
Sixty one cases were of liver HD and 11 were of hydatid lung disease. Fifty cases were with right lobe involvement and rest 11 were with both lobe involvement. Out of 11 lung hydatid only one case was with bilateral lung involvement. Only eight cases of hydatid cysts at uncommon locations and presentations were recorded as shown in Table 1. Eosinophilia was present in all eight cases. IHA test (serology) was positive in seven cases out of eight cases. Five cases were of primary HD of uncommon site and in rest three cases there was past history of liver HD.
Table 1.
Location, different form of presentations, eosinophilia, serology, imaging investigations, and management of all the nine cases

Figure 1.

Contrast-enhanced computed tomography showing splenic hydatid cyst
Figure 2.

(a) Contrast-enhanced computed tomography showing pelvic hydatid cyst, (b) Contrast-enhanced computed tomography showing B/L hydronephrosis
Figure 3.

Contrast-enhanced computed tomography showing B/L psoas muscle hydatid disease with right giant hydronephrosis
Figure 4.

(a) Ultrasonography showing big mesenteric hydatid cyst, (b) Histopathology of wall of hydatid cyst
Figure 5.

Ultrasonography showing right ovarian hydatid cyst
Figure 6.

Contrast-enhanced computed tomography showing liver hydatid with left subdiaphragmatic cyst
Figure 7.
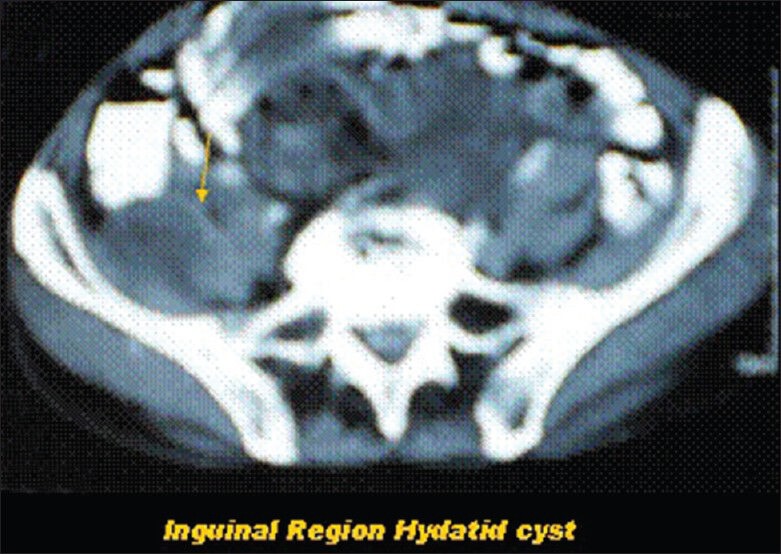
Contrast-enhanced computed tomography showing hydatid cyst of right inguinal region
Figure 8.
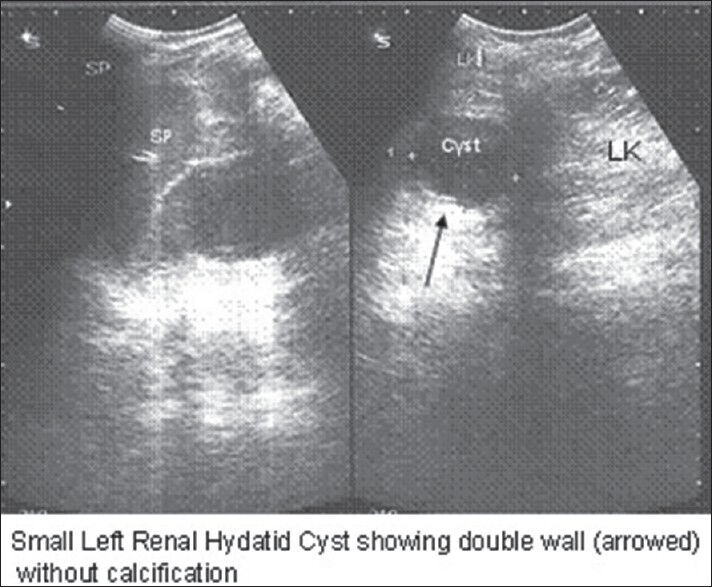
Ultrasonography showing small hydatid cyst of left kidney with typical “rim sign”
Open surgery was done in all cases except in one case in which spleen sparing laparoscopic peri-splenectomy was carried out. Total cystectomy was done in most of the cases except in cases where cyst was adherent with important structure such as ureter, blood vessels or nerves. Marsupialization with omentoplasty was carried out in cases with residual cyst cavity. Nephrectomy was done along with total cystectomy in one case.
