In this multinational, phase III study, the safety and efficacy of NEPA, a convenient, fixed-dose antiemetic combination of netupitant, a highly selective NK1 receptor antagonist (RA), and palonosetron, a distinct 5-HT3 RA, were evaluated over multiple cycles of highly and moderately emetogenic chemotherapy. NEPA was shown to be safe, well tolerated and highly effective over 1961 chemotherapy cycles.
Keywords: neurokinin-1 receptor antagonist, NEPA, netupitant, palonosetron, CINV, multiple chemotherapy cycles
Abstract
Background
Safe, effective and convenient antiemetic regimens that preserve benefit over repeated cycles are needed for optimal supportive care during cancer treatment. NEPA, an oral fixed-dose combination of netupitant, a highly selective NK1 receptor antagonist (RA), and palonosetron (PALO), a distinct 5-HT3 RA, was shown to be superior to PALO in preventing chemotherapy-induced nausea and vomiting after a single cycle of highly (HEC) or moderately (MEC) emetogenic chemotherapy in recent trials. This study was designed primarily to assess the safety but also to evaluate the efficacy of NEPA over multiple cycles of HEC and MEC.
Patients and methods
This multinational, double-blind, randomized phase III study (NCT01376297) in 413 chemotherapy-naïve patients evaluated a single oral dose of NEPA (NETU 300 mg + PALO 0.50 mg) given on day 1 with oral dexamethasone (DEX). An oral 3-day aprepitant (APR) regimen + PALO + DEX was included as a control (3:1 NEPA:APR randomization). In HEC, DEX was administered on days 1–4 and in MEC on day 1. Safety was assessed primarily by adverse events (AEs), including cardiac AEs; efficacy by complete response (CR: no emesis, no rescue).
Results
Patients completed 1961 total chemotherapy cycles (76% MEC, 24% HEC) with 75% completing ≥4 cycles. The incidence/type of AEs was comparable for both groups. Most frequent NEPA-related AEs included constipation (3.6%) and headache (1.0%); there was no indication of increasing AEs over multiple cycles. The majority of AEs were mild/moderate and there were no cardiac safety concerns based on AEs and electrocardiograms. The overall (0–120 h) CR rates in cycle 1 were 81% and 76% for NEPA and APR + PALO, respectively, and antiemetic efficacy was maintained over repeated cycles.
Conclusions
NEPA, a convenient single oral dose antiemetic targeting dual pathways, was safe, well tolerated and highly effective over multiple cycles of HEC/MEC.
introduction
Effective, safe and convenient antiemetic regimens that preserve benefit over repeated chemotherapy cycles are essential for optimal supportive care of patients receiving emetogenic chemotherapy. Unfortunately, most antiemetic trials assess chemotherapy-induced nausea and vomiting (CINV) only in a single cycle of treatment [1]. In the few trials evaluating efficacy over multiple cycles, methodologic problems are often found as the number of patients evaluated in subsequent cycles diminishes rapidly in most studies. When few patients remain with continuing treatment cycles, one is unsure of whether or not apparent preserved emetic efficacy is due to patient dropout from lack of control or from side-effects of the antiemetics [1, 2].
The current study was designed to investigate the safety and efficacy of a new antiemetic combination when given in multiple treatment cycles. NEPA is a single oral fixed-dose combination of netupitant (NETU), a new NK1 receptor antagonist (RA) with a long half-life of 90 h, and palonosetron (PALO), a pharmacologically distinct [3] and clinically superior [4] 5-HT3 RA. This combination is being developed to enhance the convenience of administering guideline-based antiemetic prophylaxis targeted at two critical molecular pathways involved in the neuropharmacology of CINV. Large-scale studies with NEPA have just been completed which establish the basis of usage with this all-oral combination [5, 6]. The trials have shown superior CINV prevention and similar safety with NEPA when compared with PALO during a single cycle of either highly (HEC) [5] or moderately emetic chemotherapy (MEC) [6]. In addition, one of the trials confirmed that a 300 mg dose of oral netupitant gave the highest degree of antiemetic activity, without any increase in toxicity, when combined with oral PALO at 0.50 mg [5].
