Abstract
Phosphorus (P), an element required for plant growth, fruit set, fruit development, and fruit ripening, can be deficient or unavailable in agricultural soils. Previously, it was shown that over-expression of a proton-pyrophosphatase gene AVP1/AVP1D (AVP1DOX) in Arabidopsis, rice, and tomato resulted in the enhancement of root branching and overall mass with the result of increased mineral P acquisition. However, although AVP1 over-expression also increased shoot biomass in Arabidopsis, this effect was not observed in tomato under phosphate-sufficient conditions. AVP1DOX tomato plants exhibited increased rootward auxin transport and root acidification compared with control plants. AVP1DOX tomato plants were analysed in detail under limiting P conditions in greenhouse and field trials. AVP1DOX plants produced 25% (P=0.001) more marketable ripened fruit per plant under P-deficient conditions compared with the controls. Further, under low phosphate conditions, AVP1DOX plants displayed increased phosphate transport from leaf (source) to fruit (sink) compared to controls. AVP1DOX plants also showed an 11% increase in transplant survival (P<0.01) in both greenhouse and field trials compared with the control plants. These results suggest that selection of tomato cultivars for increased proton pyrophosphatase gene expression could be useful when selecting for cultivars to be grown on marginal soils.
Key words: Fruit development, H+-pyrophosphatase, phosphorus, root development, tomato, transplant efficiency.
Introduction
The essential macronutrient phosphorus (P) is relatively unavailable to plants in many soils when present as calcium salts or iron–aluminum oxide complexes (Holford, 1997). Tomatoes grown in soils with low phosphorus availability manifest deficiency symptoms such as stunting and leaf darkening before flowering and almost always exhibit reduced fruit size and production (Havlin et al., 2005). For this reason, fertilization of tomato crops with P just before fruit set is a standard component of cultivation practices (Egel et al., 2008).
Rising fertilizer costs and environmental damage resulting from phosphorus run-off have stimulated interest in strategies to enhance the availability of existing soil phosphorus. Inoculation of soils with mycorrhizal fungi and no-till agricultural practices have made a substantial contribution to the reduction of P-fertilizer applications (Bolan et al., 1991). Tobacco plants engineered to overproduce the organic acid citrate have also been shown to enhance P uptake (López-Bucio, 2000). More recently, over-expression of the proton-pyrophosphatase in Arabidopsis, rice, tomato, and maize was reported to enhance P utilization (Yang et al., 2007; Pei et al., 2012). By contrast, the loss or the reduction of proton-pyrophosphatase AVP1 function in Arabidopsis results in reduced shoot and root growth (Li et al., 2005, Ferjani et al., 2011).
AVP1 is a proton pump that utilizes the energy released by hydrolysis of a pyrophosphate (PPi) into two molecules of phosphate (Pi) to acidify the vacuole. AVP1 is thought to function primarily in young, growing tissues where V-ATPase activity is reduced by depleted ATP reserves (Shiratake et al., 1997; Zhen et al., 1997; Maeshima, 2000; Heinonen, 2001). Although primarily localized to the tonoplast (Drozdowicz and Rea, 2001; Li et al, 2005), AVP1 was also localized to the plasma membrane (Langhans et al., 2001; Alexandersson et al., 2004; Paez-Valencia et al., 2011) and over-expression of AVP1 results in increased abundance and activity of H+-ATPase at the plasma membrane in Arabidopsis (Li et al., 2005; Yang et al., 2007; Undurraga et al., 2012). The increased H+-ATPase abundance observed in AVP1 over-expressors accounts for the increased apoplastic acidification observed in these lines. A direct result of this acidification is accelerated transport of the phytohormone auxin, increased lateral root branching, augmented exudation of organic acids, and enhanced P mobilization (Li et al., 2005; Yang et al., 2007). AVP1OX Arabidopsis and rice developed larger roots and shoots under both normal and low Pi conditions. Further, Arabidopsis AVP1OX plants displayed drought and salt tolerance (Gaxiola et al., 2001). However, tomato plants over-expressing of AVP1D, the E229D gain-of-function mutant of AVP1 that has increased PPi hydrolysis and H+-translocation activity (Zhen et al., 1997; Park et al., 2005), developed shoots and fruits comparable to control plants under normal Pi conditions (Yang et al., 2007). Further, the drought resistance observed in greenhouse trails (Park et al., 2005) could not be repeated in the field trials and long-term drought resistance was not observed in AVP1DOX tomato plants (data not shown).
The differences observed in tomato plants prompted an analysis, in larger scale and more detail, of the response of these transgenic lines to Pi deficiency under greenhouse and field conditions. The analysis of AVP1DOX tomato performance in greenhouse and field trials under moderate Pi-deficient conditions, similar to those encountered in Indiana farm practice as described by the Midwest Vegetable Production Guide for Commercial Growers 2008 (Egel et al., 2008), is reported here. In greenhouse trials, AVP1DOX plants displayed significantly increased shoot and root mass, and fruit production under low Pi conditions, but increased only in root mass under normal Pi conditions. In the field trials, the total fruit fresh weights of AVP1DOX plants were only slightly higher than those of control plants. However, compared with control plants, significantly (25%) more mature fruit per plant were harvested from AVP1DOX plants compared with control plants, which is consistent with its recently reported role in fruit development (Mohammed et al., 2012). AVP1DOX plants displayed higher phosphate transport from leaf (source) to fruit (sink) compared with the controls under Pi-deficient conditions. Further, AVP1DOX plants showed greater auxin transport and root acidification than control plants. Increased transplant survival was observed in all trials compared with the controls, suggesting that increased expression of native H+-pyrophosphatase genes may be an important marker for improved transplant survival.