All patients were given albendazole 15 mg/kg/day for 4 weeks. In most of the cases, there were no post-operative complications except in one case where there was post-operative pelvic collection which was drained percutaneous (ultrasound guided). There was no mortality and superficial wound infection was present in two cases only. All patients were followed upto 6 months only. There was no incidence of recurrence in any of the above case during this period. A long term follow could not be done due to poverty and long distance as all these patients belonged to low-economical status.
Discussion
Hippocrates was first to illustrate a liver hydatid cyst and pioneered techniques of treatment.[2] More than 80-90% of hydatid cysts occur in the liver, lungs, or both. Hydatid cysts have been reported infrequently in the spleen, kidney, peritoneal cavity, skin and muscles and rarely involve the heart, brain, vertebral column, ovaries, pancreas, gallbladder, thyroid gland, breast, and bones.[1] Clinical presentation of HD depends upon the size, site and depth of the lesion. Eosinophilia is expected in all patients with parasitic infestations this was seen in all our cases. HD is diagnosed mainly by history, examination and by radiological imaging. Imaging tools ultrasonography (USG) can diagnose HD pre-operatively and CT scan can confirm all the cases.[3] Combination of USG and CT scan is most helpful imaging diagnostic tools.[4] On CT their appearance varies: They may show a “spoke wheel” pattern or a water lily sign. When cysts are healed or in an inactive state, they appear as multiple cystic lesions or with calcification in the peritoneum.[5]
Different serological tests are done for the diagnosis, screening, and post-operative follow-up for recurrence. These tests consist of the hydatid immunoelectrophoresis, enzyme-linked immunosorbent assay (ELISA), latex agglutination and IHA test.[6] The ELISA test has a sensitivity of 80-100% and specificity of 88-96% in hepatic cysts, 50-56% sensitivity in lung HD and 25-65% in HD of other organs. But these tests may be negative because the capsule isolates the parasite from the host's immune system. In our study, serology (IHA) was positive in seven out of eight cases.
Pathophysiology of spread of disease
Usually parasites spread via portal blood stream. Other routes of spread may be lymphatic invasion by the parasite, and retrograde migration from the vena cava to the subclavian vein.[6] HD can also involve any organ of abdomen due to hematogenous route or due to peritoneal fluid circulation phenomenon.
Peritoneal cavity has a normal circulation of peritoneal fluid due to various compartments in abdomen. A small amount of peritoneal fluid continuously circulates normally in abdomen. The movement of the diaphragm and peristalsis of bowel regulate the movement of fluid in this circulatory pathway. It mostly flows up in the right paracolic gutter, which is deeper and wider than the left. It is partially cleared by the subphrenic lymphatics. Fluid stays in these watershed regions in the peritoneal cavity: The ileocolic region, the root of the sigmoid mesentery, and the Pouch of Douglas. The spread of HD can be along the areas of peritoneal fluid circulation and may result in spontaneous intraperitoneal seeding.[5]
Incidence of HD involving the spleen is about 2-2.5%. It can occur primarily or in association with hepatic, pulmonary, or multi-organ hydatidosis.[7]
A primary HD of the retroperitoneum is a distinct clinical entity that must be considered when caring for a patient with a retroperitoneal mass in endemic regions. Incidence of peritoneal involvement is about 12%. Clinical features include flank pain, abdominal mass and non-specific symptoms such as nausea and vomiting. It should be treated after the diagnosis is confirmed without any delay because of secondary spillages due to rupture and other possible complications.[8]
Diaphragmatic and subdiaphragmatic localization of hydatid cyst is very rare, with incidence of 1%, and most of these are usually associated with liver disease (as in our case).[9]
Pelvic HD is unusual, and the reported incidence is HD is about 2.25%.[10] Due to its location in a fixed cavity, it presents with pressure effects on adjacent organs such as the urinary bladder (most common) or rectum. Urinary symptoms may be retention of urine, frequency of micturation or as obstructive uropathy and renal failure.[11] We also had a case of primary pelvic hydatid cyst with obstructive uropathy with non-functioning right kidney with acute renal failure.
