Summary
The flood-tolerant genotype FR13A retains leaf gas films and its capacity for underwater net photosynthesis, whereas gas films are lost faster and photosynthesis declines markedly in sensitive genotypes.
Key words: Aerenchyma, flooding stress, leaf gas films, leaf air layer, leaf hydrophobicity, Oryza sativa, submergence tolerance, SUB1, leaf chlorophyll, survival, FR13A, IR42, Swarna, Swarna-Sub1.
Abstract
Floods can completely submerge some rice (Oryza sativa L.) fields. Leaves of rice have gas films that aid O2 and CO2 exchange under water. The present study explored the relationship between gas film persistence and underwater net photosynthesis (PN) as influenced by genotype and submergence duration. Four contrasting genotypes (FR13A, IR42, Swarna, and Swarna-Sub1) were submerged for 13 days in the field and leaf gas films, chlorophyll, and the capacity for underwater PN at near ambient and high CO2 were assessed with time of submergence. At high CO2 during the PN assay, all genotypes initially showed high rates of underwater PN, and this rate was not affected by time of submergence in FR13A. This superior photosynthetic performance of FR13A was not evident in Swarna-Sub1 (carrying the SUB1 QTL) and the declines in underwater PN in both Swarna-Sub1 and Swarna were equal to that in IR42. At near ambient CO2 concentration, underwater PN declined in all four genotypes and this corresponded with loss of leaf gas films with time of submergence. FR13A retained leaf gas films moderately longer than the other genotypes, but gas film retention was not linked to SUB1. Diverse rice germplasm should be screened for gas film persistence during submergence, as this trait could potentially increase carbohydrate status and internal aeration owing to increased underwater PN, which contributes to submergence tolerance in rice.
Introduction
Flooding severely impedes gas exchange between plants and the environment owing to the 104-fold slower diffusion of gases in water compared with in air (Armstrong, 1979). Rain-fed lowland rice is a semi-aquatic plant that often becomes submerged, but genotypes differ markedly in tolerance (Colmer et al., 2014; Ram et al., 1999). FR13A is a submergence-tolerant landrace and much of this tolerance is conferred by a major QTL (quantitative trait locus) called ‘SUB1’ (Xu and Mackill, 1996). The SUB1 QTL controls several traits contributing to submergence tolerance, including reduced shoot elongation, maintenance of higher soluble carbohydrate concentration, and less chlorophyll degradation during submergence, as well as less oxidative stress post-submergence (Ella et al., 2003a; Ella et al., 2003b). Rice genotypes with SUB1 therefore show better survival and recovery post-submergence than those lacking this QTL (Bailey-Serres et al., 2010; Ismail et al., 2013; Mackill et al., 2012). SUB1A-1 is an ERF transcriptional regulator that blocks ethylene responsiveness during submergence and thus also down-stream targets. It maintains the expression of the gibberellic acid (GA) signalling repressors SLENDER RICE1 (SLR1) and SLR1-like-1 (SLRL1) and their proteins during submergence. Expression of these repressors is associated with inhibition of GA induction of expansins required for cell wall expansion, and α-amylase and sucrose synthase required for starch and sucrose catabolism, respectively (Bailey-Serres et al., 2010; Fukao and Bailey-Serres, 2008; Fukao et al., 2006). More recently, Schmitz et al. (2013) reported that SUB1 differentially regulates genes associated with brassinosteroids (BR) synthesis, and BR induces a GA catabolic gene, GA2ox7, under submergence. Together these processes lead to suppression of GA-induced underwater elongation growth and conserve carbohydrates for maintenance metabolism and survival.
In addition to the importance placed on conserving carbohydrates during submergence (Bailey-Serres and Voesenek, 2008; Voesenek et al., 2006), many wetland plants can also produce carbohydrates through underwater photosynthesis (Colmer et al., 2011; Mommer et al., 2004). Rice, in particular, has been shown to photosynthesize under water (Raskin and Kende, 1983; Setter et al., 1989) and rice grew well when submerged in water enriched with CO2 to levels above air equilibrium to simulate some floodwaters (Pedersen et al., 2009; Setter et al., 1989). Like several other terrestrial wetland plants (Colmer and Pedersen, 2008b), rice possesses superhydrophobic, self-cleansing leaf surfaces that retain a thin gas film when immersed into water (Pedersen et al., 2009; Raskin and Kende, 1983; Setter et al., 1989). Leaf gas films markedly enhance gas exchange between leaf and floodwater so that underwater net photosynthesis (PN) is greater for leaves with gas films present, than when these are removed (Pedersen et al., 2009; Verboven et al., 2014; Winkel et al., 2013). In addition to carbohydrate production, underwater PN also results in better root aeration as much of the O2 produced in the leaves diffuses via the aerenchyma down to the roots (Colmer and Pedersen, 2008a; Pedersen et al., 2009; Waters et al., 1989; Winkel et al., 2013). As O2 production in underwater PN ceases at dusk, leaf gas films then also facilitate O2 uptake from the floodwater resulting in some internal aeration during darkness, but this is likely to be insufficient for the entire root system as root O2 decreases to very low levels and fermentation occurs during dark periods (Pedersen et al., 2009; Waters et al., 1989; Winkel et al., 2013).
SUB1 genotypes show less chlorophyll degradation during submergence (Ella et al., 2003b), but the possible benefit of this to underwater PN has not previously been evaluated. Furthermore, whether the leaves of submergence-tolerant FR13A or SUB1 lines differ from sensitive rice genotypes in formation and/or maintenance of leaf gas films should be evaluated. The issue of underwater PN in FR13A and SUB1 genotypes is important to evaluate as the SUB1 QTL accounts for 70% of the variation in submergence tolerance leaving 30% unexplained variation (Xu and Mackill, 1996). We assessed the submergence tolerance of 4 selected genotypes of rice during 13 d of complete submergence. The four genotypes were (i) FR13A (the tolerant donor of SUB1A), (ii) IR42 (submergence intolerant and lacking SUB1A), (iii) Swarna (submergence intolerant and lacking SUB1A), and (iv) Swarna-Sub1 (Swarna with SUB1A). Over the period of 13 d of complete submergence in an experimental field, we followed with time underwater PN, leaf chlorophyll concentrations, and leaf gas film thickness for the four contrasting genotypes in order to elucidate: (a) relationships between loss of chlorophyll and/or gas film persistence with underwater PN capacity (i.e. at near-saturated CO2) and at near-ambient CO2 (i.e. field-relevant), as influenced by time of submergence; and (b) if FR13A is superior in its capacity for underwater PN whether this trait is also expressed in Swarna-Sub1.
