Summary
This work reveals how tobacco pistil and style may communicate with pollen tubes and regulate their growth during fertilization via the γ-aminobutyric acid–Ca2+-permeable channel–glutamate decarboxylase–calmodulin signalling pathway.
Key words: γ-Aminobutyric acid, Ca2+-permeable channel, cell–cell communication, glutamate decarboxylase, Nicotiana tabacum, pollen tube.
Abstract
γ-Aminobutyric acid (GABA) is implicated in pollen tube growth, but the molecular and cellular mechanisms that it mediates are largely unknown. Here, it is shown that exogenous GABA modulates putative Ca2+-permeable channels on the plasma membranes of tobacco pollen grains and pollen tubes. Whole-cell voltage-clamp experiments and non-invasive micromeasurement technology (NMT) revealed that the influx of Ca2+ increases in pollen tubes in response to exogenous GABA. It is also demonstrated that glutamate decarboxylase (GAD), the rate-limiting enzyme of GABA biosynthesis, is involved in feedback controls of Ca2+-permeable channels to fluctuate intracellular GABA levels and thus modulate pollen tube growth. The findings suggest that GAD activity linked with Ca2+-permeable channels relays an extracellular GABA signal and integrates multiple signal pathways to modulate tobacco pollen tube growth. Thus, the data explain how GABA mediates the communication between the style and the growing pollen tubes.
Introduction
Fertilization during reproduction in flowering plants begins when pollen grains land on a compatible stigma. Following this, complex signal transduction networks mediate multiple developmental events including the directional outgrowth of the pollen tube, elongation of the pollen tube over long distances in the style tract, and delivery of sperm cells to the embryo sac within the ovule to complete fertilization (Sauter, 2009). Multiple signals are involved in the regulation of these events (Palanivelu and Sukamoto, 2012). For example, γ-aminobutyric acid (GABA), which acts as a neurotransmitter in animals, also functions in pollen tube migration, as discovered in Arabidopsis (Palanivelu et al., 2003). In plants, GABA is a ubiquitous molecule that acts in the responses to a variety of environmental signals, including hypoxia, acidosis, mechanical stress, and cold stress (Baum et al., 1996; Shelp et al., 1999; Bouché and Fromm, 2004; Renaul et al., 2011). The pathway of GABA metabolism (GABA shunt pathway) has been elucidated. GABA synthesis from glutamate is catalysed by cytosolic glutamate decarboxylase (GAD; EC 4.1.1.15); GABA is then oxidized in mitochondria via two successive steps, catalysed by GABA transaminase and succinic semialdehyde dehydrogenase (SSADH; EC 1.2.1.16), to form succinate, which then enters the tricarboxylic acid cycle (Shelp et al., 1999).
Arabidopsis pop2 mutants are defective in the gene that codes for GABA transaminase (GABA-T, POP2; EC 2.6.1.19). Loss of GABA transaminase function in pop2 mutants results in GABA accumulation in pistils, consistent with its role in metabolizing GABA in the GABA shunt pathway, and pop2 pollen tube growth is arrested or misguided in pop2 pistils (Palanivelu et al., 2003). The GABA accumulation in pop2 pistils abolishes the increasing GABA gradient from the stigma to the ovule micropyle that is present in wild-type pistils. These results led to the suggestion that loss of the GABA gradient in pop2 pistils results in abnormal pollen tube growth. However, the role of GABA in the regulation of pollen tube growth and how it mediates pollen tube–pistil interactions remain largely unknown.
In plants, GAD is involved in GABA accumulation in response to environmental stresses. Plant GADs possess an autoinhibitory domain in the C-terminal segment; this domain restrains GAD activity under normal growth conditions. The inhibition function can be removed by the binding of the Ca2+/calmodulin (CaM) complex to this domain (Chen et al., 1994; Snedden et al., 1996). Thus, when the steady-state level of cytosolic Ca2+ increases, GAD inhibition is rapidly released upon binding to Ca2+/CaM, leading to immediate synthesis of GABA. It is well known that Ca2+ plays a significant role in the regulation of pollen tube growth (Franklin-Tong, 1999). Previous work showed that CaM is an important intermediary in Ca2+ signalling in growing pollen tubes (Ma and Sun, 1997) and is also a modulator of GAD activity (Baum et al., 1996; Shelp et al., 1999). Therefore, examining the participation of Ca2+ in the regulation of GAD activity and its CaM binding ability is essential to understand the functions of GAD/GABA in pollen tube growth.
The rapid tip growth of tobacco pollen tubes serves as a good in vitro model system to study signal transduction in plants. Many proteins are involved in the regulation of pollen tube tip growth and vesicle trafficking, including a heterotrimeric G protein (Ma et al., 1999), phospholipase C (PLC) (Dowd et al., 2006; Helling et al., 2006), phospholipase D (PLD) (Potocky et al., 2003), mitogen-activated protein kinases (MAPK) (Wilson et al., 1995), and the Rop/RacGTPase molecular switches and related regulatory molecules (Cheung et al., 2003; Klahre et al., 2006; Klahre and Kost, 2006; Lavy et al., 2007; Szumlanski and Nielsen, 2009). To understand the function of GABA in pollen tube growth, it is therefore necessary to clarify the role of GABA in the complex network comprising these proteins and the pathway(s) in which GABA may be involved.
Here, it is demonstrated that exogenous GABA is involved in the regulation of pollen tube growth via activation of Ca2+-permeable channels at low concentration and inhibition at high concentration. It is also shown that GAD plays a critical role in regulating tobacco pollen tube growth by functioning as a downstream regulator of Ca2+/CaM and feedback control on Ca2+-permeable channels. The regulatory relationships of these molecules to the aforementioned genes were also investigated to identify the GABA signalling pathway in pollen tubes.
Materials and methods
Chemicals
All chemicals were purchased from Sigma unless otherwise stated.
Plant growth conditions and pollen germination
Plants of Nicotiana tabacum SR1 were grown at 22 °C in a greenhouse at Wuhan University, China, under a 16h photoperiod. Fresh pollen of flowers at stage 12 were cultivated in vitro in germination medium (GM) at 25 °C in darkness. GM contained 1.0mM CaCl2, 1.0mM KCl, 0.8mM MgSO4, 1.6mM H3BO4, 30.0 μM CuSO4, 5.0mM MES, and 20% sucrose, and the pH was adjusted to 5.8 with 1.0M TRIS. The images of growing pollen tubes were collected with the capture function of a Leica DMIRE 2 inverted microscope equipped with a CCD camera (RTE/CCD-1300-Y/HS, Roper Scientific Co.).
Pollen tube measurement
The time-lapse images of pollen tube growth were captured in 1h intervals for later measurement. The tube length was defined as the distance from the central point of a pollen grain to the tip of its pollen tube. The average length of 40 randomly selected pollen tubes was regarded as the pollen tube length at each time point. Different concentrations of 3-mercaptopropionic acid (3-MPA) were used within the range of 50 μM to 5.0mM. After treatment, pollen tubes were fixed in Carnoy’s solution (ethanol:glacial acetic acid, 3:1, v/v) supplemented with 20% sucrose. Lengths of pollen tubes were measured using the measure function of Metamorph Software, which is capable of measuring the length of curved pollen tubes. Each treatment was a randomized block design (RBD) with three replicates.
Pollen tube protoplast isolation
Tobacco pollen tube protoplasts were prepared as follows (Yu et al., 2006). The collected pollen grains were first germinated in GM [1.0mM KNO3, 1.0mM Ca (NO3)2, 1.0mM MgSO4, 1.0mM H3BO3, and 20% sucrose, pH 5.8] at 25 °C. When the pollen tubes had emerged and were vigorously growing, they were transferred into an enzyme solution containing 1% cellulose (Onozuka R-10) and 1% pectinase (Merck) dissolved in bath solution, and incubated at 28 °C for 1h to release pollen protoplasts.
Electrophysiology
Whole-cell voltage-clamp experiments were carried out at 20 °C using an EPC 10 patch clamp amplifier (HEKA Elekt-ronik, Lambrecht, Germany) interfaced to a computer running acquisition and analysis software (Pulse). A multichannel microperfusion system (MPS-2; INBIO Inc., Wuhan, China) was used to exchange the external recording solution. The membrane was held at a holding potential of 0 mV and liquid junction potentials were corrected according to a previous method (Neher, 1992; Wu et al., 2011). Pipettes were pulled from glass capillaries in two stages, with pipette resistance ranging from 20 MΩ to 30 MΩ when filled with pipette solutions. The standard solution used for ICa measurements contained 50mM CaCl2, 2.5mM MES, pH 5.8 adjusted with TRIS, and the pipette solution contained 0.1mM CaCl2, 4mM Ca(OH)2, 10mM HEPES, 10mM EGTA, 2mM MgATP, pH 7.2. The osmolalities of the pipette and bath solutions were adjusted to 1614 mosmol kg–1 with d-sorbitol. When GABA was added to the bathing solution, spontaneous ICa-like currents were recorded on the pollen tube protoplast membrane. Membrane currents were recorded upon application of 1000ms of voltage ramped from −200 mV to 0 mV for 50ms, repeated every 5 s.