Materials and methods
Plant material and culture conditions
The generation of AVP1D-over-expressing (AVP1DOX) and empty-vector control transgenic tomato plants (Solanum lycopersicum, cultivar Money Maker) was described in Park et al. (2005) and Yang et al. (2007). Three lines (AVP1D-1, -2, and -3) were used in the hydroponic experiments. Two lines, AVP1D-1 and AVP1D-2, were used in the large greenhouse trials and showed very similar results. The AVP1D-2 line was used for field trials.
Greenhouse trials with sterilized soil
Trials were conducted in the Purdue University Department of Horticulture and Landscape Architecture Research Growth Facility. Seeds were sown in cell packs and germinated in a mist greenhouse for 3 weeks, then transplanted to 2-gallon pots filled with sterilized loam soil that had been extensively washed, twice with 2% HCl and five times with deionized water. Plants were placed in the greenhouse using a randomized block design, black plastic was used to cover the soil surface to simulate field conditions, and drip irrigation was used for watering. Natural light was supplemented to 14-h days using high pressure sodium lights (150 µmol m–2 s–1). Plants were fertilized every 2 weeks with Pi-free medium containing 20mM 2-(N-morpholino) ethanesulphonic acid (pH 5.8), 5.0mM KNO3, 2.0mM MgSO4, 2.0mM Ca(NO3)2, 50 µM iron ethylenediaminetetraacetate, 70 µM H3BO3, 14 µM MnCl2, 0.5 µM CuSO4, 1.0 µM ZnSO4, 0.2 µM NaMoO4, 10 µM NaCl, and 0.01 µM CoCl2. The Pi concentration of medium was adjusted to 1mM with KH2PO4 for Pi-sufficient conditions. For low-Pi treatments, 0.1% water-washed rock phosphate/phosphorite (Fisher) by weight was incorporated into the pot soil.
Field trials
Experiments were conducted at the Throckmorton–Purdue Agricultural Center (TPAC) in south Tippecanoe County, Indiana. The farm has been extensively tiled for optimum drainage and set up for drip irrigation. Plants were watered and fertilized through the dripping system. A plot measuring 37×26 m was selected for tomato field trial experiments. Soils were sampled from random locations in the plot and tested for available macronutrient content by A&L Great Lakes Laboratories (Fort Wayne, IN). Very similar results were obtained from three sets of samples. The soil contained 22 µg g–1 P [Bray P1 method, medium for tomato according to Egel et al. (2008)], 3.3% organic matter, 117 µg g–1 potassium, 460 µg g–1 magnesium, 1900 µg g–1 calcium; the soil pH was 6.3 and buffer pH was 6.8. Tomato seeds were germinated and grown in cell packs for 30 d in the greenhouse. Seedlings were acclimated in a room without a roof close to the field for a week. With rows spaced 3 m apart and plants spaced 1 m apart in the row, 105 tagged control and AVP1D-2 plants, respectively, were randomly transplanted into six beds covered with black plastic with 35 plants in each row. The test plants were surrounded by four border rows of untransformed wild-type tomato plants. Normal cultural practices for tomato cultivation, including drip irrigation, weed control, and pesticide treatments, were followed during the course of the experiment (Egel et al., 2008). After 3 weeks of growth, tomato plants were supported with wires and stakes. No phosphate fertilizer was applied to the tested tomato plants during the experiments. Border plants were applied with phosphate-containing fertilizers through the drip irrigation system.
Greenhouse trials with unwashed soil of field trials
Trials were conducted in the Purdue University Department of Horticulture and Landscape Architecture Research Growth Facility. Low-phosphate soil was obtained from the field of field trials (see above for soil test results) from Throckmorton–Purdue Agricultural Center (TPAC) in south Tippecanoe County, Indiana. Tomato seeds were germinated and grown in cell packs for 30 d in the greenhouse. Plants were randomly transplanted in 2-gallon pots on 64 trays, 1 control and 1 AVP1D-2 in each tray. Plants were grown under Pi-sufficient conditions for 1 month. Then 32 trays continued growth under Pi-sufficient conditions and the other 32 trays were treated with low-Pi. Plants were fertilized every 2 weeks with Pi-free medium containing 20mM 2-(N-morpholino) ethanesulphonic acid (pH 5.8), 5.0mM KNO3, 2.0mM MgSO4, 2.0mM Ca(NO3)2, 50 µM iron ethylenediaminetetraacetate, 70 µM H3BO3, 14 µM MnCl2, 0.5 µM CuSO4, 1.0 µM ZnSO4, 0.2 µM NaMoO4, 10 µM NaCl, and 0.01 µM CoCl2. The Pi concentration of medium was adjusted to 1mM with KH2PO4 for Pi-sufficient conditions. For low-Pi treatments, The Pi concentration of medium was adjusted to 10 µM with KH2PO4.
Survival rate documentation
The living and dead plants were counted 1 week after transplantation into large pots for the greenhouse trial or soil for the field trial. Dead plants were not replaced to avoid the confounding effects of plants of different ages in subsequent analyses.
Fresh weight determination
In the greenhouse experiments, control (vector alone) and AVP1DOX plants were harvested 100 d after transplantation. The shoot and fruit fresh weights were determined using a scale. The diameter of fruits was measured with calipers. The roots were carefully washed and blotted dry with paper towels before weights were determined with a scale. In the field trials, all control (90) and AVP1DOX plants (102) were harvested manually and dried in the bags. In order to minimize the moisture loss, shoot and fruit fresh weights for each plant were determined with a field scale immediately after harvesting the plants.
Dry weight determination
In the greenhouse experiments, control and AVP1DOX plants were harvested 100 d after transplantation. The shoot and fruit were harvested. The roots were carefully washed and blotted dry with paper towels. Samples were dried in an oven 80 °C for 96h. Dry weights (DW) were determined using a balance.
