The increasing public interest in fecal microbiota transplant (FMT) raises serious concerns, when the application of this treatment is still in its infancy and long-term safety is not yet established. Indeed, there is evidence that anecdotal reports of success from initial results of uncontrolled studies are encouraging patients to try this line of treatment in the absence of medical direction or supervision. It is important that medical practitioners, the media and the general public are made aware of the safety issues involved in ‘stool transplant’. While FMT has been widely used in veterinary practice and the exchange of intestinal contents in humans is documented back to the fouth century (1), this intervention requires the exchange of body fluids with all its known and unknown risks. In the early 1980s in Canada, tainted blood and blood products for the treatment of hemophilia led to the serious outbreak of hepatitis C and HIV (2). The stool microbiome is complex, containing, bacterial, viral and fungal components in addition to prions and potentially unknown biologically active substances (3,4). Several excellent studies have indicated the important role of the microbiome in a variety of diseases. In addition to Clostridium difficile infection (CDI) and inflammatory bowel disease (IBD), these include obesity, diabetes and behavioural disorders (5). There is an urgent requirement for further careful prospective and controlled research in a variety of these diseases but especially to protect the safety of patients who, understandably, may be frustrated with the poor efficacy of their current treatments. Here, we consider the evidence for the use of FMT in resistant C difficile infection and its current status as an intervention in IBD, together with a brief summary of the current evidence for the safety of FMT and recommendations for the future.
ANTIBIOTIC-RESISTANT CDI
The incidence of CDI has increased approximately 20-fold over the past 10 years, and rates are currently approximately 20 per 100,000 population (6). There are a number of risk factors for infection including antibiotic use, inflammatory bowel disease, comorbidities and increasing age (7). Proton pump inhibitors have also been implicated in CDI (8,9) although this association remains controversial (10). The rising incidence of CDI has been associated with the emergence of more pathogenic strains and this has led to an increase in mortality related to the infection (11). The efficacy of traditional antibiotic therapy for CDI has declined in recent years and this amplifies the problems of increasing incidence and severity of the infection. Metronidazole is recommended as first line and vancomycin as second line therapy for CDI (12). The infection can recur in up to 30% of cases (13) and after one relapse the risk of a further episode is dramatically increased (14). Treatment with newer antibiotics such as fidaxomicin (15) has been suggested but a key focus for clinicians has been the emerging evidence that fecal microbiota transplant (FMT) is effective in antibiotic resistant CDI (16). A systematic review (17) of case series reported that there were 11 studies involving 273 antibiotic resistant CDI patients and FMT was successful in 89% (95% confidence intervals [CI] = 84% to 93%). We have conducted a literature search to assess the evidence that has accumulated after the search date of the original systematic review. We used the same eligibility criteria as the original systematic review and in particular we excluded case series with less than 10 patients to reduce the possibility of overestimating the treatment effect due to the inherent bias of publishing case reports and very small positive case series. We identified five additional case series (18–22) that have assessed the efficacy of FMT in antibiotic resistant CDI. Overall, there are now 526 patients with antibiotic resistant CDI described in 16 case series. Of these 459 responded to treatment, giving a response rate of 88% (95% CI = 83% to 92%) with rates varying between 69% and 100% (Figure 1). There is also one small randomized controlled trial (RCT) (23) that randomly assigned antibiotic resistant CDI patients to vancomycin alone (13 patients), vancomycin plus bowel lavage (13 patients) or FMT (16 patients). There was a statistically significant benefit in the FMT group, with 81% in remission after the first transplant and 94% if those who underwent a second transplant were included.
Figure 1).
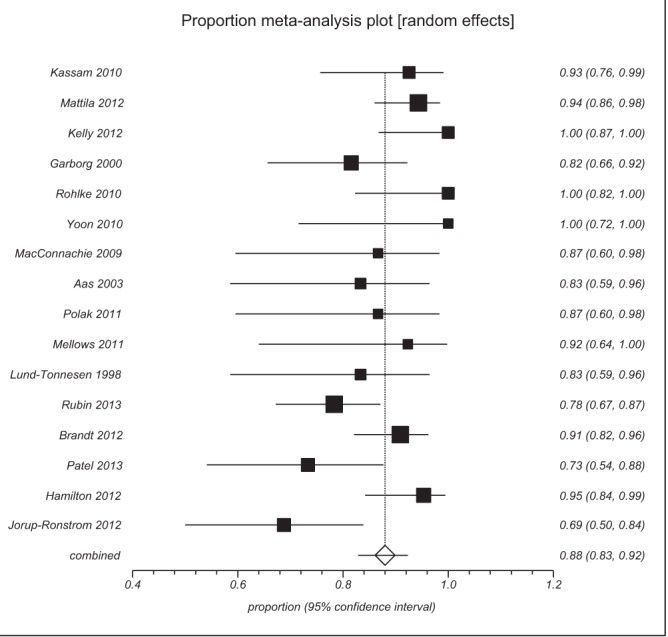
Summary of the case series reporting on the efficacy of fecal microbiota transplant therapy in Clostridium difficile infection
The evidence base for FMT consists of one small RCT and 16 case series and, therefore, it is likely that the figure of 88% response rate may be an overestimate. The effect is dramatic; however, it is very likely that FMT is effective in CDI and is a viable option in patients who experience a relapse after two courses of antibiotics.