Location in muscular tissue accounts for 2-3% of all cases. Hydatid cyst located at the right psoas muscle, obstructing the right pelviureteric junction (PUJ) and ureter resulting in giant hydronephrosis is a rare occurrence and only one case has been reported in literature so far.[12] We also present a rare case of bilateral psoas muscles hydatid cyst causing giant hydronephrotic right kidney due to compression of the right PUJ and ureter.
Hydatid cyst of the ovary is quiet uncommon, and the incidence is about 0.2-2.25%.[13]
Renal HD is also rare entity (1-3%) and cysts are usually unilateral and located in the upper or lower pole. 18% of renal hydatid cysts may rupture into the collecting system, leading to acute colic and hydatiduria. However, primary HD of these structures is extremely rare.[14]
Primary soft-tissue involvement by hydatid cyst is unusual even in endemic areas, and the incidence is about 0.5-4.7%. The frequency of osseous involvement in hydatid cyst is 1-2.4%. It is most commonly seen in the spine and pelvis.[15]
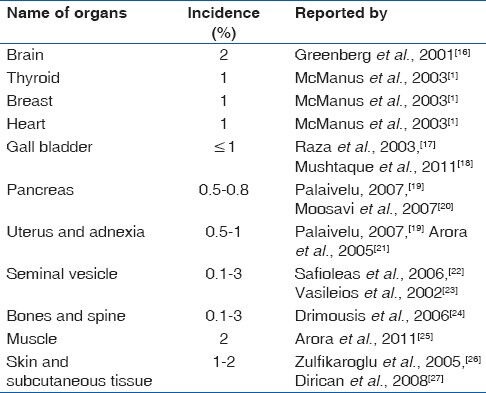
Other uncommon site, which are not encountered by us are listed in Table 2.
Table 2.
Other unusual site not in our study
Management
Treatment of HD is mainly surgical (open as well as laparoscopic). Laparoscopic procedure involves: Aspiration, installation of scolicidal, deroofing, removal of all contents and converting the cyst into a big size non-dependant cavity. However, pre- and post-operative 1-month courses of albendazole and 2 weeks of praziquantel should be considered in order to sterilize the cyst, to decrease the chance of anaphylaxis, to decrease the tension in the cyst wall (thus reducing the risk of spillage during surgery) and to reduce the recurrence rate post-operatively.[6] Intra-operatively, the use of gauge pieces soaked with hypertonic saline or 10% betadine solutions were used to surround the cyst before opening the cavities. These scolicidals kill the daughter cysts and therefore, prevent further spread or anaphylactic reaction.[6]
While surgery still remains as the standard for cystic echinococcosis treatment, there have been a number of studies that suggest that percutaneous drainage of cyst - puncture, aspiration, injection of hypertonic saline and absolute alcohol, reaspiration with chemotherapy is more effective than surgery in terms of disease recurrence, and morbidity and mortality.[28] In the case of alternative medical therapy using chemotherapy alone, albendazole is used with an adult dosage of 400 mg orally, twice a day for 1-5 months and a pediatric dosage of 15 mg/kg/day (maximum of 800 mg) for 1-6 months.[29]
In addition to the above mentioned treatments, percutaneous thermal ablation of the germinal layer in the cyst by means of a radiofrequency ablation device is a recent technique on which studies are being done to develop at it as a new treatment modality. This form of treatment is quiet new and needs much more testing before being widely used.[30]
Even though, mortality directly due to echinococcosis is very low, it can produce a very disabling morbidity. A mortality rate between 0.29% and 0.6% has been reported. The recurrence rate of this disease is still relatively high accounting for about 10%.