P transport assay
Stems (~15cm long) with 2–3 fruits and the closest compound leaf were cut from plants grown under normal and low-Pi conditions, and placed in a plastic container with 1mM and 10 µM Pi medium (above), respectively. Radiolabelled 20 µl 3.3 µM 32P-phospate solutions (1 µCi, 15 Ci mmol–1) were applied on the rachis of first two leaflets. The stem cuttings were placed under continuous light for 72h. The fruits were collected, weighed, and sliced into scintillation vials. 5ml scintillator liquid was added and the radioactivity was quantified by scintillation counting.
Auxin transport assay
Auxin transport assays were performed as described in Liu et al. (2011).
Root acidification assay
Plants were germinated in half-strength MS medium for 7 d, transferred to low-Pi medium as described above with 1mM MES, pH 6.8 and 0.04g l–1 bromocresol purple, and incubated for 3 d. The pH change was visualized via changes in medium colour. Comparisons were made with a colour bar generated by documenting the colour change of bromocresol purple in the same medium at specific pH values.
Statistical analysis
Student’s t test and one-way analysis of variance (ANOVA), followed by pair-wise Holm–Sidak post-hoc analysis, was used to compare the development of roots, shoots, and fruits in AVP1DOX tomato versus control plants.
Results
Enhanced auxin transport and root acidification in AVP1DOX plants
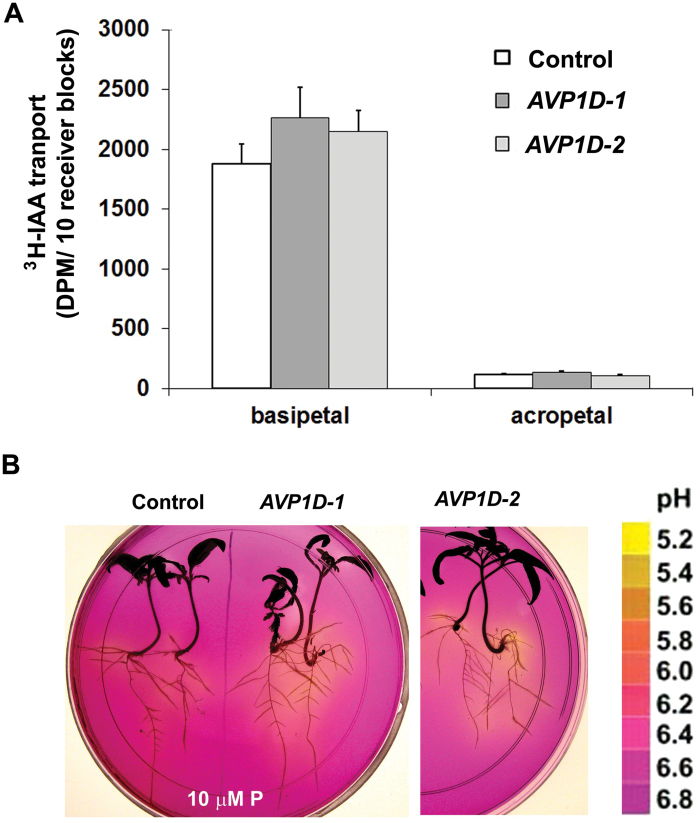
AVP1OX in Arabidopsis enhanced auxin transport and root acidification under low Pi conditions (Li et al., 2005; Yang et al., 2007). Similar results were observed in root acidification and auxin transport in AVP1DOX tomato seedlings (Fig. 1A). Enhanced auxin transport in AVP1DOX provided the explanation for larger roots measured in transgenic tomato plants. Further, AVP1D-1 and AVP1D-2 plants displayed increased acidification activity in medium under normal and low Pi conditions which also gave the basis for enhanced auxin transport (Fig. 1B). The function of AVP1OX in auxin transport and root acidification is conserved in Arabidopsis and tomato plants.
Fig. 1.
Enhanced basipetal auxin transport and root acidification in AVP1DOX plants. (A) Enhanced basipetal auxin transport in AVP1DOX plants. Values are means ±standard deviation (sd), n=3. (B) Pictures showed root acidification activity of tomato seedlings 3 d after transfer from control conditions to 10 µM Pi medium with the pH indicator bromocresol purple. (This figure is available in colour at JXB online.)
