Abstract
OBJECTIVE:
To determine practices among physicians in Canada for the assessment of liver fibrosis in patients with chronic liver diseases.
METHODS:
Hepatologists, gastroenterologists, infectious diseases specialists, members of the Canadian Gastroenterology Association and/or the Canadian HIV Trials Network who manage patients with liver diseases were invited to participate in a web-based, national survey.
RESULTS:
Of the 237 physicians invited, 104 (43.9%) completed the survey. Routine assessment of liver fibrosis was requested by the surveyed physicians mostly for chronic hepatitis C (76.5%), followed by autoimmune/cholestatic liver disease (59.6%) and chronic hepatitis B (52.9%). Liver biopsy was the main diagnostic tool for 46.2% of the respondents, Fibroscan (Echosens, France) for 39.4% and Fibrotest (LabCorp, USA) for 7.7%. Etiology-specific differences were observed: noninvasive methods were mostly used for hepatitis C (63% versus 37% liver biopsy) and hepatitis B (62.9% versus 37.1% liver biopsy). For 42.7% of respondents, the use of noninvasive methods reduced the need for liver biopsy by >50%. Physicians’ characteristics associated with higher use of noninvasive methods were older age and being based at a university hospital or in private practice versus community hospital. Physicians’ main concerns regarding noninvasive fibrosis assessment methods were access/availability (42.3%), lack of guidelines for clinical use (26.9%) and cost/lack of reimbursement (14.4%).
CONCLUSIONS:
Physicians who manage patients with chronic liver diseases in Canada require routine assessment of liver fibrosis stage. Although biopsy remains the primary diagnostic tool for almost one-half of respondents, noninvasive methods, particularly Fibroscan, have significantly reduced the need for liver biopsy in Canada. Limitations in access to and availability of the noninvasive methods represent a significant barrier. Finally, there is a need for clinical guidelines and a better reimbursement policy to implement noninvasive tools to assess liver fibrosis.
Keywords: Canadian physicians, Chronic liver diseases, Liver biopsy, Liver fibrosis, Noninvasive fibrosis methods
Abstract
OBJECTIF :
Déterminer les pratiques des médecins du Canada en matière d’évaluation de la fibrose hépatique chez des patients atteints d’une maladie hépatique chronique.
MÉTHODOLOGIE :
Les hépatologistes, les gastroentérologues, les infectiologues, les membres de l’Association canadienne de gastroentérologie et ceux du Réseau pour les essais VIH qui prenaient en charge des patients ayant une maladie hépatique ont été invités à participer à un sondage virtuel national.
RÉSULTATS :
Sur les 237 médecins invités, 104 (43,9 %) ont rempli le sondage. Les médecins demandaient une évaluation systématique de la fibrose hépatique surtout en cas d’hépatite C chronique (76,5 %), de maladie hépatique auto-immune ou de maladie cholestatique du foie (59,6 %) et d’hépatite B chronique (52,9 %). La biopsie hépatique était le principal outil diagnostique pour 46,2 % des répondants, le Fibroscan (Echosens, France), pour 39,4 % d’entre eux, et le Fibrotest (LabCorp, États-Unis), pour 7,7 % d’entre eux. Les chercheurs ont observé des différences propres à l’étiologie : les méthodes non effractives étaient surtout utilisées en cas d’hépatite C (63 % par rapport à 37 % de biopsie hépatique) et d’hépatite B (62,9 % par rapport à 37,1 % de biopsie hépatique). Chez 42,7 % des répondants, le recours à une méthode non effractive réduisait de plus de 50 % la nécessité de biopsie hépatique. Les caractéristiques des médecins associées à une plus forte utilisation de méthodes non effractives étaient le fait d’être plus âgés et de travailler dans un hôpital universitaire ou en pratique privée plutôt que dans un hôpital général. Les principales inquiétudes des médecins à l’égard de l’évaluation non effractive de la fibrose étaient l’accès ou la disponibilité (42,3 %), l’absence de directives cliniques (26,9 %) et le coût ou l’absence de remboursement (14,4 %).
CONCLUSIONS :
Les médecins qui prennent en charge les patients ayant une maladie hépatique chronique au Canada demandent une évaluation systématique du stade de fibrose hépatique. Même si la biopsie demeure le principal outil diagnostique pour près de la moitié des répondants, les méthodes non effractives, notamment le Fibroscan, ont considérablement réduit la nécessité d’utiliser la biopsie hépatique au Canada. Les limites d’accès et de disponibilité des méthodes non effractives représentent un obstacle important. Enfin, il faudrait produire des directives cliniques et prévoir une meilleure politique de remboursement pour qu’on puisse adopter les outils non effractifs dans l’évaluation de la fibrose hépatique.
Chronic liver diseases (CLDs) are a major cause of morbidity and mortality worldwide, affecting 360 per 100,000 persons and ranking as the 12th leading cause of overall mortality (1,2). In Canada, 2748 deaths were attributed to CLDs and liver cirrhosis (11th leading cause of death) in 2008 (3). The main etiologies of CLDs are chronic infections with hepatitis C and B viruses (HCV and HBV, respectively), alcoholic liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD). Chronic infections with HCV and HBV impose a significant medical and economic burden in Canada, affecting nearly 1% and 2% of the population, respectively (4,5). NAFLD is the most common liver disease in Canada, afflicting as much as 30% of the population (6). The consumption of alcohol is increasing in Canada and, as a result, the incidence of ALD is also increasing (7).