IBD
There is growing evidence that the intestinal microbiota plays a pivotal role in the pathogenesis of IBD. However, only limited evidence supports the efficacy of either probiotic or antibiotic therapy. Accordingly, there is considerable clinical interest in the use of FMT to alter the microbiome and improve the long-term course of IBD (24).
Since the first case report of successful FMT for IBD was published 25 years ago (25), only small case series have been reported, and with mixed results. Borody et al (26) reported outcomes for six patients with refractory ulcerative colitis (UC) who were treated with daily FMT for five days, administered by retention enema. Remarkably, all patients achieved drug-free remission within four months and had no recurrence over one to 13 years of follow-up. In contrast, Angelberger et al (27) reported clinical improvement (but not remission) at 12 weeks in only one of five patients with similarly refractory UC who received FMT via nasojejunal infusion and retention enema on three consecutive days. Kump et al (28) also reported disappointing results in six active UC patients given FMT by colonoscopy as a single infusion. Despite short-term clinical improvement over two weeks none of the patients achieved clinical remission. Kunde et al (29) treated 10 children and young adults (age 7 to 21 years) with refractory UC with daily FMT for five days. Of nine patients who could retain the enemas, clinical response was seen at one week in 7 (78%) and at one month in 6 (67%). Interestingly, both Angelberger and Kunde reported transient fever after FMT in 5/5 and 2/9 patients, respectively.
Although these observational data suggest some patients with UC might respond to FMT, it is impossible to make any conclusion about efficacy without properly designed randomized controlled trials. At present six such protocols are registered at www.clinicaltrials.gov (NCT01793831, NCT01896635, NCT01961492, NCT01790061, NCT01650038, NCT01545908), but no results have yet been reported. It should be noted that there is even more limited observational experience with FMT in Crohn disease, and no clinical trials for this indication have been registered. In the absence of controlled data showing clear efficacy, FMT should only be performed for treatment of IBD (CD or UC) in the setting of a clinical trial.
SAFETY OF FMT
Although the rate of adverse events appears to be low no studies to date have formally sought adverse events prospectively. A recent review suggested that FMT administered via a nasogastric or nasojejunal tube could be associated with an increased risk of adverse events, which included an upper gastrointestinal bleed, peritonitis and enteritis (17). This was based on indirect comparison between case series and more data are needed on the safety of FMT.
Currently, there is rigorous screening of stool donors to exclude infectious disease but transmission of a communicable disease remains a concern and there have been case reports of norovirus being transmitted by FMT (30,31). Screening protocols for FMT donors vary widely between reports, despite calls for standardization (32,33), and this is in contrast to protocols for blood donation. Indeed, for anonymous ‘universal’ donors it is not known how frequently they should be re-screened. Moreover, it is unclear what other conditions should be considered at screening, such as prior use of antibiotics, morbid obesity, metabolic syndrome, atopy, or mood (34). The literature is not clear regarding long-term follow-up for adverse events, which have usually been spontaneously reported rather than actively sought. This increases the risk of under reporting. RCTs and long-term adverse outcome registries have been recommended to define better the short and long-term risks of FMT (13,17).
Until the appropriate information becomes available, patients undergoing FMT, when no alternative treatment exists, should be carefully counseled on the known and possibility of unknown risks when they give informed consent to the procedure. Safety of FMT in immunosuppressed patients has not yet been formally studied and these may form a special at-risk population although some patients on a variety of immunosuppressive treatments have undergone FMT in open studies without apparent serious outcome. In one series of 77 cases, four cases of autoimmune disease were reported (34)
CONCLUSIONS
FMT is emerging as an important therapeutic option to manipulate the gut microbiome. Currently there is sufficient evidence to recommend FMT in patients with CDI that have failed or had recurrent infection after two rounds of different antibiotics (usually metronidazole and vancomycin). This intervention should only be performed by health care practitioners experienced in giving FMT using donors that are healthy and are extensively screened for communicable diseases.
There is currently insufficient evidence to recommend FMT for patients with IBD and this should only be given in the context of a clinical study. Although not considered here, other potential indications for FMT are not supported by evidence and should only be explored as part of a research protocol.
There is an urgent need to standardize how FMT donors are screened and we recommend that all groups undertaking therapeutic FMT should set up prospective adverse events registries to follow patients in the short and long term.
