Abstract
Both direct and long-range interactions between pathogenic Pseudomonas aeruginosa bacteria and their eukaryotic hosts are important in the outcome of infections. For cell-to-cell communication, these bacteria employ the quorum sensing (QS) system to pass on information of the density of the bacterial population and collectively switch on virulence factor production, biofilm formation, and resistance development. Thus, QS allows bacteria to behave as a community to perform tasks which would be impossible for individual cells, e.g., to overcome defense and immune systems and establish infections in higher organisms. This review highlights these aspects of QS and our own recent research on how P. aeruginosa communicates with human cells using the small QS signal molecules N-acyl homoserine lactones (AHL). We focus on how this conversation changes the behavior and function of neutrophils, macrophages, and epithelial cells and on how the signaling machinery in human cells responsible for the recognition of AHL. Understanding the bacteria–host relationships at both cellular and molecular levels is essential for the identification of new targets and for the development of novel strategies to fight bacterial infections in the future.
Keywords: bacteria–host cell interaction, quorum sensing, Pseudomonas aeruginosa, N-acyl homoserine lactones, epithelial cells, innate immune cells, neutrophils, macrophages
INTRODUCTION
Pseudomonas aeruginosa is an environmentally highly adaptable Gram-negative bacterium that infects different host species, including higher plants, invertebrates, and vertebrates. In humans, it elicits acute and chronic infections, typically in critically ill patients having compromised epithelial barriers and immune system or the genetic disorder cystic fibrosis. The outcome of infections and establishment of disease depends on both host defence and bacterial capacities. The latter include its autonomic efficiency to grow, divide, and adapt to the environment, and the ability to sense, and communicate with their neighbors in the population to accomplish cooperative activities, e.g., biofilm formation and production of virulence factors. To do this, P. aeruginosa uses a mechanisms of cell-to-cell communication called quorum sensing (QS). It allows the bacteria to recognize the population density by sensing and measuring the accumulation of specific small signal molecules that members of the community secrete. When the population density is high, the amount of accumulated signals in the environment is accordingly sufficient to activate signaling pathways that alter bacterial gene expression and activate cooperative responses (Rutherford and Bassler, 2012; Schuster et al., 2013; Fazli et al., 2014).
P. aeruginosa QS CONTROL OF VIRULENCE AND BIOFILM FORMATION
Being equipped with a relatively large genome, P. aeruginosa harbors three distinct but subordinated QS systems: two of LuxI/LuxR-type and a third called the Pseudomonas quinolone signal (PQS) system. The two LuxI/LuxR-type systems are N-acylhomoserine lactone (AHL) dependent. In the first, the LuxI homolog LasI produces a freely diffusible N-3-oxo-dodecanoyl-L-homoserine lactone (3O-C12-HSL) that is detected by the LuxR homolog cytoplasmic receptor LasR (More et al., 1996; Parsek et al., 1999). In the second, the LuxI homolog RhlI synthesizes another AHL, N-butyryl-L-homoserine lactone (C4-HSL) that binds to the cytoplasmic receptor RhlR (Ochsner et al., 1994; Pearson et al., 1995); LasR and RhlR are cognate transcriptional regulators. Together, the AHL–LuxR complexes of both circuits control the activation of more than 300 genes in the P. aeruginosa genome. Many of these genes code for production of extracellular products that may be considered as virulence factors, because they can damage host tissues and promote infection, and inflammation. These virulence factors include exotoxin A, elastase, proteases, pyocyanin, lectins, and toxins (Gambello and Iglewski, 1991; Toder et al., 1991; Gambello et al., 1993; Schuster et al., 2003). P. aeruginosa uses the third PQS system to control cooperative responses and gene expression of rhamnolipid, a critical biosurfactant in the late stage of biofilm formation (Ochsner et al., 1994; de Kievit, 2009). The signal molecules of this system are bicyclic compounds, 2-alkyl-4(1H)-quinolones (PQS), produced by PqsABCDH and recognized by the receptor PqsR (Deziel et al., 2004; Diggle et al., 2007). Several of PQS can act not only as a QS signals, but also possess antimicrobial, anticancer, or antiallergenic activities. Together with periplasmic components, outer membrane proteins, phospholipids, toxins, lipopolysaccharide (LPS), and DNA, PQS are typically packed into spherical 50–250 nm membrane vesicles that P. aeruginosa secrete and deliver to the environment. In this way, vesicles have a role in communication and competion in microbial communities and with host cells (Heeb et al., 2011; Tashiro et al., 2013). In other cases, P. aeruginosa can directly convey its products to other cells using the type VI secretion system. In addition, bacteria possesses an intracellular orphan receptor QscR, a LuxR homolog (Lintz et al., 2011) that can bind to 3O-C12-HSL (Oinuma and Greenberg, 2011). This natural target forms dimers with other receptors, i.e., LasR and RhlR, making them inactive and thereby repressing LasRI- and RhlRI-dependent genes leading to prevention of aberrant QS responses before the bacteria reach a quorum in a community (Ledgham et al., 2003). Furthermore, the LasR-3O-C12-HSL, RhlR-C4-HSL, and PQS-PqsR complexes target the regulation of lasI, rhlI, pqsH, and pqsR, which creates an autoinducing feed-forward loop and establishes the tightness and subordination between all three QS systems (Seed et al., 1995; Latifi et al., 1996; Deziel et al., 2004; Xiao et al., 2006; Diggle et al., 2007). Thus, with optimal precision P. aeruginosa QS system directly or indirectly controls the expression of more than 10% of genes for multiple virulence factors, secondary metabolites, swarming motility, and biofilm development (Schuster and Greenberg, 2006; Wagner and Iglewski, 2008; Williams and Camara, 2009).