Increased tolerance to Pi deficiency in transgenic tomatoes over-expressing AVP1D in greenhouse trials
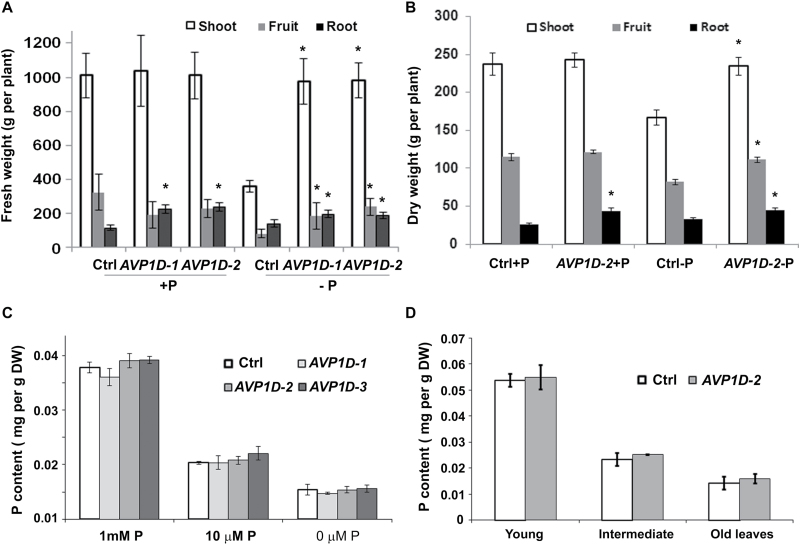
Unlike AVP1OX Arabidopsis plants, AVP1DOX tomato plants did not show long-term drought tolerance or enhanced growth under normal Pi (400 ppm) soil conditions (Yang et al., 2007). However, in this phenotypic analysis, a low Pi (0.5 ppm P) clay soil was used which has a high capacity to bind and immobilize Pi. To eliminate the Pi-sorption effect of soil, washed and sterilized loam soil was used to analyse the performance of AVP1DOX tomato plants under normal Pi (watered with 1mM Pi medium, see Materials and methods) and low Pi (rock phosphate) conditions. Two independent large greenhouse trials were performed each with 35 empty vector control plants, 35 AVP1D-1 and 35 AVP1D-2 plants. One hundred days after transplantation, fruits, shoots, and roots were harvested and weighed. Under Pi-sufficient conditions (+P), a significant increase was seen in the root fresh weight of AVP1D-1 and AVP1D-2 compared with that of control plants (Fig. 2A, Student’s t test, P <0.01) but not in shoot mass and fruit yield. Under washed rock phosphate conditions (–P), control plants had reduced shoot and fruit biomass mass compared with +P controls (Fig. 2A) which was consistent with the switch from shoot growth to root growth to enhance Pi uptake under Pi-limiting conditions. However, AVP1DOX plants produced comparable shoot, fruit, and root weights under washed rock phosphate conditions compared with +P plants (Fig. 2A), which was consistent with high acidification (Fig. 1A) and subsequent mobilization of Pi from rock phosphate, and also had increased root mass than the controls under the same conditions (Fig. 2A, P <0.05). Further, ~50% increase in fruit yield was observed in AVP1DOX plants compared with control plants under the rock phosphate conditions (Fig. 2A, P <0.01).
Fig. 2.
Performance of control and AVP1DOX plants under autoclaved loam soil, none-autoclaved low-P clay soil and hydroponic low-P conditions. (A) Greenhouse trials with washed and sterilized soil. The fresh weights of control and AVP1DOX plants under P-sufficient (+P, 1mM KH2PO4) and P-deficient (–P, 0.1% water-washed rock phosphate/phosphorite) conditions. Values are means ±standard deviation (sd), n=75. Asterisks indicate significance, ANOVA followed by Holm–Sidak post-hoc analysis, P <0.01, values from AVP1DOX plants were compared with values from control plants. (B) Greenhouse trials in untreated field soil. The dry weights of control and AVP1D-2 plants under P-sufficient (+P, 1mM KH2PO4) and P-deficient (–P, 10 µM KH2PO4) conditions. Values are means ±standard deviation (sd), n=5. Asterisks indicate significance, Student’s t test, P <0.05, values from AVP1D-2 plants were compared with values from control plants. (C) Average leaf P content in hydroponic control and AVP1DOX plants after 1 month treatment of 1mM, 10 µM, and 0 µM KH2PO4. Values are means ±standard deviation (sd), n=3. (D) P content in young, intermediate, and old leaves in hydroponic control and AVP1D-2 plants after 1 month treatment of 1mM, 10 µM, and 0 µM KH2PO4. Values are mean ±standard deviation (sd), n=3.
The performance of AVP1DOX plants was also tested using moderate low-P soil (22 µg g–1 P, Bray P1 method) from a field and watered with 1mM (+P) or 10 µM KH2PO4 (–P). The control and AVP1D-2 plants were grown under normal or low Pi conditions for 3 months and the dry weights were compared between AVP1D-2 and control plants. Similar to the fresh weight results in loam soil trials (Fig. 2A), AVP1D-2 roots had the significantly greater dry weight (Fig. 2B, P <0.05), which indicates that AVP1DOX promotes root development under normal and low-Pi conditions. Greater shoot and fruit dry weights in AVP1D-2 (Fig. 2B, P <0.05) supports the hypothesis that AVP1DOX increased Pi acquisition from high P-sorption clay soils, which was also consistent with higher rhizosphere acidification and a larger root system in AVP1D-2 plants.
Since AVP1DOX increased shoot biomass and fruit weight compared with the control tomato plants in low-P conditions, it was hypothesized that AVP1DOX increased the Pi usage efficiency in plants through the translocation of Pi from older tissues to younger tissues. To test this hypothesis, control plants and three independent AVP1DOX transgenic lines AVP1D-1, -2, and -3 were grown under hydroponic conditions in which Pi concentration and availability could easily be controlled. After treatment under the same low Pi conditions, no significant difference was seen in average leaf P content in AVP1DOX plants compared with the control plants (Fig. 2C). These results indicated that control plants can take up soluble Pi as efficiently as AVP1DOX plants under hydroponic conditions. Further, the P content in young, intermediate, and old leaves did not show a difference between AVP1D-2 and control plants (Fig. 2D). These results indicate that AVP1DOX did not increase the Pi translocation (Pi usage efficiency) from old leaves to young leaves.