Independent of etiology, the progression of all CLDs occurs via a common histopathological pathway characterized by the formation of fibrosis leading to a progressive distortion of the hepatic architecture, the hallmark of evolution to cirrhosis. T he accumulation of fibrosis in the liver is the event with the greatest impact on the prognosis of CLDs. Natural history studies indicate that approximately 20% to 40% of patients with CLDs will develop significant liver fibrosis (stage F2 according to METAVIR histological classification [8]), 10% will progress to cirrhosis and 1% to 5% will develop hepatocellular carcinoma (HCC) within two to three decades (9,10). Cirrhosis requires specific follow-up including screening for esophageal varices and HCC with periodic gastroscopy and ultrasound (11,12).
The diagnosis of liver fibrosis stage is also critical for reinforcing behavioural interventions in patients with NAFLD and ALD, and for a definitive indication for antiviral therapy in individuals with chronic viral hepatitis.
For HCV, although guidelines from the Canadian Association for the Study of the Liver (CASL) state that there is no absolute fibrosis threshold to preclude antiviral therapy, prompt initiation of treatment should be considered in patients with advanced liver fibrosis (F3 or F4 according to METAVIR classification, corresponding to bridging fibrosis or cirrhosis [8]), who are at risk for end-stage hepatic complications (4). The landscape of antiviral therapy is rapidly changing in chronic hepatitis C. Until recently, the standard of care was dual therapy with pegylated interferon and ribavirin. Recently, the direct antiviral agents boceprevir and telaprevir have offered substantial improvements in response rates for patients infected with HCV genotype 1 (4). The future looks even brighter, considering the new compounds that will soon lead to interferon-free regimens (13). In this dynamic landscape, the assessment of liver fibrosis stage will remain of paramount importance for determining prognosis and guide HCV treatment. The CASL guidelines include liver fibrosis stage in the decisional algorithm for the treatment of hepatitis B (5). As such, an assessment for liver fibrosis stage is recommended for all patients infected with HBV and HCV (4,5).
In NAFLD, the recent guidelines from the American Association for the Study of Liver Diseases (AASLD) recommend histological assessment in individuals at risk for nonalcoholic steatohepatitis (NASH), such as individuals with the metabolic syndrome (14,15). Liver fibrosis assessment also has a prognostic and diagnostic role in ALD and autoimmune/cholestatic liver diseases (16–18).
Liver biopsy has long been the gold standard to stage liver fibrosis. This procedure, however, is invasive, costly, impractical as a screening tool and prone to sampling error (11,19). In recent years, noninvasive tools for liver fibrosis have been proposed. Fibrosis can be measured noninvasively based on a biological approach (serum biomarkers) or on a physical approach (liver stiffness). The most validated serum biomarkers include Fibrotest (LabCorp, USA, combining gamma-glutamyl transpeptidase, total bilirubin, α-2-macroglobulin, apolipo-protein A1, haptoglobin, age and sex) and aspartate aminotransferase-to-platelet ratio index (APRI) (20–22). Transient elastography (Fibroscan; Echosens, France) is an ultrasound-based device that measures liver stiffness as a surrogate of liver fibrosis (23). Other methods include magnetic resonance elastography and acoustic radiation force impulse elastography (24,25). Noninvasive methods for liver fibrosis diagnosis are gaining more credibility across guidelines and experts’ recommendations, particularly in chronic HCV and HBV infections (4,5,21,26). However, there are no data regarding the adherence of Canadian physicians to guidelines with regard to routine assessment of liver fibrosis. Moreover, information regarding implementation of noninvasive methods instead of liver biopsy among Canadian physicians who manage CLD patients are lacking.
We conducted a web-based survey aimed at investigating practices of liver fibrosis assessment among physicians who manage CLD patients across Canada.
METHODS
The present study was a cross-sectional survey of Canadian specialists who manage patients with CLDs. The survey was developed by the first author (GS) and first distributed among the coauthors to assess satisfaction and face validity. Modifications were made based on feedback and comments. The final version consisted of six pages and 21 items divided into two sections: clinician profile; and practice profile and liver fibrosis assessment (Online Appendixes A to F [go to www.pulsus.com]).
The first section included questions about age, sex, primary specialty, years of practice, time dedicated to patient care, practice location and province of practice. The second section included questions regarding CLDs followed in practice, etiologies of CLDs for which assessment of liver fibrosis stage was requested, the main tool used to diagnose liver fibrosis, opinion on the best noninvasive tool to diagnose liver fibrosis, impact of noninvasive tools on the number of liver biopsies performed, impact of noninvasive methods for liver fibrosis on the number of HCV- and HBV-infected patients treated, and concerns regarding noninvasive tools for liver fibrosis. A link to the web-based survey was sent by e-mail between August 2012 and January 2013 to members of the Canadian Association of Gastroenterology (CAG) and Canadian HIV Trial Network who manage patients with CLDs. Confidentiality was preserved by the fact that e-mails were sent through the CAG National Office. All responses were anonymous and the investigators received no information that would identify the respondent or their site of practice. Ethics approval was sought from the McGill University Health Centre Research Ethics Office (Montreal, Quebec); an official research ethics board approval was not required.
Requested answers were either oriented (different items to choose from), based on a grading scale (ranking from 0 to 6 scale) or a matrix of choices with multiple answers per row. Standard descriptive statistics were used to describe response frequency. The χ2 test was used to compare categorical variables; a two-sided P<0.05 was considered to be statistically significant.
RESULTS
Of 237 invited physicians, 104 (43.9%) completed the survey. Of these, 83 (79.8%) were members of the CAG and 21 (20.2%) were members of the Canadian HIV Trial Network. Table 1 summarizes the demographics of the surveyed physicians. Overall, most respondents were male (78.8%), had gastroenterology as their primary specialty (64.4%), were based at a university hospital (51%) and dedicated >75% of their time to patient care activities (71.1%). The majority of respondents were from Ontario (40.4%) and Quebec (36.6%).