The current phase III study assessed the safety and described the efficacy of NEPA over multiple cycles in patients receiving HEC or MEC. The inclusion of aprepitant (APR) plus PALO as a control was intended to help interpret any unexpected safety finding in the NEPA group.
patients and methods
study design
This was a phase III, multinational, multicenter, randomized, double-blind, double-dummy, parallel group study conducted at 59 enrolling sites in 10 countries (Bulgaria, Czech Republic, Germany, Hungary, India, Poland, Russia, Serbia, Ukraine and the United States) between July 2011 and September 2012. The protocol was approved by ethical review committees for each center, all patients provided written informed consent and all investigators and site personnel followed GCP, ICH E6, Declaration of Helsinki (2008) ethical principles, local laws and regulations.
patients
Eligible patients were ≥18 years, diagnosed with a malignant tumor, naïve to chemotherapy and scheduled to receive repeated consecutive courses of chemotherapy. A single intravenous (i.v.) dose of one or more of the following agents administered on day 1 was necessary for inclusion:
HEC: cisplatin, mechlorethamine, streptozocin, cyclophosphamide ≥1500 mg/m2, carmustine, dacarbazine; MEC: oxaliplatin, carboplatin, epirubicin, idarubicin, ifosfamide, irinotecan, daunorubicin, doxorubicin, cyclophosphamide (<1500 mg/m2), cytarabine (>1 g/m2), azacitidine, alemtuzumab, bendamustine or clofarabine.
Patients with breast cancer scheduled to receive anthracycline–cyclophosphamide (AC) chemotherapy were not eligible. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2. Patients were not eligible if they were scheduled to receive MEC or HEC from day 2 to day 5, a bone marrow transplant or stem cell rescue therapy. They were also ineligible if they had previously received an NK1 RA, or if they had known hypersensitivity or contraindication to 5-HT3 RAs or dexamethasone (DEX).
Exclusions included patients with a history or predisposition to cardiac conduction abnormalities, torsade de point or severe cardiovascular diseases. Since netupitant is a moderate inhibitor of CYP3A4, it was prohibited to use any CYP3A4 inducer within 4 weeks, a strong/moderate inhibitor within 1 week, certain substrates or scheduled to receive CYP3A4 inhibitors/inducers as concomitant medication.
treatment
Patients were randomly allocated (stratified by HEC/MEC and gender) in a 3:1 ratio to receive one of the following treatments:
Oral NEPA (NETU 300 mg + PALO 0.50 mg) + DEX
Oral APR (125 mg day 1, 80 mg days 2–3) + oral PALO 0.50 mg day 1 + DEX
Oral DEX was open label and identical in both groups; the dose/schedule was based on the emetogenicity of the chemotherapeutic regimen and according to antiemetic guidelines (HEC: 12 mg day 1 and 8 mg days 2–4; MEC: 12 mg day 1). NEPA, APR and PALO were administered 60 min and DEX 30 min before chemotherapy on day 1. APR and DEX were administered in the morning on subsequent days. Blinding was maintained in all groups with the use of matching identical placebos.
The 0.50 mg PALO dose was chosen based on an efficacy trial evaluating the noninferiority of three oral PALO doses, 0.25, 0.50 and 0.75 mg, compared with i.v. PALO 0.25 mg [7].
Rescue medication was permitted for treatment of established, refractory or persistent nausea and vomiting; however, its use was considered treatment failure. Metoclopramide tablets were provided, although the investigator was allowed the discretion to use alternative rescue medications (excluding 5-HT3 or NK1 RAs). There was no prespecified limit to the number of consecutive chemotherapy cycles for patients.
assessments
Safety was assessed primarily by treatment-emergent adverse events (TEAEs: defined as those occurring after the first dose of study drug), but also by clinical laboratory evaluations, physical examinations and vital signs. Cardiac safety was assessed by 12-lead electrocardiograms (ECGs) (screening, predose, 5, 24 and 120 h post-dose each cycle), cardiac troponin (cTnl) levels (screening, 24 and 120 h post-dose each cycle) and left ventricular ejection fraction (LVEF) (screening and between day 14 and 21 of last cycle). The ECGs were digitally recorded and read by a cardiologist blind to study treatment, cTnl levels were analyzed by a central laboratory as exact values, and LVEF was assessed using either the multiple gated acquisition scan or echocardiography, but the same tool at both times for each patient.