QS-DRIVEN INTER-KINGDOM SIGNALING
Prokaryotes and eukaryotes have coexisted for many years, during which time they have been exposed to the signals produced and released by the other. The organisms of two kingdoms have also learnt to sense their various molecules including QS signals to influence gene expression and behavior in a process called inter-kingdom signaling (Pacheco and Sperandio, 2009; Gonzalez and Venturi, 2013).
The QS signal molecule AHL affects mammalian host cells and its signaling pathways; this was shown using both in vivo and in vitro models for immune cells, fibroblasts, and epithelial cells. For the biological activity of AHL, a long acyl chain with more than 10 C-atoms, an intact homoserine lactone ring, and oxo- or hydroxyl substitutions are important. AHL triggers and acts through multiple signaling pathways, e.g., calcium mobilization, activation of Rho GTPases, MAPK, and NFκB that control diverse functions and behaviors in host cells, like cytoskeleton remodeling, chemotaxis, migration, phagocytosis, epithelial barrier function, differentiation, proliferation, apoptosis, and production of immune mediators. This topic has been thoroughly investigated by many research groups during the last decade and extensively reviewed recently (Williams and Camara, 2009; Jarosz et al., 2011; Teplitski et al., 2011).
Many organisms, including bacteria, fungi, plants, and mammalian can disturb and inactivate AHL by enzymes in a way called quorum quenching (Czajkowski and Jafra, 2009; Chen et al., 2013). Humans have also developed an ability to destroy AHL via a class of quorum quenching enzymes called paraoxonases (Amara et al., 2011).
Here, we will further focus on mostly our research on how bacterial QS conversation changes the behavior and function of human neutrophils, macrophages, and epithelial cells, and the signaling responsible for the recognition of AHL.
EPITHELIAL BARRIER INTEGRITY AND 3O-C12-HSL
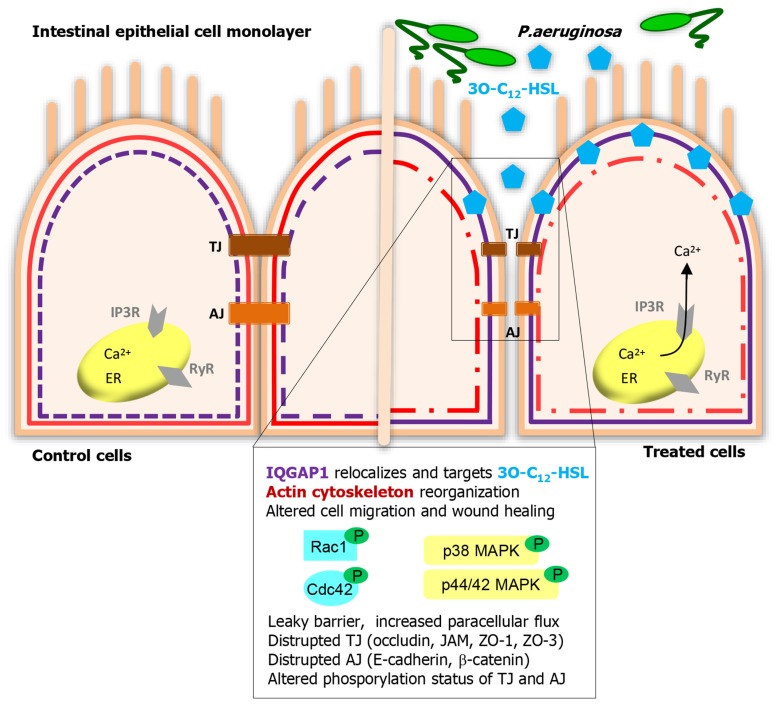
Epithelial cells are positioned strategically to provide both physical and immune barriers to pathogens and other environmental agents. The physical barrier is created by epithelial cell-to-cell junctions that prevent for instance invasion of pathogens and food constituents. The junctions are multiprotein associations of transmembrane proteins connected to cytoplasmic plaque proteins and the actin cytoskeleton (Van Itallie and Anderson, 2004; Balda and Matter, 2008; Furuse, 2010; Ivanov et al., 2010; Capaldo et al., 2014). In a model of polarized epithelial cells, 3O-C12-HSL alters their barrier integrity (Figure 1), as evidenced by decreased transepithelial electrical resistance and increased paracellular flux of different-sized dextrans (Vikstrom et al., 2006). The cell junction complexes occludin–ZO-1, JAM–ZO-1 and E-cadherin–β-catenin were disrupted and the expression and distribution of proteins were affected (Vikstrom et al., 2006, 2009, 2010). Some junctional cytoplasmic proteins play a scaffolding role in linking the actin cytoskeleton and helping to recruit an array of signaling pathways, for example the MAPK cascade molecules, protein kinases, and protein phosphatases (Balda and Matter, 2009; Rodgers and Fanning, 2011). Both p38 and p42/44 MAPK are involved in the 3O-C12-HSL-induced leaky barrier (Vikstrom et al., 2006). Moreover, the disrupted cell junction associations and enhanced paracellular permeability are paralleled by alterations in the phosphorylation status of TJ and AJ proteins (Figure 1; Vikstrom et al., 2009, 2010). Ca2+ is another important component in the regulating of immune and physical barriers of the epithelium. Mucosal epithelial cells are as other cells, equipped with Ca2+-dependent signaling which allows them to initiate immune response to bacteria and their products. The participation of Ca2+ as a second messenger is thus vital to many physiological processes of the epithelia including response to bacteria (Vandenbroucke et al., 2008; Varani, 2011). 3O-C12-HSL can mobilize intracellular calcium through influx from surrounding and release from thapsigargin-sensitive stores via inositol 1,4,5-triphosphate receptors, IP3R (Figure 1; Vikstrom et al., 2010). These receptors are based in the endoplasmatic reticulum (ER) and regulated by their messengers IP3 (Ivanova et al., 2014). Together with ER, the actin cytoskeleton is also critically involved in Ca2+ storage and release as well as in the regulation of store-coupled Ca2+ entry (Lange and Gartzke, 2006).