Materials and methods
Experimental design and harvest procedures
The submergence experiment was conducted in the wet season (Oct to Nov) in the submergence field facilities at the International Rice Research Institute at Los Baños, the Philippines, with field and soil type described previously (Singh et al., 2009). Rice genotypes (Oryza sativa L.; FR13A, IR42, Swarna and Swarna-Sub1) were sown in a seedbed in September 2011 and 21-d-old seedlings were transplanted at 20×20cm spacing into a waterlogged paddy field surrounded by bunds to enable submergence to be imposed. FR13A is a landrace from eastern India with exceptional submergence tolerance and is the donor of SUB1, a major QTL associated with submergence tolerance on chromosome 9; IR42 is a submergence-intolerant variety (Mackill et al., 2012). Swarna is a dwarf rain-fed lowland Indian variety and Swarna-Sub1 is Swarna with the SUB1 QTL introgressed through marker assisted backcrossing for improvement of submergence tolerance (Xu et al., 2006). Experiments commenced 14 d after transplanting, so that plants were 5 weeks old. Plants were completely submerged with about 1.25 m of water head and remained inundated through to the end of the experiment.
Plants were sampled at various times after submergence (see Figures) for analyses of underwater net photosynthesis (PN), leaf (lamina) chlorophyll concentrations, and lamina gas film thickness. Measurements were also taken of lamina sugar and starch concentrations, tissue porosity, and of whole shoot dry mass (DM); these supporting data are in the Supplementary Materials. A floating air-filled mattress was used to access plants in the submergence pond as this avoided disturbance of the soil that would have resulted in suspended particles and murky water; plants were gently pulled out of the soil and immediately submerged in floodwater from the same field in a plastic container to prevent air contact. This procedure did not capture all root material and thus roots were not included in any tissue analyses. Immediately after collection, plants were brought to the laboratory for analyses.
Environmental conditions
Water used to submerge the paddy field came from an adjacent reservoir; see Winkel et al. (2013) for key water chemical parameters. Morning water temperature in the paddy field was measured between 9.00h and 10.00h each day and ranged from 28–30 °C; the average O2 concentration (for the 12 mornings) was 195 mmol m–3 (17 kPa); air-equilibrium at 30 °C is 254 mmol m–3 or 20.6 kPa. Average alkalinity in the water was 5.4mol m–3 and pH was 7.9, resulting in an average dissolved CO2 concentration of 130 mmol m–3 for the 12 mornings of the experiment. The CO2 concentration in the study of Winkel et al. (2013) declined, relative to the morning value, to 71% by midday and then further to 53% by dusk. Light extinction in the water ranged from 1.1–1.9 m–1 with an average of 48% of surface light remaining at 50cm of depth (depth of floodwater was approximately 1.25 m, average initial plant height varied from 37 to 77cm). During the 13 d of submergence, the average air temperature was 26.7 °C, and varied from 23.3–32.7 °C. Average incident radiation was 403W m–2 in the period from 10.00 h–14.00h for the 13 days of submergence.
Net photosynthesis under water and in air
Underwater PN was measured on excised leaf (lamina) segments at 0.2 and 5mol m–3 of CO2. These two CO2 concentrations were chosen based on: (i) 0.2mol m–3 represents a reasonable near-ambient CO2 concentration in rice floodwaters—these waters typically contain CO2 above air-equilibrium concentrations during early mornings owing to night-time CO2 production, although CO2 can be depleted below air-equilibrium by the afternoon (summarized in Colmer et al. (2011), dynamics in Winkel et al. (2013)); (ii) five mol m–3 CO2 saturates underwater PN of rice, irrespective of leaf gas films presence or absence (Swarna-Sub1; Winkel et al., 2013) and so these measurements enabled the evaluation of the maximum capacity for underwater PN in the present system, and how this changed with time. Although 5mol m–3 CO2 would be regarded as a very high level of CO2 (possibly with some adverse effects on cellular metabolism) if in a gas phase (viz. 5mol m–3 is equivalent to 17.2 kPa CO2 in equilibrium with air at 30 °C), the CO2-response curve for underwater PN did not show any adverse effects of this high CO2 (Winkel et al., 2013). The resistance of transversing an aqueous diffusive boundary layer (DBL) is 10 000 times that of an equivalent gaseous DBL and so the CO2 concentration experienced by the cells of photosynthesizing leaves (consuming CO2) would be substantially lower when submerged than if in a gas phase of equivalent CO2.
Four replicate leaves (the second youngest fully expanded from four different plants) were taken from each of the four genotypes. Twenty mm-long leaf segments (projected area of approximately 200mm2) were excised from the top third of the lamina. Underwater PN (n=4) was measured at 30 °C using 25ml glass vials with two glass beads added to ensure mixing according to the method of Pedersen et al. (2013) with PAR inside the vials of 760±60 µmol m–2 s–1 (mean±SE, n=10). The incubation medium was artificial floodwater based on a general purpose culture medium of Smart and Barko (1985) modified by Colmer and Pedersen (2008a), with initial O2 near half air-equilibrium. To prepare artificial floodwater with a final concentration of 0.2 or 5mol m–3 CO2 and an alkalinity of 5mol m–3 (mostly bicarbonate and carbonate), we added KHCO3 at 5.2 or 10.0mol m–3 in the general purpose medium. We subsequently added known volumes of 0.1M HCl to convert the desired portion of the HCO3 – into CO2, resulting in pH values of 7.7 and 6.3 for the 0.2 and 5mol CO2 m–3, respectively (Mackereth et al., 1978). Vials without leaf segments served as blanks.
Following incubations of known durations (30–50min), the dissolved O2 concentration in each vial was measured using an O2 minielectrode (OX-500, Unisense A/S, Aarhus, Denmark) connected to a multimeter (MicroSensor Multimeter, Unisense A/S, Aarhus, Denmark). Fresh mass (FM) was then taken before samples were flash frozen in liquid N2 and freeze-dried and DM recorded. A relationship between DM and area, and also for FM and area, was established for segments from the same type of leaves for each individual genotype, for plants when waterlogged with leaves in air and also when submerged, using digital photos and ImageJ (Schneider et al., 2012), so that the projected area of each leaf segment used in underwater PN could be calculated from its DM. Using the differences between DM to area ratio from the field plants in waterlogged soil with shoots in air or when completely submerged, a linear correction was calculated to estimate the change in DM to area ratio during the submergence.
PN in air by plants in waterlogged soil with shoots that always remained in air was measured on each of the four genotypes on the second youngest fully expanded leaf using an IRGA (LI-6400, Li-Cor) at PAR of 750 µmol m–2 s–1 and CO2 (380 µL L–1) at 30 °C between 10 and 11 am in the adjacent waterlogged paddy field; for details see Winkel et al. (2013).
Gas film thickness
Gas film volume was measured by determining buoyancy of lamina samples before and after gas film removal. Measurements were taken on three segments of 50mm length of the lamina from the top third of the youngest fully expanded leaf of 3 tillers. After the first measurement of buoyancy (gas films intact) segments were brushed with a dilute solution of Triton X (0.01% v/v of Triton X-100 in artificial floodwater, composition given above) to eliminate hydrophobicity so that gas films were removed (c.f. Colmer and Pedersen, 2008b; Pedersen et al., 2009) and thereafter buoyancy was again measured. The samples were then vacuum infiltrated with water and again measured for buoyancy, to enable calculation of tissue porosity (gas-filled volume per unit tissue volume; Raskin, 1983). Segment area was calculated from the area to FM ratio, which was established for similar tissues (described above). Mean gas film thickness was calculated by dividing gas film volume (mm3) with the two-sided area (mm2), i.e. rice leaves possess gas films on both the adaxial and abaxial sides (Pedersen et al., 2009). In the present study, the detection limit of gas film thickness was approximately 2 µm and so measurements giving values below 2 µm were classified as “gas films absent”.
