We report significantly improved survival for patients with human papillomavirus (HPV)-positive compared with HPV-negative recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN) in a retrospective analysis of two Eastern Cooperative Oncology Group (ECOG) trials. These findings demonstrate that tumor HPV status, which is known to be of prognostic significance in locally advanced SCCHN, remains a strong prognostic factor in the setting of recurrent/metastatic disease.
Keywords: human papillomavirus, head and neck cancer
Abstract
Background
The purpose of this article was to study the association of human papillomavirus (HPV) with clinical outcomes in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN).
Patients and methods
Archival baseline tumor specimens were obtained from patients treated on two clinical trials in recurrent or metastatic SCCHN: E1395, a phase III trial of cisplatin and paclitaxel versus cisplatin and 5-fluorouracil, and E3301, a phase II trial of irinotecan and docetaxel. HPV DNA was detected by in situ hybridization (ISH) with a wide-spectrum probe. p16 status was evaluated by immunohistochemistry. Clinical outcomes of interest were objective response, progression-free survival (PFS) and overall survival (OS).
Results
We analyzed 64 patients for HPV ISH and 65 for p16. Eleven tumors (17%) were HPV+, 12 (18%) were p16+, whereas 52 (80%) were both HPV− and p16−. The objective response rate was 55% for HPV-positive versus 19% for HPV-negative (P = 0.022), and 50% for p16-positive versus 19% for p16-negative (P = 0.057). The median survival was 12.9 versus 6.7 months for HPV-positive versus HPV-negative patients (P = 0.014), and 11.9 versus 6.7 months for p16-positive versus p16-negative patients (P = 0.027). After adjusting for other covariates, hazard ratio for OS was 2.69 (P = 0.048) and 2.17 (P = 0.10), favoring HPV-positive and p16-positive patients, respectively. The other unfavorable risk factor for OS was loss of ≥5% weight in previous 6 months (P = 0.0021 and 0.023 for HPV and p16 models, respectively).
Conclusion
HPV is a favorable prognostic factor in recurrent or metastatic SCCHN that should be considered in the design of clinical trials in this setting.
Clinical Trial Identifier
NCT01487733 Clinicaltrials.gov.
introduction
Squamous cell carcinoma of the head and neck (SCCHN) affects ∼550 000 patients worldwide annually [1]. An increasing subset of SCCHN, in particular oropharyngeal cancers, are associated with human papillomavirus (HPV) infection, especially among younger patients who do not have a significant history of tobacco and alcohol use [2]. HPV status may be evaluated in archival tumor specimens by in situ hybridization (ISH) [2]. High concordance between HPV status as assessed by ISH using wide-spectrum probes and HPV16 E7 PCR and their agreement with high levels of HPV16 sequence reads has been previously reported by our group [3]. Positive tumor p16 immunohistochemical (IHC) staining has been identified as a viable surrogate for functional HPV infection for tumors arising in the oropharynx [4–6]. HPV has emerged as an important prognostic factor in locally advanced SCCHN [7]. Several analyses of prospective clinical trials, including cooperative group trials E2399 and RTOG 0129, have consistently demonstrated a survival benefit for patients with HPV-positive versus HPV-negative oropharyngeal cancers treated with chemoradiotherapy [8–11] or surgery with or without postoperative radiotherapy for locally advanced SCCHN [12]. The prognostic significance of HPV in the recurrent/metastatic disease setting remains unknown.
In order to learn more about the potential prognostic role of p16 and HPV status among patients who underwent treatment for recurrent/metastatic SCCHN, we studied tumors obtained from patients who were treated on recent Eastern Cooperative Oncology Group (ECOG) trials of first-line chemotherapy in recurrent/metastatic SCCHN.
patients and methods
patient selection
We selected patients from two ECOG clinical trials of first-line treatment in recurrent/metastatic SCCHN. ECOG protocol 1395 (E1395) was a randomized phase III trial that compared the combination of paclitaxel and cisplatin with a standard cisplatin and 5-fluorouracil (5-FU) regimen [13]. A total of 204 patients were analyzed on this study (NCT01487733 Clinicaltrials.gov), which showed no statistically significant difference in response rates or survival between the two regimens. The response rate was 29.8% versus 26.0% and the median overall survival (OS) was 8.1 versus 8.7 months in cisplatin/paclitaxel versus cisplatin/5-FU. Tumor samples from 124 patients on E1395 were accessible in the ECOG tumor repository.