FIGURE 1.
Human intestinal epithelial cells listen to and act on Pseudomonas aeruginosa 3O-C12-HSL signaling. IQGAP1 is a novel membrane-associated target for 3O-C12-HSL that helps human epithelial cell recognize and response to bacterial QS signaling. Further, 3O-C12-HSL triggers multiple signaling pathways, those include calcium mobilization, activation of Rho GTPases Rac1/Cdc42, MAPK, protein kinases, and protein phosphotases. These can control diverse functions and behaviors in epithelial cells, like cytoskeleton remodeling, migration, wound healing, barrier integrity, and paracellular permeability.
3O-C12-HSL-MEDIATED EPITHELIAL MIGRATION AND WOUND HEALING
Establishing and contributing to both physical and immune barriers, the epithelial cells also have to be constantly renewed and prepared to move. After injury, caused by for example oxidative stress, inflammation and infection, the epithelium undergo a wound-healing process. This is dependent on the balance of migration, proliferation, and differentiation of the cells within the wound area (Sturm and Dignass, 2008). Restitution of the epithelium requires extensive reorganization of the cytoskeleton and cellular junctions, regulated by the Rho family of small GTPases, like Rho, Rac, and Cdc42 (Kjoller and Hall, 1999; Evers et al., 2000). 3O-C12-HSL modulates epithelial cell migration in a dose- and time-dependent manner (Karlsson et al., 2012b) inducing remodeling of cytoskeletal actin (Vikstrom et al., 2006). The upstream effectors of this, and thereby regulators of cell shape and motility are the previously mentioned Rho family GTPases Rac1 and Cdc42. The Rho GTPases cycle between an active and inactive status by binding GTP and by hydrolysis of GTP to GDP, acting as molecular “on-off” switch (Wennerberg and Der, 2004). The signaling can also be modulated by their phosphorylation state via AKT1 kinase (Kwon et al., 2000). Together with the effect of 3O-C12-HSL on cell migration, low doses of 3O-C12-HSL over shorter time spans initiated phosphorylation of Rac1/Cdc42, whereas high concentrations rapidly decrease the level of phosphorylated Rac/Cdc42 (Karlsson et al., 2012b). Taken together, 3O-C12-HSL can alter barrier functions and migration in epithelial cells (Figure 1).
The immune barrier of epithelial cells is potentiated through detection of antigens and rapid signaling to recruit phagocytes to the site of infection and tissue damage. They paracellulary transmigrate across the epithelium, and cell junction protein JAM mediate this process as it is also receptor for leukocyte integrins (Zen and Parkos, 2003). Phagocytes, like neutrophils and macrophages, are key players in the innate immune defenses, providing protection from invading bacteria and tissue damage. Bacterial QS conversation may change their behavior and function and the signaling responsible for the sensing of AHL.
AHL AS A STRONG CHEMOATTRACTANT FOR NEUTROPHILS
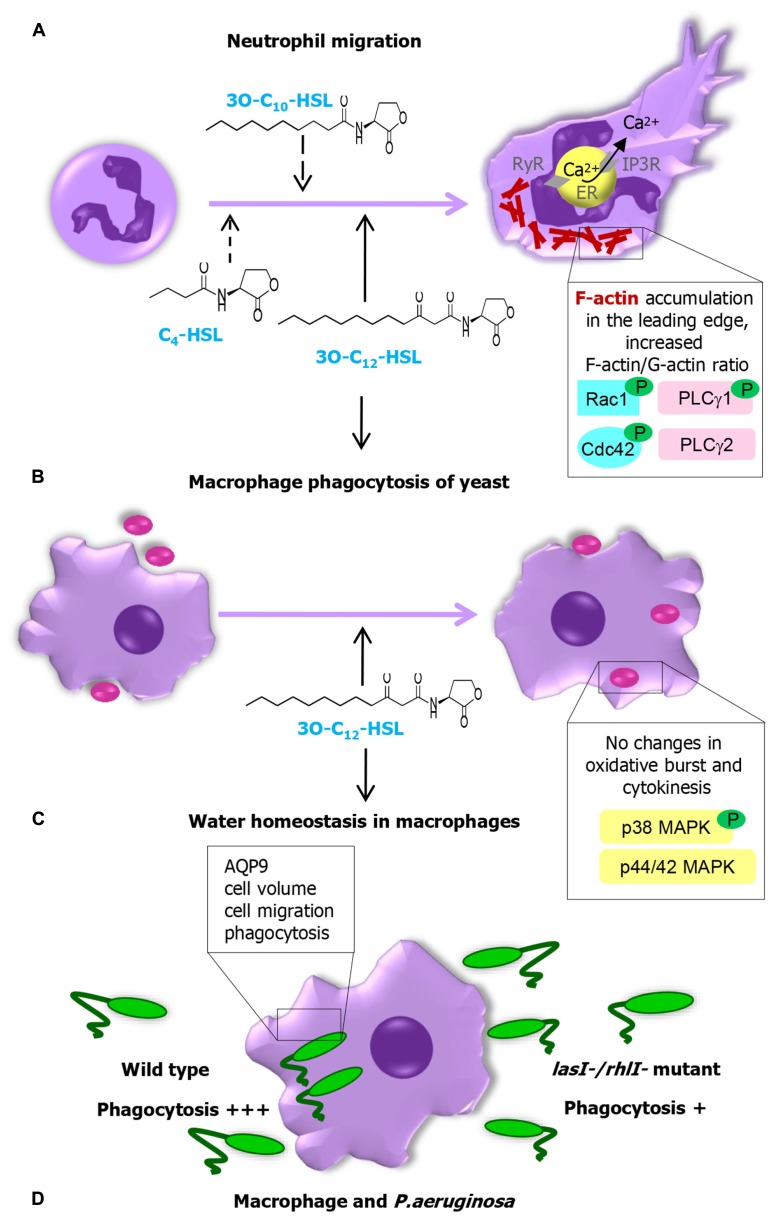
Neutrophils are small and rapidly moving phagocytes with a short life span in circulation, always appearing at the early onset of infection. They sense the bacteria very well, which gives them a head start in controlling inflammation, infection, and biofilm formation. Following activation by bacterial products or immune stimuli, they execute specialized functions of chemotaxis, phagocytosis, and generation of reactive oxygen species (ROS). 3-oxo-C12-HSL and 3-oxo-C10-HSL, but not C4-HSL, can act as a strong chemoattractants for human neutrophils and induce their migration to the site of AHL in a dose-dependent manner (Zimmermann et al., 2006; Karlsson et al., 2012a). This put long chain AHL in a position among potent bona fide chemoattractants, such as chemokines, cytokines (IL-8 and GM-CSF), leukotriene B4, platelet activating factor, products from bacteria (formylated peptides and LPS), signals from dying cells (such as TNF-α), although higher molar concentrations of AHL were required (Figure 2A). Chemotaxis and migration towards AHL are paralleled by cytoskeletal F-actin accumulation in the leading edge of neutrophils, by increased F-actin-to-G-actin ratio and via activation of Rho GTPases Rac1 and Cdc42 (Karlsson et al., 2012a). Rac1 regulates the formation of lamellipodia protrusions and membrane ruffles, and Cdc42 triggers filopodial extensions (Kjoller and Hall, 1999; Evers et al., 2000). Two long acyl chain AHL with 10 and 12 C-atoms activated phospholipase Cγ1 and mobilized intracellular calcium via the IP3R at endoplasmic reticulum, whereas an AHL with a short acyl chain failed to do this (Karlsson et al., 2012a; Figure 2A). Thus, recognition of AHL QS signals by neutrophils may play a critical role in their recruitment during infections and early stage of biofilm formation.
