Celiac disease (CD) is a gluten-sensitive enteropathy characterized by diffuse damage to the proximal small intestinal mucosa that results in malabsorption of most nutrients. CD is considered to affect at least 1% of the Western population. However, CD remains frequently missed and untreated (1).
Endoscopic findings in CD include a mosaic pattern in the duodenal mucosa, scalloping and loss of duodenal folds. However, the overall sensitivity and positive predictive value of these endoscopic signs for the diagnosis of CD is poor (2). Therefore, the diagnosis of CD relies on histological assessment of duodenal biopsies supporting positive serology.
CASE PRESENTATION
A 44-year-old woman was referred to the authors’ division with a history of chronic iron deficiency anemia. She had a family history of CD. She was started on iron supplements two years previously, but without benefit. CD screening was positive, with tissue transglutaminase immunoglobulin A antibody >200 kU/L. Esophagogastroduodenoscopy was requested to confirm CD.
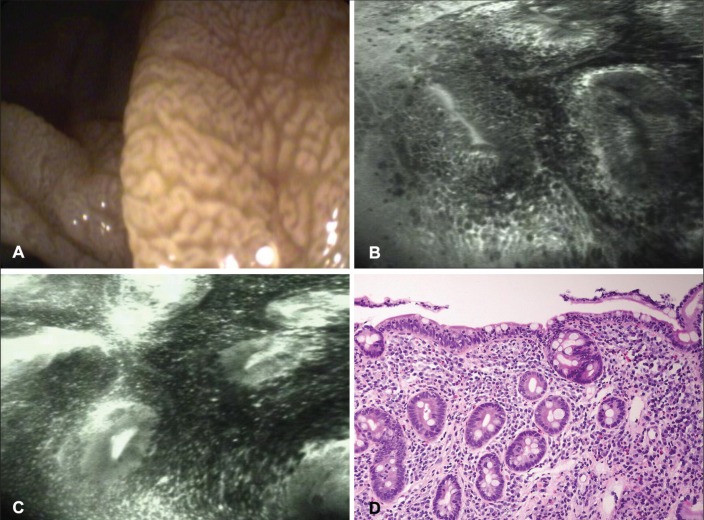
Upper gastrointestinal endoscopy was performed using a confocal laser endoscope (CLE) (EC-3870CIFK, Pentax, Japan). Endoscopic examination of the duodenum revealed scalloping and a mosaic mucosal pattern on white-light endoscopy. CLE revealed total villous atrophy, with collars of enterocytes around the crypt openings, an increased number of intraepithelial lymphocytes and leakage of fluorescein (Figure 1). Targeted biopsies were obtained.
Figure 1).
A White-light endoscopy revealing mosaic and nodular pattern. B and C Total villous atrophy with collars of enterocytes around the crypt openings and an increased number of intraepithelial lymphocytes and leakage of fluorescein. D Corresponding histological specimen reveals a Marsh IIIb focally IIIc lesion with total absence of villi and numerous intraepithelial lymphocytes (hematoxylin and eosin stain, original magnification ×20)
Histological examination revealed modified Marsh IIIb and focally IIIc lesions in the bulb and second portion of the duodenum (Figure 2).
Figure 2).
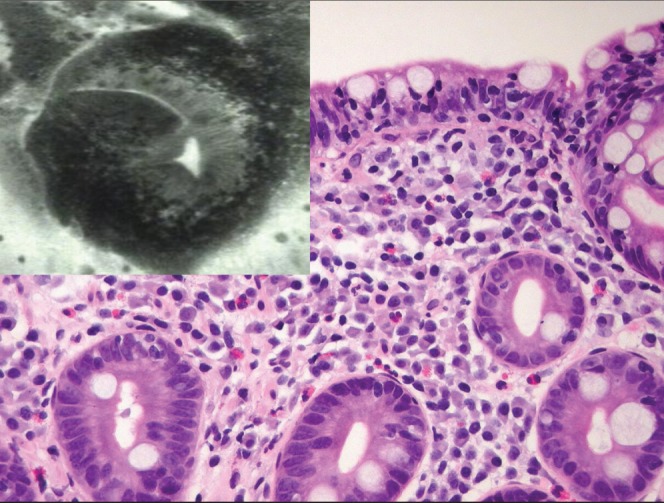
Villous atrophy and the base of the crypts appears as roundish glands resembling colonic daisy crypts. Corresponding histological specimen reveals a Marsh IIIb focally IIIc lesion. Hematoxylin and eosin stain, original magnification ×20
DISCUSSION
CLE is a novel endoscopic imaging technique that provides in vivo histology during ongoing endoscopy (3). A miniaturized laser confocal microscope is incorporated into the tip of a flexible endoscope. The 1000-fold magnified image of in situ living tissue histology has a sufficiently high resolution to distinguish cellular and subcellular structures.