Chlorophyll
Chlorophyll concentration was measured on the middle portion of the 2nd youngest fully expanded leaf of individual plants harvested from the submerged field. The samples were flash frozen in liquid N2, freeze-dried for 48h, stored at –80 °C and then ground. Chlorophyll was extracted in 80% acetone at 5 °C for 12h in darkness and then absorbance in extracts was measured at 645, 652, and 663nm on a spectrophotometer (UV-VIS 1800, Shimadzu, Nishinokyo, Kyoto, Japan). Chlorophyll concentrations were calculated using equations of Mackinney (1941).
Statistical analyses
GraphPad Prism 6 (GraphPad Software Inc., http://www.graphpad.com) was used for data analysis and two-way ANOVA with Bonferroni post hoc test to compare means of the differences in sugar, starch (in Supplementary Data, only), underwater PN, gas film thickness, and chlorophyll of the leaves of the four genotypes. Analyses of two-way ANOVA were performed separately for FR13A versus IR42 and Swarna versus Swarna-Sub1 to enable better interpretation of potential factorial interactions. Correlations between underwater PN at the two CO2 concentrations and gas film thickness and tissue chlorophyll concentration were also performed using GraphPad Prism 6 (Spearman non-parametric correlation).
Results
Capacity for underwater net photosynthesis; measurements at high dissolved CO2 (5mol m–3)
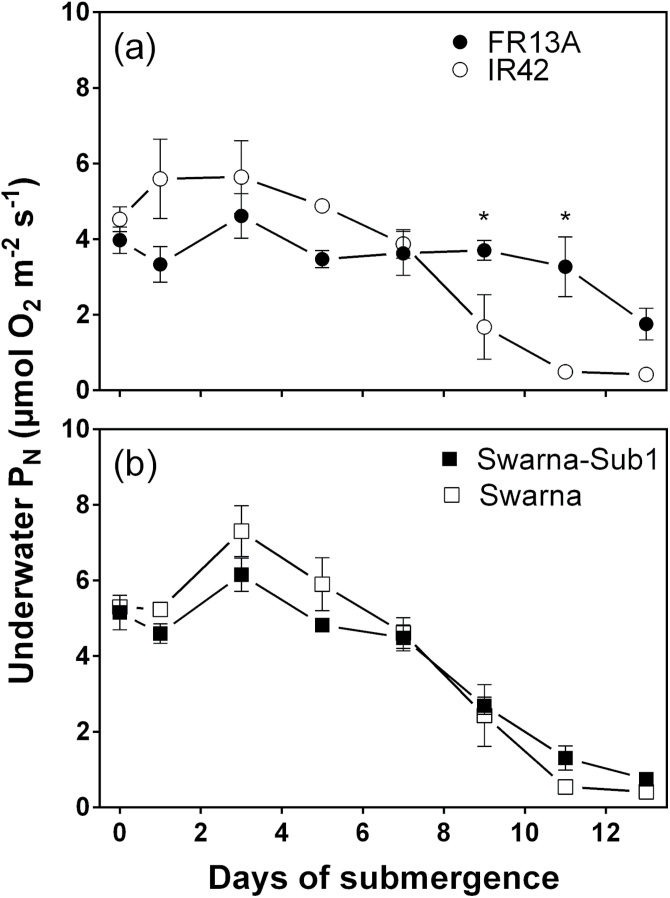
Measurements of underwater PN with 5mol CO2 m–3, a level that saturates underwater PN of Swarna-Sub1 (irrespective of leaf gas films presence or absence) in the present system (Winkel et al., 2013), was used to evaluate changes in capacity for underwater PN with time after submergence. All four genotypes had initial maximal underwater PN values between 4.0 and 5.3 µmol O2 m–2 s–1 (no significant difference; Fig. 1a, b). Capacity for underwater PN by FR13A and IR42 was significantly affected by time of submergence but maximal underwater PN of IR42 declined faster during the second week of submergence so that by the 13th day the rate was only 9% of the initial capacity (Fig. 1a; Table 1). Thus, during the latter part of the submergence treatment, capacity for underwater PN by FR13A was 6.7-fold higher than in IR42 (Fig. 1a). This superior performance of FR13A for retention of underwater photosynthetic capacity was not evident in Swarna-Sub1, which contains the SUB1 QTL from FR13A (Fig. 1b). The declines in capacity for underwater PN with time of submergence, in both Swarna-Sub1 and Swarna were equal to that in IR42 (Fig. 1a, b and Table 1). With high external CO2 in the floodwater, PN under water was 13.4–19.5% of ambient rates in air (rates of PN in air are given in the caption of Fig. 1). The lower PN rates under water than in air probably results from a combination of high resistance to gas exchange even in the presence of leaf gas films (Verboven et al., 2014) impeding O2 exit that is further reduced by the relatively low solubility of O2 in water, which would result in O2 build-up inside the tissues, and thus high photorespiration under water, as previously discussed for rice by Setter et al. (1989).
Fig. 1.
Underwater net photosynthesis (PN) of four genotypes of 5–7 weeks old rice (Oryza sativa) with time of submergence. (a) FR13A (submergence tolerant and donor of SUB1) and IR42 (submergence intolerant) and (b) Swarna (submergence intolerant) and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed). Lamina segments of 蝤200mm2 were incubated in rotating glass vials with 5mol CO2 m–3 and PAR of 760 µmol photons m–2 s–1 at 30 °C and PN was measured as O2 evolution (mean±SE, n=4). Underwater PN decreased significantly with time of submergence (Table 1); asterisk denotes significant differences between the two genotypes in each panel (Bonferroni test). Photosynthetic rates in air by FR13A, IR42, Swarna-Sub1 and Swarna, were 32.9±2, 40.3±3.4, 33.8±2.3, and 37.0±1.3 µmol CO2 m–2 s–1, and were not significantly different (1-way ANOVA, means±SE, n=3–9).
Table 1.