FIGURE 2.
Pseudomonas aeruginosa AHL changes the behavior and function of human innate immune cells and the signaling machinery responsible for the recognition of AHL. (A) Long chain AHLs are strong chemoattractants for neutrophils. Chemotaxis and migration towards these AHLs are paralleled by cytoskeletal F-actin accumulation in the leading edge and increased F-actin-to-G-actin ratio via activation of small GTPases Rac1/Cdc42, phosphorylation of PLCγ1 and mobilization of calcium via IP3R at ER. (B) 3O-C12-HSL and macrophage activity. 3O-C12-HSL increases phagocytic capacity of macrophages via the p38, but not p42/44 MAPK, having no influence on their oxidative metabolism, or production of cytokines. (C) 3O-C12-HSL-mediated water homeostasis in macrophages. (D) Macrophage phagocytosis of P. aeruginosa wild type and lasI-/rhlI- mutant lacking production of QS molecules 3O-C12-HSL and C4-HSL.
AHL AND MACROPHAGE ACTIVITY
Other important players in the innate immune system are macrophages, cells differentiated from monocytes in tissues and stimulated by neutrophils to come to the site of infection after them. Macrophages harbor the characteristics of innate immune cells and traits that initiate mechanisms of adaptive immunity. In response to microbial antigens, they can display strong phagocytic activity, generation of “killer” anti-microbial ROS, NO, “tissue repair” ornithine, pro-inflammatory cytokines stimulating other immune cells to respond to pathogens. In a macrophage phagocytosis model with Saccharomyces cerevisiae as preys, 3O-C12-HSL increased phagocytic capacity via the p38, but not the p42/44 MAPK signaling pathway (Figure 2B). It had no influence on macrophages oxidative metabolism, the level of ROS or production of cytokines (Vikstrom et al., 2005). In P. aeruginosa, two functional QS genes, lasI, and rhlI, which are responsible for synthesis of signal molecules 3O-C12-HSL and C4-HSL strongly contribute to effective macrophage phagocytosis of this pathogen. AQP9 controls cell migration by accumulation in membrane protrusions and domains preceding their expansions (Karlsson et al., 2011, 2013a, b). This trigger actin cytoskeleton to be remodeled and further regulate macrophage shape, migration, and phagocytosis of microorganisms (Figure 2D). 3O-C12-HSL caused a rapid and prolonged cell-volume increase controlled by AQP9 in human macrophages (Figure 2C), which can be a danger signal and protection mechanism (Hoffmann et al., 2009; Compan et al., 2012). AQP9 is involved in Escherichia coli LPS-enhanced brain water content and blood-brain barrier permeability (Wang et al., 2009) and has been identified as a one of markers of chronic inflammation in patients with psoriasis, rheumatoid arthritis, and inflammatory bowel disease (Mesko et al., 2010).
HUMAN CELL TARGETS FOR 3O-C12-HSL
Identifying targets for AHL allows better understanding of QS communication during host–bacteria interactions. The recognition of 3O-C12-HSL by mammalian cells probably does not rely on pattern-recognition receptors (PRRs) that usually sense invariant microbial motifs (PAMPs, pathogen-associated molecular patterns) present on or shed from bacteria (LPS, lipoteichoic acid, flagellin, and DNA). The canonical class of PRRs, the membrane-bound toll-like receptors (TLRs) located on immune cells, do not interact with 3O-C12-HSL (Kravchenko et al., 2006). Still, as for TLR activation, AHL can trigger and act through multiple signaling pathways, which include calcium signaling, activation of Rho GTPases, MAPK, and transcription factor NFκB that control expression of pro-inflammatory mediators, cytokines, chemokines, enzymes, and interferones (Smith et al., 2002; Shiner et al., 2006; Kravchenko et al., 2008; Mayer et al., 2011; Karlsson et al., 2012a; Glucksam-Galnoy et al., 2013). These mediators are involved in the coordination of innate immune response and recruit effector cells of the adaptive immune system to the site of the infection to combat the invading bacteria.