E3301 was a phase II trial of docetaxel and irinotecan in recurrent/metastatic SCCHN that was recently reported [14]. Patients received docetaxel 35 mg/m2 and irinotecan 60 mg/m2, intravenously, on days 1 and 8, every 21 days, until disease progression. Fifty-two patients were analyzable, 20 chemotherapy naive (Group A) and 32 previously treated with one chemotherapy regimen (Group B). In Group A, three (15%) patients achieved a partial response; in Group B, one (3%) patient achieved a partial response. The median progression-free survival (PFS) and OS were 3.3 and 8.2 months in Group A and 1.9 and 5.0 months in Group B, respectively. Thirty-one patients in this study had baseline tumor available for analysis.
HPV and p16 in tumor tissue
For p16INK4 (p16) immunohistochemical (IHC) staining, 5 μm sections were de-paraffinized, antigen retrieval was carried out using heat-induced epitope retrieval with 10 mM citrate buffer and tissue sections were incubated with a mouse monoclonal antibody against p16 (MTM Laboratories, Westborough, MS) at a 1:500 dilution. The p16 antibody was visualized using the avidin–biotin-peroxidase technique (LSAB* Kit, DAKO Carpenteria, CA). Staining was considered positive if a strong and diffuse staining of more than 80% of tumor cells was present and scored as negative if absent or focal.
Tumor HPV status (presence versus absence) was determined by ISH as previously reported [3, 15, 16]. HPV DNA was detected in tumors by use of the ISH catalyzed signal amplification method for biotinylated probes (Dako, GenPoint). Briefly, tissue sections underwent deparaffinization, heat-induced target retrieval and digestion with Proteinase K (Roche Diagnostics, Indianapolis, IN). Slides were hybridized to a biotinylated, wide-spectrum HPV probe that targets HPV types 6, 11, 16, 18, 30, 31, 33, 35, 45, 51 and 52 (Code Y1404, DAKO). One positive control slide, a known HPV + tumor probed with the wide-spectrum HPV probe, and one negative control slide, the same HPV+ control tissue probed with non-specific DNA probe, were included in each series of hybridization and processing reactions. After low and high stringent wash, DAKO TSA System Kit (K0620) was used for signal amplification. Slides were scored as positive for HPV ISH + if a punctate signal specific to tumor cell nuclei was present. An H&E-stained slide was concurrently reviewed to confirm the presence of the tumor in the specimen.
statistical methods
The primary end point was objective response. OS and PFS were also explored as secondary end points. Patients were classified into two categories based on their biomarker status: HPV ISH tumor status (negative versus positive) and p16 expression (negative versus positive). Fisher's exact test was used to compare categorical patient characteristics between groups and to examine the association between the dichotomized biomarkers and treatment response. The Kaplan–Meier estimates of the survival end points according to biomarker status were calculated. The log-rank test was used to examine the association between the biomarkers and the survival end points. Further, logistic regression and Cox's proportional hazards models stratified by primary tumor status and ECOG performance status (stratification factors in E1395) were employed to assess the association of the biomarkers with clinical end points after adjusting for other covariates, i.e. treatment (cisplatin/5-FU versus cisplatin/paclitaxel versus docetaxel/irinotecan), primary site (oropharynx versus larynx versus other), weight loss in previous 6 months (≥5% versus <5%), prior radiotherapy (yes versus no) and cell differentiation (well/moderately differentiated versus poorly differentiated versus other) [14]. Due to the small sample size of the study, these analyses were considered exploratory.