TABLE 1.
Characteristics of the surveyed physicians (n=104)
| Age group, years | |
| <40 | 30 (28.8) |
| 40–50 | 33 (31.7) |
| 50–60 | 27 (26) |
| >60 | 14 (13.5) |
| Sex | |
| Male | 82 (78.8) |
| Female | 22 (21.2) |
| Primary specialty | |
| Gastroenterology | 67 (64.4) |
| Hepatology | 17 (16.4) |
| Infectious disease | 10 (9.6) |
| Other (family medicine, internal medicine) | 10 (9.6) |
| Years of practice | |
| <5 | 14 (13.5) |
| 5–10 | 20 (19.2) |
| 10–20 | 30 (28.8) |
| >20 | 40 (38.5) |
| Practice location | |
| University-based hospital | 53 (51) |
| Community hospital | 29 (27.9) |
| Private practice | 22 (21.1) |
| Time dedicated to patients’ care | |
| <50 | 11 (10.6) |
| 50–75 | 19 (18.3) |
| >75 | 74 (71.1) |
| Province of practice | |
| Ontario | 42 (40.4) |
| Quebec | 38 (36.6) |
| British Columbia | 10 (9.6) |
| Alberta | 7 (6.7) |
| Rest of Canada | 7 (6.7) |
Data presented as n (%)
Etiology of CLDs and practices of responding physicians
The etiologies of CLDs seen by the respondents were as follows: 87 (83.7%) physicians managed NAFLD patients; 84 (80.8%) saw patients with autoimmune/cholestatic liver disease; 83 (79.8%) saw ALD cases; 81 (77.9%) physicians managed patients with HCV; 70 (67.3%) managed HBV patients; and 33 (31.7%) saw HIV patients coinfected with HBV and/or HCV. The primary specialty impacted the etiology of CLDs managed by the responding physician (Table 2). As such, respondents with gastroenterology as their primary specialty managed fewer HBV patients compared with hepatologists (P=0.02). Similarly, gastroenterologists saw fewer HIV-coinfected patients than hepatologists or infectious diseases specialists (P<0.0001). Conversely, infectious diseases specialists were less likely to manage NAFLD patients compared with hepatologists (P=0.0004) and gastroenterologists (P<0.0001). Infectious diseases specialists saw fewer ALD cases compared with hepatologists (P=0.0002) and gastroenterologists (P<0.0001). Interestingly, hepatologists saw significantly fewer NAFLD (P=0.04) and ALD patients (P=0.05) than gastroenterologists. Finally, infectious diseases specialists managed fewer patients with autoimmune/cholestatic liver disease compared with hepatologists and gastroenterologists (P<0.0001).
TABLE 2.
Etiology of chronic liver disease managed by surveyed physicians according to their primary specialty
| Chronic liver disease |
Primary specialty
|
P* | ||||
|---|---|---|---|---|---|---|
| Gastroenterology | Hepatology | Infectious diseases | ||||
| Hepatitis C | 53 (79.1) | 16 (94.1) | 9 (90) | 0.14 | 0.41 | 0.69 |
| Hepatitis B | 45 (67.2) | 16 (94.1) | 8 (80) | 0.02 | 0.41 | 0.25 |
| HIV coinfection | 9 (13.4) | 12 (70.6) | 8 (80) | <0.0001 | <0.0001 | 0.58 |
| Nonalcoholic fatty liver disease | 67 (100) | 16 (94.1) | 3 (30) | 0.04 | <0.0001 | 0.0004 |
| Alcoholic liver disease | 64 (95.5) | 14 (82.4) | 1 (10) | 0.05 | <0.0001 | 0.0002 |
| Autoimmune/cholestatic liver disease | 63 (94) | 16 (94.1) | 2 (20) | 0.98 | <0.0001 | <0.0001 |
Data presented as n (%). Bolded values indicate statistical significance.
χ2 test between Gastroenterology and Hepatology in the first column; between Gastroenterology and Infectious diseases in the second column; and between Hepatology and Infectious diseases in the third column
The remainder of the surveyed physicians (9.6%) had family medicine or internal medicine as their primary specialty. They managed fewer HBV patients compared with gastroenterologists (20% versus 67.2%; P=0.004), hepatologists (20% versus 94.1%; P<0.0001) and infectious diseases specialists (20% versus 80%; P=0.007) (data not shown). When compared with gastroenterologists and hepatologists, they also managed fewer patients with NAFLD (20% versus 100% and 94.1%, respectively; P<0.0001) and ALD (40% versus 95.5%; P<0.0001; and versus 82.4%, respectively; P=0.02). Conversely, they saw more HIV-coinfected patients than gastroenterologists (60% versus 13.4%; P=0.0005), and managed fewer patients with autoimmune/cholestatic liver disease compared with gastroenterologists and hepatologists (20% versus 94% and 94.1%, respectively; P<0.0001).
The effect of CLD etiology and practice characteristics on diagnosing liver fibrosis
Figure 1 depicts the rate of physicians requiring routine assessment of liver fibrosis stage according to the etiology of CLD. Chronic HCV was the most common etiology (76.9%), followed by autoimmune/cholestatic liver disease (59.6%) and chronic HBV (52.9%). Figure 2 shows the primary method used by respondents for the assessment of liver fibrosis. Liver biopsy was the primary diagnostic tool for 46.2% of the physicians, followed by Fibroscan (39.4%) and Fibrotest (7.7%). When asked which tool was used as the secondary test, 35.6% answered liver biopsy, 32.7% Fibroscan, 13.5% none, 9.6% APRI and other simple biomarkers, 2.9% Fibrotest and 5.7% other (magnetic resonance, computed tomography scan, comparative radiological examinations).