Key-results of 2-way ANOVA tests related to data shown in Figures 1, 2, 4, and 6. Analyses were performed for each parameter studied (underwater PN at 5 and 0.2mol CO2 m–3, gas film persistence, and leaf chlorophyll) with two genotypes (FR13A versus IR42 or Swarna versus Swarna-Sub1). P- and F-values are given for “genotype”, “time” and “genotype × time”. A P-level of 0.05 was used, but P-values for P<0.1 are also shown in italics; n.s.=not significant. Abbreviations: UW=underwater; PN=net photosynthesis; Chl=total chlorophyll.
| Parameters and genotype pairs in comparisons | “genotype” | “time” | “genotype × time” | Data in Figure number | |||
|---|---|---|---|---|---|---|---|
| P-value | F-value | P-value | F-value | P-value | F-value | ||
| UW PN 5 FR13A vs. IR42 | n.s. | 0.1 | <0.0001 | 11.7 | 0.0003 | 5.0 | 1 |
| UW PN 5 Swarna vs. Swarna-Sub1 | n.s. | 1.2 | <0.0001 | 60.6 | n.s. | 1.4 | 1 |
| Chl FR13A vs. IR42 | 0.0009 | 12.1 | <0.0001 | 52.9 | <0.0001 | 17.4 | 2 |
| Chl Swarna vs. Swarna-Sub1 | 0.030 | 4.9 | <0.0001 | 69.9 | 0.0003 | 4.3 | 2 |
| UW PN 0.2 FR13A vs. IR42 | 0.058 | 8.3 | <0.0001 | 46.1 | <0.0001 | 5.7 | 4 |
| UW PN 0.2 Swarna vs. Swarna-Sub1 | n.s. | 0.8 | <0.0001 | 45.3 | n.s. | 0.4 | 4 |
| Gas film FR13A vs. IR42 | 0.071 | 3.9 | <0.0001 | 50.5 | <0.0001 | 5.9 | 6 |
| Gas film Swarna vs. Swarna-Sub1 | 0.069 | 3.4 | <0.0001 | 62.3 | 0.052 | 2.0 | 6 |
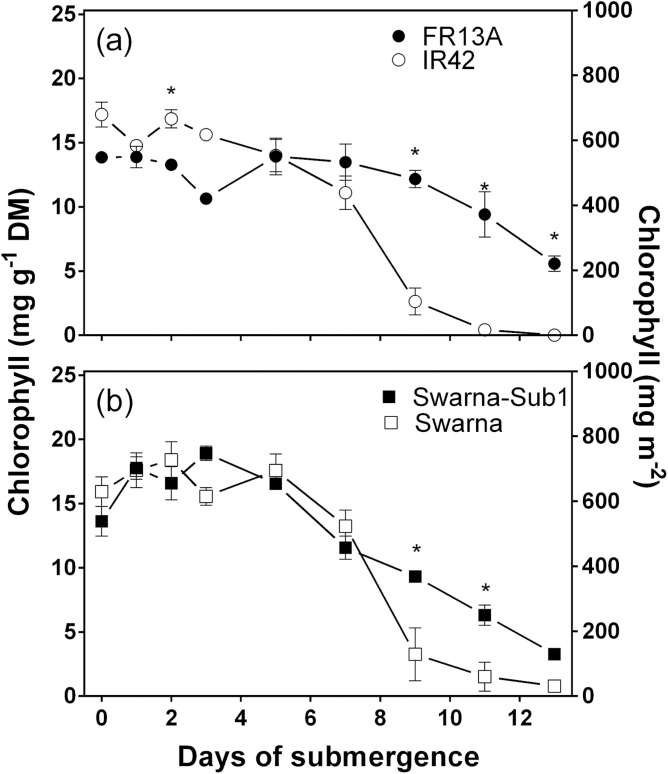
Declines in leaf chlorophyll concentrations with time of submergence (Fig. 2a, b), as well as other possible changes in the photosynthetic apparatus (not studied here), presumably contributed to the decline in photosynthetic capacity (Fig. 1a, b). Genotypes did not differ significantly in initial chlorophyll concentration. In all four genotypes, leaf chlorophyll declined with time of submergence but the patterns of these declines differed (Fig. 2a, b). Similar with the pattern for underwater photosynthetic capacity, FR13A and IR42 did not differ in chlorophyll concentrations during the first 8 days of submergence, but later in the submergence period the values in IR42 fell well below those in FR13A (Fig. 2a and Table 1). Interestingly, the superior chlorophyll retention of FR13A was conferred by the SUB1 QTL when in the Swarna background (Fig. 2b; i.e. Swarna-Sub1). The decline in leaf chlorophyll with time of submergence in Swarna did not differ from that in IR42 (Fig. 2a, b), whereas in Swarna-Sub1 it was more similar to FR13A.
Fig. 2.
Total chlorophyll concentration of four genotypes of 5–7 weeks old rice (Oryza sativa) with time of submergence. (a) FR13A (submergence tolerant and donor of SUB1) and IR42 (submergence intolerant) and (b) Swarna (submergence intolerant) and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed). Chlorophyll concentration was measured on the middle portion of the 2nd youngest fully expanded leaf (mean±SE, n=4). Chlorophyll concentration decreased significantly with time of submergence for all four genotypes (Table 1); asterisk denotes significant differences between the two genotypes in each panel (Bonferroni test).
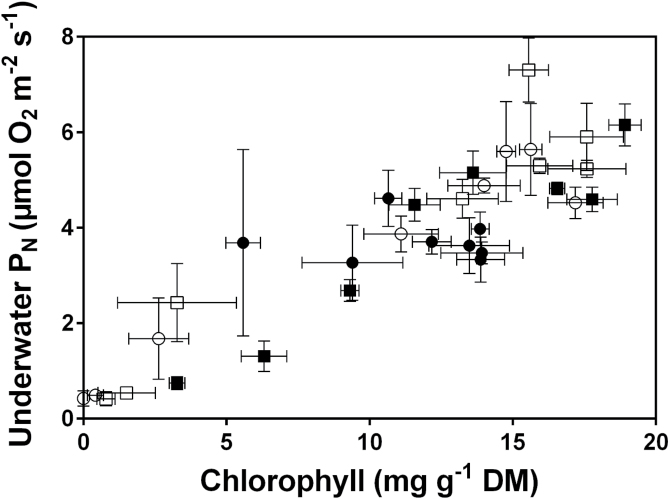
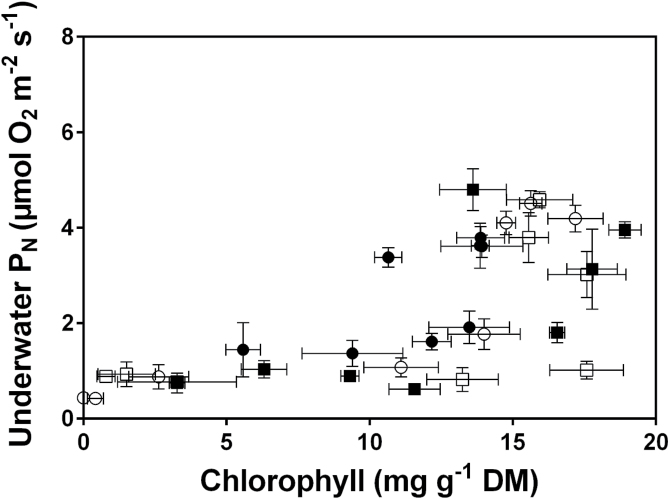
Correlation analyses were used to evaluate the relationships between leaf chlorophyll concentrations and capacity for underwater PN (Fig. 3). Underwater PN was positively correlated with leaf chlorophyll concentration for IR42, Swarna-Sub1, and Swarna, but not for FR13A. FR13A, in contrast with the other three genotypes, did not show a decline in underwater PN (Fig. 1a) despite that leaf chlorophyll decreased to 68% of its initial concentration on day 11 and to 40% on day 13 (Fig. 2a). If the submergence period was extended, so FR13A suffered greater declines in chlorophyll similar to those already apparent in the other three genotypes, then underwater PN would presumably decline and also result in a positive correlation between chlorophyll and underwater PN in FR13A.