results
Tissue was evaluable from 64 patients for HPV detection by ISH, and from 65 patients for p16 analysis (Figure 1). The concordance between p16 and HPV ISH results was high; of 65 patients with available p16 data, 52 were both HPV ISH and p16 negative (80%), 11 were both HPV ISH and p16 positive (17%) and only one oropharynx tumor was p16+ yet negative for the high-risk HPV strains tested with the broad-spectrum probe we employed; one p16− case did not have tissue available for HPV analysis. The proportion of larynx cancer was significantly lower in HPV ISH+/p16+ patients compared with HPV ISH−/p16− patients (0% versus 38%, P = 0.02), higher proportion of HPV ISH−/p16− patients received prior radiotherapy (92% versus ∼65%, P < 0.04), and HPV ISH+ patients were more likely to have poorly differentiated carcinoma (45% versus 19%, P = 0.075) (Table 1). To account for the imbalances, these three variables were adjusted for in the regression models in addition to primary tumor status, weight loss in previous 6 months, treatment regimen and cell differentiation. More detailed information of HPV ISH/p16 status by primary tumor site is given in supplementary Table S1, available at Annals of Oncology online. Overall, HPV ISH and p16 positivity were found mainly in oropharyngeal and hypopharyngeal primaries. However, the majority of oropharyngeal tumors were both HPV ISH and p16 negative. To evaluate whether the patient subset included in this analysis was representative of the whole patient population enrolled on the trials, baseline characteristics were examined between the selected subset and the patients enrolled on E1395 and E3301 but not included in analysis. Most baseline characteristics were comparable except that the proportion of female was higher in the selected subset (P = 0.041, supplementary Table S2, available at Annals of Oncology online).
Figure 1.
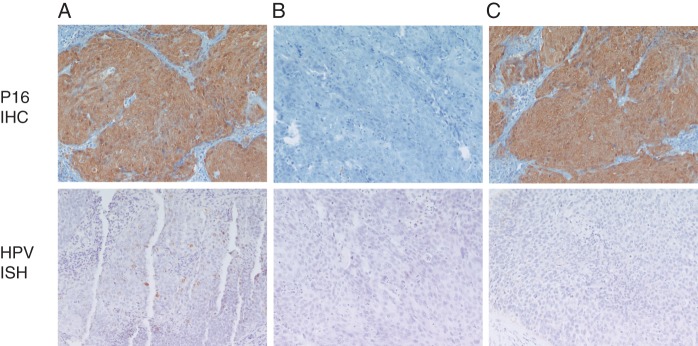
Representative images of p16 immunohistochemistry and human papillomavirus in situ hybridization results. (A) p16+/HPV+, (B) p16−/HPV− and (C) p16+, HPV− (one case).
Table 1.
Patient characteristics for samples tested for p16 and human papillomavirus
| HPV |
p16 |
|||||
|---|---|---|---|---|---|---|
| Negative (n = 53) | Positive (n = 11) | P-value | Negative (n = 53) | Positive (n = 12) | P-value | |
| Age, median (range) | 65 (43, 85) | 59 (47, 80) | 0.61 | 64 (43, 85) | 61.5 (47, 80) | 0.97 |
| Treatment | ||||||
| E1395: cisplatin + 5-FU | 11 (21%) | 4 (36%) | 0.44 | 11 (21%) | 4 (33%) | 0.70 |
| E1395: paclitaxel + cisplatin | 17 (32%) | 2 (18%) | 16 (30%) | 3 (25%) | ||