During the recognition of 3O-C12-HSL by mammalian cells, this lipophilic molecule with a long acyl chain and an intact homoserine lactone ring may interact directly with phospholipids in model membrane systems and in T-cell membranes (Davis et al., 2010). On entering host cells (Shiner et al., 2004; Ritchie et al., 2007), 3O-C12-HSL can utilize intracellular nuclear peroxisome proliferator-activated receptors (PPAR) to affect NF-κB signaling (Jahoor et al., 2008; Cooley et al., 2010). The binding of 3O-C12-HSL to nuclear PPAR does not exclude the existence of cell surface or membrane-associated receptors, which after binding to 3O-C12-HSL likely help phosphorylate phospholipase C and evoke an increase in intracellular calcium (Shiner et al., 2006; Davis et al., 2010; Karlsson et al., 2012a).
Several groups have designed probes and affinity matrixes which could be utilized to detect the mammalian target of 3O-C12-HSL (Dubinsky et al., 2009; Garner et al., 2011; Praneenararat et al., 2011; Dubinsky et al., 2013). Using a biotin-based 3O-C12-HSL probe, LC-MS/MS, and super-resolution microscopy, the IQ-motif-containing GTPase-activating protein IQGAP1 was identified as a putative human target for 3O-C12-HSL in epithelial cells (Figure 1; Karlsson et al., 2012b). IQGAP1 contains multiple domains for binding other proteins and localizes in the leading edge of migrating cells (Briggs and Sacks, 2003; Noritake et al., 2004; Bensenor et al., 2007). It directly interacts with and stabilizes the Rho-family GTPases, Rac1, and Cdc42 in their GTP-bound state (Swart-Mataraza et al., 2002; Briggs and Sacks, 2003), playing an essential role in cell shape, vesicle trafficking, and directional migration (Bensenor et al., 2007). It likely mediates these processes through its other domains, linking it to actin, myosin, β-catenin, E-cadherin, calmodulin, and MAPK (Noritake et al., 2005; Brandt and Grosse, 2007), which allows it to function as a true scaffolding protein (Figure 1). The interaction of 3O-C12-HSL with the cell membrane, diffusion and entering into the cytoplasm, targeting of IQGAP1 and PPAR do not exclude each other. It has, for example been shown that different types of lipids, such as chemoattractant leukotriene B4, can bind to both the cell surface receptor LTB4, and nuclear PPAR.
CONCLUDING REMARKS
During the last decade of research remarkable insight has been gained into the mechanisms of bacterial QS communication and that P. aeruginosa 3O-C12-HSL plays at least two distinct roles. Besides regulating bacterial social behavior and offering density-dependent fitness advantages, expression of virulence factors, and biofilm development in bacteria, it also plays a crucial role in the behavior of eukaryotic host cells regulating various vital functions. Moreover, as QS circuits often control virulence and biofilm, there is a high interest in interfering with QS as a new strategy to overcome infectious diseases and biofilm formation (Amara et al., 2011; Heeb et al., 2011; Jakobsen et al., 2013; LaSarre and Federle, 2013).
Conflict of Interest Statement
The authors are not aware of any affiliations, memberships, patents, copyrights, funding, payment, service or other activities or relationships that might be perceived as an affecting the objectivity of this review.
Acknowledgments
We apologize to those colleagues whose work could not be cited owing to space constraints. We thank our colleagues at University of Linköping for joint work, collaborations, insightful discussions and helpful suggestions, and especially Professor Karl-Eric Magnusson, Professor Peter Konradsson, Dr. Lan Bui, Dr. Maria Turkina, Dr. Thommie Karlsson, Dr. Augustas Pivorunas, Ms. Farah Musse, Ms. Olena Yakymenko, Ms. Joanna Gradin, Ms. Susanna Bohman, Mr. Risul Amin, and Ms. Andrea Molinas. We also thank Dr. Hans Blom and Professor Hjalmar Brismar (Advanced Light Microscopy Group, SciLifeLab Stockholm, Sweden). We recognize the help from Cayman Chemical (Ann Arbor, MI, USA) in the design and synthesis of AHL probes according to our suggestions. The work was supported by grants from the Swedish Research Council, the European Science foundation (TraPPs Euromembrane project), Euro-BioImaging Proof-of Concept Studies, the King Gustaf V 80 Year Foundation, Magnus Bergvalls Foundation and the Faculty of Health Sciences, Linköping University.
