Summary
This review article addresses the controversy as to whether the adult heart possesses an intrinsic growth reserve. If myocyte renewal takes place in healthy and diseased organs, the reconstitution of the damaged tissue lost upon pathological insults might be achieved by enhancing a natural occurring process. Evidence in support of the old and new view of cardiac biology is critically discussed in an attempt to understand whether the heart is a static or dynamic organ. According to the traditional concept, the heart exerts its function until death of the organism with the same or lesser number of cells that are present at birth. This paradigm was challenged by documentation of the cell cycle activation and nuclear and cellular division in a subset of myocytes. These observations raised the important question of the origin of replicating myocytes. Several theories have been proposed and are presented in this review article. Newly formed myocytes may derive from a pre-existing pool of cells that has maintained the ability to divide. Alternatively, myocytes may be generated by activation and commitment of resident cardiac stem cells or by migration of progenitor cells from distant organs. In all cases, parenchymal cell turnover throughout lifespan results in a heterogeneous population consisting of young, adult, and senescent myocytes. With time, accumulation of old myocytes has detrimental effects on cardiac performance and may cause the development of an aging myopathy.
Keywords: aging heart, cardiac niches, cardiac stem cells, myocardial regeneration, myocyte turnover
The recognition that the adult heart possesses a stem cell compartment that can regenerate myocytes and coronary vessels has changed the perennial view of the heart as a post-mitotic organ and has formed the basis of a new paradigm in which multipotent cardiac stem cells (CSCs) are implicated in the physiological turnover of myocytes and vascular endothelial cells (ECs) and smooth muscle cells (SMCs) organised in coronary vessels [1,2]. Understanding the mechanisms of cardiac homeostasis would offer the extraordinary opportunity to potentiate this natural occurring process and promote myocardial regeneration following injury. However, the field of regenerative cardiology is in its infancy and great caution has to be exercised in the implementation of any cell type in humans. Additionally, in humans, multiple variables may negatively interfere with the therapeutic efficacy of the harvested cells. Ischaemic and non-ischaemic pathologic states, co-morbidities, duration and severity of the cardiac disease may have profound implications on the availability and functional competence of CSCs. Also, the age and gender of patients have to be regarded as important determinants of CSC growth and lineage commitment.
Cell-based cardiac repair
Different cell types have been proposed experimentally for the reconstitution of the damaged heart: skeletal myoblasts [3,4], fibroblasts [5], SMCs [6], foetal myocytes [7,8], embryonic stem (ES) cells [9], bone marrow-derived cells (BMCs) [10–14], and induced pluripotent stem (iPS) cells [15]. Fibroblasts, SMCs, and foetal myocytes form a passive graft, which, by decreasing the stiffness of the scarred portion of the wall, has a transient positive effect on ventricular remodelling and function. Totipotent ES cells appear to engraft into the myocardium but die rapidly because of the lack of vessel formation and immune rejection [16]. Additionally, ES cells give rise to teratomas and teratocarcinomas [17]. Similarly, the recently identified iPS cells have a tumorigenic potential [18]. These cell types have never been employed clinically.
The first attempt to replace infarcted myocardium with a patch of skeletal muscle was performed in the 1930’s. Fifty years later, large sheets of skeletal muscle tissue were positioned on the epicardial surface of the ischaemic area and stimulated to contract with a pacemaker. This surgical procedure, known as dynamic cardiomyoplasty, has prompted investigators to utilise individual myogenic cells. The new approach has been called cellular cardiomyoplasty and consists of the direct injection of isolated skeletal myoblasts into the ischaemic area [3,4]. The autologous origin of the cells to be implanted constitutes an obvious advantage of this form of cardiac repair. Moreover, skeletal myoblasts are more resistant to ischaemia than cardiomyocytes, enhancing their possibility of survival in the necrotic myocardium. For these reasons, experimental skeletal myoblast implantation has been translated to the clinical arena, and infarcted patients have been subjected to this kind of treatment [19,20].
However, the lack of integration of skeletal myoblasts within the myocardium represents a reason of concern for the therapeutic implementation of skeletal myoblasts. Analysis of the graft-host myocardium interface has failed to document any evidence of mechanical or electrical coupling between skeletal and cardiac muscle [21]. Connexin 43 and N-cadherin are consistently absent in injected skeletal muscle cells and a layer of dense scar tissue often separates the cardiomyocytes from the skeletal muscle further opposing the incorporation process. As documented in animals [21,22], the absence of synchronous contraction may be one of the factors responsible for the arrhythmic manifestations observed in patients treated with skeletal myoblasts [19,20].
Moreover, skeletal myoblast implantation is preceded by an in vitro step of amplification. This necessity represents a limitation for the clinical application of this procedure. Adult human myoblasts divide only 20–25 times in vitro, before becoming senescent [23]. This issue is particularly relevant since it has been documented that the success of skeletal myoblast implantation is directly correlated with the number of donor cells [24]. The need of a large quantity of cells to be implanted is dictated by massive and rapid cell death that occurs over a week after administration. During in vitro expansion and following the introduction in the heart, myoblasts withdraw from the cell cycle and form myotubes. The state of terminal differentiation rapidly acquired by skeletal myoblasts opposes any possible proliferation of the implanted cells. Damaged cells within the graft cannot be replaced impairing the mechanical and elastic properties of the graft and, ultimately, its effects on cardiac function. An important argument that speaks against the utilisation of skeletal myoblasts in cardiac repair is that the injured portion of the ventricular wall is replaced by a tissue that is far from being similar to the myocardium. Regenerative medicine should target the restoration of tissue with the same functional and structural properties of the damaged organ. However, transdifferentiation of skeletal myoblast in cardiac myocytes has never been observed [16]. These numerous problems have resulted in an early termination of the enrolment of patients in clinical trials [19,20].