To evaluate the efficacy of NEPA, each patient completed a diary from the start of chemotherapy on day 1 through the morning of day 6 (0–120 h) of each cycle, documenting the time of onset and duration of each emetic episode, severity of nausea and rescue medications taken. An emetic episode was defined as a single vomiting episode, a single retching or any retching combined with vomiting. Severity of nausea was evaluated on a daily basis (for the preceding 24 h) using a 100-mm horizontal visual analog scale (VAS). The left end (0 mm) was labeled as ‘no nausea,’ and the right end (100 mm) was labeled as ‘nausea as bad as it could be’. Efficacy endpoints were complete response (CR: no emesis, no rescue medication) and no significant nausea (VAS score of <25 mm) during the acute (0–24 h), delayed (25–120 h) and overall (0–120 h) phase after the start of chemotherapy.
statistical analysis
The goal of this multiple cycle registration trial was to characterize the safety profile of NEPA over a duration of at least six cycles. It was expected that 300 patients treated with NEPA during cycle 1 would allow more than 100 patients to be treated for six cycles. If a given AE was not observed in 100 NEPA-treated patients, an incidence of ≥3% could be excluded with 95% confidence. As the study's focus was on the safety of NEPA, the APR + PALO group provided context although no formal comparison was planned.
PALO was selected to be used in conjunction with APR to standardize the 5-HT3 RA component of the regimens and decrease the variability among the treatments. Four hundred patients were to be randomized at a 3:1 ratio; 300 (225 MEC, 75 HEC) to NEPA and 100 (75 MEC, 25 HEC) to APR + PALO.
The safety analysis population consisted of all patients who received study treatment and had at least one safety assessment after treatment administration. The full analysis set (FAS) population used for the efficacy analyses was defined as all patients randomized who received the MEC/HEC regimen and study treatment.
The number of patients who experienced TEAEs was listed and summarized by treatment arm. AEs were considered treatment-related if the relationship to study drug was assessed as definite, probable, possible, unassessable or missing.
Observed values, changes from baseline and outliers were summarized for ECGs. A potential safety signal included a change from baseline in the heart-rate corrected QT interval by the Fredericia formula (QTcF) to >500 ms in more than 5% of patients or to >60 ms in more than 15% of patients. CTnls and LVEF values were summarized and changes from baseline calculated; a threshold of 0.12 ng/ml was considered an ‘alert value’.
Only descriptive statistics were provided for safety assessments and no formal testing was done for between group comparisons. The proportion (including 95% confidence intervals) of patients with CR and with no significant nausea was summarized for each cycle by treatment and by treatment and chemotherapy emetogenicity.
results
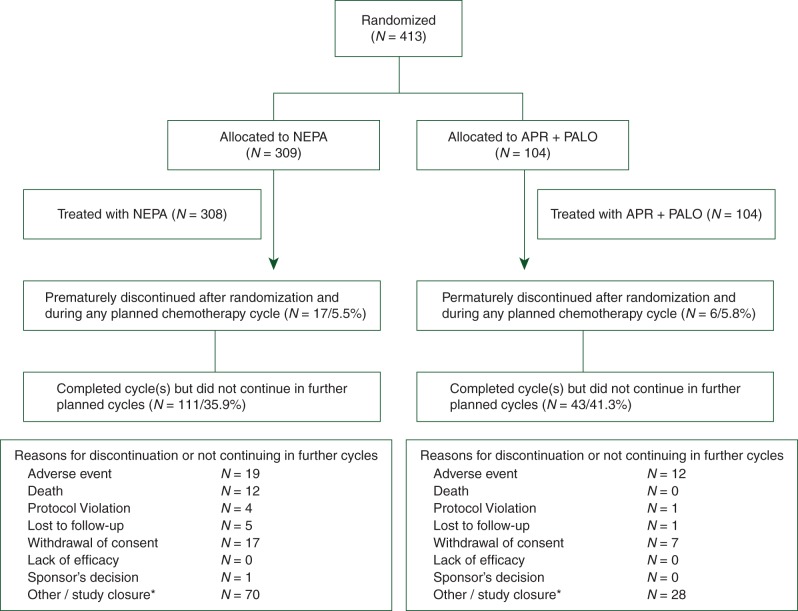
A total of 413 patients were randomized (Figure 1); 412 patients were treated for a total of 1961 cycles (1446 NEPA, 515 APR + PALO); 98% of patients completed cycle 1, 75% completed at least four cycles and 40% completed six cycles.
Figure 1.