| E3301: docetaxel + irinotecan | 25 (47%) | 5 (45%) | 26 (49%) | 5 (42%) | ||
| Sex | ||||||
| Male | 37 (70%) | 9 (82%) | 0.71 | 38 (72%) | 9 (75%) | 1.00 |
| Female | 17 (30%) | 2 (18%) | 15 (28%) | 3 (25%) | ||
| PS | ||||||
| 1 | 38 (72%) | 9 (82%) | 0.71 | 38 (72%) | 10 (83%) | 0.49 |
| 0 | 15 (28%) | 2 (18%) | 15 (28%) | 2 (17%) | ||
| Primary tumor status | ||||||
| Eradicated | 12 (23%) | 2 (18%) | 0.23 | 12 (23%) | 2 (17%) | 0.29 |
| Eradicated but recurred locally | 28 (53%) | 5 (45%) | 28 (53%) | 6 (50%) | ||
| Residual disease | 7 (13%) | 1 (9%) | 7 (13%) | 1 (8%) | ||
| Untreated | 3 (6%) | 3 (27%) | 3 (6%) | 3 (25%) | ||
| Unknown | 3 (6%) | 0 (0%) | 3 (6%) | 0 (0%) | ||
| Smoking history | ||||||
| ≤40 pack-years | 25 (47%) | 7 (64%) | 0.43 | 25 (47%) | 7 (58%) | 0.61 |
| >40 pack-years | 24 (45%) | 3 (27%) | 24 (45%) | 4 (33%) | ||
| Pipe or cigar smoker only | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | ||
| Unknown | 3 (6%) | 1 (9%) | 3 (6%) | 1 (8%) | ||
| Primary site | ||||||
| Oropharynx | 12 (23%) | 3 (27%) | 0.024 | 12 (23%) | 4 (33%) | 0.022 |
| Larynx | 20 (38%) | 0 (0%) | 20 (38%) | 0 (0%) | ||
| Other | 21 (41%) | 8 (72%) | 21 (41%) | 8 (66%) | ||
| Cell differentiation | ||||||
| Well/moderately differentiated | 37 (70%) | 4 (36%) | 0.075 | 37 (70%) | 5 (42%) | 0.14 |
| Poorly differentiated | 10 (19%) | 5 (45%) | 10 (19%) | 5 (42%) | ||
| Unknown | 6 (11%) | 2 (18%) | 6 (11%) | 2 (17%) | ||
| Weight loss in previous 6 months | 0.55 | 0.80 | ||||
| <5% | 29 (55%) | 8 (73%) | 29 (55%) | 8 (67%) | ||
| ≥5% | 19 (36%) | 2 (18%) | 19 (36%) | 3 (25%) | ||
| Unknown | 5 (9%) | 1 (9%) | 5 (9%) | 1 (8%) | ||
| Prior radiotherapy | 0.024 | 0.033 | ||||
| Yes | 49 (92%) | 7 (64%) | 49 (92%) | 8 (67%) | ||
| No | 4 (8%) | 4 (36%) | 4 (8%) | 4 (33%) | ||
overall response
HPV ISH-positive/p16-positive tumors had a greater response to chemotherapy with a response rate (RR) of 55% for HPV ISH-positive versus 19% for HPV ISH-negative (P = 0.022), and 50% for p16-positive versus 19% for p16-negative (P = 0.057) (Table 2). After adjusting for treatment regimen, primary tumor site, weight loss in previous 6 months, prior radiotherapy and cell differentiation in the stratified regression analysis by ECOG performance status and primary tumor status, the odds ratio for response was 9.05 [95% confidence interval (CI): (0.95, 86.22)] for HPV ISH-positive versus HPV ISH-negative (P = 0.056) and 4.78 for p16+ versus p16− [95% CI (0.71, 31.94), P = 0.11]. No other variables carried predictive significance for objective response in these models.
Table 2.