BMCs may translocate to the heart, form temporary niches and participate in the homeostasis of the healthy organ or the regeneration of the injured tissue [25]. The contribution of this cell class to cardiomyogenesis and coronary vasculogenesis is currently unknown and remains an important unanswered question. The involvement of BMCs in cardiac chimerism has been proposed [26]. Interestingly, a comparison has been made between the degree of chimerism in cardiac allografts and in hearts of patients who received allogeneic bone marrow transplantation [27]. In the latter case, only 2–5% chimeric myocytes were detected, while 14–16% of chimeric myocytes and endothelial cells were found in transplanted hearts. These observations suggest the intracardiac origin of the recipient cells in the donor heart and the extracardiac origin of chimeric cells in the resident heart following bone marrow transplantation. In the first case, host cells may have migrated from the residual atrial stumps to the donor heart [28] and, in the second, donor cells may have reached the myocardium because of the high level of blood chimerism [27]. Thus blood-borne cardiac cells may be detected exclusively when the peripheral blood contains a large number of haematopoietic stem cells (HSCs). Experimental results support this contention [10,29].
Whether BMCs drive the regenerative response of the damaged heart remains an unresolved issue. The striking discrepancy between the incidence of heart failure and bone marrow failure and the lack of co-morbidity of these disease stated in the same patient indicates that HSCs do not typically migrate from the bone marrow and repopulate the decompensated heart. If the bone marrow continuously replenishes the heart with new functionally competent HSCs, the decline in myocyte number with cardiac diseases would not occur, and the poorly contracting myocytes would be constantly replaced by a bone marrow-derived progeny.
Shortly after the experimental evidence that HSCs induce myocardial regeneration after infarction [10], unfractionated mononuclear BMCs and CD34-positive cells have been administered to patients affected by acute and chronic myocardial infarction, dilated cardiomyopathy, and refractory angina [30–34]. Although the individual outcomes have been inconsistent and variability exists among trials, meta-analyses of pooled data indicate that BMC therapy results in a 3–4% increase in ejection fraction [35]. Allogeneic and autologous mesenchymal stromal cells (MSCs) have also been employed in small clinical trials with encouraging results [36–38]. Although the benefits may seem modest, these initial data have favoured the conduct of larger randomised trials designed to critically evaluate the long-term effects of BMC therapy on a broader patient population. The mechanisms involved in the positive impact of BMC therapy on human beings remains to be identified. Measurements of coronary flow suggest that vasculogenesis may be operative while the contribution of de novo myocyte formation is uncertain. Additionally, the injected BMCs activate the growth and differentiation of resident CSCs via a paracrine effect, mediated by the release of a multiplicity of cytokines [39,40]. Importantly, the recent identification of CSCs has shifted the attention to endogenous cell mechanisms as a novel target of cell therapy for the failing heart.
Cardiac progenitor cell types
Dynamic cardiomyogenesis characterises the response of the damaged heart prenatally and shortly after birth. Additionally, in the adult zebrafish, cardiac regeneration in the absence of scar formation takes place after resection of up to 20% of the ventricle. Traditionally, muscle reconstitution in this model has been considered to be mediated by cardiomyocyte proliferation [41]. A similar regenerative response has been observed after surgical resection of the apex of the left ventricle in the neonatal mouse heart [42]. Again, cardiomyocyte proliferation was viewed as the crucial cell process, promoting cardiac repair. However, investigators in several laboratories concur with the notion that the adult heart contains a compartment of stem/ progenitor cells [43–53]. Distinct protocols based on the recognition of surface markers and transcription factors, and functional assays have been employed for the isolation of stem cells from the myocardium. The stem cell antigens c-kit and Sca-1 are expressed in partially overlapping pools of cardiac primitive cells. The presence of Sca-1 has been reported [45– 47,51–53] and the expression of c-kit has been detected in multiple studies [44,46,52,53] and found to be absent in another [45]. c-kit-positive cells are an established component of the canine [49] and human [28,48,50,54] heart, and their activation leads to the formation of new myocardium. The regenerative potential of c-kit-positive and Sca-1-positive cells after ischaemic injury differs significantly; a robust regenerative response occurs with c-kit-positive cells [44,55] whereas little engraftment and repair takes place with Sca-1-positive cells [45]. Whether the modality of administration of cells, the animal model, and the properties of the injected cells are responsible for the different outcome is presently unclear.
The developing heart and adult heart typically contains a CSC pool that has the ability to efflux the Hoechst 33342 dye [43,45,47,51,56]. Similar cells, which express an ATP-binding cassette transporter, have been identified in other organs and termed side-population (SP) cells [51,57]. The classicmember of this family is a P-glycoprotein that confers to the cells multidrug resistance by extruding anticancer drugs and rhodamine 123. These putative CSCs are 93% Sca-1-positive and appear to represent a small subset of the Sca-1-positive cells in the mouse heart [45]. According to a different study, however, SP cells comprise 2% of all cells (2 per 100 cardiac cells) [47]. Cardiac SP cells appear to express CD31 and form haematopoietic colonies in vitro [47]. The presence of CD31, common to bone marrow SP cells [29,57] together with the peculiar growth behaviour of these cells in vitro, raises questions concerning their actual origin and suggests the possibility of a colonisation to the heart from the haematopoietic system.
