Abstract
A recombinant inbred durum wheat population was grown under three contrasting regimes: long days following vernalization (LDV), long days without vernalization (LD), and short days following vernalization (SDV). The length of several pre-anthesis stages and the number of leaves and the phyllochron were measured. Different groups of genes were involved in determining the phenology in the three treatments, as demonstrated by a quantitative trait locus (QTL) analysis. The length of the period required to reach the terminal spikelet stage was correlated with the time to anthesis only in the case of LDV- and LD-grown plants where the timing of anthesis depended on the final leaf number. However, for SDV-grown plants, anthesis date was more dependent on the length of the period between the terminal spikelet stage and anthesis and was independent of leaf number. The involvement of the phyllochron in determining the duration of pre-anthesis development was also treatment-dependent. QTL mapping of the various flowering time associated traits uncovered some novel loci (such as those associated with the phyllochron), in addition to confirming the presence of several well-established loci.
Key words: Durum wheat, leaf number, phenology, phyllochron, pre-anthesis, QTL.
Introduction
Flowering time is an important determinant of grain yield (Reynolds et al., 2009), and its manipulation is a common breeding target. Studies of the genetic determination of flowering time in wheat have demonstrated that it is controlled by at least 20 genes, scattered over the whole genome (Snape et al., 1996; Koornneef et al., 1998); these genes have been classified according to whether they respond to vernalization or to photoperiod or whether they confer earliness per se (EPS; Worland, 1996; Law and Worland, 1997; Laurie et al., 2004). Much less information, however, has been gathered concerning the genetic basis of the duration of the various pre-anthesis stages of spike growth (Borràs-Gelonch et al., 2011) and the extent to which these vary in a genetically determined manner remains unclear (Gonzáles et al., 2005a; Whitechurch et al., 2007; Borràs-Gelonch et al., 2011). The period during which most of the growth of the wheat spike occurs coincides with the stem elongation stage, so lengthening the latter can be expected to increase the size of the spike and, by implication, also the number of potential grains that are set (Halloran and Pennell, 1982; Slafer et al., 1996; Slafer and Whitechurch, 2001). According to Fischer (1983), the period between the emergence of the penultimate leaf and anthesis is the most critical stage of the spike’s growth. Thus, the ability to fine-tune crop phenology offers some potential to increase spike fertility (Fischer, 2011; Foulkes et al., 2011; García et al., 2011).
The main stem of the wheat plant develops via the accumulation of primordia at the stem apex and their subsequent differentiation into either vegetative (leaf) or reproductive (spikelet) structures, thereby providing a mechanism whereby the duration of the various growth stages can vary (Jamieson et al., 1998). The inverse of the rate of leaf appearance (the ‘phyllochron’) is about double the inverse of the rate of primordium production (the ‘plastochron’) (Kirby, 1990). The period over which primordia are initiated depends both on the plastochron and the number of primordia actually initiated, while the period over which leaves emerge depends on the phyllochron and the final leaf number. The (thermal) time interval separating the appearance of the flag leaf ligula and anthesis is less dependent on either genotype or environment than is the duration of the preceding stages (Amir and Sinclair, 1991). Final leaf number, the phyllochron, and the length of the interval between flag leaf ligula appearance and anthesis interval are considered as being the major determinants of development (Jamieson et al., 1998). The duration of the various pre-anthesis stages can similarly be analysed in terms of final leaf number and the phyllochron, given the strong association between the length of time required to reach the terminal spikelet stage and the final leaf number (Jamieson et al., 2007).
The current experiments were designed to characterize variability in the duration of the period between consecutive various pre-anthesis phases, the final leaf number, and the phyllochron in durum wheat (Triticum turgidum ssp. durum), with a focus both on identifying the nature of the genetic control over the duration of the various pre-anthesis phases, and on defining the environmental cues (day length and vernalization), if any, which underlie them. An additional focus was to determine whether the genotypic relationships between pre-flowering phases, final leaf number, phyllochron, and anthesis date are differentially affected by EPS, sensitivity to photoperiod, or sensitivity to vernalization.
Materials and methods
Plant materials and experimental design
The mapping population was a set of 100 recombinant inbred lines (RILs) bred from the cross cv. Ofanto (an early flowering, semi-dwarf cultivar released in 1990) × cv. Cappelli (a late flowering, tall, vernalization-requiring cultivar released in 1915). The experimental site was at Ottava, Sardinia (41° N 8° E; 225 metres above sea level). A set of similarly sized vernalized and nonvernalized seedlings was potted on 24 May (day length 14.8h), and a second sowing of vernalized seedlings on 23 December of the same year. Vernalization was achieved by imbibing the grain for 24h at room temperature, and then growing the seedlings in the dark at 4 °C for 40 days. Between 6 and 8 weeks below 5 °C is assumed to be sufficient for the full vernalization of most wheat cultivars (Davidson et al., 1985; Griffiths et al., 1985). Two pots (each containing three plants) were assigned to each RIL/treatment combination and were arranged in a completely randomized design. The May-sown plants (long days, vernalized plants: LDV, long days, nonvernalized: LD) were maintained outdoors and the December-sown ones (short days, vernalized) were kept in a greenhouse. The pots were watered and fertilized as required.
The LDV treatment was characterized by the least limiting conditions in both cold and day length, as confirmed by its mean transplanting–terminal spikelet period (TRA-TS) of 449 °Cd, close to the minimum of 400 °Cd proposed by Ritchie (1991) for fully vernalized wheat plants grown under long days. Differences in EPS among the RILs were therefore estimated from this treatment. The comparison between LDV and LD allowed for the separation of the effect of vernalization from that of photoperiod, while the comparison between LDV and SDV allowed for the separation of photoperiod from vernalization effects (Herndl et al., 2008).
Phenotyping
Although the timing of arrival at TS is most accurately assessed destructively, a simpler, nondestructive means of assay has been based on observing the timing of elongation of the first internode (Hay, 1978). The latter data were obtained by a twice-weekly measurement of the height above the ground of the ligule of the youngest fully emerged leaf on the main stem. The relationship between this height and the thermal time from TRA took the form of two linear segments of nonidentical slope: the shallower one reflected growth prior to stem elongation and the steeper one post stem elongation. The accumulated thermal time at this inflexion point was quantified using a ‘segmented regression’ approach, implemented in the split-line regression procedure within GENSTAT (2008). The plants were monitored on the same twice-weekly basis to allow the timing of the emergence of the penultimate leaf (PEN) and the flag leaf (FLA), booting (BOOT) and anthesis (ANT) to be recorded on the main stem. FLA, BOOT, and ANT correspond to stages 39, 45, and 65, respectively, of Zadoks et al. (1974). These timings defined the lengths of the TRA–TS, TS–ANT, FLA–ANT, PEN–ANT, and FLA–BOOT intervals, expressed in thermal time according to Weir et al. (1984), based on measured daily values of minimum and maximum temperature. The length of the photoperiod was based on the period between daybreak and when the sun had set 6 ° below the horizon (Weir et al., 1984). Following Herndl et al. (2008) and White and Laing (1989), the relative response to vernalization (RRV) was computed from R (the inverse of the duration in °Cd of the various intervals) in the form 1 – (RLD/RLDV), and similarly RRP was given by 1 – RSDV/RLDV). These two indices enabled the quantification of photoperiod sensitivity and vernalization requirement in the form of deviations from the EPS response. The number and length of the leaves which had emerged on the main stem were recorded twice weekly until the flag leaf had become fully extended, following Haun (1973). A rate of leaf emergence was calculated for each plant from the slope of the regression between the Haun stage and the thermal time from TRA. Two separate regressions were performed for each plant: one included all the leaves and the other included only leaves 2–8, as recommended by Jamieson et al. (1995). For all plants, the linear regressions were both statistically significant, explaining >90% of the phenotypic variation. An average phyllochron (AvgPHY) was calculated as the reciprocal of the rate of leaf emergence obtained from the former regression, and a second phyllochron (PHY28) from the latter one. The total number of leaves borne by the main stem (LNANT) and that of the leaves which had emerged by TS (LNTS) was recorded. The latter was calculated by substituting the thermal time elapsed from TRA to TS into the above regressions. The number of leaves which had emerged after TS (LNafterTS) was given by the difference LNANT – LNTS.
Statistical treatment of phenotypic data
The magnitudes of the treatment, genotypic, and genotype × treatment interaction effects were obtained from a mixed-model analysis of variance (ANOVA) obtained by implementing the REML procedure within GENSTAT (2008). The same type of analysis was applied to each environment separately. The variance components and best linear unbiased predictors (BLUPs) related to each RIL and trait were calculated, and heritabilities were estimated from the resulting variance components on a line mean basis. The BLUPs were used to visualize frequency distributions across the set of RILs and to estimate genetic correlations, following Borràs-Gelonch et al. (2011).