Fig. 3.
Total chlorophyll concentration versus underwater net photosynthesis (PN) measured at 5mol CO2 m–3 of four genotypes of 5–7 weeks old rice (Oryza sativa). Genotypes were: FR13A (submergence tolerant and donor of SUB1; solid circle), IR42 (submergence intolerant; open circle), Swarna (submergence intolerant; open square), and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed; solid square). Spearman rank correlation analyses (one-tailed) of chlorophyll concentration versus underwater PN showed: all genotypes pooled, P<0.0001; FR13A P=0.3517; IR42 P=0.0054; Swarna P=0.0140, and Swarna-Sub1 P=0.0023. Means±SE, n=5.
Although changes in leaf chlorophyll concentration, and possibly other changes in the photosynthetic machinery, presumably were the major factors contributing to declines in capacity for underwater PN (Fig. 3), it should also be noted that towards the end of the submergence period (day 10 onwards), the previously gas-filled volume of the tissue had been infiltrated by water in three of the four genotypes (Supplementary Fig. S1 available at JXB online), the exception was FR13A. Water infiltration of the leaf tissue is an indication of structural degradation; any such tissue degradation would also have contributed to the low chlorophyll concentrations (Fig. 2) and very low rates of underwater PN (even at 5mol CO2 m–3) of IR42, Swarna, and Swarna-Sub 1 at the end of the treatment period (Fig. 1a, b).
Underwater net photosynthetic rates at near-ambient dissolved CO2 (0.2mol m–3)
Measurements of underwater PN with 0.2mol CO2 m–3, a near ambient concentration in a similar field situation (Winkel et al., 2013), was used to evaluate field relevant rates of underwater PN with time after submergence. At this CO2 concentration, underwater PN is limited by CO2 entry owing to the high resistance to diffusion from the bulk medium into the submerged leaf (Pedersen et al., 2009; Winkel et al., 2013). Therefore, gas film presence, a feature which reduces gas exchange resistance of submerged leaves (Colmer and Pedersen, 2008b; Raskin and Kende, 1983; Verboven et al., 2014), is of importance. Thus, the relationship of gas film persistence with underwater PN, and decline in leaf chlorophyll concentrations, both as influenced by time of submergence, are of importance to characterize for contrasting genotypes. To facilitate comparison with non-limiting CO2 conditions, we first consider the photosynthetic rates at near-ambient dissolved CO2 as related to the decline in leaf chlorophyll (Fig. 2a, b) and then followed by consideration of the role of leaf gas films.
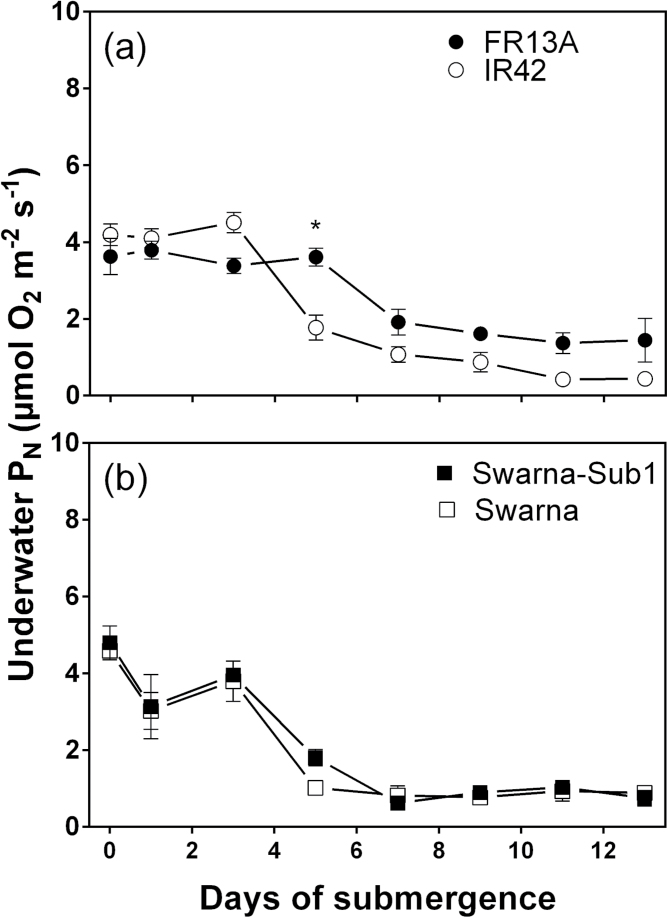
All four genotypes had initial underwater PN rates of 3.6–4.8 µmol O2 m–2 s–1 (no significant difference) when supplied with 0.2mol CO2 m–3, and these rates all declined significantly with time of submergence (Fig. 4a, b and Table 1). On the last day of submergence, underwater PN by FR13A was 3.3-fold higher than in IR42 (Fig. 4a). This higher rate in FR13A was again not evident in the SUB1 introgression line in Swarna background (Fig. 4b; i.e. Swarna-Sub1). Although underwater PN in FR13A was significantly higher than in the three other genotypes, even in FR13A towards the end of the submergence treatment the rate had declined to 40% of the initial rate (the other three genotypes had 11–19% of their initial rates). There was a positive relationship between leaf chlorophyll concentration and underwater PN for three of the genotypes, but less so for Swarna (Fig. 5). As in the CO2 saturated condition, leaf chlorophyll concentration was positively correlated with underwater PN, but closer examination of the dynamics in the changes in chlorophyll as compared with changes in underwater PN indicate there must also be an additional factor(s); here we assessed the potential influence of leaf gas films.
Fig. 4.
Underwater net photosynthesis (PN) of four genotypes of 5–7 weeks old rice (Oryza sativa) with time of submergence. (a) FR13A (submergence tolerant and donor of SUB1) and IR42 (submergence intolerant) and (b) Swarna (submergence intolerant) and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed). Lamina segments of 蝤 200mm2 were incubated in rotating glass vials with 0.2mol CO2 m–3 and PAR of 760 µmol photons m–2 s–1 at 30 °C and PN was measured as O2 evolution (mean±SE, n=4). Underwater PN decreased significantly with time of submergence for all four genotypes (Table 1); asterisk denotes significant differences between the two genotypes in each panel (Bonferroni test).
Fig. 5.