Consort diagram of the disposition of patients. One patient randomized/allocated to NEPA received APR + PALO throughout the study. Following the intent-to-treat principle, this patient was analyzed in the NEPA group for the full analysis set (FAS; N = 309) and the APR + PALO group for the safety population (N = 104). One patient randomized/allocated to APR + PALO did not receive any treatment and was therefore excluded from both the FAS (N = 103) and safety population. *The vast majority discontinued due to the study closure which occurred when the last patient enrolled had completed his/her final chemotherapy cycle; these patients were allowed to complete their current cycle but not enter any further cycles.
The patients' baseline and disease characteristics are reported in Table 1; these remained consistent across cycles. The study was not designed to control for type of cancer in each randomization arm. It should be noted that poorer cancer prognostic variables were found in the NEPA-treated patients with a greater proportion with lung/respiratory tract cancers (40% versus 31%), with metastatic cancer (52% versus 43%), and with concomitant disease (78% versus 62%) when compared with those patients who were randomly assigned to the APR + PALO study arm.
Table 1.
Patient baseline and disease characteristics
| Characteristic | NEPA (N = 308) | APR + PALO (N = 104) | Overall (N = 412) |
|---|---|---|---|
| Gender | |||
| Male (%) | 49.7 | 51.0 | 50.0 |
| Female (%) | 50.3 | 49.0 | 50.0 |
| Median age (years) | 57.0 | 58.5 | 58.0 |
| Cancer type (%) | |||
| Lung/respiratory | 39.6 | 30.8 | 37.4 |
| Othera | 23.4 | 15.4 | 21.4 |
| Ovarian | 10.7 | 17.3 | 12.4 |
| Colon | 7.8 | 12.5 | 9.0 |
| Head and neck | 6.5 | 10.6 | 7.5 |
| Colorectal | 5.5 | 4.8 | 5.3 |
| Rectal | 2.9 | 4.8 | 3.4 |
| Gastric | 2.3 | 1.0 | 1.9 |
| Bladder | 1.3 | 2.9 | 1.7 |
| Extent of cancer at entry (%) | |||
| Primary | 43.8 | 51.9 | 45.9 |
| Metastatic | 51.9 | 43.3 | 49.8 |
| Local recurrence | 4.2 | 4.8 | 4.4 |
| Site of metastasis (%) | |||
| Lymph nodes | 33.1 | 21.2 | 30.1 |
| Other | 15.6 | 19.2 | 16.5 |
| Lung | 14.0 | 13.5 | 13.8 |
| Liver | 12.0 | 12.5 | 12.1 |
| Bone | 5.8 | 4.8 | 5.6 |
| Brain | 1.6 | 2.9 | 1.9 |
| ECOG Performance Status (%) | |||
| 0 | 47.4 | 48.1 | 47.6 |
| 1 | 51.0 | 50.0 | 50.7 |
| 2 | 1.6 | 1.9 | 1.7 |
| Chemotherapyb (%) | |||
| MECc | 75.7 | 75.7 | 75.7 |
| Carboplatin | 60.3 | 61.5 | 60.6 |
| Oxaliplatin | 20.1 | 24.4 | 21.2 |
| Doxorubicin | 11.1 | 6.4 | 9.9 |
| Cyclophosphamide | 3.4 | 2.6 | 3.2 |
| Irinotecan | 3.0 | 3.8 | 3.2 |
| Epirubicin | 1.7 | 1.3 | 1.6 |
| Daunorubicin | 0.4 | 0 | 0.3 |
| HECc | 24.3 | 24.3 | 24.3 |
| Cisplatin | 96.0 | 92.0 | 95.0 |
| Dacarbazine | 4.0 | 4.0 | 4.0 |
| Carmustine | 0 | 4.0 | 1.0 |
aOther as a category included any other type of cancer not listed in the prespecified categories, including, but not limited to those of the uterus, breast, larynx and endometrium.
bBased on efficacy (full analysis set) population, while all others based on safety population.
cCycle 1 chemotherapy.
safety
The overall incidence, type and frequency of TEAEs were comparable for both treatment groups throughout the study (Table 2). Similar proportions of patients reported AEs regardless of chemotherapy emetogenicity or gender. There were no notable differences between the groups in overall percentages of patients who experienced severe or serious AEs or events leading to discontinuation.
Table 2.