Overall response rates by human papillomavirus and p16 status
| Response |
P-value | ||
|---|---|---|---|
| HPV | Complete response/ partial response | Other | 0.022 |
| Negative | 10 (19%) | 43 (81%) | |
| Positive | 6 (55%) | 5 (45%) | |
| P16 | 0.057 | ||
| Negative | 10 (19%) | 43 (81%) | |
| Positive | 6 (50%) | 6 (50%) | |
survival outcomes
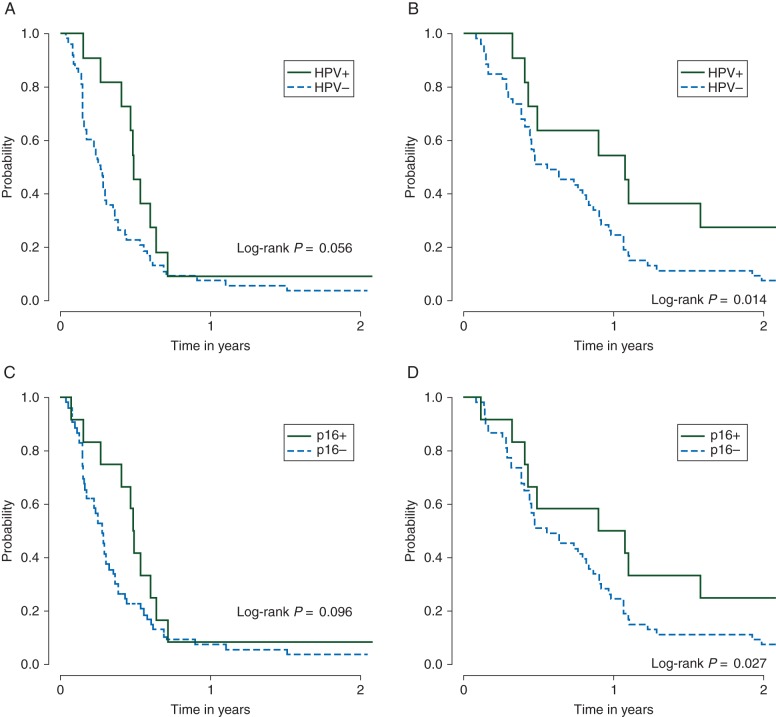
The median OS was 12.9 months [95% CI (4.9, 43.9)] for HPV ISH+ versus 6.7 months [95% CI (5.3, 10.0)] for HPV ISH− (log-rank P = 0.014) and 11.9 months for p16+ [95% CI (3.9, 43.9)] versus 6.7 months for p16− patients [95% CI (5.3, 10.0), log-rank P = 0.027] (Figure 2). The median PFS was 5.9 months for HPV ISH+ [95% CI (3.2, 7.7)] versus 3.2 months for HPV ISH− patients [95% CI (2.0, 3.8), log-rank P = 0.056] and 5.9 months for p16+ [95% CI (1.9, 7.7)] versus 3.4 months for p16− [95% CI (2.1, 3.9), log-rank P = 0.096] (Figure 2). In a multivariate analysis that included primary tumor status, ECOG performance status, treatment, primary tumor site and cell differentiation, hazard ratio (HR) for OS was 2.69 for HPV ISH− versus HPV ISH+ [95% CI (1.01, 7.20), P = 0.048] and 2.17 for p16− versus p16+ [95% CI (0.85, 5.51), P = 0.10], favoring HPV ISH+/p16+ patients. The other unfavorable risk factor for OS was loss of ≥5% weight in previous 6 months (P = 0.0021 and 0.023 for HPV and p16 models, respectively). HR for PFS was 1.91 for HPV ISH− versus HPV ISH+ [95% CI (0.74, 4.89), P = 0.18] and was 1.57 for p16− versus p16+ [95% CI (0.64, 3.88), P = 0.33]. No other variables carried prognostic significance in the PFS models. Of note, one patient died at 6 years without documented progression (although disease evaluation is censored at 3.3 months).
Figure 2.