Total chlorophyll concentration versus underwater net photosynthesis (PN) measured at 0.2mol CO2 m–3 of four genotypes of 5–7 weeks old rice (Oryza sativa). Genotypes were: FR13A (submergence tolerant and donor of SUB1; solid circle), IR42 (submergence intolerant; open circle), Swarna (submergence intolerant; open square), and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed; solid square). Spearman rank correlation analyses (one-tailed) of chlorophyll concentration versus underwater PN showed: all genotypes pooled, P<0.0001; FR13A P=0.0077; IR42 P=0.0006; Swarna P=0.0575, and Swarna-Sub1 P=0.0347. Means±SE, n=5.
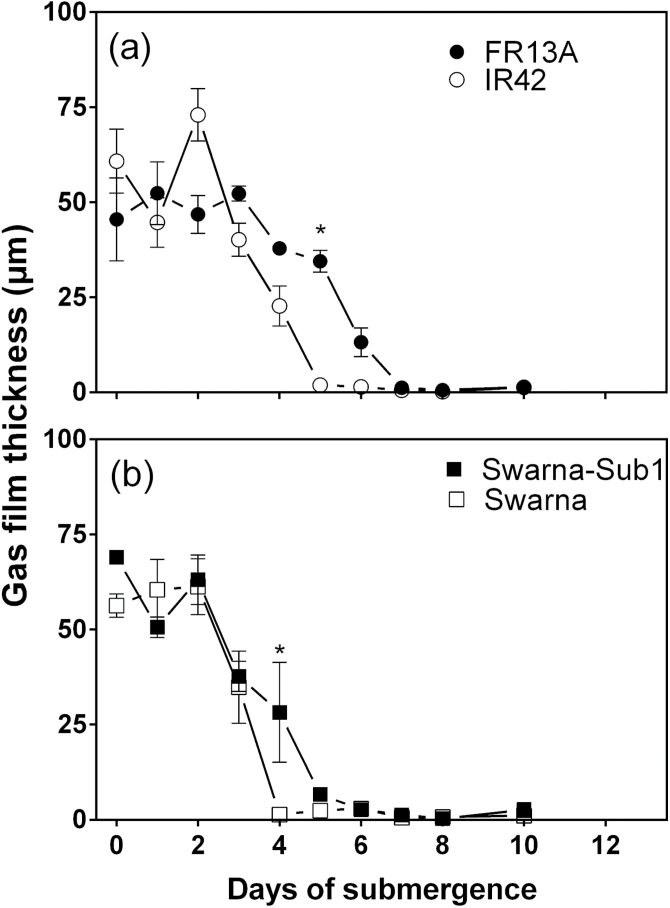
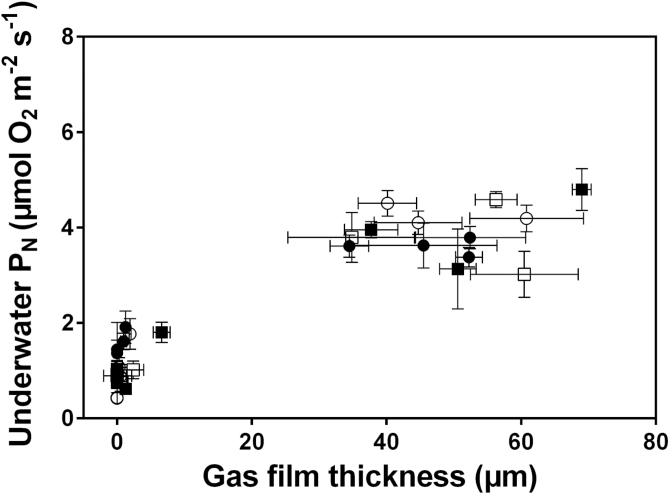
All four genotypes initially possessed gas films on both leaf sides when submerged. These gas films were maintained near the initial thickness for the first 4 days in FR13A and IR42, and then declined with time of submergence (Fig. 6a, Table 1). The decline, however, was initially faster for IR42 than FR13A, so that gas films were lost by the 5th day in IR42 and by the 7th in FR13A. The dynamics in the reductions in thickness of the gas films were, with exception of day 4, essentially the same for Swarna-Sub1 and Swarna (Fig. 6b, “genotype × time” interactions listed in Table 1); these declines resembled those of IR42. Fig. 7 evaluates the relationship between leaf gas films thickness and underwater PN using the data up to day 7 by which time gas films had been lost for all genotypes but leaf chlorophyll had not yet significantly declined; this ensures that the effect of gas films is not confounded at this stage by changes in chlorophyll concentrations. This analysis shows that the initial declines in leaf gas film thickness hardly influenced underwater PN whereas underwater PN was markedly lower when gas films were no longer present (Fig. 7).
Fig. 6.
Leaf gas film thickness of four genotypes of 5–7 weeks old rice (Oryza sativa) with time of submergence. (a) FR13A (submergence tolerant and donor of SUB1) and IR42 (submergence intolerant) and (b) Swarna (submergence intolerant) and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed). Gas film volume was measured by determining tissue buoyancy before and after gas film removal using the method of Raskin (1983) and then divided by two-sided leaf area to obtain mean thickness (mean±SE, n = 4). Gas film thickness decreased significantly with time of submergence (Table 1); asterisk denotes significant differences between the two genotypes in each panel (Bonferroni test).
Fig. 7.
Leaf gas film thickness versus underwater net photosynthesis (PN) measured at 0.2mol CO2 m–3 of four genotypes of 5–7 weeks old rice (Oryza sativa). Genotypes were: FR13A (submergence tolerant and donor of SUB1; solid circle), IR42 (submergence intolerant; open circle), Swarna (submergence intolerant; open square), and Swarna-Sub1 (submergence tolerant with SUB1 QTL introgressed; solid square). Spearman rank correlation analyses (one-tailed) of gas film thickness versus underwater PN showed no significant correlations of neither all genotypes pooled nor for each individual genotype when excluding gas film thicknesses below 2 µm (the detection limit of the present method of gas film quantification). Means±SE, n=5.
Growth, leaf sugars/starch, and survival
The present study was in a field with simulated flash-flooding causing complete submergence of 13 days. In addition to our focus here to fill the knowledge gap on underwater PN and gas film persistence for these contrasting genotypes, growth during submergence, leaf sugars/starch, and survival were also evaluated. As earlier work has focused on these aspects (Mackill et al., 2012), here we relegate those data to the supplementary materials (Supplementary Figs S2 and S3 available at JXB online).