More recently, a novel Sca-1-positive, Hoechst 33342 dyelow, and CD31-negative cardiac SP cell has been identified [51,56]. The modest expression of c-kit in these cells was attributed to methodological limitations inherent in the enzymatic cleavage of this receptor during digestion of the myocardium and cell isolation [51]. There is 1 SP cell per 30 000 cardiac cells in the mouse heart. CD31-negative cardiac SP cells form beating cardiomyocytes in vitro and acquire the adult phenotype in vivo through cellular coupling with differentiated cardiomyocytes [51]. These results strengthen the notion of a functional role for resident SP cells in the heart.
The Isl1 transcription factor is associated with the commitment to the myocyte lineage of cardiac cells that have lost their undifferentiated stem cell state. Isl1 and GATA4 are transcriptional coactivators of the myocyte transcription factor MEF2C [58]. Homozygous deletion of Isl1 alters the development of the heart, affecting the atria, right ventricle, and outflow tract [59,60]. However, these cells disappear after birth, which raises serious questions on the possibility of employing Isl1-positive cells for therapeutic purpose.
Cell culture in serum-free media on non-adhesive substrates has been employed for the isolation of primitive cells in several organs including the brain and the heart [61]. The formation of spherical clusters of cells known as floating spheres is achieved by a suspension culture method which is used for large-scale amplification of stem/progenitor cells as an alternative technique to single-cell deposition and clonal expansion [44,49]. The suspension protocol does not reflect the formation of multicellular clones from single founder cells. Spheres are highly motile structures, prone to fuse [62] and may, therefore, correspond to aggregate non-homogeneous cells. This peculiar form of anchorage-independent growth typically occurs with neural stem cells [63]. A central core of proliferating cells is commonly surrounded by quiescent cells with restricted developmental options. This dynamic phenotypic transition from a ‘mesenchymal’ monolayer state to an ‘epithelial’ floating state is commonly seen in culture of bone marrow MSCs [64].
Cardiospheres possess similar characteristics. Whether cardiospheres are clonal or oligoclonal in nature, cardiosphere-derived cells represent the progeny of the most primitive cells within the aggregates. A fraction of cells located in the core of the cardiospheres express the stem cell antigen c-kit and is surrounded by an outer layer composed of cells positive for CD105, a membrane glycoprotein commonly expressed in bone marrow MSCs [64]. Cardiosphere-derived cells undergo spontaneous maturation toward the myocyte lineage, and the process of commitment can be coaxed by co-culture with neonatal ventricular myocytes [65]. Connexin 43 is expressed between highly dividing cells within the cardiospheres and in the expanded differentiating cardiosphere-derived cells [66]. The presence of gap junctions between uncommitted and differentiated cells mimics the organisation of stem cell niches in vivo raising the possibility that the differentiated cells may function as supporting cells [67,68].
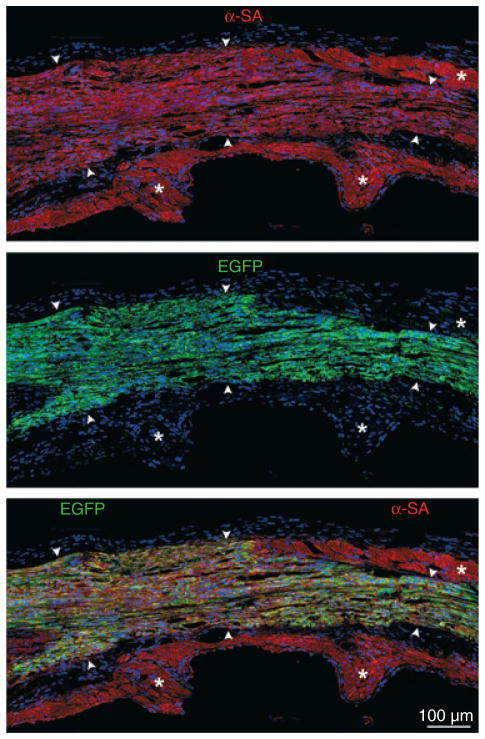
The c-kit receptor tyrosine kinase originally was detected in a class of murine HSCs with long-term reconstituting ability in irradiated recipients [69]. More recently, c-kit has been found in several populations of stem cells in the adult liver, brain, and pancreas [70]. In the heart, this stem cell antigen identifies a pool of resident CSCs that are self-renewing, clonogenic, and multipotent in vitro and in vivo [44,49]. These CSCs replace infarcts with functionally competent myocardium restoring ventricular performance experimentally. Importantly, an identical cell with similar biological and functional characteristics exists in the human heart (Fig. 1) [2]. c-kit-positive CSCs and cardiosphere-derived cells are currently employed in clinical trials for the treatment of ischaemic cardiomyopathy (ClinicalTrials.gov Identifier: NCT00474461, NCT00893360). Encouraging results have been obtained [71] although the small number of treated patients and the short duration of the follow-up preclude definitive conclusions concerning the efficacy of cardiac progenitors in the treatment of heart failure. Together, these observations are consistent with the notion that the heart possesses an intrinsic capacity for regeneration, but whether differences in the expression of surface antigens reflect CSC subclasses, which are functionally distinct, is difficult to ascertain.
Fig. 1.
Viral tagging of cultured hCSCs and myocardial regeneration. Band of regenerated myocardium (arrowheads) within the infarcted region of the rat left ventricle. Newly formed myocytes express α-SA (upper panel, red) and EGFP (central panel, green). Lower panel, merge of upper and central panel. *Spared myocytes.