REFERENCES
- Amara N., Krom B. P., Kaufmann G. F., Meijler M. M. (2011). Macromolecular inhibition of quorum sensing: enzymes, antibodies, and beyond. Chem. Rev. 111 195–208 10.1021/cr100101c [DOI] [PubMed] [Google Scholar]
- Balda M. S., Matter K. (2008). Tight junctions at a glance. J. Cell Sci. 121(Pt22) 3677–3682 10.1242/jcs.023887 [DOI] [PubMed] [Google Scholar]
- Balda M. S., Matter K. (2009). Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta 1788 761–767 10.1016/j.bbamem.2008.11.024 [DOI] [PubMed] [Google Scholar]
- Bensenor L. B., Kan H. M., Wang N., Wallrabe H., Davidson L. A., Cai Y., et al. (2007). IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J. Cell Sci. 120(Pt 4) 658–669 10.1242/jcs.03376 [DOI] [PubMed] [Google Scholar]
- Brandt D. T., Grosse R. (2007). Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 8 1019–1023 10.1038/sj.embor.7401089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. W., Sacks D. B. (2003). IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 542 7–11 10.1016/S0014-5793(03)00333-8 [DOI] [PubMed] [Google Scholar]
- Capaldo C. T., Farkas A. E., Nusrat A. (2014). Epithelial adhesive junctions. F1000Prime Rep. 6:1 10.12703/P6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Gao Y., Chen X., Yu Z., Li X. (2013). Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int. J. Mol. Sci. 14 17477–17500 10.3390/ijms140917477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V., Baroja-Mazo A., Lopez-Castejon G., Gomez A. I., Martinez C. M., Angosto D., et al. (2012). Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37 487–500 10.1016/j.immuni.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Cooley M. A., Whittall C., Rolph M. S. (2010). Pseudomonas signal molecule 3-oxo-C12-homoserine lactone interferes with binding of rosiglitazone to human PPARgamma. Microbes Infect. 12 231–237 10.1016/j.micinf.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Czajkowski R., Jafra S. (2009). Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 56 1–16 [PubMed] [Google Scholar]
- Davis B., Jensen M. R., Williams P, O’Shea P. (2010). The interaction of N-acylhomoserine lactone quorum sensing signaling molecules with biological membranes: implications for inter-kingdom signaling. PLoS ONE 5:e13522 10.1371/journal.pone.0013522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kievit T. R. (2009). Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 11 279–288 10.1111/j.1462-2920.2008.01792.x [DOI] [PubMed] [Google Scholar]
- Deziel E., Lepine F., Milot S., He J., Mindrinos M. N., Tompkins R. G., et al. (2004). Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. U.S.A. 101 1339–1344 10.1073/pnas.0307694100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle S. P., Matthijs S., Wright V. J., Fletcher M. P., Chhabra S. R., Lamont I. L., et al. (2007). The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 14 87–96 10.1016/j.chembiol.2006.11.014 [DOI] [PubMed] [Google Scholar]
- Dubinsky L., Delago A., Amara N., Krief P., Rayo J., Zor T., et al. (2013). Species selective diazirine positioning in tag-free photoactive quorum sensing probes. Chem. Commun. (Camb.) 49 5826–5828 10.1039/c3cc43092h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky L., Jarosz L. M., Amara N., Krief P., Kravchenko V. V., Krom B. P., et al. (2009). Synthesis and validation of a probe to identify quorum sensing receptors. Chem. Commun. (Camb.) 47 7378–7380 10.1039/b917507e [DOI] [PubMed] [Google Scholar]
- Evers E. E., Zondag G. C., Malliri A., Price L. S., ten Klooster J. P., van der Kammen R. A., et al. (2000). Rho family proteins in cell adhesion and cell migration. Eur. J. Cancer 36 1269–1274 10.1016/S0959-8049(00)00091-5 [DOI] [PubMed] [Google Scholar]
- Fazli M., Almblad H., Rybtke M. L., Givskov M., Eberl L., Tolker-Nielsen T. (2014). Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 10.1111/1462-2920.12448 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Furuse M. (2010). Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2:a002907 10.1101/cshperspect.a002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. (1991). Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173 3000–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Kaye S., Iglewski B. H. (1993). LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61 1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A. L., Yu J., Struss A. K., Lowery C. A., Zhu J., Kim S. K., et al. (2011). Synthesis of ‘clickable’ acylhomoserine lactone quorum sensing probes: unanticipated effects on mammalian cell activation. Bioorg. Med. Chem. Lett. 21 2702–2705 10.1016/j.bmcl.2010.11.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glucksam-Galnoy Y., Sananes R., Silberstein N., Krief P., Kravchenko V. V., Meijler M. M., et al. (2013). The bacterial quorum-sensing signal molecule N-3-oxo-dodecanoyl-L-homoserine lactone reciprocally modulates pro- and anti-inflammatory cytokines in activated macrophages. J. Immunol. 191 337–344 10.4049/jimmunol.1300368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. F., Venturi V. (2013). A novel widespread interkingdom signaling circuit. Trends Plant Sci. 18 167–174 10.1016/j.tplants.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Heeb S., Fletcher M. P., Chhabra S. R., Diggle S. P., Williams P., Camara M. (2011). Quinolones: from antibiotics to autoinducers. FEMS Microbiol. Rev. 35 247–274 10.1111/j.1574-6976.2010.00247.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009). Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89 193–277 10.1152/physrev.00037.2007 [DOI] [PubMed] [Google Scholar]
- Ivanov A. I., Parkos C. A., Nusrat A. (2010). Cytoskeletal regulation of epithelial barrier function during inflammation. Am. J. Pathol. 177 512–524 10.2353/ajpath.2010.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova H., Vervliet T., Missiaen L., Parys J. B., De Smedt H., Bultynck G. (2014). Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys. Acta 10.1016/j.bbamcr.2014.03.007 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jahoor A., Patel R., Bryan A., Do C., Krier J., Watters C., et al. (2008). Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J. Bacteriol. 190 4408–4415 10.1128/JB.01444-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen T. H., Bjarnsholt T., Jensen P. O., Givskov M., Hoiby N. (2013). Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol. 8 901–921 10.2217/fmb.13.57 [DOI] [PubMed] [Google Scholar]