Measurements of Ca2+ fluxes with non-invasive micromeasurement technology (NMT)
The procedure was done according to a previous report (Michard et al., 2011). Tobacco pollen was germinated in GM for 3h. Pollen tubes 300 μm in length were selected for Ca2+ flux measurement. Net fluxes of Ca2+ were measured non-invasively using an NMT system BIO-IM (Younger USA, LLC, Amherst, MA, USA). The concentration gradients of the target ions were measured by moving the ion-selective microelectrode between two positions close to the plant material in a pre-set excursion (10 μm for tobacco pollen tubes in the present experiment) at a programmable frequency in the range of 0.3–0.5 Hz. The NMT can measure ionic fluxes down to picomolar levels, but must be measured slowly at ~1–2 s per point.
Pre-pulled and silanized glass micropipettes (2–4 μm aperture, XYPG120-2; Beijing Xuyue Sci. and Tech. Co., Ltd) were first filled with a backfilling solution (Ca2+: 100mM CaCl2, pH 7.0) to a length of ~1cm from the tip. Then the micropipettes were front filled with ~15 μm columns of selective calcium ionophore I–cocktail A (Sigma 21048, Sigma-Aldrich, St Louis, MO, USA). An Ag/AgCl wire electrode holder (XYEH01-1; Beijing Xuyue Sci. and Tech. Co., Ltd) was inserted in the back of the electrode to make electrical contact with the electrolyte solution. DRIREF-2 (World Precision Instruments) was used as the reference electrode. Ion-selective electrodes of Ca2+ and K+ were calibrated prior to flux measurements. The calibration media for Ca2+ were: 10 μM CaCl2 (100 μM KCl, 1.6mM H3BO4, 50 μM MES, 1% sucrose, pH 5.8); and 100 μM CaCl2 (100 μM KCl, 1.6mM H3BO4, 50 μM MES, 1% sucrose, pH 5.8). Calibration solution for K+ are 50 μM KCl (50 μM CaCl2, 1.6 mM H3BO4, 50 μM MES, 1 % sucrose, pH 5.8); and 500 μM KCl (50 μM CaCl2, 1.6 mM H3BO4, 50 μM MES, 1% sucrose, pH 5.8). The detailed calibration method is as follows: the electrode was put into germination medium and calibration solution of Ca2+, K+, respectively, to obtain the voltage number, and then to carry out a linear fit for the voltage and the logarithm of the concentration to obtain the slope and intercept. Only electrodes with Nernstian slopes >26 mV per decade were used in this study. Ion flux was calculated by Fick’s law of diffusion: J= –D (dc/dx), where J represents the ion flux in the x direction, dc/dx is the ion concentration gradient, and D is the ion diffusion constant in a particular medium. Data and image acquisition, preliminary processing, control of the three-dimensional electrode positioner, and stepper motor-controlled fine focus of the microscope stage were performed with Mageflux online software (www.xuyue.net).
Additional methods are provided as supplementary information available at JXB online.
Statistical analysis
The data are expressed as the mean ±SE. Experiments are conducted by the RBD method with three replicates. P-values were determined by a one-way analysis of variance (ANOVA) combined with post-hoc analysis in the SPSS 20.0 software.
Results
Exogenous GABA modulates tobacco pollen tube growth in vitro
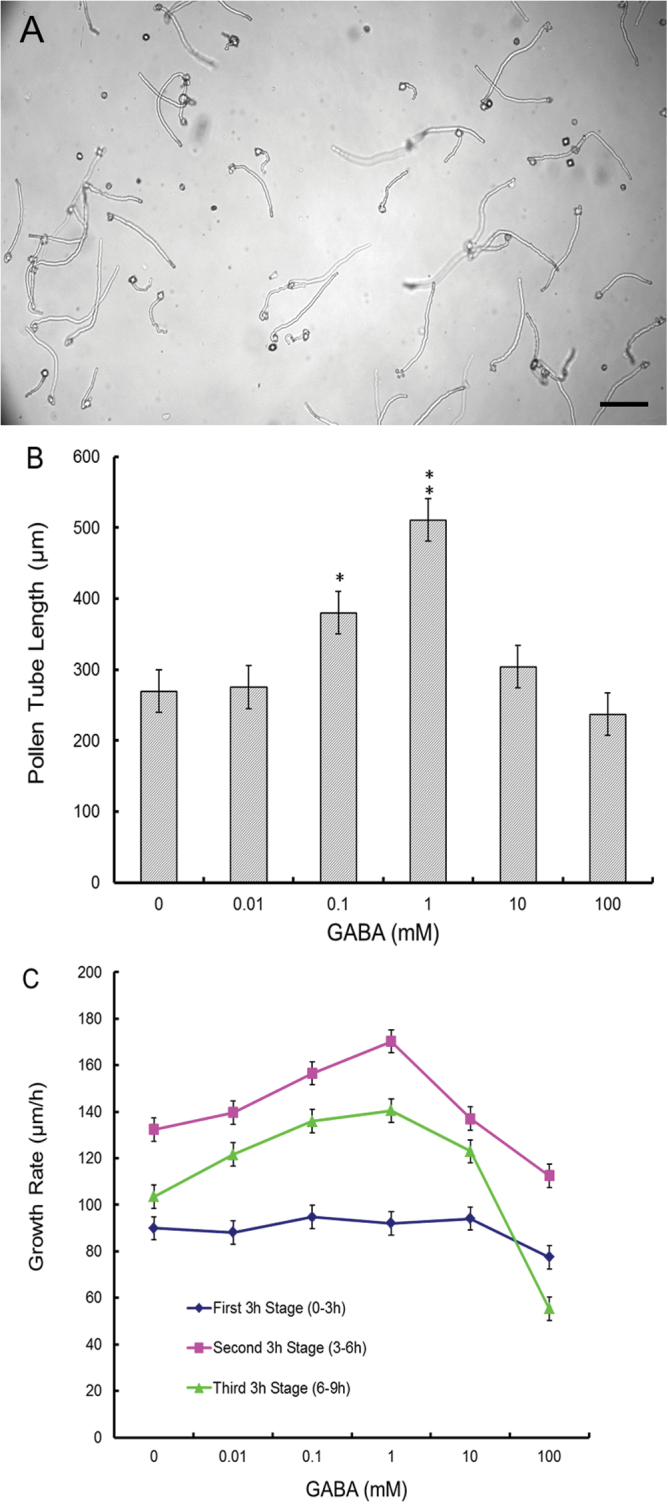
To assess the effect of exogenous GABA on tobacco pollen germination and pollen tube growth, pollen grains were germinated in culture media containing different concentrations of GABA. In the GM, pollen tubes grew rapidly and reached 120 μm in length within 1h (Fig. 1A). Significant differences in pollen tube length were observed in the presence of 0.1mM GABA (P=0.038) and 1mM GABA (P=0.006) after cultivation for 6h (Fig. 1B; Supplementary Fig. S1 at JXB online). The average growth rate of pollen tubes treated with 1.0mM GABA was higher than that of pollen tubes treated with GABA at other concentrations (P=0.006) (Fig. 1C). However, higher concentrations of GABA (≥10mM) inhibited pollen tube growth as early as the second 3h of cultivation. These results indicate that exogenous GABA stimulates pollen tube growth at lower concentrations, but inhibits pollen tube growth at higher concentrations.
Fig. 1.
Effect of GABA on in vitro cultivated pollen tube growth. (A) Pollen tubes cultured in germination medium for 1h. Scale bar=60 μm. (B) Concentration-dependent effect of GABA on pollen tube growth after 6h culture. (C) GABA dose-dependent growth rate assay. Images of growing pollen tubes were taken via a CCD-coupled microscope at 3h intervals, and then the length of at least 120 pollen tubes was measured with Metamorph software. The average growth rate was calculated every 3h. Means ±SE represent three independent experiments. Significant differences were determined by a one-way ANOVA function combined with post-hoc analysis in SPSS 20.0 software (*P<0.05; **P< 0.01).
To check whether GABA has a general role in pollen tube growth, pollen of lily (Lilium concolor), another widely used model plant, was used to perform a similar experiment. The results indicate that the average length of the GABA-treated pollen tubes (n≥40) is much longer than that of the control (without GABA treatment) after 6, 24, and 26h culture (Supplementary Fig. S2 at JXB online). These results suggest that GABA is a common signal to regulate pollen tube growth in multiple species with remote evolutionary relationships.