Discussion
FR13A has high tolerance of submergence (Singh et al., 2001) and a large proportion of this tolerance is associated with the SUB1 QTL (Mackill et al., 2012). The SUB1 QTL confers submergence tolerance in rice, assessed as survival and recovery of growth and/or yield following transient complete submergence (Jagadish et al., 2012). This tolerance is associated with less elongation during submergence, higher soluble carbohydrates in shoots, and less oxidative damage post-submergence (Fukao et al., 2009; Xu and Mackill, 1996; Xu et al., 2006). These traits are well studied in FR13A and SUB1 genotypes, whereas the known ability of FR13A to retain chlorophyll when submerged (Ella et al., 2003b) and its influence on underwater PN had not previously been evaluated. The present study shows that when submerged, FR13A retains its capacity for underwater PN (CO2 saturated rate), whereas this capacity declined markedly in sensitive genotypes (IR42 and Swarna, Fig. 1). Nevertheless, at near ambient CO2 levels in floodwater, underwater PN had declined in all genotypes during the second week of submergence, as leaf gas films only persisted for the first several days (Fig. 6). Regarding the SUB1 QTL, Swarna-Sub1 also showed improved chlorophyll retention, but its capacity for underwater PN was not improved, indicating that other components of the photosynthetic machinery must have been compromised. The changes in gas film presence and leaf chlorophyll concentration (and presumably other components of the photosynthetic machinery) with duration of submergence both contribute to the decline in rates of underwater PN of submerged rice.
The impressive maintenance by FR13A of capacity for underwater PN (CO2 saturated rate) during 13 days of submergence adds to the list of known traits associated with submergence tolerance in this genotype, being much higher than in Sub1 introgression lines (Neeraja et al., 2007; Singh et al., 2009). FR13A is known to possess four more, but minor, QTLs associated with submergence tolerance (Nandi et al., 1997). Submerged rice can suffer leaf chlorosis, a condition triggered by ethylene accumulation, but chlorosis is less in tolerant (e.g. FR13A) as compared with sensitive (e.g. IR42) genotypes (Ella et al., 2003b; Jackson et al., 1987). The present underwater PN measurements add functional data to extend the previous observation of better chlorophyll retention in FR13A as compared with IR42 (Ella et al., 2003b). An earlier study had indicated a significant decline in photosynthetic capacity already after 1 day (IR42) and 3 days (FR13A) of submergence (Smith et al., 1988), but this earlier work used an IRGA to measure leaves soon after return to air. By contrast, the present study measured photosynthesis under water (Pedersen et al., 2013) and IR42 declined in photosynthetic capacity (i.e. CO2 saturated rate) only during the second week of submergence (Fig. 1). The fast declines in photosynthetic rates observed by Smith et al. (1988) were not associated with changes in leaf chlorophyll, whereas in our longer term study there were strong positive correlations between reductions in leaf chlorophyll concentrations and reduced capacity for underwater PN (Fig. 3).
Underwater PN at 0.2mol CO2 m–3 (representative of ambient in submergence situations) was not, however, preserved as well as underwater PN at high CO2 (5mol CO2 m–3) for the leaves of submerged rice. The declines with time in underwater PN of the various genotypes at 0.2mol CO2 m–3 were probably due to the loss of leaf gas films after 4–6 days of submergence; loss of gas films would decrease the uptake of CO2 from the floodwater (c.f. Pedersen et al., 2009). Gas films persisted on the submerged leaves for 4–6 days depending on genotype and the loss of leaf gas films were strongly linked to a steep decline in underwater PN at 0.2mol CO2 m–3 for all four genotypes (Figs 4 and 6). By contrast, lamina chlorophyll concentration did not significantly decrease until after the leaf gas films had disappeared and so the substantial declines in underwater PN during the initial 5 days of submergence were therefore unlikely to have been caused by chlorophyll degradation. Leaf gas films increase underwater gas exchange and thus CO2 entry to sustain rates of underwater PN (Colmer and Pedersen, 2008b; Pedersen et al., 2009; Winkel et al., 2011). Moreover, modelling of O2 entry during darkness into respiring rice leaves with or without gas films has further demonstrated that the resistance to O2 exchange with the floodwater is reduced by the presence of gas films, with assessments also of the various resistance components in the pathway(s) (Verboven et al., 2014).
Leaf gas films have been shown to enhance internal aeration of belowground tissues during complete submergence (Pedersen et al., 2009; Winkel et al., 2013; Winkel et al., 2011). It was recently shown that even low rates of underwater PN greatly influence root O2 status during daytime for Swarna-Sub1 during 2 days of submergence in a field (Winkel et al., 2013). Thus, retention and persistence of leaf gas films by submerged plants is likely to be beneficial, but factors involved in the degradation of leaf gas films during prolonged submergence require additional study. Leaf gas films might also be an effective barrier against infections and we speculate when lost this will facilitate contact and colonization by microorganisms in the floodwater. It can be hypothesized that once the leaf gas films have been lost the process of tissue deterioration speeds up, eventually leading to tissue death. Superhydrophobic leaf surfaces are hypothesized to be an adaptation for leaves to self-clean and facilitate water to roll off leaves in air when it rains to prevent covering of leaves by a film of water (Neinhuis and Barthlott, 1997), as a water layer on a leaf surface would reduce gas exchange and thus photosynthesis, and also enhance the likelihood of bacteria and fungi infecting leaves (Koch et al., 2009). The leaf gas film persistence was moderately longer in FR13A and our data show that underwater PN at a near ambient CO2 concentration was strongly enhanced by leaf gas film presence. Thus, we wonder if there is larger diversity of gas film retention and persistence in lowland rice than documented in the present study.
Pedersen et al. (2009) demonstrated the essential role of leaf gas films on sugar status of completely submerged rice and Winkel et al. (2013) showed the importance of underwater PN for internal aeration in roots of submerged rice. The mechanisms determining longevity of leaf gas films should be further elucidated and rice germplasm screened for longer leaf gas film persistence during submergence, as this trait could potentially increase carbohydrate status and internal aeration owing to increased underwater PN during prolonged submergence. Furthermore, studies are needed to investigate the extent of gas films persistence as related to various weather and floodwater characteristics that affects survival in the field e.g. conditions as noted in Das et al. (2009) and in Colmer et al. (2014).
Supplementary data
Supplementary data are available at JXB online
Figure S1. Leaf lamina porosity
Figure S2. Leaf lamina sugars and starch
Figure S3. Relative growth rate and survival
Acknowledgements
We thank Anja Fløytrup, Melencio Apostol, James Egdane, and Vichelle Dastas for their technical assistance in setting up the trials and collecting and analysing the samples for sugars and chlorophyll. This work was funded by a University of Western Australia International Postgraduate Research Scholarship to Anders Winkel, the Danish Council for Independent Research grant no. 09-072482, the Crawford Fund for International Agricultural Research, and the International Rice Research Institute. We thank Unisense A/S for the use of equipment and the UWA Institute of Advanced Studies for hosting Ole Pedersen during his visits to UWA.