Overview of adverse events
| Type of adverse event number of patients (%) |
NEPA (N = 308) |
APR + PALO (N = 104) |
NEPA (N = 308) |
APR + PALO (N = 104) |
|---|---|---|---|---|
|
Cycle 1 |
Entire multiple cycle study perioda |
|||
| Any ‘treatment-emergent’ adverse event | 199 (64.6%) | 64 (61.5%) | 265 (86.0%) | 95 (91.3%) |
| ‘Treatment-related’ adverse event | 16 (5.2%) | 3 (2.9%) | 31 (10.1%) | 6 (5.8%) |
| ‘Severe’ treatment-related adverse event | 1 (0.3%) | 0 | 1 (0.3%) | 0 |
| ‘Serious’ treatment-related adverse event | 1 (0.3%) | 0 | 2 (0.6%) | 0 |
| Treatment-related adverse event ‘leading to discontinuation’ | 1 (0.3%) | 0 | 1 (0.3%) | 0 |
| ‘Total deaths’ (all unrelated to study drug) | 7 (2.3%) | 0 | 16 (5.2%) | 1 (1.0%) |
Treatment-emergent adverse event: any AE reported after first study drug intake.
Treatment-related adverse event: AEs deemed definitely, probably or possibly related to study drug.
Safety population.
aIncludes cycle 1 through all cycles.
The majority of TEAEs reported were of mild/moderate intensity; 25.0% and 32.7% of patients experienced severe TEAEs with NEPA and APR + PALO, respectively. The most commonly reported severe TEAEs were neutropenia (11.7% NEPA, 10.6% APR + PALO) and leukopenia (4.5% NEPA, 4.8% APR + PALO). Only one AE was considered severe and possibly antiemetic treatment-related and was the only one leading to discontinuation (acute psychosis in a NEPA patient in cycle 1; also considered to be possibly related to DEX).
There was no indication of increasing AEs over multiple cycles (Table 3). The proportion of patients with AEs considered study drug-related was relatively low in both treatment groups (10.1% NEPA, 5.8% APR + PALO). The most frequent treatment-related AEs for NEPA included constipation (3.6%) and headache (1.0%) (Table 4).
Table 3.
Patients with any treatment-related adverse events across cycles
| % of patients with adverse event |
Treatment-emergent adverse event |
Treatment-related adverse event |
||
|---|---|---|---|---|
| Cycle (N = NEPA/APR) | NEPA (N = 308) | APR + PALO (N = 104) | NEPA (N = 308) | APR + PALO (N = 104) |
| Cycle 1 (N = 308/104) | 64.6% | 61.5% | 5.2% | 2.9% |
| Cycle 2 (N = 279/96) | 54.8% | 57.3% | 4.3% | 1.0% |
| Cycle 3 (N = 258/91) | 50.4% | 58.2% | 1.9% | 2.2% |
| Cycle 4 (N = 232/81) | 43.5% | 48.1% | 1.3% | 1.2% |
| Cycle 5 (N = 156/57) | 45.5% | 57.9% | 0.6% | 1.8% |
| Cycle 6 (N = 124/43) | 34.7% | 32.6% | 1.6% | 0.0% |
Safety population.
Table 4.
Summary of most common treatment-related adverse events
| Adverse event |
NEPA (N = 308) |
APR + PALO (N = 104) |
NEPA (N = 308) |
APR + PALO (N = 104) |
|---|---|---|---|---|
|
Cycle 1 |
Entire Multiple Cycle Study Perioda |
|||
| Constipation | 7 (2.3%) | 0 | 11 (3.6%) | 1 (1.0%) |
| Dyspepsia | 0 | 1 (1.0%) | 1 (0.3%) | 1 (1.0%) |
| Eructation | 1 (0.3%) | 1 (1.0%) | 1 (0.3%) | 1 (1.0%) |
| Headache | 3 (1.0%) | 1 (1.0%) | 3 (1.0%) | 1 (1.0%) |
aIncludes cycle 1 through all cycles; Safety population.