Quantitative trait locus mapping
Quantitative trait locus (QTL) analysis was performed using the software package MapQTL version 5.0 (van Ooijen and Voorips, 2004) and was based on the genotypic data and derived genetic map described by Marone et al. (2012) and Panio et al. (2013). Limit of detection (LOD) profiles obtained from simple interval mapping were used to identify the marker closest to each predicted QTL position, and this was then used as a cofactor to perform multiple QTL mapping analysis. LOD significance threshold levels were calculated via a 10 000 permutation test, provided within MapQTL. The length of the genetic interval between the flanking markers of each QTL was determined using the ΔLOD-1 support interval criterion. A number of QTL associated with a LOD scoring marginally below the significance threshold (LOD=3) were included only where they colocalized with one or more statistically significant QTL.
Results
Day length and temperature
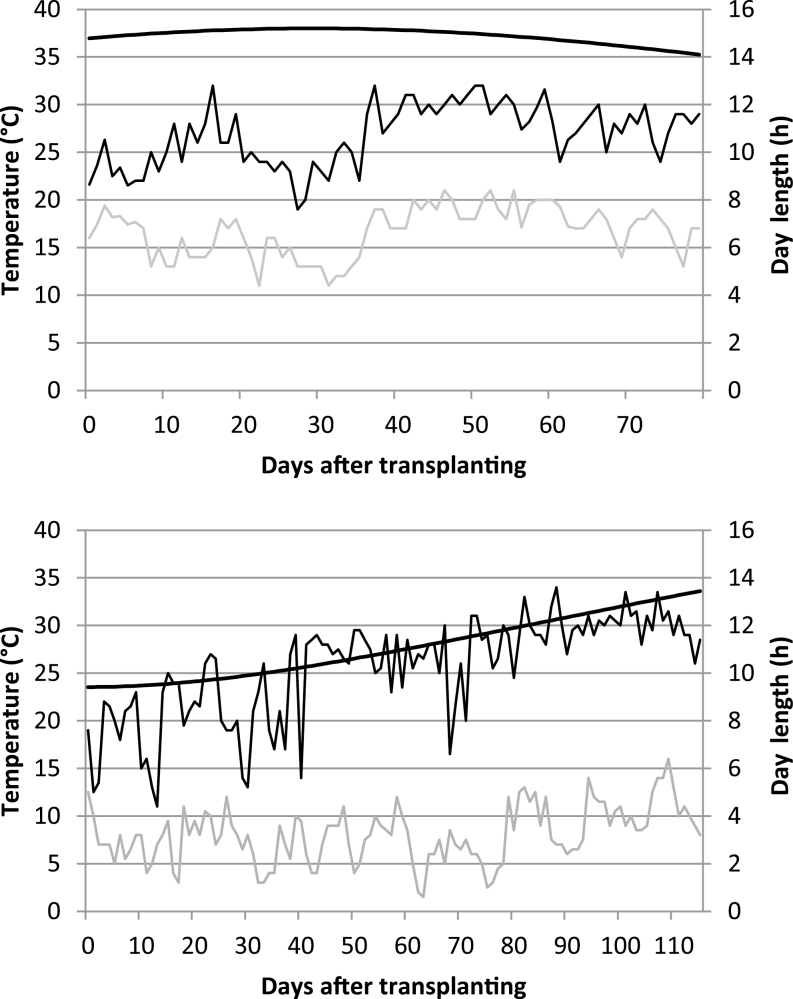
The photoperiod at the time of the May transplanting was 14.8h, while at the time of the December transplanting, it was 9.4h (Fig. 1). Following the May transplanting (LDV and LD), it peaked at 15.2h, around the mean of the time when the RILs had reached TS. At the mean anthesis date, the photoperiod was 15.1h for LDV-grown plants and 14.6h for the LD-grown ones. The SDV-grown plants experienced a lengthening photoperiod, reaching 10.3h around TS, 12.4h around FLA, and 13.1h around anthesis. The mean air temperature was about 20.3 °C over the TRA–TS interval for the LD- and LDV-grown plants, but only 13.9 °C for the SDV-grown ones. Over the period TS–ANT, the mean air temperature rose to 24.2 °C for the LDV-grown plants and to 18.1 °C for the SDV-grown plants.
Fig. 1.
Maximum and minimum air temperature and the variation in day length between transplanting and anthesis in LDV- and LD-grown plants (top) and SDV-grown plants (bottom).
Trait analysis
The ANOVA derived from the full data set indicated the absence of a main genetic effect for most of the traits, but the presence of a major genotype × treatment interaction (Table 1). On the other hand, the genetic component was significant for most of the traits when each set of plants (LDV-, LD-, and SDV-grown) was analysed separately. The broad sense heritability was >90% with respect to the length of the TRA–ANT period for each set of plants, and less (but still high) for the duration of the other pre-anthesis stages. Comparable levels of heritability were observed for both LN and the phyllochron.
Table 1.
ANOVA for and heritability of the various phenological traits expressed in the cv. Ofanto × cv. Cappelli RIL population
| Trait | LDV | LD | SDV | Combined analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Residual | h2 | Genotype | Residual | h2 | Genotype | Residual | h2 | Genotype | GxE | Residual | h2 | |
| Anthesis (°Cd) | 1138±179 | 438±32 | 92.6 | 22 951±3610 | 4118±439 | 94.2 | 3567±578 | 1490±117 | 91.4 | 147±579 | 8865±966 | 1639±78 | 4.4 |
| TRA–TS (°Cd) | 1472±243 | 1010±72 | 88.2 | 17 266±2593 | 1164±123 | 97.8 | 3270±639 | 4364±339 | 77.0 | 0±449 | 7522±848 | 2784±128 | 0.0 |
| TS–ANT (°Cd) | 268±65 | 824±61 | 61.1 | 3580±746 | 3583±386 | 74.0 | 8656±1411 | 3822±299 | 91.0 | 46±307 | 4070±518 | 3571±165 | 2.3 |
| PEN–ANT (°Cd) | 196±59 | 1004±72 | 49.4 | 1941±603 | 5715±582 | 49.5 | 290±79 | 1021±79 | 55.0 | 0±81 | 806±140 | 2054±96 | 0.0 |
| FLA–BOOT (°Cd) | 259±66 | 927±67 | 58.4 | 335±146 | 1723±177 | 36.5 | 355±84 | 874±68 | 64.3 | 150±45 | 165±45 | 1057±48 | 39.4 |
| FLA–ANT (°Cd) | 60±26 | 533±40 | 35.1 | 2363±577 | 3700±401 | 64.1 | 280±83 | 1190±93 | 51.1 | 0±71 | 680±125 | 2053±94 | 0.0 |
| LNANT (no) | 0.20±0.04 | 0.38±0.03 | 73.1 | 2.12±0.35 | 0.61±0.1 | 90.8 | 0.12±0.03 | 0.36±0.03 | 58.8 | 0.07±0.06 | 0.67±0.08 | 0.42±0.02 | 19.4 |
| LNTS (no) | 0.12±0.02 | 0.16±0.01 | 78.6 | 0.89±0.15 | 0.34±0.0 | 87.6 | 0.11±0.02 | 0.22±0.02 | 67.7 | 0.00±0.03 | 0.36±0.04 | 0.22±0.01 | 0.0 |
| LNafterTS (no) | 0.03±0.02 | 0.38±0.03 | 28.2 | 0.35±0.10 | 0.77±0.1 | 54.5 | 0.14±0.04 | 0.43±0.03 | 59.9 | 0.01±0.02 | 0.14±0.03 | 0.48±0.02 | 9.4 |
| AvgPHY (°Cd) | 5.74±2.80 | 66.1±4.6 | 30.4 | 21.42±5.47 | 41.2±4.2 | 61.1 | 53.9±8.89 | 26.5±2.1 | 89.9 | 2.07±2.32 | 21.8±3.57 | 47.3±2.2 | 12.0 |
| PHY28 (°Cd) | 5.39±3.33 | 87.1±6.0 | 24.7 | 16.79±7.52 | 95.8±9.4 | 34.9 | 15.1±4.29 | 60.4±4.6 | 52.8 | 4.4±2.1 | 7.01±2.77 | 79.5±3.6 | 22.2 |
Values are variance components±SE. Analysis was performed both within each treatment (LDV: long days, vernalized plants; LD: long days, nonvernalized plants; SDV: short days, vernalized plants) separately, and also on the combined data set. ANT: anthesis; AvgPHY: phyllochron relative to all the leaves BOOT: booting; FLA: flag leaf; LN: leaf number; PEN: penultimate leaf; PHY28: phyllochron relative to the leaves 2–8; TRA: transplanting; TS: terminal spikelet.