A GABA gradient exists in tobacco pistils from the stigma to the ovary
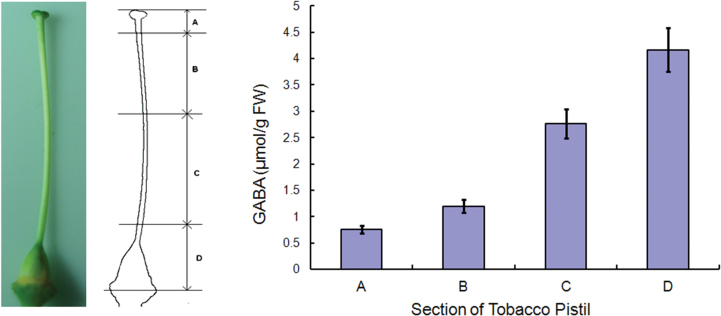
A previous investigation showed that Arabidopsis pistils exhibit a gradient of GABA levels that increases from the stigma to the ovule micropyle (Palanivelu et al., 2003). To assay whether a GABA gradient exists in tobacco pistils as in Arabidopsis pistils, and to obtain evidence for the in vivo relevance of exogenous GABA and its effect on pollen tubes demonstrated in this study, physiological concentrations of GABA were further evaluated in tobacco pistils. Tobacco pistils were separated into four sections from stigma to ovary, total free amino acids were extracted from each section, and GABA content was measured using an amino acid analyser (Fig. 2). In this analysis system, the retention time of the derivatized GABA was 23min in the 570nm channel (Supplementary Fig. S3 at JXB online). The GABA concentration in the uppermost section (Fig. 2, Section A) was ~0.75 μmol g–1 FW (fresh weight). From the top to the bottom of the pistil, the GABA concentration gradually increased ~2-fold in the lower style section and 5-fold in the ovary section (Fig. 2). The calculations indicate that the physiological concentration of GABA in the pistil is in the range of 0.75–4.2mM (Supplementary Note 1). These results demonstrate that a GABA gradient is present in the pistil of tobacco, and might function as an extracellular signal in regulating pollen tube growth.
Fig. 2.
A GABA gradient is present along the length of the pistil. (A–D) Sections of tobacco pistil used for analysis. (A) Uppermost section of the pistil including the stigma; (B) upper section of the style; (C) bottom section of the style; (D) bottom section of the pistil including the ovary. The GABA concentration in each section was determined using an L-8800 automatic amino acid analyser. Means ±SE represents three independent repeats. (This figure is available in colour at JXB online.)
Exogenous GABA modulates Ca2+-permeable channels in the plasma membrane of pollen tube protoplasts
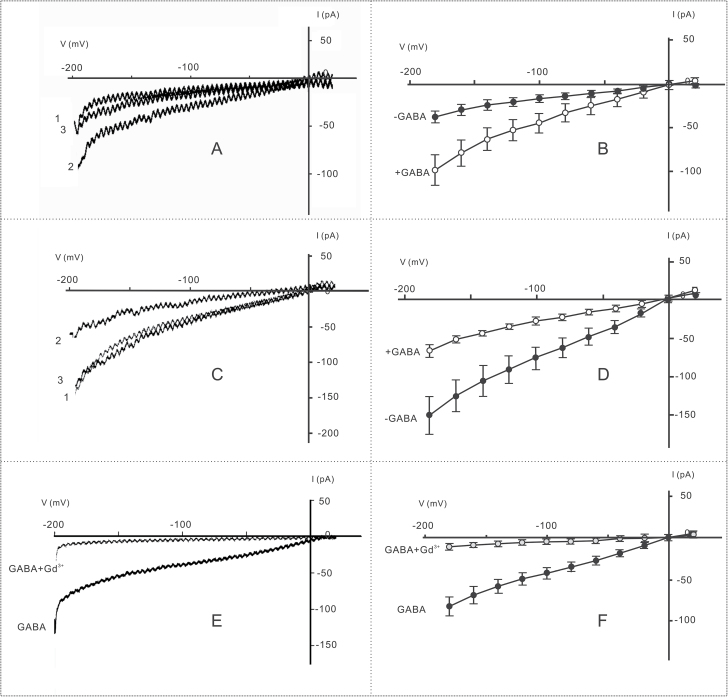
Previous work demonstrated that exogenous GABA binds to the surfaces of cell membranes of protoplasts and triggers fluctuations of cytosolic Ca2+ in these cells (Yu et al., 2006). However, the underlying Ca2+ mobilization pathways have remained unclear. To understand further how exogenous GABA modulates Ca2+ flow in pollen tubes, protoplasts isolated from germinated pollen grains with growing tubes were used to conduct whole-cell patch-clamp experiments to examine the effects of external GABA on dynamic Ca2+ currents. To ensure that the recorded currents were indeed Ca2+ currents, the constituents of the bathing and pipette solutions were adjusted in such a way that only Ca2+ and Cl– could transit the plasma membrane. The reversal potential of –2.1±0.9 mV (n=18) was close to the equilibrium potential for Ca2+ (E Ca2+=31.6 mV) and far from the Cl– equilibrium potential (E Cl–= –156.5 mV), suggesting that the hyperpolarization-activated currents are predominantly caused by Ca2+ influx.
The whole-cell Ca2+ currents of pollen tube protoplasts are shown in Fig. 3. The membrane potential was ramped from –200 mV to 0 mV within 1 s. When 1.0mM GABA was added to the bathing solution, the Ca2+ currents increased within 2min (Fig. 3A), indicating that 1.0mM GABA is sufficient to activate Ca2+-permeable channels in the plasma membrane. On average, the Ca2+ currents induced by 1.0mM GABA increased from 36.9±7.2 pA to 97.9±18.1 pA at –180 mV (Fig. 3B). Upon washout, the inward current returned to pre-stimulation levels, and this stimulation and restoration pattern could be repeated by adding or removing (by washout) GABA from the bathing solution. This confirmed that Ca2+ current alternations were indeed induced by GABA.
Fig. 3.
GABA influenced hyperpolarization-activated whole-cell Ca2+ currents in tobacco pollen tube protoplasts. (A) Current fluctuation in response to 1.0mM GABA: 1, before GABA addition; 2, treated with 1.0mM GABA; 3, washed with bath solution until the current was stable (n=6). (B) Current–voltage relationship of cells treated with 1.0mM GABA (+GABA, n=4–6; –GABA, n=4–6). (C) Pollen tube protoplasts were treated with 100mM GABA: 1, before GABA addition; 2, treated with 100mM GABA; 3, washed with bathing solution until the current was stable (n=6). (D) Current–voltage relationship of cells treated with 100mM GABA (+GABA, n=4–6; –GABA, n=4–6). (E) Currents induced by 1.0mM GABA before and after addition of 100 μM Gd3+ to the bathing solution. (F) Current–voltage relationship of protoplasts before and after adding 100 μM Gd3+ to the bathing solution (n=5).
Next, Ca2+ currents were tested in response to different GABA concentrations. It was found that 0.1mM GABA had no effect on Ca2+ currents (n=5; Supplementary Fig. S4 at JXB online). The 1.0mM GABA treatment, which elicited the maximal stimulatory effect on pollen tube length and growth rate, also significantly enhanced the Ca2+ currents (n=6; Supplementary Fig. S4). Although the Ca2+ currents were observed with 10mM GABA, the extent of the enhancement of Ca2+ currents was lower than that observed with 1.0mM GABA. Notably, when 100mM GABA was applied, a concentration that inhibited pollen tube growth, the Ca2+ currents were also obviously inhibited (n=6; Fig. 3C; Supplementary Fig. S4). On average, compared with the level observed with 1.0mM GABA, currents at –180 mV were inhibited from 153.0±24.8 pA to 68.7±8.7 pA in response to 100mM GABA (Fig. 3D). These results suggest that the enhancement and inhibition of Ca2+ currents in response to GABA were consistent with the stimulation and inhibition of pollen tube growth in response to GABA.
To confirm the specificity of GABA-induced Ca2+ currents, control experiments were performed by adding gadolinium (Gd3+), which specifically blocks Ca2+ channels, into the bathing solution. The results showed that GABA-induced currents were significantly inhibited after addition of 100 μM Gd3+ (Fig. 3E). On average, the currents at –180 mV were inhibited from 104.5±11.8 pA to 19.58±4.32 pA by Gd3+ (Fig. 3F). These results confirm that GABA indeed induces Ca2+ current alterations in pollen protoplasts. In addition, these results show that GABA produces biphasic effects on Ca2+ currents, activating them at low concentrations and inhibiting them at high concentrations by modulating Ca2+-permeable channels in the plasma membrane of pollen tube protoplasts.