Conclusion
We conclude that E. granulosus can affect any organ in the body from head to toe except hair and nails. A high index of suspicion is required for pre-operative diagnosis of HD in unusual locations, and it should be considered in the differential diagnosis for any cystic mass found in patients from endemic areas. Moreover, medical treatment should precede and follow the surgical intervention.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 2.Kouskos E, Chatziantoniou J, Chrissafis I, Anitsakis C, Zamtrakis S. Uncommon locations of hydatid cysts. Singapore Med J. 2007;48:e119–21. [PubMed] [Google Scholar]
- 3.Czermak BV, Unsinn KM, Gotwald T, Niehoff AA, Freund MC, Waldenberger P, et al. Echinococcus granulosus revisited: Radiologic patterns seen in pediatric and adult patients. AJR Am J Roentgenol. 2001;177:1051–6. doi: 10.2214/ajr.177.5.1771051. [DOI] [PubMed] [Google Scholar]
- 4.Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS. Hydatid disease: Radiologic and pathologic features and complications. Radiographics. 2000;20:795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 5.Thambidurai L, Santhosham R, Dev B. Hydatid cyst: Anywhere, everywhere. Radiol Case Rep. 2011;6:486. doi: 10.2484/rcr.v6i3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mushtaque M, Mir MF, Malik AA, Arif SH, Khanday SA, Dar RA. Atypicallocalizations of hydatid disease: Experience from a single institute. Niger J Surg. 2012;18:2–7. doi: 10.4103/1117-6806.95466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murtaza B, Gondal ZI, Mehmood A, Shah SS, Abbasi MH, Tamimy MS, et al. Massive splenic hydatid cyst. J Coll Physicians Surg Pak. 2005;15:568–70. [PubMed] [Google Scholar]
- 8.Aydinli B, Ozturk G, Polat KY, Atamanalp SS, Ozbey I, Onbas O, et al. Extravisceral primary hydatid cyst of the retroperitoneum. ANZ J Surg. 2007;77:455–9. doi: 10.1111/j.1445-2197.2007.04094.x. [DOI] [PubMed] [Google Scholar]
- 9.Isik AF, Sagay S, Ciftci A. Diaphragmatic hydatid disease. Acta Chir Belg. 2006;106:96–7. doi: 10.1080/00015458.2006.11679844. [DOI] [PubMed] [Google Scholar]
- 10.Emir L, Karabulut A, Balci U, Germiyanoðlu C, Erol D. An unusual cause of urinary retention: A primary retrovesical echinococcal cyst. Urology. 2000;56:856. doi: 10.1016/s0090-4295(00)00759-7. [DOI] [PubMed] [Google Scholar]
- 11.Kohli A, Gupta RK, Poptani H, Roy R. In vivo proton magnetic resonance spectroscopy in a case of intracranial hydatid cyst. Neurology. 1995;45:562–4. doi: 10.1212/wnl.45.3.562. [DOI] [PubMed] [Google Scholar]
- 12.Kandhrah E, Semercioz A, Metin A, Eroglu M, Uysal B. Non-functioning kidney resulted from primary hydatid cyst of the psoas muscle. MMJ. 2006;19:145–6. [Google Scholar]
- 13.Tampakoudis P, Assimakopoulos E, Zafrakas M, Tzevelekis P, Kostopoulou E, Bontis J. Pelvic echinococcus mimicking multicystic ovary. Ultrasound Obstet Gynecol. 2003;22:196–8. doi: 10.1002/uog.172. [DOI] [PubMed] [Google Scholar]