Phenology
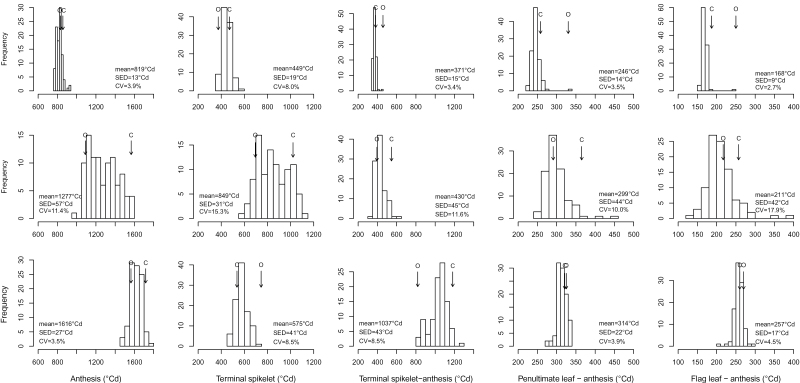
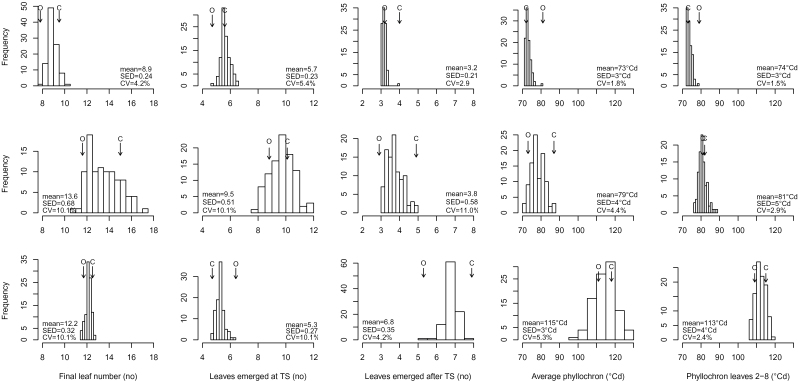
The mean TRA–ANT period for the LDV-grown RILs was 42 days (equivalent to 819 °Cd), ranging from 40–48 days; Fig. 2). Across the set of RILs, the TRA–TS period was more variable than the TS–ANT one, and this stage represented about 50% of the whole pre-anthesis period. The mean LNANT was 8.9, with the lowest number (8) produced by cv. Ofanto (Fig. 3). More leaves were produced (mean 5.7) and the genetic variation was larger (5–6.5) before TS than after it. AvgPHY and PHY28 were very similar to one another, both with respect to their magnitude and their variation across the RIL population.
Fig. 2.
Distribution of RIL means (BLUPs, Best Linear Unbiased Predictors) for the lengths of the various pre-anthesis intervals. Top: long days, vernalized plants; middle: long days, nonvernalized plants; bottom: short days, vernalized plants. Arrows indicate the performance of the parents (O: cv. Ofanto; C: cv. Cappelli). CV: coefficient of variation; SED: standard error of the difference between BLUPs.
Fig. 3.
Distribution of RIL means (BLUPs, Best Linear Unbiased Predictors) for the number of leaves emerged prior to and after terminal spikelet and at anthesis, and for the two measured phyllochrons (relative to all the leaves and to the leaves from the second to the eighth). Top: long days, vernalized plants; middle: long days, nonvernalized plants; bottom: short days, vernalized plants. Arrows indicate the performance of the parents (O: cv. Ofanto; C: cv. Cappelli). CV: coefficient of variation; SED: standard error of the difference between best linear unbiased predictors.
All LD-grown plants reached anthesis despite their lack of vernalization, but they flowered a mean of 22 days (457 °Cd) later than did the LDV-grown plants. The lack of vernalization had a large effect on the variation in the length of TRA–ANT interval among the RILs (29 days, 600 °Cd). The LD-grown plants needed almost twice the time to reach TS compared to the LDV-grown ones (43 vs. 22 days, 849 vs. 450 °Cd). The LD-grown material exhibited the most genetic variation with respect to the length of the TRA–TS interval (30–55 days, equivalent to 514 °Cd). The lengthened TRA–TS interval resulted in a reduction of the contribution of the TS–ANT interval to 34–36% of the whole pre-anthesis period. The PEN–ANT and FLA–ANT intervals were prolonged by, respectively, 50 and 40 °Cd, and genetic variation for these times became clear. The range in length of the PEN–ANT interval across the RILs was 252–365 °Cd (excluding two outliers), corresponding to 5 days, while that of the FLA–ANT interval varied from 132 to 395 °Cd (8–20 days). Nonvernalized plants developed many more leaves than did the vernalized ones; the range in LNANT across the LD-grown RILs was 11–17 (mean 14), while LNTS ranged from 8 to 12 and LNafterTS from 3 to 5. The mean values of AvgPHY and PHY28 were close to one another, and the difference between the LDV- and LD-grown RILs was greater for AvgPHY (ranging from about 70–90 °Cd) than for PHY28.
The SDV-grown plants took 796 °Cd longer than the LDV-grown ones to reach anthesis. The shorter photoperiod experienced by the SDV-grown plants strongly affected the length of the TS–ANT interval, inducing a mean increase of about 2.8-fold compared to the length of the same interval in the LDV-grown plants. The range in length of the TS–ANT interval among the RILs was 464 °Cd (30 days), and it comprised 45–64% of the whole pre-anthesis period. The FLA–ANT interval (258 °Cd) was longer than for either the LD- or LDV-grown plants. Only a narrow range in LNANT and LNTS was recorded, and AvgPHY and PHY28 were each increased by about 30 °Cd compared to the plants grown under either LD or LDV. The clearest genetic variation for AvgPHY was displayed by the SDV-grown plants.
Vernalization and photoperiod sensitivity
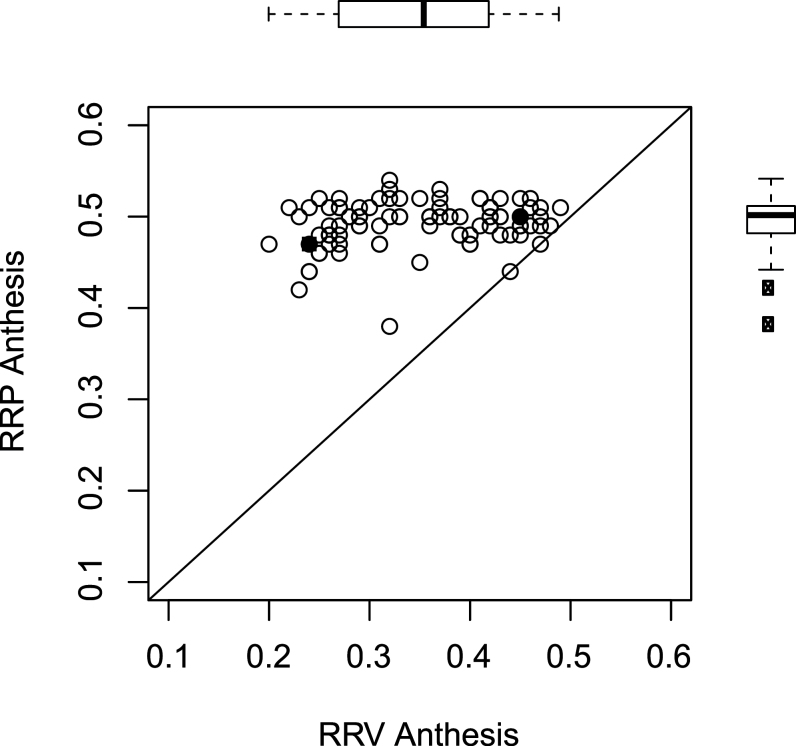
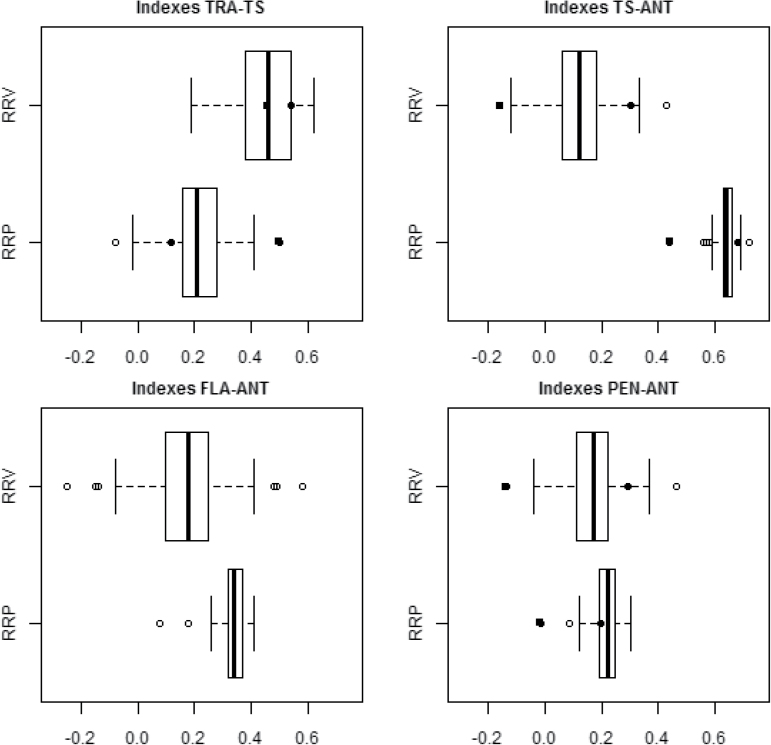
The calculation of RRV and RRP allowed the effects of EPS on phenology to be recognized and thus made it possible to quantify the sensitivity of each RIL to vernalization and photoperiod. Across the whole population, the RRP for TRA–ANT was 0.49 (Fig. 4). In absolute terms this RRP value corresponded to a mean difference of 92 °Cd h–1 of day length between LDV (range 52–63 °Cd h–1) and SDV (range 133–160 °Cd h–1). RRV was lower but more variable than RRP, ranging from 0.20 to 0.49, as against 0.38–0.54. The two indices were correlated to one another, but only explained less than 6% of the overall variation (R 2=0.058, P<0.05). The triggering of most of the pre-anthesis stages was more sensitive to vernalization than to photoperiod (Fig. 5). The interval most strongly affected by vernalization, in terms of both mean effect and variability, was TRA–TS, but the FLA–ANT interval was also markedly affected. Photoperiod acted most strongly on the TS–ANT interval, although variability among the RILs was scarce. The TS–ANT interval was a mean of 70 °Cd h–1 of day length longer and more variable in the SDV plants (range 73–115 °Cd h–1) than in the LDV-grown ones (range 23–27 °Cd h–1).