A similar proportion of patients experienced serious TEAEs (N = 50/16.2% NEPA, N = 19/18.3% APR + PALO); two of these in the NEPA group (0.6%) were deemed treatment-related (probably related ventricular systoles in cycle 6 and acute psychosis mentioned above). Seventeen patients (4.1%) died during the study or the follow-up period with 5.2% in the NEPA assigned treatment arm and 1.0% in the APR + PALO arm. The most common cause of death was disease progression; none of these events was attributed to study drug. As noted above, several poorer disease prognostic characteristics differences, including proportion with metastatic or concomitant disease, occurred according to randomized treatment arm assignment.
cardiac safety
Changes from baseline in 12-lead ECGs at 5 and 24 h after dose were similar between the groups, with any corrected QT (QTc) interval prolongation being transient and returning to predose measurements within 120 h after dose across all cycles. The percentage of patients with a >60 ms increase in QTcF was low in both groups [1 NEPA patient (0.3%) in each of cycles 1, 3, 4 and 5; 1 APR + PALO patient (1.0%) in each of cycles 1 and 4]. A >500 ms change in QTcF was observed for a NEPA patient in cycles 1, 3 and 4 and an APR + PALO patient in cycle 6. The percentage of patients who developed ECG abnormalities was comparable for the treatment groups throughout the study. The most frequently reported abnormalities were flat T waves (16.9% and 13.5% NEPA and APR + PALO, respectively) and ST depression (11.7% and 16.3%, respectively). Only two (0.6%) NEPA-treated and one (1.0%) APR-treated patients experienced abnormal U waves.
Throughout the study, there were seven (2.3%) NEPA-treated patients and three (2.9%) APR-treated patients with high postdose troponin values. Mean LVEF was comparable at screening and at the end of the study with small changes in both groups.
efficacy
Antiemetic efficacy (CR rates) during the overall phase across six cycles of chemotherapy is summarized in Figure 2. Overall CR rates were high and were maintained across cycles for both treatment groups, with NEPA showing a small but consistent numerical advantage (2%–7%) over APR + PALO during each cycle. CR rates were similar between the two antiemetic treatment groups in the acute phase while rates during the delayed phase of each cycle mimicked the overall phase with differences ranging from 2% to 6%.
Figure 2.
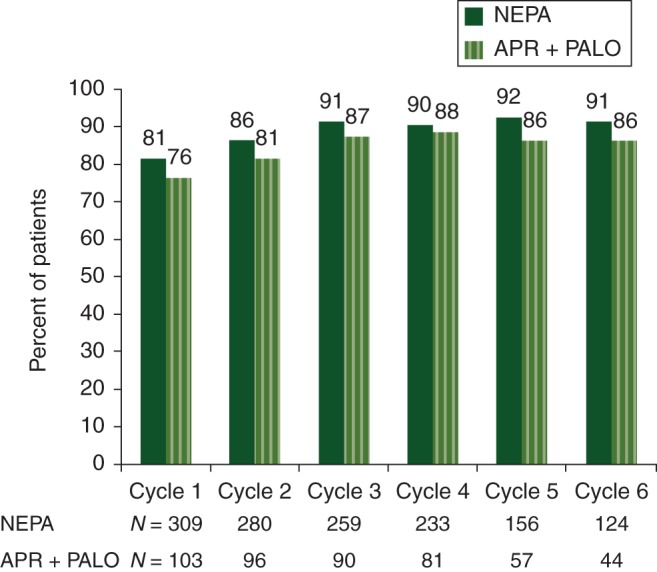
Primary analysis: complete response (no emesis, no rescue medication) (overall 0–120 h). Full analysis population.
The proportions of patients with no significant nausea were high in both treatment groups, with overall rates ranging from 84% to 92% across cycles for NEPA and from 81% to 87% for APR + PALO. A similar numerical advantage over APR + PALO was shown in the delayed phase.
In the NEPA group, the percentage of patients with an overall CR across cycles was similar for the patients receiving HEC (79%–91%) and MEC (80%–93%). Observed CR rates were modestly lower in the HEC (58%–86%) subgroup when compared with the MEC (82%–89%) subgroup for APR + PALO. Overall CR rates in the NEPA group were generally similar for males and females across cycles.
discussion
This study including 413 patients receiving 1961 chemotherapy cycles offers compelling evidence of the continuous safety of NEPA and support for its efficacy in patients receiving multiple cycles of either MEC or HEC.