References
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7, 225–332 [Google Scholar]
- Bailey-Serres J, Fukao T, Ismail AM, Heuer S, Mackill DJ. 2010. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice 3, 138–147 [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: Acclimations and genetic diversity. Annual Review of Plant Biology 59, 313–339 [DOI] [PubMed] [Google Scholar]
- Colmer TD, Armstrong W, Greenway H, Ismail AM, Kirk GJD, Atwell BJ. 2014. Physiological mechanisms of flooding tolerance in rice: transient complete submergence and prolonged standing water. Progress in Botany 75, 255–307 [Google Scholar]
- Colmer TD, Pedersen O. 2008a. Oxygen dynamics in submerged rice (Oryza sativa). New Phytologist 178, 326–334 [DOI] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O. 2008b. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177, 918–926 [DOI] [PubMed] [Google Scholar]
- Colmer TD, Winkel A, Pedersen O. 2011. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB PLANTS 2011, plr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KK, Panda D, Sarkar RK, Reddy JN, Ismail AM. 2009. Submergence tolerance in relation to variable floodwater conditions in rice. Environmental and Experimental Botany 66, 425–434 [Google Scholar]
- Ella ES, Kawano N, Ito O. 2003a. Importance of active oxygen-scavenging system in the recovery of rice seedlings after submergence. Plant Science 165, 85–93 [Google Scholar]
- Ella ES, Kawano N, Yamauchi Y, Tanaka K, Ismail AM. 2003b. Blocking ethylene perception enhances flooding tolerance in rice seedlings. Functional Plant Biology 30, 813–819 [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. 2008. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proceedings of the National Academy of Sciences, USA 105, 16814–16819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Harris T, Bailey-Serres J. 2009. Evolutionary analysis of the Sub1 gene cluster that confers submergence tolerance to domesticated rice. Annals of Botany 103, 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. 2006. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell Online 18, 2021–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Singh US, Singh S, Dar MH, Mackill DJ. 2013. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Research 152, 83–89 [Google Scholar]
- Jackson M, Waters I, Setter T, Greenway H. 1987. Injury to rice plants caused by complete submergence; a contribution by ethylerie (ethene). Journal of Experimental Botany 38, 1826–1838 [Google Scholar]
- Jagadish S, Septiningsih E, Kohli A, Thomson M, Ye C, Redona E, Kumar A, Gregorio G, Wassmann R, Ismail A. 2012. Genetic advances in adapting rice to a rapidly changing climate. Journal of Agronomy and Crop Science 198, 360–373 [Google Scholar]
- Koch K, Bhushan B, Barthlott W. 2009. Multifunctional surface structures of plants: An inspiration for biomimetics. Progress in Materials Science 54, 137–178 [Google Scholar]
- Mackereth FJH, Heron J, Talling JF. 1978. Water analysis: some revised methods for limnologists. UK: Freshwater Biological Association [Google Scholar]
- Mackill DJ, Ismail AM, Singh US, Labios AV, Paris TR. 2012. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. In: Sparks DL, ed. Advances in Agronomy Vol. 115. San Diego: Academic Press, 299–352 [Google Scholar]
- Mackinney G. 1941. Absorption of light by chlorophyll solutions. The Journal of Biological Chemistry 140, 315–322 [Google Scholar]
- Mommer L, Pedersen O, Visser EJW. 2004. Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant, Cell and Environment 27, 1281–1287 [Google Scholar]
- Nandi S, Subudhi P, Senadhira D, Manigbas N, Sen-Mandi S, Huang N. 1997. Mapping QTLs for submergence tolerance in rice by AFLP analysis and selective genotyping. Molecular and General Genetics MGG 255, 1–8 [DOI] [PubMed] [Google Scholar]
- Neeraja C, Maghirang-Rodriguez R, Pamplona A, Heuer S, Collard B, Septiningsih E, Vergara G, Sanchez D, Xu K, Ismail A. 2007. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theoretical and Applied Genetics 115, 767–776 [DOI] [PubMed] [Google Scholar]
- Neinhuis C, Barthlott W. 1997. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Annals of Botany 79, 667–677 [Google Scholar]
- Pedersen O, Colmer TD, Sand-Jensen K. 2013. Underwater photosynthesis of submerged plants—recent advances and methods. Frontiers in Plant Science 4, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Colmer TD. 2009. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58, 147–156 [DOI] [PubMed] [Google Scholar]
- Ram PC, Singh AK, Singh BB, Singh VK, Singh HP, Setter TL, Singh VP, Singh RK. 1999. Environmental characterization of floodwater in eastern India: relevance to submergence tolerance of lowland rice. Experimental Agriculture 35, 141–152 [Google Scholar]
- Raskin I. 1983. A method for measuring leaf density, thickness and internal gas. Hortscience 18, 698–699 [Google Scholar]
- Raskin I, Kende H. 1983. How does deep water rice solve its aeration problem? Plant Physiology 72, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz AJ, Folsom JJ, Jikamaru Y, Ronald P, Walia H. 2013. SUB1A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytologist 198, 1060–1070 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Waters I, Wallace I, Bekhasut P, Greenway H. 1989. Submergence of rice. I. Growth and photosynthetic response to CO2 enrichment of floodwater. Australian Journal of Plant Physiology 16, 251–263 [Google Scholar]
- Singh HP, Singh BB, Ram PC. 2001. Submergence tolerance of rainfed lowland rice: search for physiological marker traits. Journal of Plant Physiology 158, 883–889 [Google Scholar]
- Singh S, Mackill DJ, Ismail AM. 2009. Responses of SUB1 rice introgression lines to submergence in the field: Yield and grain quality. Field Crops Research 113, 12–23 [Google Scholar]
- Smart R, Barko J. 1985. Laboratory culture of submersed freshwater macrophytes on natural sediments. Aquatic Botany 21, 251–263 [Google Scholar]
- Smith PA, Kupkanchanakul K, Emes MJ, Cutter EG. 1988. Changes in fluorescence and photosynthesis during submergence of deepwater rice. In: Proceedings of the 1987 International Deepwater Rice Workshop. Los Banos: International Rice Research Institute, 327–335 [Google Scholar]
- Verboven P, Pedersen O, Ho QT, Nicolaï BM, Colmer TD. 2014. The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant, Cell and Environment, doi: 10.1111/pce.12300. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. 2006. How plants cope with complete submergence. New Phytologist 170, 213–226 [DOI] [PubMed] [Google Scholar]
- Waters I, Armstrong W, Thomson C, Setter T, Adkins S, Gibbs J, Greenway H. 1989. Diurnal changes in radial oxygen loss and ethanol metabolism in roots of submerged and non-submerged rice seedlings. New Phytologist 113, 439–451 [Google Scholar]
- Winkel A, Colmer TD, Ismail AM, Pedersen O. 2013. Internal aeration of paddy field rice (Oryza sativa L.) during complete submergence—importance of light and floodwater O2 . New Phytologist 197, 1193–1203 [DOI] [PubMed] [Google Scholar]
- Winkel A, Colmer TD, Pedersen O. 2011. Leaf gas films of Spartina anglica enhance rhizome and root oxygen during tidal submergence. Plant, Cell and Environment 34, 2083–2092 [DOI] [PubMed] [Google Scholar]
- Xu K, Mackill DJ. 1996. A major locus for submergence tolerance mapped on rice chromosome 9. Molecular Breeding 2, 219–224 [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. 2006. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442, 705–708 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.