Enhanced Pi translocation from leaves to fruits in AVP1DOX plants
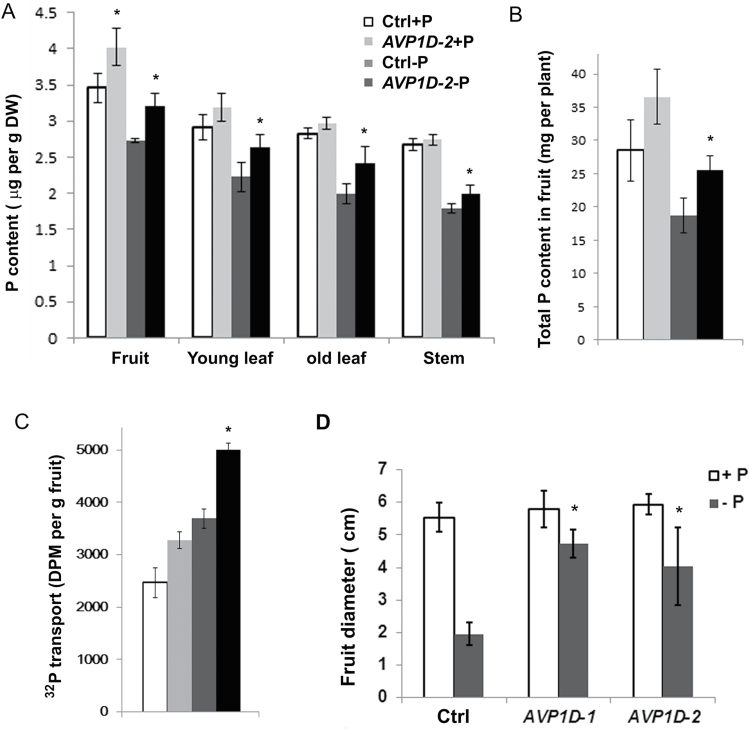
To test the hypothesis that AVP1DOX enhances the Pi translocation to fruits, P contents in fruits were determined and Pi transport assays were performed using 32P-phosphate. Higher fruit P contents (µg g–1 dry weight) were measured in AVP1D-2 compared with the controls under both normal and low Pi conditions (Fig. 3A, P <0.05). The P contents in AVP1D-2 were also higher than the controls in leaves and stems when grown under low Pi soil conditions (Fig. 3A, P <0.05). Total P content in fruits (mg per plant) were higher in AVP1D-2 than controls under low-Pi conditions (Fig. 3B, P <0.05). Enhanced transport of Pi detected in AVP1DOX stem cuttings, compared with the controls under low-Pi conditions, indicated the transport of 32P-phosphate from neighboring compound leaves into fruit (Fig. 3C, P <0.05). These data supported that AVP1DOX enhanced Pi translocation to fruits, which is consistent with the important role of type-I pyrophosphatases in tomato fruit development (Mohammed et al., 2012). To confirm the role of AVP1DOX in fruit development further, the diameter of fruits was measured after 100 d growing in the greenhouse. AVP1D-1 and AVP1D-2 displayed enhanced fruit development compared with control plants under low-P conditions (Fig. 3D).
Fig. 3.
Enhanced P transport to fruits in AVP1D-2. (A) The P content of control and AVP1D-2 plants under P-sufficient (+P) and P-deficient (–P) conditions. Values are means ±standard deviation (sd), n=4. Asterisks indicate significance, Student’s t test, P <0.05. (B) The total P content in fruits of control and AVP1D-2 plants under P-sufficient (+P) and P-deficient (–P) conditions. Values are means ±standard deviation (sd), n=4. Asterisks indicate significance, Student’s t test, P <0.05. (C) Radiolabelled 32P-phospate transport from leave to fruits. Values are means ±standard deviation (sd), n=3. Asterisks indicate significance, Student’s t test, P <0.05. (D) Enhanced fruit development in AVP1DOX plants under P deficient conditions. Values are means ±standard deviation (sd), n=7. Asterisks indicate significance, Student’s t test, P <0.05.
Performance of AVP1DOX tomatoes grown without additional phosphorus in field trials
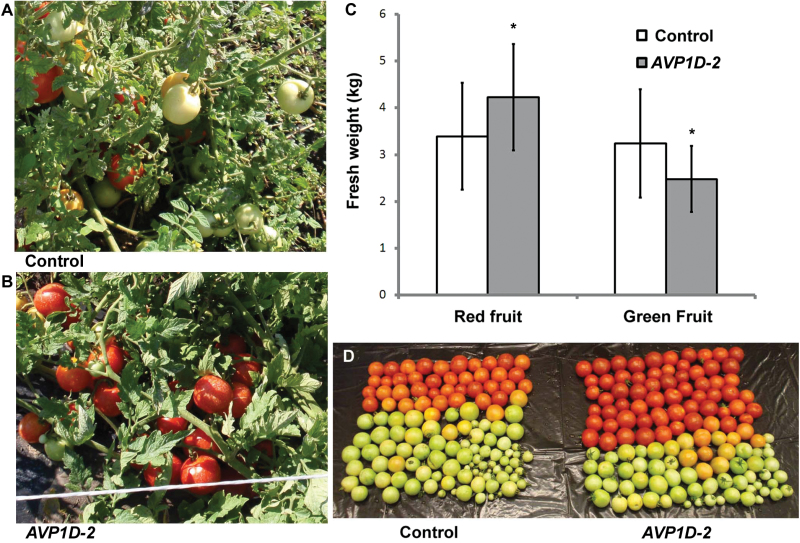
The large greenhouse trial results were consistent with the results obtained in the small greenhouse trial with the low-P clay soil experiments (Yang et al., 2007). However, plants may develop different root systems when grown under field conditions as space limitation is different in pots and field, and the response of control and AVP1DOX plants to low Pi may be different under field conditions. To test this possibility, the control and AVP1D-2 plants were planted randomly in the field with 105 plants of each genotype distributed in six rows (see Supplementary Fig. S1A at JXB online). Shoots and fruits were harvested after 3 months in the fields (when >95% of the fruits were red in border plants with normal Pi conditions), and fresh weights were documented. Shoot fresh weight per plant of AVP1D-2 plants was slightly greater than that of control plants (see Supplementary Fig. S1B at JXB online, P=0.06, Student’s t test). Both control and AVP1D-2 plants showed low-P symptoms such as purple leaves after 3 months in field. The leaves and stems of control plants were more chlorotic than those of AVP1DOX plants (see Supplementary Fig. S1C–F at JXB online). These results were consistent with enhanced Pi acquisition by AVP1DOX in the clay soil greenhouse experiments (Fig. 2B, 3A). Fruit fresh weights of AVP1OX plants were not different from those of the control plants (see Supplementary Fig. S1B at JXB online, P=0.7). However, AVP1D-2 plants produced more mature fruits that control plants (Fig. 4A, B), and 0.85kg more mature fruit per plant were harvested from AVP1DOX plants, 25% more than that harvested from control plants (Fig. 4C, P <0.01). The weight ratio of red fruits to green fruits was 1:1 for controls and 1.7:1 for AVP1OX plants, respectively. These results indicate that AVP1DOX enhanced fruit development under limiting P field conditions. These results were also consistent with enhanced Pi transport from leaves to fruit and fruit development under low-P conditions (Fig. 3C, D). A recent report showed that tomato vacuolar H+-pyrophosphatases play a role in early fruit development (Mohammed et al., 2012). Knock-down of pyrophosphatase in tomato resulted in fruit growth retardation (Mohammed et al., 2012), and fruit growth retardation was more obvious in control plants than AVP1D-2 plants (Fig. 4D). Further, phosphate is required for phosphate esterification during fruit ripening (Marks et al., 1957). Our results suggest that AVP1DOX plants can transport more phosphate for fruit development and maturation under limiting phosphate conditions.