Fig. 4.
Sensitivity of RILs to photoperiod (RRP), estimated from anthesis date plotted against relative sensitivity to vernalization (RRV) and the corresponding boxplots. The RIL parents are indicated by either squares (cv. Ofanto) or circles (cv. Cappelli).
Fig. 5.
Boxplots illustrating the variation among the RILs with respect to relative photoperiod and vernalization sensitivity for the length of the various pre-anthesis intervals. The RIL parents are indicated by either squares (cv. Ofanto) or circles (cv. Cappelli).
Genetic correlations
The observed genetic correlations between traits differed among the three treatments (Table 2). The traits most strongly correlated with the length of the TRA–ANT interval in the LDV-grown material were LNTS (R 2=0.34, P<0.001), LNANT (R 2=0.36, P<0.001), and TS (R 2=0.61, P<0.001). The length of the TRA–TS interval was negatively correlated with that of TS–ANT, as was LNTS with LNafterTS, and the phyllochron explained a lower (but nevertheless significant) proportion of the variation in time to reach anthesis, and was strongly correlated with the FLA–ANT and PEN–ANT phases. TS and LNANT were the main determinants of the length of the TRA–ANT interval for the LD-grown plants (R 2=0.79, P<0.001 and 0.66, P<0.001, respectively), while significant correlations also existed between the length of the TRA–ANT interval and all the traits except for PHY28. Among the LD-grown plants, there was no correlation between the length of the TRA–TS interval and any of the post-TS stages, while all three LN variables were positively correlated with AvgPHY (but not with PHY28). Finally, for the SDV-grown plants, both the length of the TS–ANT interval (R 2=0.46, P<0.001) and AvgPHY (R 2=0.34, P<0.001) were strongly associated with the length of the TRA-ANT interval. The length of the TRA–TS interval was negatively correlated that of the TS–ANT interval, as was LNTS with LNafterTS.
Table 2.
Genetic correlations among the measured phenological traits for LDV-, LD- and SDV- grown plants
| TRA–ANT | TRA–TS | TS–ANT | FLA–ANT | PEN–ANT | LNANT | LNTS | LNafterTS | AvgPHY | |
|---|---|---|---|---|---|---|---|---|---|
| Long days, vernalized plants | |||||||||
| TRA–TS | 0.78*** | ||||||||
| TS–ANT | 0.05 | –0.50*** | |||||||
| FLA–ANT | 0.08 | –0.28** | 0.70*** | ||||||
| PEN–ANT | 0.20* | –0.20* | 0.65*** | 0.88*** | |||||
| LNANT | 0.60*** | 0.69*** | –0.26** | –0.33*** | –0.36*** | ||||
| LNTS | 0.58*** | 0.77*** | –0.45*** | –0.38*** | –0.30** | 0.72*** | |||
| LNafterTS | 0.09 | –0.02 | 0.15 | 0.04 | –0.12 | 0.45*** | –0.17 | ||
| AvgPHY | 0.24* | 0.06 | 0.32** | 0.44*** | 0.54*** | –0.44*** | –0.12 | –0.47*** | |
| PHY28 | 0.30** | 0.22* | 0.14 | 0.35*** | 0.44*** | –0.32*** | 0.00 | –0.47*** | 0.91*** |
| Long days, nonvernalized plants | |||||||||
| TRA–TS | 0.89*** | ||||||||
| TS–ANT | 0.53*** | 0.10 | |||||||
| FLA–ANT | 0.42*** | 0.07 | 0.81*** | ||||||
| PEN–ANT | 0.43*** | 0.11 | 0.77*** | 0.86*** | |||||
| LNANT | 0.81*** | 0.91*** | 0.10 | –0.01 | 0.02 | ||||
| LNTS | 0.67*** | 0.79*** | –0.01 | 0.00 | 0.04 | 0.81*** | |||
| LNafterTS | 0.63*** | 0.64*** | 0.21* | –0.02 | –0.02 | 0.74*** | 0.26** | ||
| AvgPHY | 0.68*** | 0.63*** | 0.30** | 0.17 | 0.23* | 0.44*** | 0.32*** | 0.43*** | |
| PHY28 | 0.17 | 0.11 | 0.16 | 0.21* | 0.21* | –0.01 | –0.03 | 0.04 | 0.47*** |
| Short days, vernalized plants | |||||||||
| TRA–TS | –0.09 | ||||||||
| TS–ANT | 0.68*** | –0.78*** | |||||||
| FLA–ANT | 0.33** | –0.18 | 0.33*** | ||||||
| PEN–ANT | 0.48*** | –0.32*** | 0.54*** | 0.82*** | |||||
| LNANT | 0.20 | –0.07 | 0.19 | –0.05 | –0.08 | ||||
| LNTS | –0.15 | 0.65*** | –0.53*** | –0.07 | –0.13 | 0.16 | |||
| LNafterTS | 0.27** | –0.55*** | 0.55*** | 0.02 | 0.03 | 0.66*** | –0.63*** | ||
| AvgPHY | 0.58*** | –0.34*** | 0.62*** | 0.24* | 0.50*** | –0.40*** | –0.23** | –0.14 | |
| PHY28 | 0.31** | 0.00 | 0.17 | –0.04 | 0.12 | –0.37*** | –0.21* | –0.12 | 0.55*** |
ANT: anthesis; AvgPHY: phyllochron relative to all the leaves; BOOT: booting; FLA: flag leaf; LN: leaf number; PEN: penultimate leaf; PHY28: phyllochron relative to the leaves 2–8; TRA: transplanting; TS: terminal spikelet.
*P<0.05; **P<0.01; ***P<0.001.
QTL analysis
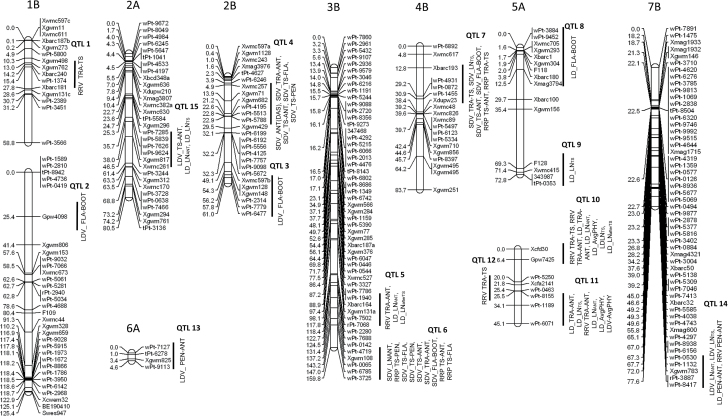
A total of 15 significant QTLs was identified, mapping to chromosomes 1B, 2A, 2B, 3B, 4B, 5A, 6A, and 7B (Table 3, Fig. 6). Variation for some of the traits was associated with a different spectrum of QTLs, depending on the treatment; for example, that for LNANT was determined by QTLs 5, 10, 11, and 15 in the LD-grown plants, by QTL 14 in LDV-grown ones, and by QTL 6 in the SDV-grown ones. Each locus (except for QTL 14) was specific to a treatment. Three loci underlay variation for the length of the TRA–ANT interval (QTLs 4, 5, 10, and 11); they were expressed by either the LD- or the SDV-grown plants, but not by the LDV-grown ones. The LOD values associated with these loci ranged from 4.5 to 12.3, and the proportion of the phenotypic variance explained (PVE) by each ranged from 17 to 46%. The two largest effect loci (QTLs 10 and 11) both mapped to chromosome arm 5AL and had a pleiotropic effect on some of the other traits. QTL 4 (mapping to chromosome arm 2BS) was the only locus for the TRA–ANT interval identified among the SDV-grown population, and QTL 5 (chromosome arm 3BL), identified in the LD-grown plants, the only one specific to the TRA–ANT interval. The latter locus was associated with a PVE of 36% and a LOD of 4.8.