Cardiac stem cell niches
Stem cells are sheltered in specialised structures called niches, which provide a microenvironment designed to preserve the survival and replication potential of the primitive cells [72]. The concept of niches was introduced in 1978 by Schofield, who defined niche as ‘a stable microenvironment that might control haematopoietic stem cell behaviour’ [73]. Currently, the niche is viewed as ‘a subset of tissue cells and extracellular substrates that can indefinitely house one or more stem cells and control their self-renewal and progeny production in vivo’ [72]. Interstitial structures with the architectural organisation of niches have been found in the adult heart [1,67]. CSCs, progenitors, precursors and early differentiating cells are clustered together in the niche and are coupled with the surrounding cells through the expression of gap and adherens junctions [74,75]. Gap junctions are intercellular channels formed by individual structural units called connexins [76,77] while adherens junctions are composed of cadherins. Gap junctions allow cells to communicate with each other and to exchange small molecules. CSCs utilise gap junctions to transmit and receive signals from the surrounding tissue for cell survival, proliferation or differentiation [68,74,75,78,79]. Adherens junctions appear to be involved in the preservation of the undifferentiated state of CSCs. The function of gap junctions is probably cell and tissue specific. However, deregulation of the expression of connexins and cadherins, and alterations in the configuration of the junctional complexes affect the growth and commitment of stem cells.
Resident CSCs accumulate in areas exposed to low levels of haemodynamic load and wall stress. These properties are commonly found in the atria and in the apical portion of the ventricle. However, niches have been detected in several sites of the free wall of the left ventricle indicating that CSCs are preferentially but not exclusively present in protected areas of the heart. The formation of ventricular niches may be dictated by the migration of CSCs from their sites of storage to the regions of damage, which are typically located in the ventricular wall. Physical forces in the ventricular wall can be transduced in intracellular responses that regulate cell behaviour and fate. The consequences of mechanical factors on CSC function are unknown. The low haemodynamic stress in the atria and apex may facilitate the preservation of the CSC pool while the high degree of stress at the base andmid-region of the left ventricular wall may condition CSC commitment. Importantly, abnormal pathologic loads are coupled with the initiation of myocyte regeneration, which is heterogeneous and tends to parallel the alterations in the distribution of stresses in the damaged heart [80,81]. The effects of strain on CSCs are currently unknown. However, the peculiar topographical distribution of CSCs in the heart suggests that a relationship may exist between the function of CSCs and the level of haemodynamic stress.
Growth kinetics of c-kit-positive cardiac stem cells
If the heart is a dynamic organ, the replenishment of its parenchymal and non-parenchymal cells is regulated by a stem cell compartment and by the ability of these primitive cells to self-renew and differentiate. Regeneration conforms to a hierarchical archetype: slowly dividing stem cells give rise to highly proliferating, lineage-restricted progenitor cells, which then become committed precursors that, eventually, reach growth arrest and terminal differentiation. Stem cells divide rarely while committed transient amplifying cells are the actual group of replicating cells in self-renewing organs. The short-lived amplifying cells possess a unique property; they undergo a finite number of doublings and simultaneously differentiate until they withdraw from the cell cycle and reach terminal full maturation.
Stem cells divide symmetrically and asymmetrically. When stem cells divide symmetrically, two self-renewing daughter stem cells may be formed. The purpose of this modality of replication is the expansion of the stem cell pool, which occurs in active phases of growth during prenatal organ development. Stem cells can also divide symmetrically into two committed daughter cells; this type of cytokinesis is triggered by an emergent situation, that requires restoration of the lost tissue upon a pathological insult. This process may decrease the number of primitive cells. With asymmetric division, two differently fated sibling cells are generated: one daughter-stem cell and one daughter-committed cell. The non-stem cell sister is a short-life committed progenitor cell that divides and simultaneously differentiates, i.e. the amplifying cell [70,82–84]. When the amplifying cell acquires complete maturation, the cell cannot divide further and reaches the terminal state of differentiation. The objective of asymmetric division is to maintain a steady state in which organ homeostasis is conditioned by a tight balance between stem cell formation and the production of a committed progeny. The developmental choice made by CSCs at any given time has a direct impact on the number of stem cells, progenitors, precursors, amplifying cells and, ultimately, mature cells. The size of the amplifying myocyte pool conditions the magnitude of the homeostatic and regenerative response in damaged heart.
The organisation of the stem cell compartment in niches is crucial for the maintenance of the primitive cell pool and for the preservation of organ homeostasis [67,72,82,83,85,86]. The niche microenvironment controls the number of stem cells and their progeny by influencing the pattern of stem cell division. The inhomogeneous intracellular segregation of selective proteins in daughter cells at the time of mitosis constitutes the intrinsic determinants of CSC fate in animals and humans. Genes, including numb, α-adaptin and members of the Notch pathway, interact to enable single primitive cells to produce differently destined sibling cells [87–90]. Numb can segregate to one of the two daughter cells or be equally distributed in the cytoplasm of both daughter cells [87–90]. Numb is expressed during mitosis, from late prophase to telophase, and in the early stages of life of the new daughter cell [91]. Numb localises to endocytic vesicles and binds to the endocytic protein α-adaptin inducing the internalisation and inactivation of the Notch receptor [92].