- Jarosz L. M., Ovchinnikova E. S., Meijler M. M., Krom B. P. (2011). Microbial spy games and host response: roles of a Pseudomonas aeruginosa small molecule in communication with other species. PLoS Pathog. 7:e1002312 10.1371/journal.ppat.1002312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson T., Bolshakova A., Magalhaes M. A., Loitto V. M., Magnusson K. E. (2013a). Fluxes of water through aquaporin 9 weaken membrane-cytoskeleton anchorage and promote formation of membrane protrusions. PLoS ONE 8:e59901 10.1371/journal.pone.0059901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson T., Lagerholm B. C., Vikstrom E., Loitto V. M., Magnusson K. E. (2013b). Water fluxes through aquaporin-9 prime epithelial cells for rapid wound healing. Biochem. Biophys. Res. Commun. 430 993–998 10.1016/j.bbrc.2012.11.125 [DOI] [PubMed] [Google Scholar]
- Karlsson T., Glogauer M., Ellen R. P., Loitto V. M., Magnusson K. E., Magalhaes M. A. (2011). Aquaporin 9 phosphorylation mediates membrane localization and neutrophil polarization. J. Leukoc. Biol. 90 963–973 10.1189/jlb.0910540 [DOI] [PubMed] [Google Scholar]
- Karlsson T., Musse F., Magnusson K. E., Vikstrom E. (2012a). N-Acylhomoserine lactones are potent neutrophil chemoattractants that act via calcium mobilization and actin remodeling. J. Leukoc. Biol. 91 15–26 10.1189/jlb.0111034 [DOI] [PubMed] [Google Scholar]
- Karlsson T., Turkina M. V., Yakymenko O., Magnusson K. E., Vikstrom E. (2012b). The Pseudomonas aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migration. PLoS Pathog. 8:e1002953 10.1371/journal.ppat.1002953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjoller L., Hall A. (1999). Signaling to Rho GTPases. Exp. Cell Res. 253 166–179 10.1006/excr.1999.4674 [DOI] [PubMed] [Google Scholar]
- Kravchenko V. V., Kaufmann G. F., Mathison J. C., Scott D. A., Katz A. Z., Grauer D. C., et al. (2008). Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science 321 259–263 10.1126/science.1156499 [DOI] [PubMed] [Google Scholar]
- Kravchenko V. V., Kaufmann G. F., Mathison J. C., Scott D. A., Katz A. Z., Wood M. R., et al. (2006). N-(3-oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J. Biol. Chem. 281 28822–28830 10.1074/jbc.M606613200 [DOI] [PubMed] [Google Scholar]
- Kwon T., Kwon D. Y., Chun J., Kim J. H., Kang S. S. (2000). Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J. Biol. Chem. 275 423–428 10.1074/jbc.275.1.423 [DOI] [PubMed] [Google Scholar]
- Lange K., Gartzke J. (2006). F-actin-based Ca signaling-a critical comparison with the current concept of Ca signaling. J. Cell. Physiol. 209 270–287 10.1002/jcp.20717 [DOI] [PubMed] [Google Scholar]
- LaSarre B., Federle M. J. (2013). Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 77 73–111 10.1128/MMBR.00046-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi A., Foglino M., Tanaka K., Williams P., Lazdunski A. (1996). A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21 1137–1146 10.1046/j.1365-2958.1996.00063.x [DOI] [PubMed] [Google Scholar]
- Ledgham F., Ventre I., Soscia C., Foglino M., Sturgis J. N., Lazdunski A. (2003). Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol. Microbiol. 48 199–210 10.1046/j.1365-2958.2003.03423.x [DOI] [PubMed] [Google Scholar]
- Lintz M. J., Oinuma K., Wysoczynski C. L., Greenberg E. P., Churchill M. E. (2011). Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc. Natl. Acad. Sci. U.S.A. 108 15763–15768 10.1073/pnas.1112398108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sheridan J. A., Blohmke C. J., Turvey S. E., Hancock R. E. (2011). The Pseudomonas aeruginosa autoinducer 3O-C12 homoserine lactone provokes hyperinflammatory responses from cystic fibrosis airway epithelial cells. PLoS ONE 6:e16246 10.1371/journal.pone.0016246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesko B., Poliska S., Szegedi A., Szekanecz Z., Palatka K., Papp M., et al. (2010). Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med. Genomics 3:15 10.1186/1755-8794-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More M. I., Finger L. D., Stryker J. L., Fuqua C., Eberhard A., Winans S. C. (1996). Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272 1655–1658 10.1126/science.272.5268.1655 [DOI] [PubMed] [Google Scholar]
- Noritake J., Fukata M., Sato K., Nakagawa M., Watanabe T., Izumi N., et al. (2004). Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell-cell contact. Mol. Biol. Cell 15 1065–1076 10.1091/mbc.E03-08-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake J., Watanabe T., Sato K., Wang S., Kaibuchi K. (2005). IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 118(Pt 10) 2085–2092 10.1242/jcs.02379 [DOI] [PubMed] [Google Scholar]
- Ochsner U. A., Koch A. K., Fiechter A., Reiser J. (1994). Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176 2044–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinuma K., Greenberg E. P. (2011). Acyl-homoserine lactone binding to and stability of the orphan Pseudomonas aeruginosa quorum-sensing signal receptor QscR. J. Bacteriol. 193 421–428 10.1128/JB.01041-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A. R., Sperandio V. (2009). Inter-kingdom signaling: chemical language between bacteria and host. Curr. Opin. Microbiol. 12 192–198 10.1016/j.mib.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsek M. R., Val D. L., Hanzelka B. L., Cronan J. E., Jr., Greenberg E. P. (1999). Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. U.S.A. 96 4360–4365 10.1073/pnas.96.8.4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Passador L., Iglewski B. H., Greenberg E. P. (1995). A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 92 1490–1494 10.1073/pnas.92.5.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praneenararat T., Beary T. M., Breitbach A. S., Blackwell H. E. (2011). Synthesis and application of an N-acylated l-homoserine lactone derivatized affinity matrix for the isolation of quorum sensing signal receptors. Bioorg. Med. Chem. Lett. 21 5054–5057 10.1016/j.bmcl.2011.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. J., Whittall C., Lazenby J. J., Chhabra S. R., Pritchard D. I., Cooley M. A. (2007). The immunomodulatory Pseudomonas aeruginosa signalling molecule N-(3-oxododecanoyl)-L-homoserine lactone enters mammalian cells in an unregulated fashion. Immunol. Cell Biol. 85 596–602 10.1038/sj.icb.7100090 [DOI] [PubMed] [Google Scholar]