CANADIAN ASSOCIATION OF GASTROENTEROLOGY STATEMENT
This position paper has been developed under the direction of Drs Paul Moayyedi, John Marshall, Yuhong Yuan and Richard Hunt in accordance with the policies and procedures of the Canadian Association of Gastroenterology (CAG) and under the direction of CAG Clinical Affairs. It has been reviewed by the CAG Practice Affairs and Clinical Affairs Committees and the CAG Board of Directors. The paper was developed following a thorough consideration of medical literature and the best available evidence and clinical experience. It represents the consensus of a Canadian panel comprised of experts on this topic. This position paper aims to provide a reasonable and practical approach to care for specialists and allied health professionals obliged with the duty of bestowing optimal care to patients and families, and can be subject to change as scientific knowledge and technology advance and as practice patterns evolve. The position paper is not intended to be a substitute for physicians using their individual judgment in managing clinical care in consultation with the patient, with appropriate regard to all the individual circumstances of the patient, diagnostic and treatment options available and available resources. Adherence to these recommendations will not necessarily produce successful outcomes in every case.
The CAG is proud to acknowledge its Benefactor Corporate Sponsors:
AbbVie Corporation
Olympus Canada Inc
Pentax Canada Inc
Janssen Inc
Takeda Canada Inc
REFERENCES
- 1.Zhang F, Luo W, Shi Y, et al. Should we standardize the 1700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 2.Krever H. Government of Canada Publications. 1997. Commission of Inquiry on the Blood System in Canada. [Google Scholar]
- 3.El-Matary W. Fecal microbiota transplantation: Long-term safety issues. Am J Gastroenterol. 2013;108:1537–8. doi: 10.1038/ajg.2013.208. [DOI] [PubMed] [Google Scholar]
- 4.Kassam Z, Lee CH, Yuan Y, Hunt RH. Navigating long-term safety in fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1538. doi: 10.1038/ajg.2013.214. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J, Palva AM, de Vos WM, Satokari R. Contribution of the intestinal microbiota to human health: From birth to 100 years of age. Curr Topics Microbiol Immunol. 2013;358:323–46. doi: 10.1007/82_2011_189. [DOI] [PubMed] [Google Scholar]
- 6.Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti M, Villa G, Pecori D, Arzese A, Wilcox M. Epidemiology, diagnosis and treatment of Clostridium difficile infection. Exp Rev Antiinfect Ther. 2012;10:1405–23. doi: 10.1586/eri.12.135. [DOI] [PubMed] [Google Scholar]
- 8.Janarthanan S, Ditah I, Phil M, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am J Gastroentrol. 2012;107:1001–10. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 9.Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: Meta-analysis. Am J Gastroenterol. 2012;107:1011–9. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 10.Leontiadis GI, Miller MA, Howden CW. How much do PPIs contribute to C. difficile infections? Am J Gastroenterol. 2012;107:1020–1. doi: 10.1038/ajg.2012.174. [DOI] [PubMed] [Google Scholar]
- 11.Lamontagne F, Labbe AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–72. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 13.Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15:285–9. doi: 10.1016/j.anaerobe.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 14.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: Treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 15.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–9. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 16.Borody TJ, Warren EF, Leis SM, et al. Bacteriotherapy using fecal flora: Toying with human motions. J Clin Gastroenterol. 2004;38:475–83. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 17.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 18.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 19.Jorup-Ronstrom C, Hakanson A, Sandell S, et al. Fecal transplant against relapsing Clostridium difficile-associated diarrhea in 32 patients. Scand J Gastroenterol. 2012;47:548–52. doi: 10.3109/00365521.2012.672587. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–7. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 21.Rubin TA, Gessert CE, Aas J, Bakken JS. Fecal microbiome transplantation for recurrent Clostridium difficile infection: Report on a case series. Anaerobe. 2013;19:22–6. doi: 10.1016/j.anaerobe.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Patel NC, Griesbach CL, DiBaise JK, Orenstein R. Fecal microbiota transplant for recurrent Clostridium difficile infection: Mayo clinic in Arizona experience. Mayo Clinic Proc. 2013;88:799–805. doi: 10.1016/j.mayocp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Eng J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 24.Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: Is there a role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452–9. doi: 10.1038/ajg.2012.93. [DOI] [PubMed] [Google Scholar]
- 25.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 26.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–7. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Angelberger S, Reinisch W, Makrislathis A, et al. Temporal bacterial community dynamics vary among ulcerative patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–30. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 28.Kump PK, Grochenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–65. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 29.Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108:1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 31.Brandt LJ. FMT: First step in a long journey. Am J Gastroenterol. 2013;108:1367–8. doi: 10.1038/ajg.2013.165. [DOI] [PubMed] [Google Scholar]
- 32.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–9. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen-Vercoe E, Reid G, et al. A Canadian Working Group report on fecal microbial therapy: Microbial ecosystems therapeutics. Can J Gastroenterol. 2012;26:457–62. doi: 10.1155/2012/213828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt LJ. American Journal of Gastroenterology Lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am J Gastroenterol. 2013;108:177–85. doi: 10.1038/ajg.2012.450. [DOI] [PubMed] [Google Scholar]