Exogenous GABA increases Ca2+ influx in tobacco pollen tubes
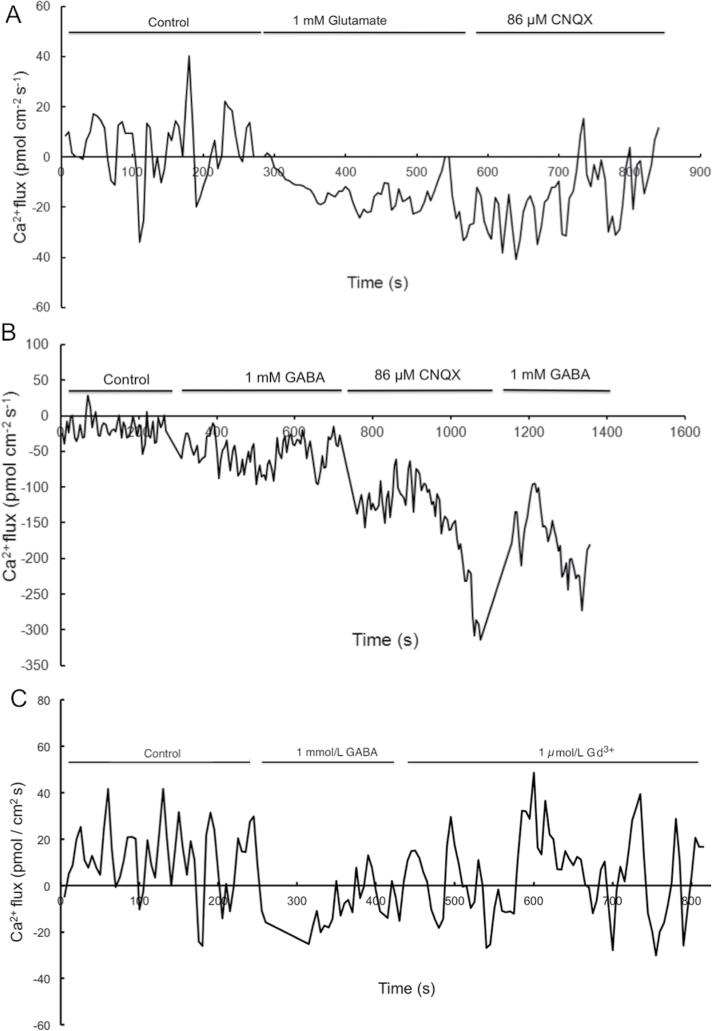
To assay dynamic Ca2+ flux in growing pollen tubes in response to exogenous GABA, Ca2+ influx was measured by NMT (Chen et al., 2009; Michard et al., 2011). This NMT method is based on Fick’s law of diffusion and measures ion flux using specific liquid ion exchanger (LIX) electrodes. To detect Ca2+ flux across the cell membrane of pollen tubes in response to exogenous GABA, an electrode containing 10 μM calcium ionophore I (Sigma-Aldrich) was used in stretched micropipettes (Supplementary Fig. S5A at JXB online). The results demonstrated that exogenous GABA can indeed trigger the influx of Ca2+ in growing pollen tubes; however, the Ca2+ oscillation pattern differed in response to different GABA concentrations, and significant oscillation was noticeable with 1.0mM GABA (Supplementary Fig. S5B).
To clarify the specific role of GABA in triggering Ca2+ influx, the Ca2+ oscillation patterns in pollen tubes exposed to GABA were compared with that in pollen tubes treated with glutamate, the precursor of GABA biosynthesis. The results indicated that 1.0mM glutamate could also trigger the influx of Ca2+. However, this influx could be reversed or inhibited by 6-cyano-nitroquinoxaline-2, 3-dione (CNQX) (at 86 μM), a specific inhibitor of one group of ionotropic glutamate receptors (Fig. 4A). This result suggests that tobacco pollen tubes probably express at least one kind of glutamate receptor. However, the oscillation amplitude triggered by 1.0mM GABA was larger than that triggered by glutamate and this was not affected by the addition of CNQX (Fig. 4B). This implied that Ca2+ influx caused by GABA is independent of that caused by glutamate. Interestingly, subsequent addition of 1.0mM GABA further triggered Ca2+ influx again (Fig. 4B), indicating that GABA-triggered Ca2+ influx was not inhibited by CNQX. These data demonstrate that the Ca2+ oscillation pattern induced by 1.0mM GABA is different from that triggered by 1.0mM glutamate.
Fig. 4.
Different patterns of Ca2+ flux in response to GABA and glutamate. Data from three experimental blocks were best fit using regression of the moving average model. (A) Ca2+ oscillations affected by treatment with 1.0mM glutamate and then with 86 μM CNQX; (B) Ca2+ flux recorded at the pollen tube treated first with 1.0mM GABA, then with 86 μM CNQX, and further with 1.0mM GABA; (C) Ca2+ oscillations affected by treatment first with 1.0mM GABA and then with 1.0 μM Gd3+.
To confirm that Ca2+ influx triggered by GABA occurs via Ca2+-permeable channels, Gd3+, a specific inhibitor of Ca2+-permeable channels, was used and Ca2+ current influx through pollen tubes was monitored. The results showed that the inward current of Ca2+ was markedly reversed by the application of Gd3+ at 1.0 μM (Fig. 4C). Thus, the results from both whole-cell patch-clamp and NMT experiments indicate that the inward current of Ca2+ induced by 1.0mM GABA is linked to the activation of Ca2+-permeable channels in tobacco pollen tube protoplasts and growing pollen tubes.
GAD is expressed in mature pollen and pollen tubes and co-localizes with GABA at the pollen tube tip
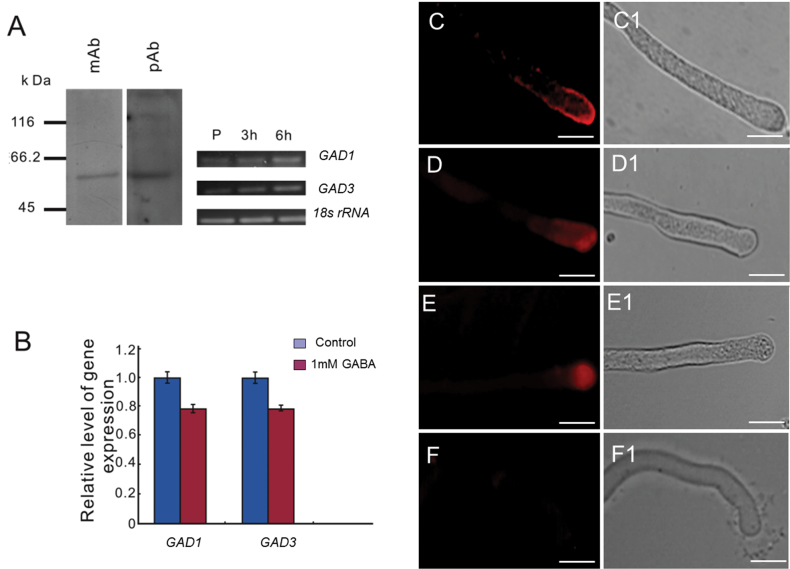
As pollen tubes can grow in the absence of exogenous GABA, it was next questioned how intracellular GAD plays a balancing role in responding to exogenous GABA. GAD is a key rate-limiting enzyme in the GABA biosynthetic pathway and has a domain that interacts with CaM (Baum et al., 1996). Therefore, it was hypothesized that GAD might be a downstream effector of Ca2+/CaM to modulate GABA’s effect on pollen tube growth. To test whether GAD accumulates in pollen, total proteins of germinated tobacco pollen were extracted and examined by immunoblotting with either monoclonal (mAb) or polyclonal (pAb) antibodies raised against tobacco GADs (mAb GAD-107.1 and pAb-GAD). mAb GAD-107.1 detected a major band with a mol. wt of ~58kDa, consistent with the expected size of GAD (Chen et al., 1994). In addition, an ~58kDa band was also detected using pAb-GAD (Fig. 5A, left panel). These results indicate that GADs are indeed expressed in tobacco pollen. Real-time PCR (RT-PCR) results provided further evidence that GAD1 (NCBI Genbank accession no. AF352732) and GAD3 (accession no. AF353615) are expressed in pollen and pollen tubes; other tobacco GAD isoform gene were not detected in pollen tubes. In comparison with the internal control of 18S rRNA expression, the expression of GAD genes was enhanced during pollen tube growth (Fig. 5A, right panel). Quantitative RT-PCR (RT-qPCR) showed that the expression of GAD genes in the growing pollen tubes was inhibited by the application of exogenous GABA, suggesting a negative feedback regulation between GABA levels and expression of GAD genes (Fig. 5B).