CSCs that receive Numb become unresponsive to Notch while Numb-negative CSCs retain their responsiveness to Notch and adopt the phenotype associated with Notch activation [87,93]. Signalling through the Notch receptor can occur only between closely adjacent stem cells and supporting cells. The Notch ligands are transmembrane proteins, which, upon binding, cleave the Notch receptor so that its intracellular domain is translocated to the nucleus where it forms complexes with transcription factors of the recombinant DNA binding protein RBP-Jk [94–96]. These effector pathways are operative in the heart and Notch1 activation by the Jagged1 ligand promotes the commitment of CSCs to the myocyte lineage within the cardiac niches in the mouse heart [97]. A similar behaviour has been observed in human CSCs (hCSCs). The progressive dropout of myocytes with aging and pathological states may activate an emergency response of CSCs towards the differentiating pathway to generate rapidly a large number of muscle cells. The long-term outcome of CSC growth is a reduction of the stem cell pool and, ultimately, the exhaustion of their replicating reserve.
Cardiomyogenesis and myocyte turnover in the aging heart
The adult heart is largely composed of terminally differentiated myocytes. Damaged and old units of this highly specialised compartment of contracting cells are constantly replaced by new younger elements. Mitosis and cytokinesis have been recognised in poorly differentiated myocytes with a thin subsarcolemmal halo of myofibrils (Fig. 2) [50], in combination with telomerase activity [50,54,98,99]. These in vivo findings, together with observations in vitro [44], have indicated that replicating myocytes are transit amplifying cells derived from lineage commitment of CSCs. The ability to replenish old and damaged cells is maintained by the existence of telomerase-competent CSCs. Telomerase activity has been documented in the decompensated old rat heart [100], in the failing canine heart [101], and in the human myocardium. In humans, cardiac hypertrophy with modest ventricular dysfunction [54], and prematurely aged heart with severe functional impairment [102] are characterised by an increase in telomerase activity. This enzyme, however, cannot prevent telomere erosion [54]. In hCSCs, telomere shortening occurs at a rate of approximately 130 base pairs per population doublings, possibly limiting CSC lifespan. Thus, aging effects on CSCs lead to an imbalance between telomerase activity and length of telomeres, resulting in critical telomeric shortening, permanent withdrawal from the cell cycle, and CSC senescence.
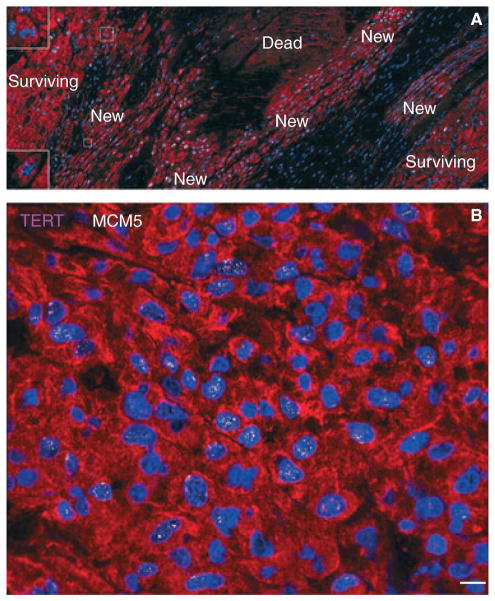
Fig. 2.
Spontaneous myocyte regeneration within the infarcted human myocardium. (A) Clusters of highly proliferating small developing myocytes are visible. Myocytes are labelled by cardiac myosin (red) and nuclei by DAPI (blue). Most of these cells are positive for MCM5 (white). Two dividing small myocytes are shown in insets. (B) Small developing myocytes within the infarct are positive for telomerase (magenta) and MCM5 (white) in the nuclei (Scale bars: A, 100 μm; B, 10 μm).
The controversy on the growth reserve of the adult human heart has not been resolved, and the extent of myocyte renewal claimed by different groups varies dramatically. A recent study, based on retrospective carbon 14 (14C) birth dating of cells, has claimed that 1% and 0.45% replacement of myocytes occurs annually in the human heart at 25 and 75 years of age, respectively [103]. These findings indicate that only 50% of myocytes are renewed during the entire life of the human heart, from birth to death, whereas an equal number lives as long as the organ and organism, up to 100 years of age and longer. Although the possibility of myocyte regeneration was confirmed, the actual magnitude of the process is in contrast with the level of myocyte apoptosis found in the adult human heart [104] and the progressive increase in myocyte loss that occurs with aging in humans [105]. Myocyte regeneration increases as a function of age, and the age of cardiomyocytes does not coincide with the age of the organ and organism. This discrepancy becomes more apparent in the senescent myocardium in which a large proportion of myocytes is 5 years old or younger in both women and men [106]. The older the human heart, the younger is its myocyte compartment. From 19 to 104 years of age, essentially none of the myocytes present at birth is preserved in the young adult, middle-aged, and senescent heart [106]. These findings question the contention that 50% of cardiomyocytes are not replaced during the entire lifespan in humans [103], suggesting that a large proportion of cells survives and retains its function for more than 100 years.
The presence of hCSCs throughout the lifespan of the human heart is apparently at variance with the limited capacity of endogenous tissue repair after infarction [50]. This phenomenon has been interpreted as the unequivocal documentation of the inability of the adult heart to create cardiomyocytes [107– 110]. A possible explanation for this apparent paradox has been obtained in animal models in which dead stem cells have been found throughout the infarct, indicating that the fate of hCSCs is comparable to that of the other cells located in the ischaemic region [84]. It might come as a surprise, but a similar event occurs in other solid and nonsolid organs including the skin, bone marrow, liver, intestine, and kidney. In all cases, occlusion of a supplying artery leads to scar formation mimicking cardiac pathology [1,84].