Fig. 4.
Field trials of control and AVP1DOX plants. Phosphate fertilizer was not applied during the course of the field trial. Field soil contained 22 µg g–1 P. (A) Control plant shows more green fruits. (B) AVP1D-2 plant shows more red fruits. (C) AVP1D-2 plants produce significantly more red fruit fresh weight per plant than control plants. Values are means ±standard deviation (sd), n=85. Asterisks indicate significance, Student’s t test, P <0.01. (D) Total fruit from a representative control and an AVP1D-2 plant, respectively.
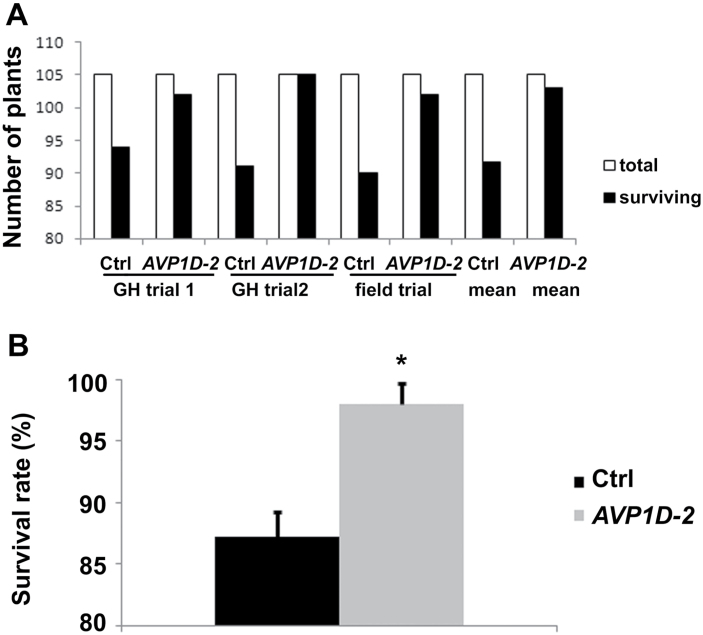
Increased transplant survival rates of AVP1DOX transgenic plant in large-scale greenhouse and field trials
Interestingly, transplant survival rate of AVP1DOX plants was higher than control plants in both large-scale greenhouse and field trials. 94 and 91 out of 105 (89% and 86%, respectively) control plants survived transplantation to large pots, while 102 (97%) and 105 (100%) of the 105 AVP1OX plants survived transplantation to large pots, respectively, in two large-scale greenhouse trials (Fig. 3A). In the field trials, 92 out of 105 control plants (87%) and 102 out of 105 AVP1OX plants (97%) survived transplantation to the field (Fig. 5A). Overall, transplant-survival rate for AVP1OX plants was 98%, which is significantly higher than the 87% transplant-survival rate in control tomato plants (Fig. 5B, P=0.002). Subsequent transplantation experiments performed in the greenhouse and in the field confirmed that those survival rates are consistently observed (data not shown). Although under our conditions control plants displayed decreased transplantation survival rates than expected, these conditions revealed differences between control and AVP1DOX plants. These results suggest that AVP1 expression can be used as a selective marker in breeding efforts to identify lines with enhanced P utilization capacity and improved transplantation efficiency. The approach of AVP1OX can be applied to improve transplantation success for crops or trees with low transplantation efficiency.
Fig. 5.
Survival rates of control and AVP1OX plants after transplantation. (A) Number of living plants after transplantation in large-scale greenhouse (GH) and field trails as indicated. Values are number of individuals. (B) Total transplant survival rate for both greenhouse and field trials. Values are means ±standard deviation (sd), n=3 trials. Asterisk indicates significance, Student’s t test, P <0.01.
Discussion
AVP1 enhancement of plant performance in low-P soils: biomass versus fruit production
In both the large-scale greenhouse and the field trials, AVP1DOX tomato plants developed more biomass under P-deficient conditions than control plants. However, this biomass increase did not translate into fruit quantity, but fruit quality was enhanced as more mature fruit were developed in AVP1DOX plants (Fig. 4). In other words, AVP1DOX plants under low P produced the same amount of mature fruit as the wild type under P-sufficient conditions. AVP1 over-expression has been shown to increase overall biomass accumulation and seed production by Arabidopsis, rice, and maize under both P-sufficient and P-deficient laboratory conditions (Yang et al., 2007; Pei et al., 2012). However, biomass and fruit production were not significantly increased in AVP1OX tomato plants under P-sufficient conditions, presumably due to species-specific developmental factors (reviewed in McSteen and Leyser, 2005). A recent study showed the localization of all three tomato pyrophosphatases to fruits and showed that their activity was critical for fruit development (Mohammed et al., 2012) which supports this hypothesis. The diversification of pyrophosphatase in fruit plants suggests that tissue- or organ-specific AVP1 over-expression may be valuable for enhancement of specific traits.