Fig. 5.
GAD expression analysis in pollen and pollen tubes. (A) Left panel: western blot analysis of GAD protein in tobacco pollen grain extracts. mAb and pAb indicate monoclonal and polyclonal GAD antibodies, respectively; Right panel: RT-PCR detection of GAD genes in pollen and pollen tubes. P indicates pollen grains, and 3h and 6h indicate the times of in vitro cultivation in germination medium. The 18S rRNA gene was used as the internal control. (B) The RT-qPCR assay for expression of GAD genes in response to treatment with 1.0mM GABA after 6h culture. Means ±SE represent three independent biological repeats. Pollen tubes without GABA treatment and cultured in the same conditions for the same time were used as controls. (C) Fluorescent signal showing localization of GAD (antibody: mAb-GAD 107.1). (D) Fluorescent signal showing localization of CaM (antibody: mAb CaM72-17.28); (E) Fluorescent signal showing localization of GABA. (F) As a control, tobacco pollen tubes were first incubated with immunodepleted antibody and then with TRITC-conjugated secondary antibody in B, C, D. Light microscopy views of the same images are shown in C1–F1. Scale bar=100 μm.
Immunolocalization was carried out to assess the spatial distribution of GAD in pollen tubes using the mAb against GADs. The immunofluorescence signal was preferentially localized in pollen tube tips (Fig. 5C). When pollen tubes were probed with an anti-CaM mAb (CaM72-17.28) that specifically recognizes the GAD-binding domain in CaM (Baum et al., 1996), the fluorescence was also preferentially localized in pollen tube tips (Fig. 5D). Pollen tubes were also stained with an mouse anti-GABA mAb, and it was found that GABA-related fluorescence is primarily found in the pollen tip region (Fig. 5E). No fluorescent signal was detected in control experiments when pollen tubes were treated with immunodepleted antibody plus the secondary antibody (Fig. 5F). These results show that in pollen tubes, GADs and their catalytic product GABA are primarily localized in pollen tube tips, suggesting a regulatory role for GAD/GABA in pollen tube tip growth.
Pollen tube growth and tip morphology can be altered by a specific inhibitor of GAD
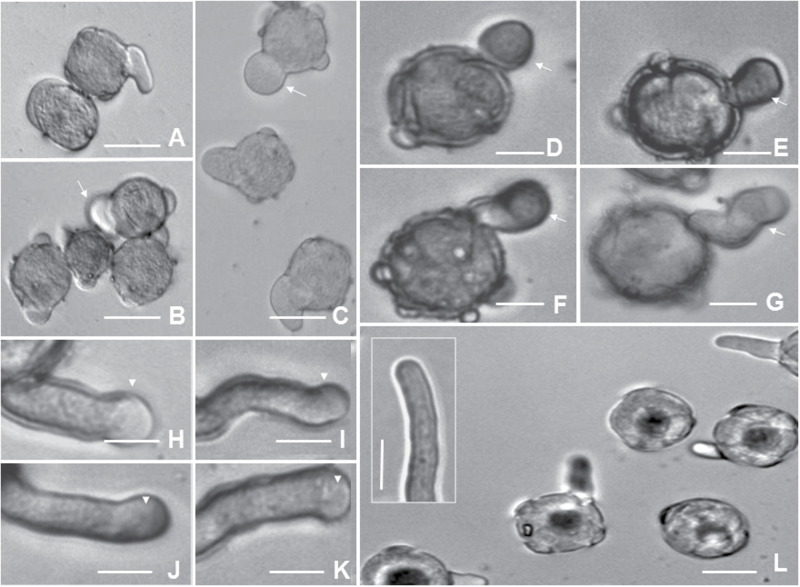
To find evidence of GAD’s involvement in pollen tube growth, 3-MPA, an inhibitor of GAD in neurons (Netopilova et al., 1997), was first evaluated for its role in specific inhibition of plant GAD (Supplementary Fig. S6, Supplementary Note 2 at JXB online). 3-MPA was next used in an in vitro pollen germination assay to examine the role of GAD in pollen germination and tube growth. At concentrations of 2.0mM and 5.0mM, 3-MPA completely inhibited pollen germination. At 1.0mM 3-MPA, the pollen grains appeared to initiate germination through multiple pores, even though the tube eventually emerged only from one pore. Furthermore, 1.0mM 3-MPA altered the morphology and behaviour of the pollen tubes, which expanded or swelled, and grew in a ‘zigzag’ pattern (Fig. 6A–K). About 68% of the pollen tubes had bulged tips and 24% had broadened shanks even at the first 3h cultivation stage (n=400; Fig. 6A–F; Supplementary Fig. S7).
Fig. 6.
Higher concentrations of 3-MPA altered the morphogenesis of pollen tubes. (A–F) Pollen tubes were treated with 1.0mM 3-MPA. White arrows indicate bulbous pollen tube tips. Scale bar=25 μm. (G–K) Pollen was pre-germinated for 30min and then treated with 1.0mM 3-MPA. Swollen protrusions at the tips of pollen tubes are indicated by arrows. Scale bar=50 μm. (L) Normal pollen tubes in the absence of 3-MPA. Scale bar=50 μm. The insert is a 1/5 magnified image of the pollen tubes. Scale bar=25 μm.
To test further the effect of 3-MPA on pollen tube growth, pollen grains were pre-cultivated in GM for 30min and then treated with 1.0mM 3-MPA. Over 90% of the pollen tubes had abnormalities, with balloon-like protrusions at the tips (Fig. 6G–K). Although 3-MPA at a lower concentration (50 μM) did not noticeably affect the morphology of the pollen tubes, it significantly slowed down pollen tube growth, leading to shorter pollen tubes. Interestingly, treatment with 50 μM 3-MPA supplemented with 1.0mM of exogenous GABA alleviated the inhibitory effect of 3-MPA (P=0.042; Supplementary Fig. S8 at JXB online). Together, these results indicate that GAD activity is critical for pollen tube growth.
3-MPA disrupts actin organization and vesicle trafficking in pollen tubes
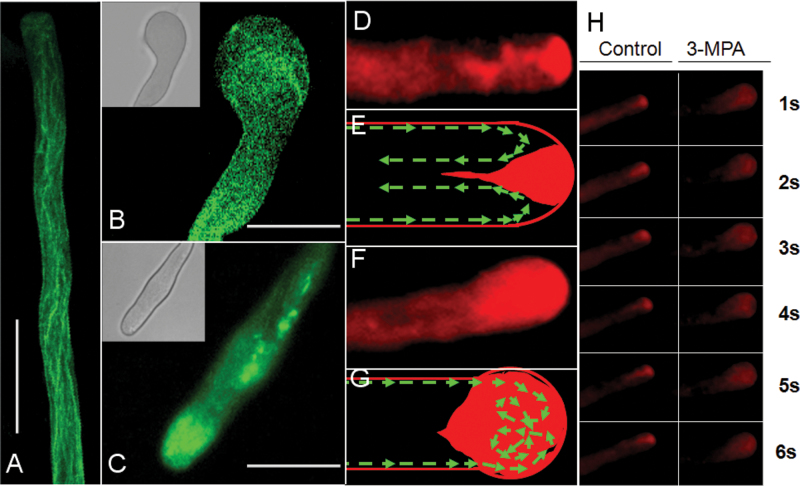
To investigate the possible mechanisms of GAD/GABA action in pollen tube growth regulation, abnormally growing pollen tubes treated with 3-MPA were further analysed. As described above, 1.0mM 3-MPA altered the morphology of pollen tubes within 30min, causing either balloon-shaped or swollen tips, and broadened pollen tube shanks (Fig. 7, insets). Actin plays a critical role in the morphogenesis of pollen tubes (Foissner et al., 2002; Cvrckova et al., 2004); therefore, it was investigated whether the morphological alteration observed upon 3-MPA treatment was caused by disruption of actin organization. In untreated samples, longitudinal bundles of F-actin were distributed along the shanks of the pollen tubes and were absent near the pollen tube apex (Fig. 7A). After application of 1.0mM 3-MPA, actin became disorganized in the shanks of the pollen tubes, particularly in the balloon-shaped tip (Fig. 7B), perhaps causing broadening of the pollen tube shank (Fig. 7C). These results imply that GAD is required for maintenance of dynamic actin organization and the normal morphology of pollen tubes.
Fig. 7.