hCSC aging conditions myocardial aging; chronological age leads to telomeric attrition in hCSCs, which generate a progeny that rapidly attains the senescent phenotype. Daughter cells acquire the shortened telomeres of maternal hCSCs and, after a few rounds of division and terminal differentiation, express p16INK4a in nearly 2 years [106]. The pool of old cardiomyocytes progressively increases, defining the aging myopathy. Telomere length reflects the past replicative history and cumulative oxidative DNA damage occurring during the life cycle of the cell [111]. Telomerase activity delays but does not prevent telomere erosion, which is mediated by downregulation of telomerase, reactive oxygen species, and loss of telomere-related proteins [50,112]. Shortening of telomeres beyond a critical length triggers cellular senescence, which corresponds to irreversible growth arrest in G1 with loss of specialised functions, including cell proliferation, migration, and differentiation. Suggestive evidence in humans and genetically manipulated mice [113–115] points to shortening of telomeres as a critical determinant of cellular senescence and, possibly, organ aging. In this regard, a lineage relationship was found between hCSCs with short telomeres and the formation of old myocytes. In a mouse model of accelerated aging, the deletion of the RNA competent of telomerase leads to cardiac hypertrophy, cavitary dilation and heart failure [113]. These pathological alterations are dictated by excessive myocyte apoptosis and defective cardiomyogenesis. In analogy with HSCs [116], forced expression of telomerase in CSCs may prevent replicative senescence. Although it is difficult to establish whether telomeric shortening is a consequence of aging or a primary event conditioning the aging myopathy, telomerase deletion is coupled with telomere shortening and the manifestation of a premature aging cardiac phenotype [113].
Importantly, the female heart possesses a superior ability to sustain the multiple variables associated with the aging process and the development of the senescent myopathy. At all ages, the female heart is equipped with a larger pool of functionally competent hCSCs and younger myocytes than the male myocardium. The replicative potential is higher and telomeres are longer in female hCSCs than in male hCSCs. Animal studies have shown that the insulin-like growth factor (IGF)-1– IGF-1 receptor system is present in CSCs at very old age [117] and overexpression of IGF-1 in cardiomyocytes prevents the manifestations of the senescent cardiac phenotype and heart failure [118]. Additionally, the IGF-1–IGF-1 receptor axis is enhanced in female myocytes [119], and it may condition the favourable outcome of age in this gender. Importantly, premature cardiac aging appears to affect predominantly men than women [102]. Additionally, oestrogens phosphorylate IGF-1 receptors [120] mimicking the effects of IGF-1, a powerful inducer of CSC division, survival, and maintenance of telomere length [117,118]. In postmenopausal women and throughout life in men, oestrogen is synthesised in extragonadal organs including bone, cartilage, adipose tissue, skin, breast, and heart [121]. With aging, oestrogen loses its circulating generalised function and works mainly at the local level as a paracrine, autocrine, or intracrine factor. Oestrogen induces transcription of the catalytic subunit of the telomerase protein (TERT), because an oestrogen response element is present in the TERT promoter [122]. Additional downstream effector pathways of oestrogen involve the activation of phosphatidylinositol 3-kinase/Akt cascade, which exerts multiple beneficial effects on cardiac performance and biology [123].
Myocyte regeneration in the physiologically aging heart takes place at previously unexpected levels in both women and men. In the female heart, myocyte replacement occurs at a rate of 10%, 14%, and 40% per year at 20, 60, and 100 years of age, respectively. Corresponding values in the male heart are 7%, 12%, and 32% per year [106], documenting that myocyte turnover involves a large and progressively increasing number of parenchymal cells with aging. From 20 to 100 years of age, the myocyte compartment undergoes 15 cycles of complete replacement in women, and 11 cycles in men [106].
This high degree of myocyte turnover, which increases further with age, is strikingly different from the data derived from the integration of 14C into the DNA of myocyte nuclei [103]. Retrospective birth dating of human myocardium DNA by 14C mirrors the incorporation of thymidine analogues in animal models, an analysis that has only been rarely possible in human beings and, thus far, has been restricted to the brain [124] and more recently to the heart [125]. An inherent limitation of 14C birth dating is related to the need to introduce mathematical models with assumptions that affect the computed cell turnover values [103]. The scenario chosen presumed that the number of myocytes in the heart was constant and that cells turned over at a near constant rate. This form of invariant growth defines parenchyma in a steady state in which cell death is compensated by cell regeneration in young healthy individuals. However, this steady-state scenario poorly represents the biology of aging in men where nearly 64 × 106 cardiomyocytes are lost per year. Furthermore, because women do not lose cardiomyocytes as they age like men [105] different models should be used to reflect the changing cell populations. Importantly, myocyte number increases postnatally [125,126] and cell loss typically occurs with cardiac diseases [104].
Another problem with the study of retrospective 14C birth-dating involves the use of troponin I expression as a marker for the isolation of a representative pool of myocyte nuclei. The presence of troponin I almost exclusively identifies a population of p16INK4a-positive senescent cells that exhibit marked alterations in the permeability of nuclear pore complexes [125]. Additionally, the percentage of p16INK4a-positive myocytes varies dramatically with age and cardiac diseases [50,102]. Multiple markers in a large number of healthy hearts spanning a large age range are required to measure and model the actual degree of cardiac cell turnover [106].