In general, the AE profile seen is typical for a diverse cancer population receiving chemotherapy. As demonstrated in the single cycle trials [5, 6], NEPA was well tolerated with a comparable and low AE profile, as was seen with APR + PALO. The majority of AEs were mild/moderate in intensity and only two patients experienced serious treatment-related AEs. While the proportion of patients reporting treatment-related AEs was greater in the NEPA group compared with APR + PALO, the incidence was relatively low in both groups (10.1% versus 5.8%), with constipation being the only AE exceeding 1% (3.6% versus 1.0%). Reassuringly, there was no indication of increasing AEs, whether treatment-related or not, over multiple cycles.
None of the deaths occurring in either treatment arm in this trial, largely in patients with advanced stages of cancer, was attributed to or related to study drug. The difference between groups in number of deaths is potentially due to the combination of a greater number of patients randomly assigned to the NEPA treatment arm together with poorer prognostic variables (lung/respiratory cancer, metastatic disease and concomitant disease) compared with APR-treated patients.
There were no cardiac safety concerns for NEPA based on cardiac AEs and ECGs. This was supported by the very low proportions of patients with a change in QTcF of >60 ms or to a value >500 ms, combined with the low proportion of patients with abnormal U waves.
These efficacy results provide evidence of the benefits of the preservation of excellent emetic control over multiple chemotherapy cycles with the new antiemetic combination, NEPA. Not only were the CR rates high, exceeding 90% in the acute phase and 80% in the delayed/overall phases in the first cycle, but these rates for NEPA were maintained throughout six cycles. Having over 75% of patients in the efficacy evaluation after four cycles provides confidence in the preservation of antiemetic effect over multiple cycles. As in all trials, the control rates for nausea were less than the rates for vomiting; however, the reported control of nausea was high as well. Although the differences were small, the numerically better control in the NEPA group compared with APR + PALO is important in the development of a new antiemetic agent, demonstrating that there is no suggestion of loss of efficacy in exchange for a regimen of potentially greater convenience. Further studies should be carried out to determine whether the highly convenient concept of NEPA will result in greater adherence, fostering improved emetic control.
In conclusion, NEPA, a convenient single-dose oral antiemetic targeting two critical antiemetic pathways, was safe, well tolerated and highly effective over multiple cycles of HEC or MEC. NEPA offers an opportunity to provide effective antiemetic care which is consistent with guideline recommendations in a maximally convenient combination.
funding
This work was supported by Helsinn Healthcare, SA who provided the study drugs and the funding for this study.
disclosure
The authors have the following conflicts of interest to disclose: RG: advisor for Merck, Helsinn Healthcare and Eisai. KJ: speakers bureau for Merck and Helsinn Healthcare. GR, GR and MEB: employees of Helsinn Healthcare. All remaining authors have declared no conflicts of interest.
acknowledgements
The authors express our sincere appreciation to the late Steven Grunberg, our esteemed colleague whose contributions to supportive care and to this study were of great significance. They thank the clinical investigators, patients and site personnel who participated in the study. They acknowledge the editorial support of Jennifer Vanden Burgt during the writing of this manuscript, Silvia Olivari, Silvia Sebastiani and Marco Palmas from Helsinn Healthcare SA and Norman Nagl from Eisai, Inc., for critically reviewing the manuscript, and the NEPA Publication Steering Committee (Paul Hesketh, Richard Gralla, Matti Aapro, Karin Jordan, and Steven Grunberg) for their leadership and guidance.
references
- 1.De Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol. 2003;22:4105–4111. doi: 10.1200/JCO.2003.10.128. [DOI] [PubMed] [Google Scholar]
- 2.De Wit R, Schmitz PIM, Verweij J, et al. Analysis of cumulative probabilities shows that the efficacy of 5-HT3 antagonist prophylaxis is not maintained. J Clin Oncol. 1996;14:644–651. doi: 10.1200/JCO.1996.14.2.644. [DOI] [PubMed] [Google Scholar]
- 3.Rojas C, Slusher BS. Pharmacological mechanism of 5-HT3 and tachykinin NK-1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2012;684(1–3):1–7. doi: 10.1016/j.ejphar.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol. 2011;22(1):30–38. doi: 10.1093/annonc/mdq600. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh P, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340–1346. doi: 10.1093/annonc/mdu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aapro M, Rugo H, Rossi G, et al. A randomized phase 3 study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boccia R, Grunberg S, Franco-Gonzales E, et al. Efficacy of oral palonosetron compared to intravenous palonosetron for prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trial. Support Care Cancer. 2013;21(5):1453–1460. doi: 10.1007/s00520-012-1691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]