- Rodgers L. S., Fanning A. S. (2011). Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton (Hoboken) 68 653–660 10.1002/cm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S. T., Bassler B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med 2:a012427 10.1101/cshperspect.a012427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M., Greenberg E. P. (2006). A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296 73–81 10.1016/j.ijmm.2006.01.036 [DOI] [PubMed] [Google Scholar]
- Schuster M., Lostroh C. P., Ogi T., Greenberg E. P. (2003). Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185 2066–2079 10.1128/JB.185.7.2066-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M., Sexton D. J., Diggle S. P., Greenberg E. P. (2013). Acyl-homoserine lactone quorum sensing: from evolution to application. Annu. Rev. Microbiol. 67 43–63 10.1146/annurev-micro-092412-155635 [DOI] [PubMed] [Google Scholar]
- Seed P. C., Passador L., Iglewski B. H. (1995). Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177 654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner E. K., Reddy S., Timmons C., Li G., Williams S. C., Rumbaugh K. P. (2004). Construction of a bacterial autoinducer detection system in mammalian cells. Biol. Proced. Online 6 268–276 10.1251/bpo98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner E. K., Terentyev D., Bryan A., Sennoune S., Martinez-Zaguilan R., Li G., et al. (2006). Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell Microbiol. 8 1601–1610 10.1111/j.1462-5822.2006.00734.x [DOI] [PubMed] [Google Scholar]
- Smith R. S., Kelly R., Iglewski B. H., Phipps R. P. (2002). The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169 2636–2642 10.4049/jimmunol.169.5.2636 [DOI] [PubMed] [Google Scholar]
- Sturm A., Dignass A. U. (2008). Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 14 348–353 10.3748/wjg.14.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart-Mataraza J. M., Li Z., Sacks D. B. (2002). IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 277 24753–24763 10.1074/jbc.M111165200 [DOI] [PubMed] [Google Scholar]
- Tashiro Y., Yawata Y., Toyofuku M., Uchiyama H., Nomura N. (2013). Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 28 13–24 10.1264/jsme2.ME12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitski M., Mathesius U., Rumbaugh K. P. (2011). Perception and degradation of N-acyl homoserine lactone quorum sensing signals by mammalian and plant cells. Chem. Rev. 111 100–116 10.1021/cr100045m [DOI] [PubMed] [Google Scholar]
- Toder D. S., Gambello M. J., Iglewski B. H. (1991). Pseudomonas aeruginosa LasA: a second elastase under the transcriptional control of lasR. Mol. Microbiol. 5 2003–2010 10.1111/j.1365-2958.1991.tb00822.x [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M. (2004). The molecular physiology of tight junction pores. Physiology (Bethesda) 19 331–338 10.1152/physiol.00027.2004 [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E., Mehta D., Minshall R., Malik A. B. (2008). Regulation of endothelial junctional permeability. Ann. N. Y. Acad. Sci. 1123 134–145 10.1196/annals.1420.016 [DOI] [PubMed] [Google Scholar]
- Varani J. (2011). Calcium, calcium-sensing receptor and growth control in the colonic mucosa. Histol. Histopathol. 26 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom E., Bui L., Konradsson P., Magnusson K. E. (2009). The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation-dependent mechanisms. Exp. Cell Res. 315 313–326 10.1016/j.yexcr.2008.10.044 [DOI] [PubMed] [Google Scholar]
- Vikstrom E., Bui L., Konradsson P., Magnusson K. E. (2010). Role of calcium signalling and phosphorylations in disruption of the epithelial junctions by Pseudomonas aeruginosa quorum sensing molecule. Eur. J. Cell Biol. 89 584–597 10.1016/j.ejcb.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Vikstrom E., Magnusson K. E., Pivoriunas A. (2005). The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone stimulates phagocytic activity in human macrophages through the p38 MAPK pathway. Microbes Infect. 7 1512–1518 10.1016/j.micinf.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Vikstrom E., Tafazoli F., Magnusson K. E. (2006). Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-l-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. FEBS Lett. 580 6921–6928 10.1016/j.febslet.2006.11.057 [DOI] [PubMed] [Google Scholar]
- Wagner V. E., Iglewski B. H. (2008). P. aeruginosa Biofilms in CF Infection. Clin. Rev. Allergy Immunol. 35 124–134 10.1007/s12016-008-8079-9 [DOI] [PubMed] [Google Scholar]
- Wang H., Jin R., Tian P., Zhuo Z. (2009). Enhanced expression of aquaporin-9 in rat brain edema induced by bacterial lipopolysaccharides. J. Huazhong Univ. Sci. Technolog. Med. Sci. 29 150–155 10.1007/s11596-009-0203-4 [DOI] [PubMed] [Google Scholar]
- Wennerberg K., Der C. J. (2004). Rho–family GTPases: it’s not only Rac and Rho (and I like it). J. Cell Sci. 117(Pt 8) 1301–1312 10.1242/jcs.01118 [DOI] [PubMed] [Google Scholar]
- Williams P., Camara M. (2009). Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 12 182–191 10.1016/j.mib.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Xiao G., He J., Rahme L. G. (2006). Mutation analysis of the Pseudomonas aeruginosa mvfR and pqsABCDE gene promoters demonstrates complex quorum-sensing circuitry. Microbiology 152(Pt 6) 1679–1686 10.1099/mic.0.28605-0 [DOI] [PubMed] [Google Scholar]
- Zen K., Parkos C. A. (2003). Leukocyte-epithelial interactions. Curr. Opin. Cell Biol. 15 557–564 10.1016/S0955-0674(03)00103-0 [DOI] [PubMed] [Google Scholar]
- Zimmermann S., Wagner C., Muller W., Brenner-Weiss G., Hug F., Prior B., et al. (2006). Induction of neutrophil chemotaxis by the quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Infect. Immun. 74 5687–5692 10.1128/IAI.01940-05 [DOI] [PMC free article] [PubMed] [Google Scholar]