AVP1DOX enhances fruit growth and ripening in tomatoes under P-deficient conditions
In field trials, AVP1DOX plants produced more mature fruits both in number and weight than control tomato plants without P supplementation (Fig. 4E, F). Fruit development can be divided into distinct periods: (1) cell division, (2) cell enlargement, (3) maturation, (4) ‘autogenous climacteric’, and (5) senescence. The climacteric stage is characterized by a sudden, sharp increase in respiratory rate and ethylene production in fruit ripening of climacteric fruits such as tomatoes, apples, bananas, melons, and apricots. Phosphate is required for fruit growth and phosphate esterification during fruit ripening (Marks et al., 1957) and is consistent with the observation here that P deficiency resulted in more small, green tomatoes in control plants. Although other nutrients are also important for fruit development, P is critical for fruit set and growth, and P fertilizer application is common practice prior to fruit set in tomato fields. Our data indicated that AVP1 plays an important role in the translocation of P for tomato fruit development. However, over-expression of AVP1 in Lactuca sativa also enhances nitrogen use efficiency in leaves (Paez-Valencia et al., 2013).
AVP1D over-expression enhances root growth and transplantation survival
AVP1DOX tomato plants had higher transplantation survival rates (Fig. 5). Similar results were seen for AVP1OX rice plants under semi-dry conditions (data not shown). However, enhanced transplantation survival could be the result of enhanced root growth in AVP1DOX tomato plants as indicated by root weights (Fig. 2). Plants encounter multiple stresses during transplantation including root loss, wilting, wounding, drought, nutrition, disease, and other stresses (reviewed in Koller, 1977). Transplantation success rate is an important component of production methods, and cultivars with robust root systems are more likely to survive transplantation (Harris and Bassuk, 1993). AVP1OX in many species, Arabidopsis, alfalfa, rice, tomato, barley, maize, tobacco, and cotton developed larger root systems and are more resistant to salt and drought stress, and/or limiting Pi conditions (Gaxiola et al., 2001; Park et al., 2005; Zhao et al., 2006; Bao et al., 2008; Li et al., 2008; Lv et al., 2008, 2009; Pasapula et al., 2010; Pei et al., 2012; Schilling et al., 2013). Enhanced short-term drought tolerance in AVP1DOX plants was consistent with higher transplantation survival rates (Park et al., 2005). Increased auxin fluxes in AVP1DOX plants (Fig. 1) may also facilitate adventitious root initiation during transplantation. Increased auxin transport and root acidification were also observed in Arabidopsis AVP1OX plants which indicated that AVP1OX share similar roles in root development in Arabidopsis and tomato plants. However, the unique shoot and fruit phenotype in AVP1DOX tomato plants suggested that AVP1D plays a specific role in the transport of Pi between leaves (source) and fruits (sink tissue).
Supplementary data
Supplementary data can be found at JXB online
Supplementary Fig. S1. Field trials of control and AVP1OX plants.
Acknowledgements
This work was supported by a USDA grant to ASM and RAG. Xiao Zhang was supported by the China Scholarship Council. Thanks to Plant-Growth-Facilities Manager Robert Eddy and undergraduate students Heather Areus, Bill Maher, and Eric O’Brien for their help in the large-scale greenhouse trials. Thanks to Purdue Agricultural Center’s Jerry Frankhauser, Jay Young, and Nathan Linder for their help in the field trials. Thanks to Zuozhao Li, Fazeeda Hosein, Hee Jin Park, Anne S Knoeller, Yan Cheng, and Bharat Bangari for their help in harvesting the tomato plants in the field trials.