Progression-free survival (PFS) and overall survival (OS) between HPV ± and p16 ± . (A) PFS by human papillomavirus status, (B) OS by human papillomavirus status, (C) PFS by p16 status and (D) OS by p16 status.
discussion
We report HPV ISH and p16 results across two ECOG trials for recurrent/metastatic SCCHN. Despite a small sample size (n = 65), we have demonstrated a statistically significant improvement in response rate and survival for the HPV ISH-positive/p16-positive population when compared with patients with non-HPV-associated SCCHN treated on the same clinical trials. We previously reported prognostic factors in patients with recurrent/metastatic SCCHN [17], such as primary tumor site, performance status, prior radiotherapy and cell differentiation, which were not found to be significant in our current models, possibly due to small sample size. On the other hand, HPV status as well as the presence of weight loss emerged as strong independent prognostic factors in these models. In our study, HPV-positive tumors were well represented among anatomic sites in addition to the oropharynx, in particular from the hypopharynx. Anatomic allocation has been previously reported to be inaccurate and this may have contributed to these findings [18]. However, p16-positive/HPV-positive SCCHN have been observed to occur at sites other than the oropharynx in studies of locally advanced SCCHN, and is associated with a favorable prognosis as well [5, 19]. Chung et al. [19] reported reduced prevalence of HPV-positive tumors compared with p16-positive tumors in non-oropharyngeal SCCHN, indicating that only a subset of these p16-positive tumors resulted from HPV infection. Although p16 may not be a reliable surrogate for oncogenic HPV infection outside of the oropharynx, by using a wide-spectrum probe, we found high concordance between HPV ISH and p16 IHC across all anatomic sites. Based on our findings, an appropriate strategy in this setting will be to test all primary sites for p16, possibly followed by confirmatory HPV testing for the p16-positive tumors.
Our observations regarding the prognostic impact of HPV in the recurrent/metastatic clinical setting are consistent with findings from other similar analyses in locally advanced, potentially curable SCCHN. In a planned prospective analysis of the ECOG trial 2399, in which patients were treated with induction chemotherapy followed by chemoradiotherapy, tumors were analyzed for HPV16, 33 and 35 DNA by ISH in addition to multiplex PCR [20]. Among the 96 tumors tested, 38 were HPV-positive; this population was associated with a statistically significant improvement in PFS and OS. Subsequent studies have looked at prognostic models that incorporated HPV, smoking history and stage [8, 21].
There are emerging data regarding the outcome of patients with HPV-positive tumors in the recurrent/metastatic setting from the analysis of two randomized trials, ‘SPECTRUM’ and ‘EXTREME’. The ‘SPECTRUM’ trial randomized patients with recurrent/metastatic SCCHN to a platinum doublet either with or without the fully humanized EGFR (epidermal growth factor receptor) inhibitor panitumumab [22]. The primary end point, OS, was not met. In a planned secondary analysis, available specimens (67%) were assayed for p16 [23]. The definition of positive (>10%) of tumor cells departed from the previously used clinical definition of strong staining in >70% of tumor cells. Twenty-eight percent of tumors tested were from the oropharynx. Among the patients who received chemotherapy only, there was a non-significant trend toward a survival benefit among the p16-positive patients (HR 0.70). Interestingly, improved survival was observed for patients with p16-negative tumors who received chemotherapy plus panitumumab (HR 0.73, P = 0.01) [5, 22]. The inclusion in the SPECTRUM analysis of p16-positive non-oropharyngeal cancers without confirmatory HPV results, i.e. utilizing the highly specific HPV ISH or a more sensitive assay, such as HPV E6/E7 mRNA expression, is a limitation of that analysis.
In a retrospective analysis of the ‘EXTREME’ trial [24], which showed superiority of chemotherapy plus cetuximab over chemotherapy alone, HPV status was evaluated with p16 IHC [25] and also using an HPV ISH, amplification and fluorescence technique [26]. In contrast to the SPECTRUM trial data, a more widely accepted definition of p16-positivity was utilized. Tissue was available for analysis from 421 patients; 10% of patients were found to be p16-positive and, even less, 6% HPV-positive; only 56% of the p16-positive tumors were HPV-positive. Both p16-positive and p16-negative patients and HPV-positive and HPV-negative patients benefited from the addition of cetuximab. In addition, there were non-significant trends toward better OS for the p16-positive and HPV-positive patients [26]. The results of both the SPECTRUM and EXTREME data sets could in part be influenced by the use of EGFR inhibitor therapy, whereas our study examined the prognostic benefit of HPV in a population of patients naïve to EGFR inhibitor therapy, thus limiting the potential confounding effects of this targeted therapy [5].