3-MPA altered F-actin organization and vesicle trafficking in pollen tube tips. (A–C) Pollen tubes were treated with 1.0mM 3-MPA at 30min before chemical fixation and FITC–phalloidin staining. (A) Bundles of actin filaments in control cells. (B) Actin filaments in the ballooned tip of a pollen tube. (C) Actin filaments in the broadened shanks of a pollen tube. Inserts show the bright field view of the fluorescence images in about a 1/6 ratio. Scale bar in A=20 μm; scale bar in B, C=40 μm. (D–G) Vesicles were stained with FM4-64, which was internalized within 20min. (D) Control, typical inverse fountain vesicle trafficking (refer to Supplementary Video S1 at JXB online). (E) Sketch model of D. The green line and arrowheads indicate the direction of vesicle flow. (F) The direction of vesicle trafficking becomes random after 3-MPA treatment of the balloon-shaped swollen apical pollen tubes (see also Supplementary Video S2). (G) Sketch of vesicle trafficking depicted in F. The dotted line and arrowheads indicate the random direction of vesicle flow. (H) Control and right panels provide observations of vesicle trafficking over time within 6 s without or with 1.0mM 3-MPA treatment, respectively.
During pollen tube growth, secretory vesicles are delivered to the tip via actin microfilaments. A highly efficient vesicular transport system, mobilized by the actin cytoskeleton, supports rapid tip growth in pollen tubes (Hepler et al., 2001). To monitor the extent to which vesicle trafficking is affected by 3-MPA, vesicle trafficking was tracked with the vesicle-specific fluorescent dye FM4-64. As expected, in pollen tubes grown without 3-MPA, secretory vesicles exhibited rapid bidirectional cytoplasmic streaming, known as a reverse fountain movement (Fig. 7D–E; H, control panel; Supplementary Video S1 at JXB online). However, in the balloon-shaped tips of pollen tubes grown in the presence of 3-MPA, the direction of vesicle trafficking was random (Fig. 7F, G; H, right-hand panel; Supplementary Video S2). These results raise the possibility that actin filament disorganization and misdirected vesicle trafficking caused by 3-MPA-treatment led to the abnormal pollen tube morphology.
Ca2+ efflux caused by 3-MPA can be reversed upon GABA addition
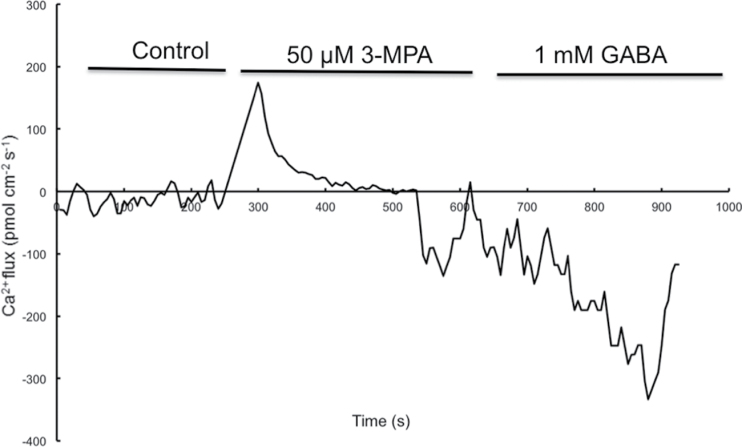
Ca2+ oscillation is a sensitive and early indicator of extracellular stimulation. Ca2+ oscillation was observed when pollen tubes were exposed to 3-MPA. This result showed that addition of 3-MPA at 50 μM triggered an obvious efflux of Ca2+ (Fig. 8); however, this Ca2+ efflux was noticeably reversed by the addition of 1.0mM GABA (Fig. 8). This result suggests that this re-triggered Ca2+ influx might be due to the function of GABA in inducing Ca2+ influx at the early time points after the addition of GABA.
Fig. 8.
Ca2+ oscillations were affected by 3-MPA. Data from three experimental blocks were best fit using a regression of moving average model. Ca2+ oscillations affected by 3-MPA and GABA compared with control. First treatment with 50 μM 3-MPA and then with 1.0mM GABA.
Expression of multiple putative downstream signalling components may be activated in response to GABA
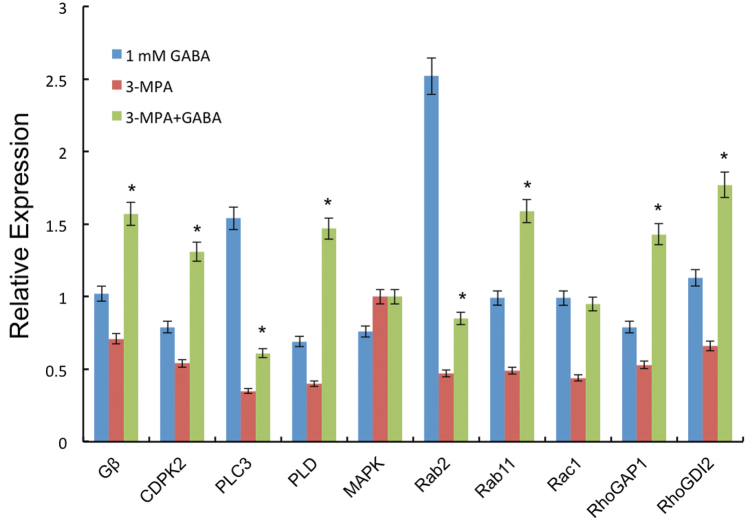
To screen the possible effectors of GABA signalling in pollen tube growth, RT-qPCR was used to measure the gene expression of multiple regulatory/signal molecules. These components are involved in vesicle trafficking and polarity signal transduction pathways that are crucial for pollen tube tip growth. The heterotrimeric Gβ subunit (Ma et al., 1999) and its possible downstream signalling components PLC3 (Dowd et al., 2006; Helling et al., 2006), PLD (Potocky et al., 2003), MAPK (Wilson et al., 1995), and CDPK2 (calcium-dependent protein kinase) (Yoon et al., 2006) are involved in the regulation of pollen tube growth. Moreover, small G proteins such as Rab2 and Rab11 participate in vesicle trafficking in tobacco pollen tube tip growth (Cheung et al., 2002; de Graaf et al., 2005). Additional regulatory molecules, such as guanine nucleotide dissociation inhibitors (GDIs) and GAP1 (GTPase-activating protein gene), play critical roles in the regulation of molecular switch activities (Cheung et al., 2003; Klahre and Kost, 2006; Klahre et al., 2006). RT-qPCRs showed that the expression of PLC3 and Rab2 increased markedly in pollen tubes grown in the presence of exogenous GABA, 0.5- and 1-fold higher than that in the control (the ratio was normalized to the internal housekeeping 18S rRNA gene), respectively. RhoGDI2 expression increased by 0.3-fold. After 3-MPA treatment, the expression of multiple genes, excluding MAPK which did not show an obvious change, was decreased (Fig. 9). However, compared with 3-MPA treatment, 3-MPA plus GABA markedly increased the expression of Gβ (1-fold higher), CDPK2 (1.5-fold higher), PLD (2.2-fold higher), Rab11 (2-fold higher), RhoGAP1 (1.5-fold higher), and RhoGDI2 (1.5-fold higher). In addition, PLC3, Rab2, and Rac1 also increased ~0.5- to 1-fold, and MAPK appeared not to be affected by GABA treatment (Fig. 9).
Fig. 9.
Expression of genes for various signalling molecules in response to different treatments. The transcript level ratio was normalized to the 18S rRNA gene; the relative expression level was calculated by the ΔΔCT method. The expression levels of Gβ, CDPK2, PLC3, PLD, MAPK, Rab2, Rab11, Rac1, and Rho GAP1RhoGDI2 upon GABA (1.0mM) and 3-MPA (50 μM) plus GABA (1.0mM) treatments, respectively. Results represent means ±SE of three independent repeats. Significant differences between different treatments were calculated via a one-way ANOVA combined with post-hoc analysis. *P<0.05. (This figure is available in colour at JXB online.)
Discussion
In their long journey to the ovules, pollen tubes must penetrate the style and respond properly to various signals from the pistil tissue to ensure their correct arrival at an ovule (Sauter, 2009). Among these signals, GABA from the pistil is a critical guidance clue (Palanivelu et al., 2003). In the present work, it was found that an optimum concentration of exogenous GABA enhanced pollen tube growth, but a high concentration of GABA inhibited growth (Fig. 1). It was further confirmed that a gradient of GABA exists in the tobacco style, and the 1.0mM exogenous GABA applied in this research also falls within the physiological concentration. This result is consistent with previous observation of Arabidopsis (Palanivelu et al., 2003). This suggests that variations in GABA concentration in the style regulate the pace of pollen tube growth (Fig. 2). Evidence is also provided for the mechanism by which exogenous GABA regulates pollen tube growth. In this contribution, Evidence is provided to show the possible mechanism of GABA involvement in the enigmatic fertilization courses of plants.