Conclusions
The human heart is a highly dynamic organ regulated by a pool of resident hCSCs that modulate cardiac homeostasis and condition of organ aging. Hopefully, recent findings will resolve the long debate that has divided the scientific community in strong opponents and passionate supporters of the regenerative potential of the human heart, offering a more biologically valid understanding of cardiac homeostasis and repair. A common ground can now be found to translate this different perspective of cardiac biology into the development of novel strategies for the management of the human disease. However, the magnitude of the process, the effects of age on the extent of myocyte renewal, and the origin of newly formed cardiomyocytes remains a matter of controversy.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–74. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–23. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–33. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 5.Galli D, Innocenzi A, Staszewsky L, Zanetta L, Sampaolesi M, Bai A, Martinoli E, Carlo E, Balconi G, Fiordaliso F, Chimenti S, Cusella G, Dejana E, Cossu G, Latini R. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms: a comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:692–7. doi: 10.1161/01.ATV.0000156402.52029.ce. [DOI] [PubMed] [Google Scholar]
- 6.Li RK, Jia ZQ, Weisel RD, Merante F, Mickle DA. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol. 1999;31:513–22. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- 7.Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 8.Koh GY, Soonpaa MH, Klug MG, Pride HP, Cooper BJ, Zipes DP, Field LJ. Stable fetal cardiomyocyte grafts in the hearts of dystrophic mice and dogs. J Clin Invest. 1995;96:2034–42. doi: 10.1172/JCI118251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–14. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair in infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 12.Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, Vajkoczy P, Hofmann WK, Peters C, Pennacchio LA, Abolmaali ND, Chavakis E, Reinheckel T, Zeiher AM, Dimmeler S. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–13. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–25. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 15.Sturzu AC, Wu SM. Developmental and regenerative biology of multipotent cardiovascular progenitor cells. Circ Res. 2011;108:353–64. doi: 10.1161/CIRCRESAHA.110.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 17.Su W, Zhou M, Zheng Y, Fan Y, Wang L, Han Z, Kong D, Zhao RC, Wu JC, Xiang R, Li Z. Bioluminescence reporter gene imaging characterize human embryonic stem cell-derived teratoma formation. J Cell Biochem. 2011;112:840–8. doi: 10.1002/jcb.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed RP, Ashraf M, Buccini S, Shujia J, Haider HK. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen Med. 2011;6:171–8. doi: 10.2217/rme.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagège AA, Marolleau JP, Vilquin JT, Alhéritière A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasché P. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114:I108–13. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 20.Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 21.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol. 2000;149:731–40. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinecke H, Minami E, Virag JI, Murry CE. Gene transfer of connexin43 into skeletal muscle. Hum Gene Ther. 2004;15:627–36. doi: 10.1089/1043034041361253. [DOI] [PubMed] [Google Scholar]
- 23.Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–38. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- 24.Pouzet B, Vilquin JT, Hagège AA, Scorsin M, Messas E, Fiszman M, Schwartz K, Menasché P. Factors affecting functional outcome after autologous skeletal myoblast transplantation. Ann Thorac Surg. 2001;71:844–50. doi: 10.1016/s0003-4975(00)01785-9. [DOI] [PubMed] [Google Scholar]
- 25.Hosoda T, Kajstura J, Leri A, Anversa P. Mechanisms of myocardial regeneration. Circ J. 2010;74:13–7. doi: 10.1253/circj.cj-09-0665. [DOI] [PubMed] [Google Scholar]
- 26.Korbling M, Estrov Z. Adult stem cells and tissue repair. Bone Marrow Transplant. 2003;32:S23–4. doi: 10.1038/sj.bmt.1703939. [DOI] [PubMed] [Google Scholar]
- 27.Thiele J, Varus E, Wickenhauser C, Kvasnicka HM, Lorenzen J, Gramley F, Metz KA, Rivero F, Beelan DW. Mixed chimerism of cardiomyocytes and vessels after allogenic bone marrow and stem-cell transplantation in comparison with cardiac allografts. Transplantation. 2004;77:1902–5. doi: 10.1097/01.tp.0000127591.34203.8e. [DOI] [PubMed] [Google Scholar]
- 28.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 29.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Godell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 31.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 32.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 33.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 34.Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, Schächinger V, Tonn T, Martin H, Dimmeler S, Zeiher AM. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail. 2009;2:417–23. doi: 10.1161/CIRCHEARTFAILURE.109.855023. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Sun A, Zhang S, Yao K, Wu C, Fu M, Wang K, Zou Y, Ge J. Efficacy and safety of intracoronary autologous bone marrow-derived cell transplantation in patients with acute myocardial infarction: insights from randomized controlled trials with 12 or more months follow-up. Clin Cardiol. 2010;33:353–60. doi: 10.1002/clc.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161:487–93. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–6. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–98. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 42.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–43. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 44.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–6. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 45.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman NL, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomycytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 47.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 48.Messina E, DeAngelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MVG, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 49.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–71. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 52.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that following activation regenerate the infarcted myocardium improving ventricular function and long-term survival. Circ Res. 2005;97:663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 53.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–33. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:10440–5. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–71. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–2. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 57.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 58.Dodou E, Verzi MP, Anderson JP, Xu SM, Balck BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–42. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 59.Yuan S, Schoenwolf GC. Islet-1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. Anat Rec. 2000;260:204–7. doi: 10.1002/1097-0185(20001001)260:2<204::AID-AR90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Cai CL, Laing X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Is11 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anversa P, Kajstura J, Leri A. If I can stop one heart from breaking. Circulation. 2007;115:829–32. doi: 10.1161/CIRCULATIONAHA.106.682195. [DOI] [PubMed] [Google Scholar]