Figure 1).
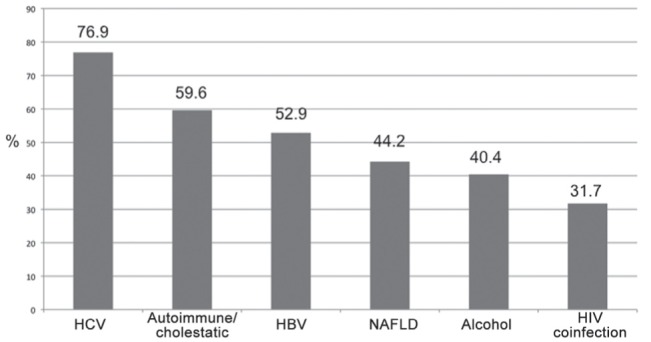
Rate of surveyed physicians requiring routine assessment of liver fibrosis stage according to etiology of chronic liver diseases. HBV Hepatitis B virus; HCV Hepatitis C virus; NAFLD Nonalcoholic fatty liver disease
Figure 2).
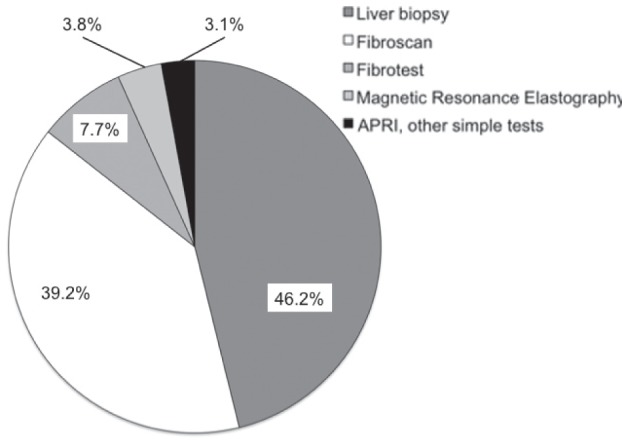
Primary method used by the responding physicians for the assessment of liver fibrosis. APRI Aspartate aminotransferase-to-platelet ratio index; Fibroscan (Echosens, France); Fibrotest (LabCorp, USA)
Figure 3 depicts the use of liver biopsy versus noninvasive methods according to etiology of CLDs. Overall, in chronic viral hepatitis, including HCV, HBV and HIV-coinfected patients, there was a tendency for a higher use of noninvasive methods for liver fibrosis staging. Autoimmune/cholestatic liver disease was the only disease in which liver biopsy was significantly more used than noninvasive tools (69% versus 31%; P<0.0001 compared with the other etiologies of CLDs). Table 3 shows the detailed distribution of methods for liver fibrosis diagnosis according to etiology of CLDs. Fibroscan was the most used noninvasive method in all etiologies of CLDs. Interestingly, the use of Fibroscan was significantly higher in HCV versus ALD and autoimmune/cholestatic liver disease (53.1% versus 33.7% and 19%, respectively; P=0.02 and P<0.0001). Simple serum biomarkers were more frequently used in ALD than HCV (16.9% versus 2.5%; P=0.0006). Figure 4 illustrates the impact of noninvasive methods in terms of reduction of liver biopsies performed in a physician’s practice. Overall, for 42.7% of respondents, the use of noninvasive methods reduced the need for liver biopsy by >50%. Table 4 summarizes the etiology-specific decrease in the need for liver biopsy. The highest reduction in liver biopsies was observed in HCV, given that a ≥50% decrease was observed in 62.9% of respondents. This figure was significantly higher than that in NAFLD (43.7%; P=0.01), ALD (39%; P=0.04) and autoimmune/cholestatic liver disease (27.8%; P<0.0001).
Figure 3).
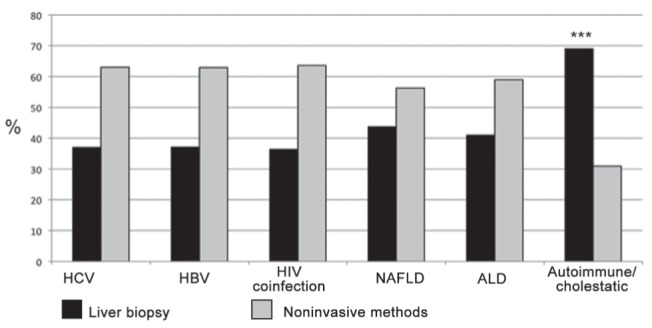
Use of noninvasive methods for assessing liver fibrosis versus liver biopsy according to etiology of chronic liver diseasaes. ***P<0.0001 compared with all other etiologies of chronic liver diseases. ALD Alcoholic liver disease; HBV Hepatitis B virus; HCV Hepatitis C virus; NAFLD Nonalcoholic fatty liver disease
TABLE 3.
Primary method for assessment of liver fibrosis according to etiology of chronic liver disease
| Chronic liver disease | Liver biopsy | Fibroscan* | Fibrotest† | APRI, other simple biomarkers | Magnetic resonance elastography |
|---|---|---|---|---|---|
| Hepatitis C | 30 (37) | 43 (53.1) | 6 (7.4) | 2 (2.5) | 0 (0) |
| Hepatitis B | 26 (37.1) | 32 (45.7) | 6 (8.6) | 6 (8.6) | 0 (0) |
| HIV coinfection | 12 (36.4) | 15 (45.4) | 3 (9.1) | 3 (9.1) | 0 (0) |
| Nonalcoholic fatty liver disease | 38 (43.7) | 33 (37.9) | 5 (4.6) | 9 (1.1) | 2 (2.3) |
| Alcoholic liver disease | 34 (41) | 28 (33.7) | 4 (4.8) | 14 (16.9) | 3 (3.6) |
| Autoimmune/cholestatic liver disease | 58 (69) | 16 (19) | 2 (2.4) | 6 (7.2) | 2 (2.4) |
Data presented as n (%).