Pistil–pollen tube communication: exogenous GABA regulates Ca2+ influx by modulating Ca2+-permeable channels
Although pistils of different species have been reported to contain many factors that play roles in pollen tube growth (Kim et al., 2003; Marton et al., 2005; Okuda et al., 2009), the molecular mechanisms of communication between the pistil and pollen tube remain largely unknown. Previously, it was found that GABA can bind to the membrane surface of Arabidopsis and tobacco pollen protoplasts, trigger fluctuations in cytosolic Ca2+, and enhance Ca2+ levels in the cells (Yu et al., 2006). In the present work, it was confirmed that GABA affects Ca2+ influx by modulating Ca2+-permeable channels (Fig. 3). The results show that exogenous GABA can affect Ca2+ currents in a concentration-dependent biphasic manner in tobacco pollen tube protoplasts and growing pollen tubes, activating the Ca2+-permeable channels at lower concentrations and inhibiting the channels at higher concentrations. Previous research confirmed that pollen tubes have the same type of Ca2+-permeable channels as pollen grains (Wang et al., 2004). Studies showed that d-serine can regulate Ca2+-permeable channels via the activation of a glutamate receptor-like receptor (a type of Ca2+ channel) in pollen tubes (Michard et al., 2011). The present results indicate that GABA can also activate Ca2+ channels. The results of NMT offer further support that this possible ligand-gated activation of Ca2+-permeable channels occurs as a specific response to GABA (Fig. 4). Therefore, it is reasonable to deduce that the effects of GABA on pollen tube growth are mainly transmitted via its modulation of Ca2+-permeable channels. This suggests that GABA, as an external cue, mediates communication between the pistil and pollen tubes.
Notably, the relatively high influx of Ca2+ was still observed in the presence of CNQX (at 86 μM) and the addition of GABA led to further Ca2+ influx (Fig. 4). This indicates that the GABA-triggered Ca2+ influx was not inhibited by CNQX and is independent of that triggered by glutamate. CNQX was used as a specific inhibitor of ionotropic glutamate receptors in previous research (Michard et al., 2011). There is a possibility that the blockage of ionotropic glutamate receptors by CNQX might stimulate GABA-triggered Ca2+ influx in compensation. Thus, CNQX treatment may have no specific influence on GABA-triggered Ca2+ influx as observed in Fig. 4. It is reasonable that multiple Ca2+ channels are present on the plasma membrane in responding to various extracellular signals during pollen tube growth.
It was also observed that GABA could trigger the tendency for K+ outflux (Supplementary Figs S9, S10 at JXB online) in addition to activating Ca2+ channels. This is consistent with a previous report showing that the outward K+ channel is reciprocally regulated by Ca2+ channel activation in Pyrus pyrifolia pollen (Wu et al., 2011). Furthermore, K+ outward rectifying flux induced by GABA is also different from that caused by glutamate (Supplementary Fig. S10, Supplementary Note 3). These results imply that the activation of Ca2+ and K+ channels might be simultaneously regulated by the binding of GABA to the putative receptor, as occurs in animal cells (Blein et al., 2000; Niswender and Conn, 2010). These data substantiate the possibility that proteins analogous to the metabotropic GABAB receptor exist on the membrane of pollen tubes and provide an important tool to understand the mechanism of GABA-mediated pollen tube–pistil communication.
GAD/CaM probably acts as a rheostat of Ca2+ to integrate its downstream signalling
GAD has an autoinhibitory domain in its C-terminal region and can bind to a Ca2+/CaM complex. When cytosolic Ca2+ increases, Ca2+/CaM binds to GAD, resulting in the rapid induction of GABA synthesis (Chen et al., 1994). It was previously shown that exogenous GABA can bind to the cell membrane, resulting in oscillation of Ca2+ in tobacco pollen, and indicating that exogenous GABA can affect the levels and distribution of Ca2+ in cells (Yu et al., 2006). In the present work, it was confirmed that GAD, GABA, and CaM are closely co-localized in the growing tips of pollen tubes (Fig. 5). It was also demonstrated that GAD is mainly localized on the membrane at the pollen tube tips. This result is consistent with the previous prediction that GAD is associated with the cell membrane (Alexandersson et al., 2004). Interestingly, it was revealed that CaM could probably form a complex with Ca2+ channels to sense the Ca2+ source (Tadross et al., 2008). This suggests that GAD and CaM may be directly associated with Ca2+ channels and function as a complex. It is possible that exogenous GABA modulates Ca2+-permeable channels by activating G protein-coupled receptors (GPCRs) and regulating GAD activity via CaM, which is associated with a Ca2+-permeable channel. To test this idea, a GAD inhibitor, 3-MPA, was applied which led to significantly slowed pollen tube growth and shorter pollen tubes with swollen and twisted tips (Figs 6, 7). The inhibition of pollen tube growth by 3-MPA treatment could be partially alleviated by exogenous GABA. Proteomic and cytological analyses have revealed the important role of the Ca2+/CaM complex in pollen tube growth (Chen et al., 2009). Although the complicated interlink of actin organization, vesicular trafficking, and Ca2+ dynamics made it difficult to distinguish the cause and the consequence among them in relation to altered morphology after inhibitor treatment, the present data clearly show that GAD seems to be a key component of the Ca2+/CaM downstream targets and plays a critical role in the regulation of pollen tube growth as a downstream modulator of Ca2+/CaM in response to exogenous GABA.
Multiple signal pathways may be involved in perceiving exogenous GABA
The mechanisms of GABA function in pollen tube growth, although interesting and important (Ma, 2003; Palanivelu et al., 2003; Yang, 2003), remain largely unknown. The present data revealed that the inhibition of GAD resulted in abnormal actin organization and vesicle trafficking, and thus abnormal pollen tube growth. To understand its relationship to known signalling pathways involved in pollen tube growth, some key components involved in signal transduction and vesicle trafficking in pollen tube growth were tested. Previous research demonstrated that PLC3, PLD, and CDPK are all involved in plasma membrane polarization and polar pollen tube growth (Potocky et al., 2003; Dowd et al., 2006; Helling et al., 2006). The present RT-qPCR results indicated that exogenous GABA significantly stimulates the expression of PLC3 and Rab2 during normal pollen tube growth (Fig. 9). However, when endogenous GABA synthesis was inhibited by 3-MPA, the expression of these genes was reduced, and exogenous GABA only markedly increased the expression of Gβ, CDPK2, PLD, Rab11, RhoGAP1, and Rho GDI2. Although the activities of these signalling effectors were not assayed at the protein level, the present data suggest that there might be at least three common signalling pathways, PLC3, PLD, and CDPK2, that respond to GABA. Moreover, in the interlinked signal pathways, GAD and its regulator CaM may act as a Ca2+ rheostat to maintain the balance of Ca2+ and modulate the GABA level in pollen tubes by the feedback modulation on the Ca2+ channel. Although exogenous and endogenous GABA signals may trigger the activation of different pathways, they finally converge to regulate F-actin and vesicle trafficking directly or indirectly, as well as regulating subsequent pollen tube tip growth (Dowd et al., 2006).
In summary, the flux of ions across membranes via ion channel activation is vital to cellular responses to internal and external stimuli, and therefore crucial for cellular survival in changing circumstances. It was previously reported that exogenous GABA can trigger an increase in cytosolic Ca2+ in tobacco pollen protoplast and it was proposed that Ca2+ influx occurs across the plasma membrane. However, the upstream stimuli that activate plasma membrane-localized Ca2+ channels and downstream signalling transduction pathway were unknown. Here evidence is provided for the activation of Ca2+-permeable channels coupling outward K+ efflux in the plasma membrane of tobacco pollen tubes in response to exogenous GABA. When pollen tubes pass through a GABA gradient in the tobacco style, this extracellular GABA might efficiently modulate pollen tube growth in a dose-dependent manner by modulating Ca2+-permeable channels on the pollen tube membrane, triggering Ca2+ influx in pollen tubes, and thus mediating GAD/CaM signalling. In addition, the Ca2+ flux pattern triggered by GABA is different from that induced by glutamate, indicating its specificity in activating Ca2+-permeable channels. In brief, GABA as a signalling molecule mediates style–pollen tube communication and regulates pollen tube growth though Ca2+ signalling.
Supplementary data
Supplementary data are available at JXB online.
Supplementary methods
Figure S1. The impact of different GABA concentrations on tobacco pollen tube growth. Photographs were taken at 6h cultivation
Figure S2. Effect of GABA on lily pollen tube growth.
Figure S3. Content of amino acids analysed by an L-8800 automatic amino acid analyser.
Figure S4. Fold change of whole-cell currents at –200 mV against different GABA concentrations. n=4–6.
Figure S5. Ca2+ oscillations recorded by non-invasive micromeasurement technology (NMT).
Figure S6. Specific inhibition by 3-MPA of the production of GABA.
Figure S7. Percentage of the different abnormal pollen tubes after the application of 1.0mM 3-MPA for 3h.