References
- Alexandersson E, Saalbach G, Larsson C, Kjellbom P. 2004. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant and Cell Physiology 45, 1543–1556 [DOI] [PubMed] [Google Scholar]
- Bao AK, Wang SM, Wu GQ, Xi JJ, Zhang JL, Wang CW. 2009. Over-expression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Science 176, 232–240 [Google Scholar]
- Bolan NS. 1991. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant and Soil 134, 189–207 [Google Scholar]
- Drozdowicz YM, Rea PA. 2001. Vacuolar H+-pyrophosphatases: from evolutionary backwaters into mainstream. Trends in Plant Science 6, 206–211 [DOI] [PubMed] [Google Scholar]
- Egel D, Foster R, Maynard E, et al. 2008. Midwest vegetable production guide for commercial growers 2008. Purdue University Press, 158–170 [Google Scholar]
- Ferjani A, Segami S, Horiguchi G, Muto Y, Maeshima M, Tsukaya H. 2011. Keep an eye on PPi: the vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. The Plant Cell 23, 2895–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR. 2001. Drought- and salt-tolerant plants result from over-expression of the AVP1 H+-pump. Proceedings of the National Academy of Sciences, USA 98, 11444–11449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR, Bassuk NL. 1993. Tree planting fundamentals. Journal of Arboriculture 19, 64–70 [Google Scholar]
- Havlin J, Beaton JD, Tisdale SL, Nelson WL. 2005. Soil fertility and fertilizers: an introduction to nutrient management , 7th edn Upper Saddle River, New Jersey: Pearson Prentice Hall, 187–190 [Google Scholar]
- Heinonen J. 2001. Biological role of inorganic pyrophosphate . Norwell, MA: Kluwer Academic Publishers Group, 1–28 [Google Scholar]
- Holford ICR. 1997. Soil phosphorus: its measurements, and its uptake by plants. Australian Journal of Soil Research 35, 227–239 [Google Scholar]
- Koller GL. 1977. Transplanting stress: a view from the plant’s perspective. Arnoldia 37, 230–241 [Google Scholar]
- Langhans M, Ratajczak R, Lutzelschwab M, Michalke W, Wachter R, Fischer-Schliebs E, Ullrich CI. 2001. Immunolocalization of plasma-membrane H+-ATPase and tonoplast-type pyrophosphatase in the plasma membrane of the sieve element–companion cell complex in the stem of Ricinus communis L. Planta 213, 11–19 [DOI] [PubMed] [Google Scholar]
- Li B, Wei A, Song C, Li N, Zhang J. 2008. Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnology Journal 6, 146–59 [DOI] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, et al. 2005. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310, 121–125 [DOI] [PubMed] [Google Scholar]
- Liu X, Cohen JD, Gardner G. 2011. Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiology 157, 891–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio J, de la Vega OM, Guevara-Garcia A, Herrera-Estrella L. 2000. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nature Biotechnology 18, 450–453 [DOI] [PubMed] [Google Scholar]
- Lv S, Zhang K, Gao Q, Lian L, Song Y, Zhang J. 2008. Overexpression of an H+-PPase gene from Thellungiella halophila in cotton enhances salt tolerance and improves growth and photosynthetic performance. Plant and Cell Physiology 49, 1150–1164 [DOI] [PubMed] [Google Scholar]
- Lv SL, Lian LJ, Tao PL, Li ZX, Zhang KW, Zhang JR. 2009. Over-expression of Thellungiella halophila H+-PPase (TsVP) in cotton enhances drought stress resistance of plants. Planta 229, 899–910 [DOI] [PubMed] [Google Scholar]
- Maeshima M. 2000. Vacuolar H(+)-pyrophosphatase. Biochimica et Biophysica Acta 1465, 37–51 [DOI] [PubMed] [Google Scholar]
- McSteen P, Leyser O. 2005. Shoot branching. Annual Review of Plant Biology 56, 353–374 [DOI] [PubMed] [Google Scholar]
- Marks JD, Bernlohr R, Varner JE. 1957. Esterification of phosphate in ripening fruit. Plant Physiology 32, 259–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed SA, Nishio S, Takahashi H, Shiratake K, Ikeda H, Kanahama K, Kanayama Y. 2012. Role of vacuolar H+-inorganic pyrophosphatase in tomato fruit development. Journal of Experimental Botany 63, 5613–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Valencia J, Patron-Soberano A, Rodriguez-Leviz A, Sanchez-Lares J, Sanchez-Gomez C, Valencia-Mayoral P, Diaz-Rosas G, Gaxiola R. 2011. Plasma membrane localization of the type I H+-PPase AVP1 in sieve element–companion cell complexes from Arabidopsis thaliana . Plant Science 181, 23–30 [DOI] [PubMed] [Google Scholar]
- Paez-Valencia J, Sanchez-Lares J, Marsh E, et al. 2013. Enhanced H+-PPase activity improves nitrogen use efficiency in romaine lettuce. Plant Physiology 161, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasapula V, Shen G, Kuppu S, et al. 2010. Expression of an Arabidopsis vacuolar H(+)-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fiber yield in the field conditions. Plant Biotechnology Journal 9, 88–99 [DOI] [PubMed] [Google Scholar]
- Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA. 2005. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proceedings of the National Academy of Sciences, USA 102, 18830–18835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Wang J, Li K, Li Y, Li B, Gao F, Yang A. 2012. Over-expression of Thellungiella halophila H+-pyrophosphatase gene improves low phosphate tolerance in maize. PLoS ONE 7(8),e43501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling RK, Marschner P, Shavrukov Y, Berger B, Tester M, Roy SJ, Plett DC. 2013. Expression of the Arabidopsis vacuolar H+ -pyrophosphatase gene (AVP1) improves the shoot biomass of transgenic barley and increases grain yield in a saline field. Plant Biotechnology Journal 10.1111/pbi.12145 [DOI] [PubMed] [Google Scholar]
- Shiratake K, Kanayama Y, Maeshima M, Yamaki S. 1997. Changes in H(+)-pumps and a tonoplast intrinsic protein of vacuolar membranes during the development of pear fruit. Plant and Cell Physiology 38, 1039–1045 [DOI] [PubMed] [Google Scholar]
- Undurraga S, P-Santos M, Paez-Valencia J, Yang H, Hepler PK, Facanha AR, Hirschi KD, Gaxiola R. 2012. Arabidopsis sodium dependent and independent phenotypes triggered by H+-PPase up-regulation are SOS1 dependent. Plant Science 183, 96–105 [DOI] [PubMed] [Google Scholar]
- Yang H, Knapp J, Koirala P, Rajagopal D, Peer WA, Silbart LK, Murphy A, Gaxiola RA. 2007. Enhanced phosphorus nutrition in monocots and dicots over-expressing a phosphorus-responsive type I H+-pyrophosphatase. Plant Biotechnology Journal 5, 735–745 [DOI] [PubMed] [Google Scholar]
- Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H. 2006. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1 . Molecular Breeding 17, 341–353 [Google Scholar]
- Zhen R, Kim EJ, Rea PA. 1997. Acidic residues necessary for pyrophosphate-energized pumping and inhibition of the vacuolar H+-pyrophosphatase by N,N’-dicyclohexylcarbodiimide. Journal of Biological Chemistry 272, 22340–22348 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.