Echosens, France;
LabCorp, USA. APRI Aspartate aminotransferase-to-platelet ratio index
Figure 4).
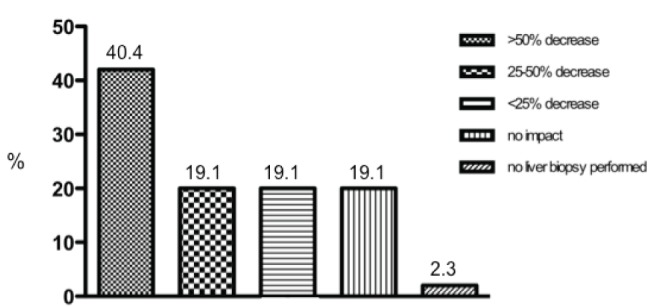
Impact of noninvasive methods on the number of liver biopsies performed by the surveyed physicians
TABLE 4.
Etiology-specific impact of noninvasive methods in terms of reductions of liver biopsies performed
| Chronic liver disease | Liver biopsy no longer performed |
Reduction, %
|
||
|---|---|---|---|---|
| >50 | 25 to 50 | <25 | ||
| Hepatitis C | 15 (18.5) | 36 (44.4) | 17 (21) | 13 (16.1) |
| Hepatitis B | 11 (15.7) | 26 (37.1) | 17 (24.3) | 16 (22.9) |
| HIV coinfection | 4 (12.1) | 14 (42.4) | 10 (30.3) | 5 (15.2) |
| Nonalcoholic fatty liver disease | 10 (11.5) | 28 (32.2) | 20 (23) | 29 (33.3) |
| Alcoholic liver disease | 10 (12) | 29 (35) | 14 (16.9) | 30 (36.1) |
| Autoimmune/cholestatic liver disease | 10 (11.9) | 15 (17.9) | 18 (21.4) | 41 (48.8) |
Data presented as n (%)
The effect of physicians’ demographics and characteristics on practices of liver fibrosis diagnosis is shown in Table 5. Older physicians used more noninvasive methods for liver fibrosis than younger respondents (P=0.02). Hepatologists and infectious diseases specialists used more noninvasive methods for liver fibrosis (particularly Fibroscan) than gastroenterologists, although this difference was not statistically significant. Respondents practicing their primary specialty for longer tended to use more noninvasive methods for liver fibrosis assessment than those practicing for shorter durations. Interestingly, physicians based at a university hospital or in private practice used more non-invasive methods than those based at a community hospital (P=0.05). Although the province of practice did not impact the use of noninvasive methods versus biopsy, a trend for regional variation in the use of noninvasive methods was observed (Table 6). In Ontario, Fibrotest was the primary noninvasive tool for fibrosis diagnosis for 30.5% of the surveyed physicians, and this figure was significantly higher than the other provinces (P=0.004).
TABLE 5.
Effect of physicians’ demographics and practice characteristics on methods used to diagnose liver fibrosis
| Noninvasive methods | Liver biopsy | P | |
|---|---|---|---|
| Age, years | 0.02 | ||
| <40 | 15 (26.8) | 15 (31.3) | |
| 40–50 | 13 (23.2) | 20 (41.7) | |
| 50–60 | 16 (28.6) | 11 (22.9) | |
| >60 | 12 (21.4) | 2 (4.1) | |
| Sex | 0.22 | ||
| Male | 47 (83.9) | 35 (72.9) | |
| Female | 9 (16.1) | 13 (27.1) | |
| Primary specialty | 0.15 | ||
| Gastroenterology | 31 (55.4) | 36 (75) | |
| Hepatology | 11 (19.6) | 6 (12.5) | |
| Infectious diseases | 6 (10.7) | 4 (8.3) | |
| Other | 8 (14.3) | 2 (4.2) | |
| Years of practice | 0.09 | ||
| <5 | 4 (7.1) | 10 (20.8) | |
| 5–10 | 12 (21.4) | 8 (16.7) | |
| 10–20 | 14 (25) | 16 (33.3) | |
| >20 | 26 (46.5) | 14 (29.2) | |
| Practice location | 0.05 | ||
| University-based hospital | 32 (57.1) | 21 (44) | |
| Community hospital | 10 (17.9) | 19 (40) | |
| Private practice | 14 (25) | 8 (17) | |
| Time dedicated to patients’ care | 0.90 | ||
| <50 | 6 (10.7) | 5 (10.4) | |
| 50–75 | 9 (16.1) | 10 (20.8) | |
| >75 | 41 (73.2) | 33 (68.8) | |
| Province | 0.62 | ||
| Ontario | 23 (41.1) | 19 (39.6) | |
| Quebec | 21 (37.6) | 17 (35.4) | |
| British Columbia | 5 (8.9) | 5 (10.4) | |
| Alberta | 6 (10.7) | 1 (2.1) | |
| Rest of Canada | 1 (1.7) | 6 (12.5) |
Data presented as n (%). Bolded values indicate statistical significance
TABLE 6.