Our results demonstrate that the difference in natural history seen in the locally advanced setting between HPV-positive and HPV-negative tumors is also found in the recurrent/metastatic disease setting. There are several limitations of our analysis, including retrospective nature of the data, the selection of a subset of available tumors from the original trials and the small sample size. In addition, although concordance of p16-positivity and HPV ISH-positive tumor status suggest reliable HPV status assignment, our study did not utilize HPV16 E6/E7 mRNA expression, which has demonstrated improved sensitivity compared with HPV ISH [4]. Retrospective data are limited by the potential for selection bias. Except for the gender distribution, we found no difference in the baseline characteristics of the original study population and our subset. Tumors were analyzed based on tissue availability, rather than based on clinical factors. In spite of the small sample size, the magnitude of effect noted contributes to the significance of our findings, and illustrates that even a small number of HPV-positive recurrent/metastatic patients can impact the results of prospective therapeutic studies. Thus, additional studies should be performed to confirm our findings, which have significant implications for discussions of patient prognosis as well as for the design of clinical trials. Optimal testing strategies will be essential for reliable patient selection [27]. Given the epidemic of HPV-positive SCCHN, it is likely that upcoming studies for recurrent/metastatic disease will have increasing numbers of HPV-positive patients, possibly from oropharyngeal and non-oropharyngeal primary sites. Stratification will be important to avoid bias. Head and neck cancers which arise because of HPV infection have distinct biology and natural history, possibly opening up the possibility of different therapeutic approaches in the future.
funding
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA39229, CA17145, CA27525, CA16116 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger PM, Yu Z, Kountourakis P, et al. Defining molecular phenotypes of human papillomavirus-associated oropharyngeal squamous cell carcinoma: validation of three-class hypothesis. Otolaryngol Head Neck Surg. 2009;141:382–389. doi: 10.1016/j.otohns.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posner MR, Lorch JH, Goloubeva O, et al. Oropharynx cancer (OPC) in TAX 324: Human papillomavirus (HPV) and survival. In ASCO; J Clin Oncol; 2010. p. A5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 13.Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 14.Argiris A, Buchanan A, Brockstein B, et al. Docetaxel and irinotecan in recurrent or metastatic head and neck cancer: a phase 2 trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4504–4513. doi: 10.1002/cncr.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28:5294–5300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler S, Siwak DR, Chai R, et al. Tumor epidermal growth factor receptor and EGFR PY1068 are independent prognostic indicators for head and neck squamous cell carcinoma. Clin Cancer Res. 2012;18:2278–2289. doi: 10.1158/1078-0432.CCR-11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–2229. doi: 10.1002/cncr.20640. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Z, Keck MK, Khattri A, et al. Multimodality determination of HPV status in head and neck cancers (HNC) and development of an HPV signature. J Clin Oncol. 2013;31((suppl)):abstr 6008. [Google Scholar]
- 19.Chung CH, Zhang Q, Kong C, et al. p16 expression as a human papillomavirus (HPV)-independent prognostic biomarker in non-oropharyngeal squamous cell carcinoma (non-OPSCC). J Clin Oncol. 2013;31((suppl)):abstr 6007. [Google Scholar]
- 20.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 22.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 24.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 25.Psyrri A, Licitra L, De Blas B, et al. Safety and efficacy of cisplatin plus 5-FU and cetuximab in HPV-positive and HPV-negative recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): analysis of the phase III EXTREME trial. ESMO 2012; Abstr 10180) [Google Scholar]
- 26.Vermorken JB, Psyrri A, Mesia R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25:801–807. doi: 10.1093/annonc/mdt574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiwert T. Accurate HPV testing: a requirement for precision medicine for head and neck cancer. Ann Oncol. 2013;24:2711–2713. doi: 10.1093/annonc/mdt417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.