On confocal imaging, normal duodenal villi appear regular and finger-like, with the surface exhibiting hexagonal-shaped enterocytes yielding a honeycomb appearance, interspersed with goblet cells (Figure 3). At a deeper plane, the single layer of brush border columnar epithelial cells lining the lamina propria is well visualized. The lamina propria demonstrates capillary vasculature in the stroma. Crypts are not normally visible. These features correspond well with histology.
Figure 3).
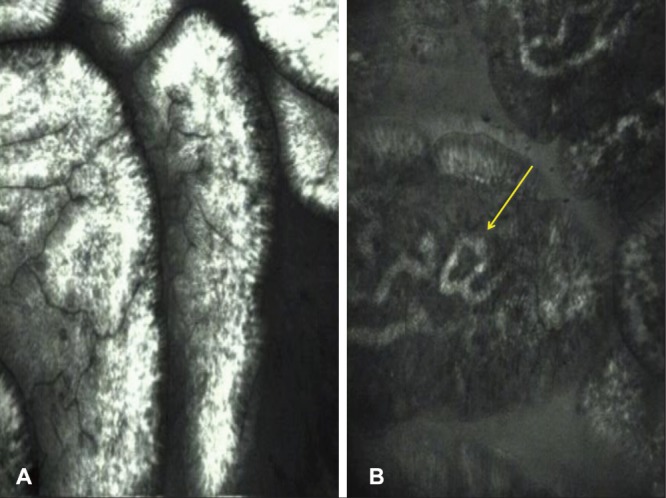
Confocal endomicroscopy images of normal duodenal mucosa. A Villi appear regular, ‘fingerlike’ with goblet cells between the enterocytes. B Endomicroscopy especially highlights capillaries within the lamina propria (arrow)
The endomicroscopic findings of CD have been described in the literature. Trovato et al (4) described the duodenal surface to be devoid of villi, with collars of enterocytes around the crypt openings and with an increased number of intraepithelial lymphocytes. More recently, a study by Leong et al (5) standardized and defined the CLE findings of CD. Villous atrophy was defined as the presence of <5 blunt-shaped villi observed on superficial scans and crypt hypertrophy was defined as presence of <1 crypts on small bowel deep CLE imaging. The confocal celiac score was defined as the ratio of images with definitive features of CD (villous atrophy or crypt hypertrophy) relative to total images. The accuracy for diagnosing CD was excellent and correlated well with Marsh grading. These results suggest that CLE has a potential clinical utility in diagnosing or excluding CD in vivo, and may minimize – if not replace – the need for biopsies. Moreover, there have been reports of a patchy duodenal involvement in CD. In this scenario, clinical awareness and adequate and precise biopsy sampling are key steps for early diagnosis. Therefore, CLE is capable of detecting typical histological signs of CD during ongoing endoscopy and it may help in direct-targeted ‘smart’ biopsies to the abnormal areas and, thus, potentially increase the diagnostic yields of CD. In the future, it may also reduce the number of the biopsies in the duodenum.
In summary, CLE is an emerging technique that enables in vivo histology assessment while ongoing endoscopy and can improve the diagnosis of CD with high accuracy.
REFERENCES
- 1.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: A large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128(Suppl 1):S19–S24. doi: 10.1053/j.gastro.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Gastrointest Endosc Clin North Am. 2005;15:715–31. doi: 10.1016/j.giec.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Trovato C, Sonzogni A, Ravizza D, et al. Celiac disease: In vivo diagnosis by confocal endomicroscopy. Gastrointest Endosc. 2007;65:1096–9. doi: 10.1016/j.gie.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Leong RW, Nguyen NQ, Meredith CG, et al. In vivo confocal endomicroscopy in the diagnosis and evaluation of celiac disease. Gastroenterology. 2008;135:1870–5. doi: 10.1053/j.gastro.2008.08.054. [DOI] [PubMed] [Google Scholar]