Distribution of noninvasive methods to diagnose liver fibrosis according to province of practice of surveyed physicians
| Province | Fibroscan* | Fibrotest† | APRI, other simple biomarkers | MRE |
|---|---|---|---|---|
| Ontario | 14 (60.9) | 7 (30.5) | 1 (4.3) | 1 (4.3) |
| Quebec | 18 (85.7) | 0 (0) | 1 (4.8) | 2 (9.5) |
| British Columbia | 4 (80) | 0 (0) | 0 (0) | 1 (20) |
| Alberta | 5 (83.3) | 1 (16.7) | 0 (0) | 0 (0) |
| Rest of Canada | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
Data presented as n (%).
Echosens, France;
LabCorp, USA. APRI Aspartate aminotransferase-to-platelet ratio index; MRE Magnetic resonance elastography
Satisfaction with and concerns about noninvasive methods for liver fibrosis
When asked whether noninvasive methods for liver fibrosis provide an accurate assessment, 86 (82.6%) of respondents agreed, nine (8.7%) disagreed and nine (8.7%) neither agreed nor disagreed. For 91 (87.5%) of the participants, Fibroscan was ranked as the best noninvasive method for staging liver fibrosis. For 35 (33.7%) of the surveyed physicians, the implementation of noninvasive methods for liver fibrosis resulted in more patients treated with antiviral therapy, while there was no change in the remaining 69 (66.3%). The major concern regarding noninvasive methods for liver fibrosis was access/availability for 44 (42.3%) of respondents, lack of guidelines for use in clinical practice for 28 (26.9%), cost/lack of reimbursement for 15 (14.4%), unsatisfactory accuracy for eight (7.7%), poor reproducibility for six (5.8%) and delayed results for three (2.9%). Overall, 62 (59.6%) of the responding physicians did not have a Fibroscan in their clinics, and 38 (61.3%) physicians in this group reported no convenient access to the device. All responding physicians who did not have a Fibroscan nor convenient access to it would increase the use of noninvasive methods for liver fibrosis if access/availability was improved.
DISCUSSION
The present study was the first to evaluate practice patterns for diagnosing and staging liver fibrosis among Canadian physicians who manage patients with CLDs. Most surveyed physicians require a systematic assessment of liver fibrosis; thus, they adhere to current guidelines that recommend assessment of liver fibrosis in patients with CLDs (4,5). However, although a number of noninvasive diagnostic methods have been developed, almost one-half of Canadian physicians still use liver biopsy as their primary diagnostic tool. Importantly, most of the surveyed physicians believe that noninvasive methods, particularly Fibroscan, provide an accurate staging of liver fibrosis in CLDs. Nevertheless, limitations in access/availability represent a significant barrier. Our results highlight the importance of improving access to noninvasive methods for liver fibrosis diagnosis. Canadian physicians have also identified the emerging need for guidelines in clinical practice and an improved reimbursement policy.
In Canada, CLDs, mainly due to HCV, HBV, NAFLD and ALD, represent the 11th leading cause of death (3). Liver fibrosis stage is the single most important factor impacting the prognosis of patients with CLDs and it has a major role in management decisions, such as initiation of antiviral therapy and implementation of interventions including alcohol abstinence and control of dysmetabolisms (4,5,10,16,26). Once cirrhosis is established, it may lead to end-stage complications including decompensation, HCC and death. Identification of patients with cirrhosis is critical to begin appropriate surveillance measures such as endoscopy for esophageal varices or ultrasound for HCC screening. However, in 20% of patients with CLDs, the diagnosis is made on presentation of the first episode of hepatic decompensation due to cirrhosis (27). Thus, implementation of liver fibrosis staging and surveillance for cirrhosis at a preclinical stage will facilitate long-term planning for these patients. Similarly, the longitudinal assessment of fibrosis in a safe, noninvasive manner is desirable. Our study highlights that most Canadian physicians who follow patients with CLDs require routine assessment of liver fibrosis stage, thus recognizing its importance for decision making. Chronic hepatitis C is the etiology for which assessment of liver fibrosis is required by most physicians, followed by autoimmune/cholestatic liver disease, chronic hepatitis B, NAFLD and ALD. This is consistent with national and international guidelines. In fact, an assessment of liver fibrosis is recommended in chronic HBV and HCV infections; however, in some conditions, such as ALD and autoimmune/cholestatic liver disease, liver biopsy still has a diagnostic role in assessing severity or ruling out other forms of liver disease (4,5,14,16–18,26,28,29). Importantly, although patients with HIV and HCV and/or HBV coinfection experience higher rates of evolution toward cirrhosis than monoinfected individuals, routine assessment of liver fibrosis was requested by only 31.7% of respondents. This may be due to the fear of performing liver biopsy in HIV-positive patients given a possible higher chance of bleeding associated with clotting abnormalities (30,31).
In our experience, the primary specialty of the respondents impacted the etiology of CLDs seen. Gastroenterologists are more likely to manage NAFLD and ALD patients than hepatologists and infectious diseases specialists, and they manage fewer patients with HBV and HIV coinfection. Conversely, infectious diseases specialists manage fewer patients with autoimmune/cholestatic disease. This practice profile reflects a subspecialty in the management of CLDs that has been already observed in other Canadian studies (32,33).
For almost one-half of the surveyed physicians, liver biopsy remains the primary tool used to diagnose liver fibrosis. This procedure, however, is invasive and accompanied by several drawbacks including pain and risk of fibrosis underestimation (19,34). Cost is also a major issue. A cost-benefit analysis (35) showed that in the United States, the cost of a liver biopsy is USD$1,032 and could rise to USD$2,745 when complications occur. In Canada, the mean cost of a complicated liver biopsy requiring hospitalization is $4,579 (36). During the past two decades, scientific interest has been focused on implementing noninvasive approaches to diagnose liver fibrosis in CLDs. The most validated among them include Fibroscan and serum biomarkers that are computed from readily available parameters, such as APRI and Fibrotest. Liver biopsy use increased by 41% between 1994 and 2002 in Canada, highlighting the increasing importance of liver fibrosis staging for clinicians managing CLD patients (36). On the other hand, at that time, noninvasive measures of fibrosis had not yet affected the utilization of liver biopsy in our health region, probably owing to their limited availability before 2003. Our data demonstrate that the introduction of noninvasive methods to assess liver fibrosis has impacted Canadian physicians’ practices considering that 42.6% of respondents have reduced the number of liver biopsies performed by >50%.