- 14.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–94. doi: 10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 15.Kizilkaya E, Silit E, Basekim C, Karsli AF. Hepatic, extrahepatic soft tissue and bone involvement in hydatid disease. Turk J Diagn Interv Radiol. 2002;8:101–4. [Google Scholar]
- 16.Greenberg SM. 1st ed. New York: Thieme Medical Publisher; 2001. Handbook of Neurosurgery; pp. 238–9. [Google Scholar]
- 17.Raza MH, Harris SH, Khan R. Hydatid cyst of gall bladder. Indian J Gastroenterol. 2003;22:67–8. [PubMed] [Google Scholar]
- 18.Mushtaque M, Malik AA, Malik RA. Hydatid cyst of the gall bladder: A rare location. East J Med. 2011;16:83–6. [Google Scholar]
- 19.Palaivelu C. Art of Laparoscopic Surgery-Textbook and Atlas. Dehli: Jaypee Publishers; 2007. Laparoscopic management of hydatid cysts of liver; pp. 757–83. [Google Scholar]
- 20.Moosavi SR, Kermany HK. Epigastric mass due to a hydatid cyst of the pancreas. A case report and review of the literature. JOP. 2007;8:232–4. [PubMed] [Google Scholar]
- 21.Arora M, Gupta CR, Jindal S, Kapoor N. An unusual case of hydatid cyst of broad ligament. J Indian Acad Clin Med. 2005;6:86–7. [Google Scholar]
- 22.Safioleas M, Stamatakos M, Zervas A, Agapitos E. Hydatid disease of the seminal vesicle: A rare presentation of hydatid cyst. Int Urol Nephrol. 2006;38:287–9. doi: 10.1007/s11255-006-6652-9. [DOI] [PubMed] [Google Scholar]
- 23.Vasileios R, Athanasios P, Stavros T. Echinococcal cyst of the seminal vesicles: A case-report and literature review. Int Urol Nephrol. 2002;34:527–30. doi: 10.1023/a:1025662818745. [DOI] [PubMed] [Google Scholar]
- 24.Drimousis PG, Stamou KM, Koutras A, Tsekouras DK, Zografos G. Unusual site of recurrent musculoskeletal hydatid cyst: Case report and brief review of the literature. World J Gastroenterol. 2006;12:5577–8. doi: 10.3748/wjg.v12.i34.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arora V, Nijjar IS, Gill KS, Singh G. Case report: Primary hydatid cyst of muscle – A rare site. Indian J Radiol Imaging. 2006;16:239–41. [Google Scholar]
- 26.Zulfikaroglu B, Koc M, Ozalp N, Ozmen MM. A rare primary location of echinococcal disease: Report of a case. Ups J Med Sci. 2005;110:167–71. doi: 10.3109/2000-1967-078. [DOI] [PubMed] [Google Scholar]
- 27.Dirican A, Unal B, Kayaalp C, Kirimlioglu V. Subcutaneous hydatid cysts occurring in the palm and the thigh: Two case reports. J Med Case Rep. 2008;2:273. doi: 10.1186/1752-1947-2-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–35. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlowski Z, Eckert SJ, Vuitton DA, Ammann RW, Kern P, Craig PS, et al. Echinococcosis in humans: Clinical aspects, diagnosis and treatment. In: Eckert J, Germmell MA, Meslin F.X, Pawlowski ZS, editors. WHO/OIE Manual on Echinococcus in Humans and Animals: A Public Health Problem of Global Concern. Paris, France: World Organisation for Animal Health; 2011. pp. 20–66. [Google Scholar]
- 30.Brunetti E, Filice C. Radiofrequency thermal ablation of echinococcal liver cysts. Lancet. 2001;358:1464. doi: 10.1016/S0140-6736(01)06518-7. [DOI] [PubMed] [Google Scholar]