- 62.Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–6. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 63.Nakayama T, Inoue N. Neural stem sphere: induction of neural stem cells and neurons by astrocyte-derived factors in embryonic stem cells in vitro. Methods Mol Biol. 2006;220:1–13. doi: 10.1385/1-59745-036-7:001. [DOI] [PubMed] [Google Scholar]
- 64.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 65.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 66.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R, Marbán E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosoda T, Zheng H, Cabral-de-Silva M, Sanada F, Ide-Iwata N, Ogórek B, Ferreira-Martins J, Arranto C, D’Amario D, DelMonte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–96. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–6. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 71.Bolli R, Chugh AR, D’Amario D, Stoddard MF, Ikram S, Wagner SG, Beache GM, Leri A, Hosoda T, Loughran JH, Goihberg P, Fiorini C, Solankhi NK, Fahsah I, Chatterjee A, Elmore JB, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Use of cardiac stem cells for the treatment of heart failure: translation from bench to the clinical setting. Circ Res. 2010;107:A23734. [Google Scholar]
- 72.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 73.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 74.Cancelas JA, Koevoet WL, de Koning AE, Mayen AE, Rombouts EJ, Ploemacher RE. Connexin-43 gap junctions are involved in multiconnexin-expressing stromal support of hemopoietic progenitors and stem cells. Blood. 2000;96:498–505. [PubMed] [Google Scholar]
- 75.Montecino-Rodriguez E, Dorshkind K. Regulation of hematopoiesis by gap junction-mediated intercellular communication. J Leukoc Biol. 2001;70:341–7. [PubMed] [Google Scholar]
- 76.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–8. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 77.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 78.Rosendaal M, Green CR, Rahman A, Morgan D. Up-regulation of the connexin43+gap junction network in haemopoietic tissue before the growth of stem cells. J Cell Sci. 1994;107:29–37. doi: 10.1242/jcs.107.1.29. [DOI] [PubMed] [Google Scholar]
- 79.Rosendaal M, Mayen A, de Koning A, Dunina-Barkovskaya T, Krenács T, Ploemacher R. Does transmembrane communication through gap junctions enable stem cells to overcome stromal inhibition? Leukemia. 1997;11:1281–9. doi: 10.1038/sj.leu.2400744. [DOI] [PubMed] [Google Scholar]
- 80.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 81.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–3. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 82.Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 83.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–63. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 85.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–40. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 86.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 87.Verdi JM, Bashirullah A, Goldhawk DE, Kubu CJ, Jamali M, Meakin SO, Lipshitz HD. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc Natl Acad Sci USA. 1999;96:10472–6. doi: 10.1073/pnas.96.18.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–53. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- 89.Shen Q, Temple S. Creating asymmetric cell divisions by skewing endocytosis. Sci STKE. 2002;2002:pe52. doi: 10.1126/stke.2002.162.pe52. [DOI] [PubMed] [Google Scholar]
- 90.Li HS, Wang D, Shen Q, Schonemann MD, Gorski JA, Jones KR, Temple S, Jan LY, Jan YN. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–18. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 91.Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–91. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 92.Berdnik D, Török T, González-Gaitán M, Knoblich JA. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002;3:221–31. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 93.Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 94.Vercauteren SM, Sutherland HJ. Constitutively active Notch4 promotes early human hematopoietic progenitor cell maintenance while inhibiting differentiation and causes lymphoid abnormalities in vivo. Blood. 2004;104:2315–22. doi: 10.1182/blood-2004-01-0204. [DOI] [PubMed] [Google Scholar]
- 95.Zhu J, Zhang Y, Joe GJ, Pompetti R, Emerson SG. NF-Ya activates multiple hematopoietic stem cell (HSC) regulatory genes and promotes HSC self-renewal. Proc Natl Acad Sci USA. 2005;102:11728–33. doi: 10.1073/pnas.0503405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:15529–34. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998;95:8801–5. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 100.Leri A, Malhotra A, Liew CC, Kajstura J, Anversa P. Telomerase activity in rat cardiac myocytes is age and gender dependent. J Mol Cell Cardiol. 2000;32:385–90. doi: 10.1006/jmcc.1999.1084. [DOI] [PubMed] [Google Scholar]
- 101.Leri A, Barlucchi L, Limana F, Deptala A, Darzynkiewicz Z, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. Telomerase expression and activity are coupled with myocyte proliferation and preservation of telomeric length in the failing heart. Proc Natl Acad Sci USA. 2001;98:8626–31. doi: 10.1073/pnas.151013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–13. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 103.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–41. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 105.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–79. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 106.Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, delMonte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–86. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 107.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110–8. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 108.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 109.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–9. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 110.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 111.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–9. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 112.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 113.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–9. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blasco MA. Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging. EMBO J. 2005;24:1095–103. doi: 10.1038/sj.emboj.7600598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allsopp RC, Morin GB, Horner JW, DePinho R, Harley CB, Weissman IL. Effect of TERT over-expression on the long-term transplantation capacity of hematopoietic stem cells. Nat Med. 2003;9:369–71. doi: 10.1038/nm0403-369. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 118.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 over-expression. Circ Res. 2007;94:514–24. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 119.Leri A, Kajstura J, Li B, Sonnenblick EH, Beltrami CA, Anversa P, Frishman WH. Cardiomyocyte aging is gender-dependent: the local IGF-1–IGF-1R system. Heart Dis. 2000;2:108–15. [PubMed] [Google Scholar]
- 120.Richards RG, DiAugustine RP, Petrusz P, Clark GC, Sebastian J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci USA. 1996;93:12002–7. doi: 10.1073/pnas.93.21.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–30. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 122.Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res. 1999;59:5917–21. [PubMed] [Google Scholar]
- 123.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–41. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 124.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 125.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–15. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Rakusan K. Cardiac growth, maturation, and aging. In: Zak R, editor. Growth of the Heart in Health and Disease. New York: Raven Press; 1984. pp. 131–64. [Google Scholar]