Significant differences exist according to the etiology of liver disease. As such, liver biopsy is used significantly more in autoimmune/cholestatic patients compared with other types of CLDs. This most likely reflects the fact that liver biopsy remains important to diagnose the cause of liver disease in these patients, while for HBV and HCV the diagnosis is established by clinical information and serological tests. Although liver biopsy remains a major diagnostic tool to differentiate NASH from simple steatosis, liver biopsy is not used significantly more in NAFLD by Canadian physicians.
The most widely used noninvasive tool in Canada is Fibroscan, followed by Fibrotest. This finding is consistent with the most recommended noninvasive methods (4,21). Several surveyed physicians use magnetic resonance elastography as the primary tool for liver fibrosis assessment. This noninvasive method has recently shown excellent accuracy to diagnose liver fibrosis (24). However, it is costly, time consuming and has limited availability. Surprisingly, simple biomarkers, such as APRI, were used by only a minority of respondents. This can be explained by the fact that, although readily available and inexpensive, these biomarkers have significant lower diagnostic accuracy and higher rates of unclassified cases compared with sophisticated methods such as Fibroscan and Fibrotest (11,21). Interestingly, there is higher use of simple biomarkers in ALD versus HCV, in which Fibroscan is the predominant noninvasive method. This likely reflects the fact that the aspartate aminotransferase to alanine aminotranferase ratio, a simple and inexpensive biomarker, has long been known as a potential indicator of ALD (37,38).
Demographics and practice characteristics of surveyed physicians impact the diagnostic methods adopted. Older physicians use more noninvasive methods than younger respondents. Similarly, and contrary to our presurvey expectations, liver biopsy was used more often by the junior (years of practice) respondents versus senior physicians, who preferred noninvasive techniques, although this difference was not statistically significant. This may be due to a greater familiarity with the noninvasive methods and the ability to use them in clinical practice. Physicians based at a university hospital or practicing in private used more noninvasive tools than respondents based at a community hospital, likely reflecting easier access and availability of the noninvasive tools in these settings. Interestingly, surveyed physicians practicing in Ontario used Fibrotest more than any other Canadian province.
Most of the surveyed physicians demonstrate satisfaction with the accuracy of the currently available noninvasive methods for staging liver fibrosis. The implementation of noninvasive tools for liver fibrosis resulted in more patients treated with antiviral therapy for one-third of respondents. We speculate that access to and availability of noninvasive methods, which were the major concern for the surveyed physicians, represent a significant barrier to increasing the number of treated patients, given the critical role of liver fibrosis stage in guiding antiviral therapy in HCV and HBV patients. An additional barrier includes a lack of guidelines for use in clinical practice. The Asian Pacific Association for the Study of the Liver produced the only consensus recommendations on liver fibrosis in 2009 (39). At that time, the consensus statement read that clinical utility of noninvasive techniques would be proven by further studies in large numbers of patients. More recent guidelines on management of specific CLDs provide a different perspective. As such, the European Association for the Study of the Liver (EASL) guidelines for HCV management state that, although liver biopsy remains the gold standard of reference, non-invasive methods can also be used (26). Similarly, the CASL guidelines state that acceptable methods to stage liver fibrosis include liver biopsy, Fibroscan and serum biomarkers (4). The EASL guidelines for HBV management state that a liver biopsy is often recommended to facilitate treatment decision (28). According to the CASL guidelines on HBV, clinicians should have access to transient elastography testing (5). In NAFLD, the AASLD guidelines recommend the use of a simple biomarker, the NAFLD fibrosis score, to direct patients at high risk for advanced fibrosis or NASH toward liver biopsy (14).
The variation in the strength of recommendation of noninvasive methods according to the etiology of CLDs and across different guidelines reflects the quality and number of validating studies and the local availability and reimbursement policy. Chronic hepatitis C is the etiology for which most studies have been conducted, and the abundance of data is reflected by the recommendation of implementing non-invasive tools.
Finally, reimbursement policy was a major concern for 14.4% of the respondents. While in France, la Haute Authorité de Santé has approved reimbursement for Fibroscan and Fibrotest since 2007, Fibroscan is currently reimbursed only in Quebec. However, work is in progress in British Columbia, Alberta, Ontario and Nova Scotia. Fibrotest is currently not reimbursed by any Canadian province.
Few studies have investigated physicians’ practices regarding liver biopsy use and fibrosis assessment before our study. A French survey that interviewed 1177 general practitioners concluded that liver biopsy may be refused by up to 59% of patients with hepatitis C, and 22% of the physicians share the same concern due to the invasiveness of the procedure (40). Another survey performed at an American centre (41) showed that among 112 clinicians, 29.5% did not perform liver biopsy for the following reasons: concern about risks (72.7%); low reimbursement (66.7%); and logistical issues with space and recovery time (45.4%). Interestingly, in France, where noninvasive methods of liver fibrosis were first marketed and reimbursement policies have been implemented since 2007, a nationwide survey regarding assessment of liver fibrosis in hepatitis C among French hepatologists showed that liver biopsy is still systematically performed by only 4% of respondents (42). In agreement with our findings, this French study showed that updated guidelines for the use of noninvasive methods in clinical practice were required by 95% of respondents. Also, a survey by Ratziu et al (43) investigating diagnostic practices for NAFLD showed that in France, liver biopsy is rarely performed as a first-line diagnostic procedure and that the large majority use serum biomarkers or Fibroscan (43). The high rate of use of noninvasive methods in France reflects the high dissemination of knowledge and the presence of key opinion leaders in this area, which likely contributed to the implementation of these methods.