Table 3.
QTLs identified in each treatment
| QTL | Chr. | Position (cM) | Peak marker | LDV | LD | SDV | LOD | R 2 (%) | Add. eff. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1BS | 15.4 | wPt-1374 | RRV (TRA–TS) | 3.00 | 9.6 | –0.03 | ||
| 2 | 1BL | 33.4 | Gpw4098 | FLA–BOOT | 3.64 | 18.5 | –6.05 | ||
| 1BL | 37.4 | Xgwm806 | RRP (FLA–BOOT) | 2.16 | 11.1 | 0.06 | |||
| 3 | 2BS | 54.3 | Xgwm128 | FLA–BOOT | 3.16 | 10.8 | –4.12 | ||
| 4 | 2BS | 22.2 | Xgwm682 | ANT (DAS) | 7.43 | 30.4 | –1.25 | ||
| 2BS | 22.2 | Xgwm682 | TRA–ANT | 4.59 | 17.5 | –25.03 | |||
| 2BS | 21.2 | Xgwm682 | TS–ANT | 3.34 | 11.8 | –30.00 | |||
| 2BS | 21.2 | Xgwm682 | TS–FLA | 3.00 | 9.6 | –24.70 | |||
| 2BS | 21.2 | Xgwm682 | TS–PEN | 2.85 | 9.2 | –22.72 | |||
| 2BS | 22.0 | Xgwm682 | LN TS | 2.62 | 10.1 | –0.10 | |||
| 2BS | 37.3 | wPt-5672 | TRA–ANT | 2.54 | 12.1 | –22.54 | |||
| 5 | 3BL | 107.1 | rPt-7068 | RRV (TRA–ANT) | 4.79 | 36.3 | –0.05 | ||
| 3BL | 97.0 | Xgwm131a | LN ANT | 3.28 | 7.2 | –0.42 | |||
| 3BL | 106.1 | wPt-7502 | LN afterTS | 2.71 | 11.8 | –0.16 | |||
| 6 | 3BL | 147.0 | wPt-6785 | LN ANT | 3.29 | 14.6 | 0.10 | ||
| 3BL | 142.2 | wPt-0065 | RRP (TS–PEN) | 3.18 | 13.2 | 0.01 | |||
| 3BL | 143.2 | wPt-0065 | TS–FLA | 3.14 | 10.4 | 25.75 | |||
| 3BL | 143.2 | wPt-0065 | TS–PEN | 3.08 | 10.1 | 23.80 | |||
| 3BL | 143.2 | wPt-0065 | TS–ANT | 2.64 | 9.2 | 26.52 | |||
| 3BL | 140.2 | wPt-0065 | TRA–ANT | 2.53 | 7.7 | 16.96 | |||
| 3BL | 128.5 | wPt-0142 | FLA–BOOT | 2.39 | 10.6 | 5.06 | |||
| 3BL | 142.2 | wPt-0065 | RRP (TS–ANT) | 2.37 | 9.9 | 0.01 | |||
| 3BL | 140.2 | wPt-0065 | RRP (TS–FLA) | 2.33 | 10.7 | 0.01 | |||
| 7 | 4BS | 29.2 | wPt-4931 | TRA–TS | 4.22 | 18.3 | 24.89 | ||
| 4BS | 22.8 | wPt-4931 | LN TS | 2.85 | 20.1 | 0.17 | |||
| 4BS | 29.2 | wPt-4931 | TS–ANT | 2.81 | 9.8 | –32.51 | |||
| 4BS | 27.8 | wPt-4931 | RRP (TS–ANT) | 2.28 | 10.3 | –0.01 | |||
| 4BS | 37.9 | Xdupw23 | FLA–BOOT | 2.25 | 8.8 | –5.34 | |||
| 4BS | 26.8 | wPt-4931 | RRP (TRA–TS) | 2.16 | 11.2 | 0.04 | |||
| 8 | 5AS | 2.0 | F118 | FLA–BOOT | 3.00 | 13.6 | –4.32 | ||
| 9 | 5AL | 72.8 | 343987 | LN TS | 3.00 | 7.4 | 0.26 | ||
| 10 | 5AL | 0.0 | Xcfd30a | RRV (TRA–TS) | 13.73 | 58.0 | –0.08 | ||
| 5AL | 0.0 | Xcfd30a | RRV (TRA–ANT) | 12.31 | 45.9 | –0.06 | |||
| 5AL | 0.0 | Xcfd30a | TRA–ANT | 10.45 | 37.5 | –105.64 | |||
| 5AL | 0.0 | Xcfd30a | LN ANT | 8.59 | 28.0 | –0.88 | |||
| 5AL | 0.0 | Xcfd30a | AvgPHY | 6.78 | 27.9 | –1.99 | |||
| 5AL | 0.0 | Xcfd30a | LN TS | 6.67 | 25.9 | –0.49 | |||
| 5AL | 0.0 | Xcfd30a | LN afterTS | 3.07 | 13.2 | –0.18 | |||
| 11 | 5AL | 45 | wPt-6071 | TRA–ANT | 5.03 | 18.6 | –72.35 | ||
| 5AL | 45.1 | wPt-6071 | LN TS | 4.56 | 18.7 | –0.42 | |||
| 5AL | 39.1 | wPt-1189 | RRV (ANT) | 4.45 | 17.5 | –0.04 | |||
| 5AL | 45.0 | wPt-6071 | LN ANT | 3.48 | 13.0 | –0.59 | |||
| 5AL | 34.0 | wPt-1189 | AvgPHY | 2.90 | 14.2 | 0.53 | |||
| 5AL | 39.1 | wPt-1189 | AvgPHY | 2.49 | 11.2 | –1.25 | |||
| 12 | 5AL | 21.8 | Xcfa2141 | RRV (TRA–TS) | 3.45 | 13.6 | –0.04 | ||
| 13 | 6AS | 1.0 | tPt-6278 | PEN–ANT | 3.3 | 14.5 | –0.93 | ||
| 14 | 7BL | 70.0 | Xgwm783 | LN ANT | 4.11 | 19.0 | –0.18 | ||
| 7BL | 69.7 | Xgwm783 | RRV (PEN–ANT) | 3.18 | 15.3 | –0.03 | |||
| 7BL | 77.0 | rPt-3887 | LN TS | 3.10 | 13.7 | –0.13 | |||
| 7BL | 69.7 | Xgwm783 | PEN–ANT | 2.39 | 10.0 | –9.99 | |||
| 15 | 2AS | 35.0 | wPt-9624 | LN ANT | 3.00 | 10.0 | 0.51 | ||
| 2AS | 38.0 | Xgwm817 | TS–ANT | 2.07 | 8.9 | –3.97 | |||
| 2AS | 46.5 | Xwmc261c | LNTS | 2.32 | 7.9 | 0.26 |
Bold indicates those associated with LOD ≥3; italic indicates those associated with LOD <3. Add. eff.: Additive effect of the Ofanto allele; ANT: anthesis; AvgPHY: phyllochron relative to all the leaves; LDV: long days, vernalized plants; LD: long days, nonvernalized plants; SDV: short days, vernalized plants; LOD: limit of detection; BOOT: booting; FLA: flag leaf; LN: leaf number; PEN: penultimate leaf; RRP: response to photoperiod; RRV: response to vernalization; TRA: transplanting; TS: terminal spikelet.
Fig. 6.
Chromosomal regions harbouring QTLs for phenological traits.