Figure S8. The growth inhibition by a low concentration of 3-MPA was ameliorated by addition of exogenous GABA.
Figure S9. K+ oscillation pattern responding to different GABA concentrations.
Figure S10. Influence of CNQX after GABA (A) and glutamate (B) treatment on K+ flux in the tip of tobacco pollen tubes.
Table S1. Gene-specific primers used in RT-qPCR assay.
Video S1. Time-lapse video showing the vesicle trafficking pattern in normal pollen tubes.
Video S2. Time-lapse video showing the vesicle trafficking pattern in pollen tubes after 1.0mM 3-MPA treatment.
Note 1. Calculation of GABA concentration in tobacco style.
Note 2. Specific inhibition by 3-MPA of GAD activity.
Note S3. K+ flux responds to GABA.
Supplementary References
Acknowledgements
We would like to express our gratitude to Professor Hillel Fromm (Tel Aviv University, Israel) for his kind offer of monoclonal and polyclonal anti-GAD antibodies and the anti-CaM antibody. This work was supported by the ‘Major Research Plan from the Ministry of Science and Technology of China’ (2013CB945100), National Natural Science Foundation of China (31270361), an OPTICHINA research fellowship in Rothamsted Research (supported by the EU and CAAS), China Scholarship Council Project for young outstanding talents, Academic Innovation Team for Plant Development and Genetics in the South-Central University for Nationalities, and by a grant from the US National Science Foundation to RP (IOS-0723421).
Glossary
Abbreviations:
- CaM
calmodulin
- CNQX
6-cyano-nitroquinoxaline-2, 3-dione
- GABA
γ-aminobutyric acid
- GAD
glutamate decarboxylase
- 3-MPA
3-mercaptopropionic acid
- TRITC
tetramethylrhodamine-5-(and 6)-isothiocyanate.
References
- Alexandersson E, Saalbach G, Larsson C, Kjellbom P. 2004. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant and Cell Physiology 45, 1543–1556 [DOI] [PubMed] [Google Scholar]
- Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Fromm H. 1996. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO Journal 15, 2988–2996 [PMC free article] [PubMed] [Google Scholar]
- Blein S, Hawrot E, Barlow P. 2000. The metabotropic GABA receptor: molecular insights and their functional consequences. Cellular and Molecular Life Sciences 57, 635–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N, Fromm H. 2004. GABA in plants: just a metabolite? Trends in Plant Science 9, 110–115 [DOI] [PubMed] [Google Scholar]
- Chen T, Wu X, Chen Y, Li X, Huang M, Zheng M, Baluska F, Samaj J, Lin J. 2009. Combined proteomic and cytological analysis of Ca2+–calmodulin regulation in Piceameyeri pollen tube growth. Plant Physiology 149, 1111–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Baum G, Fromm H. 1994. The 58-kilodalton calmodulin-binding glutamate decarboxylase is a ubiquitous protein in Petunia organs and its expression is developmentally regulated. Plant Physiology 106, 1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Chen CY, Glaven RH, de Graaf BH, Vidali L, Hepler PK, Wu HM. 2002. Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. The Plant Cell 14, 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Chen CY, Tao LZ, Andreyeva T, Twell D, Wu HM. 2003. Regulation of pollen tube growth by Rac-like GTPases. Journal of Experimental Botany 54, 73–81 [DOI] [PubMed] [Google Scholar]
- Cvrckova F, Rivero F, Bavlnka B. 2004. Evolutionarily conserved modules in actin nucleation: lessons from Dictyostelium discoideum and plants. Protoplasma 224, 15–31 [DOI] [PubMed] [Google Scholar]
- de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM. 2005. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. The Plant Cell 17, 2564–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd PE, Coursol S, Skirpan AL, Kao TH, Gilroy S. 2006. Petunia phospholipase C1 is involved in pollen tube growth. The Plant Cell 18, 1438–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Grolig F, Obermeyer G. 2002. Reversible protein phosphorylation regulates the dynamic organization of the pollen tube cytoskeleton: effects of calyculin A and okadaic acid. Protoplasma 220, 1–15 [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE. 1999. Signaling in pollination. Current Opinion in Plant Biology 2, 490–495 [DOI] [PubMed] [Google Scholar]
- Helling D, Possart A, Cottier S, Klahre U, Kost B. 2006. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. The Plant Cell 18, 3519–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY. 2001. Polarized cell growth in higher plants. Annual Review of Cell and Developmental Biology 17, 159–187 [DOI] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. 2003. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences, USA 100, 16125–16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Becker C, Schmitt AC, Kost B. 2006. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. The Plant Journal 46, 1018–1031 [DOI] [PubMed] [Google Scholar]
- Klahre U, Kost B. 2006. Tobacco RhoGTPase activating PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. The Plant Cell 18, 3033–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S. 2007. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Current Biology 17, 947–952 [DOI] [PubMed] [Google Scholar]
- Ma H. 2003. Plant reproduction: GABA gradient, guidance and growth. Current Biology 13, R834–R836 [DOI] [PubMed] [Google Scholar]
- Ma LG, Sun DY. 1997. The effects of extracellular calmodulin on initiation of pollen germination and tube growth. Planta 202, 336–340 [Google Scholar]
- Ma L, Xu X, Cui S, Sun D. 1999. The presence of a heterotrimeric G protein and its role in signal transduction of extracellular calmodulin in pollen germination and tube growth. The Plant Cell 11, 1351–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton ML, Cordts S, Broadhvest J, Dresselhaus T. 2005. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307, 573–576 [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA. 2011. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil d -serine. Science 332, 434–437 [DOI] [PubMed] [Google Scholar]
- Neher E. 1992. Correction for liquid junction potentials in patch-clamp experiments. Methods in Enzymology 207, 123–131 [DOI] [PubMed] [Google Scholar]
- Netopilova M, Drsata J, Haugvicova R, Kubova H, Mares P. 1997. Inhibition of glutamate decarboxylase activity by 3-mercaptopropionic acid has different time course in the immature and adult rat brains. Neuroscience Letters 226, 68–70 [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. 2010. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual Review of Pharmacology and Toxicology 50, 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, et al. 2009. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D. 2003. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114, 47–59 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Sukamoto T. 2012. Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. Wiley Iinterdisciplinary Reviews. Developmental Biology 1, 96–113 [DOI] [PubMed] [Google Scholar]
- Potocky M, Elias M, Profotova B, Novotna Z, Valentova O, Zarsky V. 2003. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta 217, 122–130 [DOI] [PubMed] [Google Scholar]
- Renaul H, EI Amrani A, Palanivelu R, Updegraff EP, Yu A, Renou JP, Preuss D, Bouchereau A, Deleu C. 2011. GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana . Plant and Cell Physiology 52, 894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M. 2009. A guided tour: pollen tube orientation in flowering plants. Chinese Science Bulletin 54, 2376–2382 [Google Scholar]
- Shelp BJ, Brown A, McLean MD. 1999. Metabolism and functions of gamma-aminobutyric acid. Trends in Plant Science 4, 446–452 [DOI] [PubMed] [Google Scholar]
- Snedden WA, Koutsia N, Baum G, Fromm H. 1996. Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin-binding domain. Journal of Biological Chemistry 271, 4148–4153 [DOI] [PubMed] [Google Scholar]
- Szumlanski AL, Nielsen E. 2009. The RabGTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. The Plant Cell 21, 526–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadross MR, Dick IE, Yue DT. 2008. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell 133, 1228–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Fan LM, Zhang WZ, Zhang W, Wu WH. 2004. Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiology 136, 3892–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Anglmayer R, Vicente O, Heberle-Bors E. 1995. Molecular cloning, functional expression in Escherichia coli, and characterization of multiple mitogen-activated-protein kinases from tobacco. European Journal of Biochemistry 233, 249–257 [DOI] [PubMed] [Google Scholar]
- Wu JY, Qu HY, Shang ZL, Tao ST, Xu GH, Wu J, Wu HQ, Zhang SL. 2011. Reciprocal regulation of Ca2+-activated outward K+ channels of Pyrus pyrifolia pollen by heme and carbon monoxide. New Phytologist 189, 1060–1068 [DOI] [PubMed] [Google Scholar]
- Yang ZB. 2003. GABA, a new player in the plant mating game. Developmental Cell 5, 185–186 [DOI] [PubMed] [Google Scholar]
- Yoon GM, Dowd PE, Gilroy S, McCubbin AG. 2006. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. The Plant Cell 18, 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GH, Liang JG, He ZK, Sun MX. 2006. Quantum dot-mediated detection of gamma-aminobutyric acid binding sites on the surface of living pollen protoplasts in tobacco. Chemistry and Biology 13, 723–731 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.