The duration of the TRA–TS interval was under the genetic control of four QTLs, of which three (QTL 1 on chromosome arm 4BS, QTL 7 on 1BS, and QTL 12 on 5AL) acted specifically on the length of this interval. The strongest effect was exerted by QTL 7 (PVE of nearly 20% for the SDV-grown plants), compared with QTL 1 (PVE of 10%) and QTL 12 (14%). QTL 10 also acted strongly on the length of this interval (PVE of 58%). Other significant QTLs were mapped to chromosome arms 2BS (QTL 4) and 3BL (QTL 6) for the length of the TS–ANT, TS–FLA, and TS–PEN intervals, each associated with a PVE of around 10%. The loci underlying the length of the FLA–BOOT interval were QTL 2 on chromosome arm 1BL, QTL 3 on chromosome arm 2BS, and QTL 8 on chromosome arm 5AS; while those for the length of the PEN–ANT stage were QTL 13 on chromosome arm 6AS and QTL 14 on chromosome arm 7BL. These loci were detected among either the LDV- or LD-grown plants and were associated with a PVE of 14–15%.
QTLs controlling leaf number and the phyllochron
The largest effect locus affecting LN throughout the life cycle was QTL 10 (chromosome arm 5AL). This locus was also the only one which influenced both the phyllochron and LNafterTS. Variation in LNTS was contributed by QTL 9 (chromosome arm 5AL), while LNANT was under the control of five loci (QTLs 5, 6, 11, 14, and 15) mapping to, respectively, chromosome arms 3BL, 3BL, 5AL, 7BL, and 2AS. The only one of these loci which was specific for just LNANT was QTL 15. A minor effect on AvgPHY, detected both among the LDV- and the LD-grown plants, was exerted by QTL 11; in both cases the LOD fell below the threshold of 3 (2.9 and 2.5) and PVEs were 14 and 11%, respectively.
Expression of most QTLs was treatment specific
Five of the 15 QTLs were detectable among the LDV-grown plants, of which three (QTLs 2, 3, and 13) specified variation in the length of either the FLA–BOOT or PEN–ANT intervals and were detected in neither the LD- nor the SDV-grown materials.
The highest number of QTLs was identified among the LD-grown plants. Seven of these nine QTLs were not detected in either of the other two sets of material, and two (QTLs 5 and 10) were associated with large PVEs for the length of the TRA–ANT interval, LNANT, and LNTS. The SDV-grown plants produced the fewest QTLs (three in total: QTLs 4, 6, and 7), none of which were detectable in either the LD- or the LDV-grown materials. The QTLs involved were mainly associated with the control of the lengths of the TRA–ANT interval and the post-TS stages.
Discussion
The RIL population displayed variation for the length of each of the individual pre-anthesis intervals, reflecting the divergent phenology of the parental cultivars: cv. Cappelli is a late-flowering, highly photoperiod sensitive type with a substantial vernalization requirement (Motzo and Giunta, 2007), while cv. Ofanto is relatively recent constitution, earlier than Cappelli when sown in autumn (Panio et al., 2013). The population was also expected to segregate with respect to EPS, since modern Italian cultivars such as cv. Ofanto harbour much less effective alleles for this trait than do the older traditional types such as cv. Cappelli (Motzo and Giunta, 2007). Nevertheless, the indication was that the contribution of genotype to the global variance in phenotype was low for most of the traits. This apparent anomaly was, however, balanced by the large contribution made by the genotype × treatment interaction. The interpretation is that, although the same lines were analysed in each of the three treatments, phenology was being determined by a different set of genes in each case: in the LDV-grown plants only genes controlling EPS were active, in the LD-grown ones both EPS and vernalization requirement ones were involved, while in the SDV experiment the relevant genes were those responsible for EPS and for photoperiod sensitivity. This assumption is consistent with the outcome of the QTL analysis, since a different spectrum of loci emerged from each experiment.
Some of the QTLs identified here map to genomic regions known to harbour loci affecting heading date in bread wheat. For example, the chromosome 1B region, in which QTL 1 was mapped, is similar to the one on harbouring loci specifying heading date in bread wheat (Griffiths et al., 2009; Reif et al., 2011). The site of QTL 2 probably overlaps the stem elongation QTL identified by Borràs-Gelonch et al. (2011), as does that of QTL 14 with an ear emergence QTL described by Griffiths et al., (2009). QTLs 3, 5, 9, and 13 similarly match the map position of loci specifying ear emergence (Griffiths et al., 2009), while QTLs 3, 4, 8, and 10 map to matching regions identified by Hanocq et al. (2007) as sites of heading date QTLs.
EPS QTLs
Rather few loci affecting EPS were identified, and the size of the effect of those which were identified was smaller than those associated with sensitivity to either photoperiod or vernalization. Major genes controlling EPS have only rarely been reported (Snape et al., 2001), presumably because of the difficulty of designing experiments in which their action is not confounded by other classes of gene (Laurie, 1997; Griffiths et al., 2009). Based on the length of the TRA–ANT interval, no EPS QTLs were apparent; instead, they were only detected by dividing the pre-anthesis development of the plants into discrete phases and by considering LN and the phyllochron. The LDV-grown plants were designed to identify EPS genes, since they avoided exposure to either low temperatures or short day lengths. The extent of the genetic variation caused by EPS genes with respect to the length of the TRA–ANT interval was similar to that noted by van Beem et al. (2005) in their comparison of 51 cultivars. Nevertheless, it was clear that these genes affected both LNANT and LNTS and, to a lesser extent, the phyllochron. EPS genes have been proposed to control how many leaf or spikelet primordia are initiated (Gotoh, 1977; Hoogendoorn, 1985). QTL 14 was a determinant of both LNANT and LNTS, and its location on chromosome arm 7BL fits the conclusions drawn by Flood and Halloran (1983) and Hoogendoorn (1985) regarding the site of EPS genes in bread wheat. Similarly, the location of QTL 11 may match that of EPS genes described by Kato et al. (2003), since its PVE was 14%. Its strong effect confirms that the action of EPS genes is also exerted via the control of leaf appearance rate. A role of the phyllochron in the expression of EPS has also recently been suggested (He et al., 2012). The two other EPS-specific QTLs identified mapped to chromosome arms 1BL and 2BS. EPS genes are known to reside on chromosome 2B (Scarth and Law, 1983; Shindo et al., 2003). The latter authors have suggested that a EPS QTL lying close to the major Ppd-B1 (photoperiod sensitivity) gene may represent the orthologue of the barley gene eps2S (Laurie et al., 1995).
QTLs associated with vernalization requirement
The RRV associated with the length of the TRA–ANT interval was very consistent with that reported by Herndl et al. (2008) in a study of 26 European bread wheat cultivars. The RIL parent cv. Cappelli has a high vernalization requirement, while even current Italian spring type cultivars such as cv. Ofanto have a residual requirement (Motzo and Giunta, 2007). Winter cultivars carry one or more of the major Vrn (vernalization requirement) genes, which have a large effect on the length of the sowing to TS interval (Griffiths et al., 1985; Robertson et al., 1996; Motzo and Giunta, 2007). The behaviour of the RILs suggested that much of the variation in the length of the TRA–TS interval, of the TS RRV, and in LNANT was the principal driver of variation in the length of the TRA–ANT interval and that this variation reflected genetic differences amongst the RILs with respect to their individual vernalization requirement. The time required to reach TS was the trait most often associated with the QTLs detected in the LD-grown plants. The colocation of QTLs controlling the length of the TRA–TS interval and LNTS (QTLs 10 and 11) should be viewed in the light of the much discussed physiological association between the length of the period during which primordia are produced and leaf number (Kirby, 1990; Brooking and Jamieson, 2002; Jamieson et al., 2007). The degree of variability in LNANT which was induced by the lengthening of the TRA–TS interval was mirrored in the LD-grown plants by a positive association between LNANT and AvgPHY, likely reflecting the deceleration in the rate of appearance of the leaves later than the eighth (Miglietta, 1991). As a consequence, AvgPHY (but not PHY28) was associated with many of the phenological events occurring both before and after TS. The implication is that vernalization requirement genes extend their effect beyond TS. The association between LNANT and AvgPHY resulted in the pleiotropic action of QTL 10 on AvgPHY, establishing a genetic basis for phyllochron variation when the leaf number exceeds eight. The Vrn-A1 locus is located on chromosome arm 5AL (Galiba et al. 1995), where four of the QTLs detected only in the LD-grown material were located. The ability of every RIL to flower, despite the absence of any vernalization treatment and the imposition of a long-day photoperiod, implied that none of the RILs carried the winter type (recessive) allele at either Vrn-A1 or -B1. Based on the allele present at the microsatellite locus Xcfa2141 linked to QTL 12, the indication is that QTL 12 is identical to Vrn-A1. Vernalization-responsive QTLs were also located on chromosome arms 1BS, 3BL, and 7BL, sites which are consistent with the map locations of Vrn-2 and Vrn-B3 (Goncharov, 2003; Kato et al., 2003; Yan et al., 2006).
QTLs associated with photoperiod sensitivity
The RILs displayed only a limited extent of variation for the length of the TRA–ANT interval when grown under short-day conditions. Most the variation present was associated with AvgPHY and the length of the TS–ANT interval, the variation in length of which was partly explicable by variation in the phyllochron. The notion that the prolonged sensitivity of wheat plants to photoperiod after TS can affect the phyllochron is in line with conclusions drawn by both Gonzáles et al. (2005b) and Miralles and Richards (2000). Two of the three QTLs identified (QTLs 4 and 6) controlled several substages within the TS–ANT interval, underlining the importance of the length of the TS–ANT interval for the expression of photoperiod sensitivity (Fig. 2). QTL 4 could correspond to some Ppd-B genes that have already been mapped on chromosome 2B (Hanocq et al. 2007). In particular, the presence of a common DArT marker (wPt5672) reported by Crossa et al. (2007) and Le Gouis et al. (2012) on chromosome 2B confirmed the proximity to the Ppd-B1 gene. The low variability of LNANT could be attributed to a low level of genetic variation in the timing of the response to day length in the RIL population (i.e. in the time of the final commitment of the flag leaf primordium). The initiation of the last leaf primordium can occur at any point up to and beyond TS, which provides one of the ways in which genetic polymorphisms in photoperiod sensitivity can be translated into variation in LN and hence in anthesis date (Brooking et al., 1995). On the other hand, the RIL population diverged markedly with respect to LNafterTS, resulting in a negative correlation between the number of leaves which emerged before and after TS, and this was in turn responsible for the strong negative correlation between the lengths of the two consecutive intervals TRA–TS and TS–ANT. The cv. Ofanto allele at QTL 7 delayed TS while simultaneously reducing the length of the TS–ANT interval. In contrast, Borràs-Gelonch et al. (2011) have shown that in bread wheat, there was a positive genetic correlation between the time taken to reach TS and the length of the TS–ANT interval; the underlying cause of this difference in behaviour is hard to discern, as LNANT was not monitored in the current experiment. The only way in which an almost constant LNANT can result in variation in LN before and after TS is where there is variation in the time elapsed between the commitment to the formation of the flag leaf primordium and the initiation of the TS primordium at the meristematic apex, reflecting genetic variation with respect to photoperiod sensitivity during this period (Rawson, 1970). The remarkable impact of altering the length of the TS–ANT interval must therefore represent an outcome of a magnifying effect of the high phyllochron on the number of leaves which emerge after TS. The lengthening of the TS–ANT interval induced by short-day conditions was accompanied by a lengthening of the PEN–ANT interval, implying that the period of maximum spike growth can be positively affected by the expression of genes controlling photoperiod sensitivity. In the present case, however, the extent of the variation was insufficient to enable the detection of QTLs specifically controlling the length of this stage under short-day conditions.
Conclusions
The splitting of the period between planting and anthesis into a series of physiologically based components has allowed the detection of many more genetic factors responsible for anthesis date than would have been achieved by simply searching for QTLs associated solely with anthesis date. At the same time, manipulating the environment in which the plants were grown to isolate known flowering cues has given the opportunity to define how each of the resulting QTLs identified interact with the growing environment. As well as the well-recognized impact of vernalization on plant developmental prior to TS, it was also possible to show that the vernalization effect can extend well beyond TS, due to its impact on LN and the phyllochron. EPS genes appear to affect the lengths of the pre-anthesis intervals via their independent effect on both LN and the phyllochron. For the particular RIL population in question, the length of the TRA–TS interval was correlated with that of the TRA–ANT interval only where the anthesis date was dependent on the final LN. Otherwise, it was the length of the TS–ANT interval which was the most strongly correlated with the timing of anthesis. Under an inductive photoperiod regime, the extent of genetic variation for the length of the pre-anthesis intervals was more marked than for anthesis date itself, due to the photoperiod sensitivity of the TRA–TS interval, coupled with a limited effect on total LN. The implication is that similar anthesis dates could potentially be arrived at even though the lengths of the various pre-anthesis intervals varied.
The overall result of the experiments supports the idea that, although intensive breeding and selection over the past century has succeeded in reducing the length of time between sowing and flowering of the durum wheat, there remains potential to manipulate the duration of the various pre-anthesis stages; the recognition of the genetic basis of these durations via the identification of relevant QTLs could lead to a marker-based strategy for fine-tuning the crop to its growing environment.
Acknowledgements
This work was supported by the Italian Ministry of Education, University and Research (MIUR; ISCOCEM: grant no. PON01_01145). This research represents part of a PhD project carried out by the senior author at the doctoral school of Science and Biotechnology of Agricultural and Forestry Science and of Food Production, curriculum Crop Productivity, of the University of Sassari.
References
- Amir J, Sinclair TR. 1991. A model of the temperature and solar radiation effects on spring wheat growth and yield. Field Crops Research 28, 47–58 [Google Scholar]
- Borràs-Gelonch G, Rebetzke GJ, Richards RA, Romagosa I. 2011. Genetic control of duration of pre-anthesis phases in wheat (Triticum aestivum L.) and relationships to leaf appearance, tillering, and dry matter accumulation. Journal of Experimental Botany 63, 69–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooking IR, Jamieson PD. 2002. Temperature and photoperiod response of vernalization in near-isogenic lines of wheat. Field Crops Research 79, 21–38 [Google Scholar]
- Brooking IR, Jamieson PD, Porter JR. 1995. The influence of daylength on final leaf number in spring wheat. Field Crops Research 41, 155–165 [Google Scholar]
- Crossa J, Burgueno J, Dreisigacker S, et al. 2007. Association analysis of historical bread wheat germplasm using additive genetic covariance of relatives and population structure. Genetics 177, 1889–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JL, Christian KR, Jones DB, Bremner PM. 1985. Responses of wheat to vernalization and photoperiod. Australian Journal of Agricultural Research 36, 347–359 [Google Scholar]
- Fischer RA. 1983. Wheat. In: Smith WH, Banta SJ, eds, Proceedings of symposium on potential productivity of field crops under different environments. Los Banos Laguna, Philippines: International Rice Research Institute; pp 129–154 [Google Scholar]
- Fischer RA. 2011. Wheat physiology: a review of recent developments. Crop and Pasture Science 62, 95–114 [Google Scholar]
- Flood RG, Halloran GM. 1983. The influence of certain chromosomes of the hexaploid wheat cultivar Thatcher on time to ear emergence in Chinese Spring. Euphytica 32, 121–124 [Google Scholar]
- Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP. 2011. Raising yield potential of wheat. (III) Optimizing partitioning to grain while maintaining lodging resistance. Journal of Experimental Botany 62, 469–486 [DOI] [PubMed] [Google Scholar]
- Galiba G, Quarrie SA, Sutka J, Morgounov A. 1995. RFLP mapping of the vernalization (Vrn1) and frost resistance (Fr1) genes on chromosome 5A of wheat. Theoretical and Applied Genetics 90, 1174–1179 [DOI] [PubMed] [Google Scholar]
- García GA, Serrago RA, Appendino ML, Lombardo LA, Vanzetti LS, Helguera M, Miralles DJ. 2011. Variability of duration of pre-anthesis phases as a strategy for increasing wheat grain yield. Field Crops Research 124, 408–416 [Google Scholar]
- GENSTAT. 2008. GENSTAT statistical package release 11.1. Rothamstad: Lawes Agricultural Trust. [Google Scholar]
- Goncharov NP. 2003. Genetics of growth habit (spring vs winter) in common wheat: confirmation of the existence of dominant gene Vrn4 . Theoretical and Applied Genetics 107, 768–772 [DOI] [PubMed] [Google Scholar]
- Gonzáles FG, Slafer GA, Miralles DJ. 2005a. Pre-anthesis development and number of fertile florets in wheat as affected by photoperiod sensitivity genes Ppd-D1 and Ppd-B1 . Euphytica 146, 253–269 [Google Scholar]
- Gonzáles FG, Slafer GA, Miralles DJ. 2005b. Floret development and survival in wheat plants exposed to contrasting photoperiod and radiation environments during stem elongation. Functional Plant Biology 32, 189–197 [DOI] [PubMed] [Google Scholar]
- Gotoh T. 1977. Varietal differences in photoperiodic and thermal responses of wheat. Japanese Journal of Breeding 27, 57–69 (Jap. E. summ.) [Google Scholar]
- Griffiths FEW, Lyndon RF, Bennett MD. 1985. The effects of vernalization on the growth of the wheat shoot apex. Annals of Botany 56, 501–511 [Google Scholar]
- Griffiths S, Simmonds J, Leverington M, et al. 2009. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theoretical and Applied Genetics 119, 383–395 [DOI] [PubMed] [Google Scholar]
- Halloran GM, Pennell D. 1982. Duration and rate of development phases in wheat in two environments. Annals of Botany 49, 115–121 [Google Scholar]
- Hanocq E, Laperche A, Jaminon O, Lainé AL, Le Gouis J. 2007. Most significant genome regions involved in the control of earliness traits in bread wheat, as revealed by QTL meta-analysis. Theoretical and Applied Genetics 114, 569–584 [DOI] [PubMed] [Google Scholar]
- Haun JR. 1973. Visual quantification of wheat development. Agronomy Journal 65, 116–119 [Google Scholar]
- Hay RKM. 1978. Seasonal changes in the position of the shoot apex of winter wheat and spring barley in relation to the soil surface. Journal of Agricultural Science Cambridge 91, 245–248 [Google Scholar]
- He JQ, Le Gouis J, Stratonovitch P, et al. 2012. Simulation of environmental and genotypic variations of final leaf number and anthesis date for wheat. European Journal of Agronomy 42, 22–33 [Google Scholar]
- Herndl M, White JW, Hunt LA, Graeff S, Claupein W. 2008. Field-based evaluation of vernalisation requirement, photoperiod response and earliness per se in bread wheat (Triticum aestivum L.). Field Crops Research 105, 193–201 [Google Scholar]
- Hoogendoorn J. 1985. The physiology of variation in the time of ear emergence among wheats from different regions of the world. Euphytica 34, 559–571 [Google Scholar]
- Jamieson PD, Brooking IR, Porter JR, McMaster GS, White JW, Porter JR. 2007. Reconciling alternative models of phenological development in winter wheat. Field Crops Research 103, 36–41 [Google Scholar]
- Jamieson PD, Brooking IR, Porter JR, Wilson DR. 1995. Prediction of leaf appearance in wheat—a question of temperature. Field Crops Research 41, 35–44 [Google Scholar]
- Jamieson PD, Brooking IR, Semenov MA, Porter JR. 1998. Making sense of wheat development: a critique of methodology. Field Crops Research 55, 117–127 [Google Scholar]
- Kato K, Yamashita M, Ishimoto K, Yoshino H, Fujita M. 2003. Genetic analysis of two genes for vernalization response, the former Vrn2 and Vrn4, by using PCR based molecular markers. In: Pogna NE, Romano M, Pogna EA, Galterio G, eds, Proceedings of the 10th International Wheat Genetics Symposium. Rome: Istituto Sperimentale; pp 971–973 [Google Scholar]
- Kirby EJM. 1990. Co-ordination of leaf emergence and leaf and spikelet primordium initiation in wheat. Field Crops Research 25, 253–264 [Google Scholar]
- Koornneef M, Alonso-Blanco C, Peeters AJ, Soppe W. 1998. Genetic control of flowering time in Arabidopsis . Annual Review of Plant Physiology and Plant Molecular Biology 49, 345–370 [DOI] [PubMed] [Google Scholar]
- Laurie DA, Griffiths S, Christodoulou V. 2004. Comparative genetic approaches to the study of control of flowering time in temperate cereals. Field Crops Research 90, 87–99 [Google Scholar]
- Laurie DA, Pratchett N, Bezant JH, Snape JW. 1995. RFLP mapping of 5 major genes and 8 quantitative trait loci controlling flowering time in a winter × spring barley (Hordeum vulgare L.) cross. Genome 38, 575–585 [DOI] [PubMed] [Google Scholar]
- Laurie DA. 1997. Comparative genetics of flowering time in cereals. Plant Molecular Biology 35, 167–177 [PubMed] [Google Scholar]
- Law CN, Worland AJ. 1997Genetic analysis of some flowering time and adaptive traits in wheat. New Phytologist 137, 19–28 [Google Scholar]
- Le Gouis J, Bordes J, Ravel C, et al. 2012. Genome-wide association analysis to identify chromosomal regions determining components of earliness in wheat. Theoretical and Applied Genetics 124, 597–611 [DOI] [PubMed] [Google Scholar]
- Marone D, Laidò G, Gadaleta A, et al. 2012. A high-density consensus map of A and B wheat genomes. Theoretical and Applied Genetics 125, 1619–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglietta F. 1991. Simulation of wheat ontogenesis. I. Appearance of main stem leaves in the field. Climate Research 1, 145–150 [Google Scholar]
- Miralles DJ, Richards RA. 2000. Responses of leaf and tiller emergence and primordium initiation in wheat and barley to interchanged photoperiod. Annals of Botany 85, 655–663 [Google Scholar]
- Motzo R, Giunta F. 2007. The effect of breeding on the phenology of Italian durum wheats: from landraces to modern cultivars. European Journal of Agronomy 26, 462– –470 [Google Scholar]
- Panio G, Motzo R, Mastrangelo AM, Marone D, Cattivelli L, Giunta F, De Vita P. 2013. Molecular mapping of stomatal-conductance-related traits in durum wheat (Triticum turgidum ssp durum). Annals of Applied Biology 162, 258–270 [Google Scholar]
- Rawson HM. 1970. Spikelet number, its control and relation to yield per ear in wheat. Australian Journal of Biological Science 23, 1–15 [Google Scholar]
- Reif JC, Gowda M, Maurer HP, Longin CF, Korzun V, Ebmeyer E, Bothe R, Pietsch C, Würschum T. 2011. Association mapping for quality traits in soft winter wheat. Theoretical and Applied Genetics 122, 961–970 [DOI] [PubMed] [Google Scholar]
- Reynolds MP, Foulkes MJ, Slafer GA, Berry PM, Parry MAJ, Snape JW, Angus WJ. 2009. Raising yield potential in wheat. Journal of Experimental Botany 60, 1899–1918 [DOI] [PubMed] [Google Scholar]
- Ritchie JT. 1991. Wheat phasic development. In: Hanks J, Ritchie JT, eds, Modeling plant and soil systems. Madison, WI: American Society of Agronomy; pp 31–54 [Google Scholar]
- Robertson MJ, Brooking IR, Ritchie JT. 1996. Temperature response of vernalization in wheat: modelling the effect on the final number of mainstem leaves. Annals of Botany 78: 371–381 [Google Scholar]
- Scarth R, Law CN. 1983. The location of the photoperiodic gene, Ppd2, and an additional factor for ear-emergence time on chromosome 2B of wheat. Heredity 51, 607–619 [Google Scholar]
- Shindo C, Tsujimoto H, Sasakuma T. 2003. Segregation analysis of heading traits in hexaploid wheat utilizing recombinant inbred lines. Heredity 90, 56–63 [DOI] [PubMed] [Google Scholar]
- Slafer GA, Calderini DF, Miralles D. 1996. Yield components in wheat: opportunities for further increasing yield potential. In: Reynolds MP, Rajaram S, McNab A, eds, Increasing yield potential in wheat: breaking the barriers. Mexico City: CYMMIT; pp 101–133 [Google Scholar]
- Slafer GA, Whitechurch EM. 2001. Manipulating wheat development to improve adaptation and to search for alternative opportunities to increase yield potential. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, eds, Application of physiology to wheat breeding. Mexico City: CIMMYT; pp 167–170 [Google Scholar]
- Snape JW, Butterworth K, Whitechurch EM, Worland AJ. 2001. Waiting for fine times: genetics of flowering time in wheat. Euphytica 119, 185–190 [Google Scholar]
- Snape JW, Quarrie SA, Laurie DA. 1996. Comparative mapping and its use for the genetic analysis of agronomic characters in wheat. Euphytica 89, 27–31 [Google Scholar]
- van Beem J, Mohler V, Lukman R, van Ginkel M, William W, Crossa J, Worland AJ. 2005. Analysis of genetic factors influencing the development rate of globally important CIMMYT wheat cultivars. Crop Science 45, 2113–2119 [Google Scholar]
- Van Ooijen JW, Voorips RE. 2004. JoinMap version 3.0, software for the calculation of genetic linkage maps. Wageningen: Kyazma [Google Scholar]
- Weir AH, Bragg PL, Porter JR, Rayner JH. 1984. A winter wheat crop simulation model without water or nutrient limitations. Journal of Agricultural Science Cambridge 102, 371–382 [Google Scholar]
- White JW, Laing DR. 1989. Photoperiod response of flowering in diverse genotypes of common bean (Phaseolus vulgaris). Field Crops Research 22: 113–128 [Google Scholar]
- Whitechurch EM, Slafer GA, Miralles DJ. 2007. Variability in the duration of stem elongation in wheat and barley. Journal of Agronomy and Crop Science 193, 138–145 [Google Scholar]
- Worland AJ. 1996. The influence of flowering time genes on environmental adaptability in European wheats. Euphytica 89, 49–57 [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proceedings of the National Academy of Sciences, USA 103, 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weeds Research 14, 415–421 [Google Scholar]