A limitation of our study was the relatively low response rate of 43.9%. This is similar to previous analogue surveys (43) but could bias the results because nonresponders may hold divergent views on some aspects of liver fibrosis practices and accuracy of noninvasive methods or have lower levels of overall interest in it. However, all responding and nonresponding physicians were active members of a scientific hepatogastroenterological or HIV society, thus forming a rather homogenous group in terms of training and medical interest. Also, the majority of respondents came from two provinces. The opinions and practices of physicians from the rest of Canada may, therefore, be under-represented. Nonetheless, the salient issues regarding fibrosis assessment faced by practitioners across the country are similar. Finally, we did not solicit responses from wider groups of physicians, including family medicine, infectious diseases and internal medicine specialists; therefore, there may be over- or underestimation of the use of noninvasive tests in these groups.
CONCLUSION.
The present nationwide survey showed that most Canadian physicians who manage patients with CLDs adhere to current guidelines regarding the routine assessment of liver fibrosis. Although biopsy was the primary tool for fibrosis assessment for 50% of the surveyed physicians, noninvasive tools, particularly Fibroscan, have significantly reduced the need for liver biopsy in Canada. Limitations in access to/availability of the noninvasive tools represent a significant barrier. Finally, the present study emphasizes the need for ‘ad hoc’ clinical guidelines of use and an improved reimbursement policy to implement noninvasive methods to diagnose and stage fibrosis that will ultimately minimize costs and the use of liver biopsy in the Canadian health care system.
Acknowledgments
This work was supported by start-up operating funds from the Department of Medicine of McGill University and from the Research Institute of the McGill University Health Centre. GS holds a Chercheur-Boursier career award from the Fonds de la Recherche en Santé du Quebéc (FRSQ). MBK is supported by a Chercheurs Nationaux career award from the FRSQ. The authors are grateful to Ms Kathleen Rollet for the scientific contribution to the statistical analysis of the data.
Footnotes
CONTRIBUTIONS: GS contributed to the conception, study design, data, interpretation of the data and first draft of the article. PG, PW, MBK and MD contributed with data and interpretation of the data. RPM contributed to the conception, study design, data and interpretation of the data.
DISCLOSURES: GS has acted as speaker for Merck, Vertex, Gilead, served as an advisory board member for Boheringer Ingelheim and Novartis and has received research funding from Vertex. PG has acted as speaker for Merck, Vertex and Gilead and received research funding from Merck. PW has served as speaker and consultant for Bristol Myers Squibb, Gilead, Merck, Novartis, Roche, and as investigator for Merck, Novartis, Roche and Vertex. MBK has received honoraria and acted as a consultant for viiv, Gilead, Janssen and Merck and received research funding from Merck. MD has served as an advisory board member for Roche, Merck, Janssen, Vertex and Gilead. RPM has received consulting fees from Merck Canada Inc, Roche Canada, Genentech Inc, Johnson and Johnson, Norgine Ltd, GE Healthcare and Vertex, has served as speaker for Merck Canada Inc, Roche Canada, Vertex and KNS Canada, and has received research support from the Canadian Institutes for Health Research, Alberta Heritage Foundation for Medical Research (now Alberta Innovates-Health Solutions) and Echosens.
REFERENCES
- 1.Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: A general population-based study. J Hepatol. 2008;49:732–8. doi: 10.1016/j.jhep.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: Summary of a workshop. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 3.Sanabria AJ, Dion R, Lucar E, Soto JC. Evolution of the determinants of chronic liver disease in Quebec. Chronic Dis Inj Can. 2013;33:137–45. [PubMed] [Google Scholar]
- 4.Myers RP, Ramji A, Bilodeau M, Wong S, Feld JJ. An update on the management of hepatitis C: Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol. 2012;26:359–75. doi: 10.1155/2012/947676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffin CS, Fung SK, Ma MM, Canadian Association for the Study of the Liver Management of chronic hepatitis B: Canadian Association for the Study of the Liver consensus guidelines. Can J Gastroenterol. 2012;26:917–38. doi: 10.1155/2012/506819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaton MD. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Can J Gastroenterol. 2012;26:353–7. doi: 10.1155/2012/725468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendall PRW. Public Health approach to alcohol policy: An updated report from the provincial health officer. National Library of Canada Cataloging in Publication Data. 2008 [Google Scholar]
- 8.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–40. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 11.Sebastiani G, Alberti A. How far is noninvasive assessment of liver fibrosis from replacing liver biopsy in hepatitis C? J Viral Hepat. 2012;19(Suppl 1):18–32. doi: 10.1111/j.1365-2893.2011.01518.x. [DOI] [PubMed] [Google Scholar]
- 12.de Franchis R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–8. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59:375–82. doi: 10.1016/j.jhep.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 15.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 17.EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 19.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–44. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 20.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 21.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–302. e1294. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study. Lancet. 2001;357:1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 23.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239–47. doi: 10.1002/hep.25610. [DOI] [PubMed] [Google Scholar]
- 25.Cassinotto C, Lapuyade B, Ait-Ali A, et al. Liver fibrosis: Noninvasive assessment with acoustic radiation force impulse elastography – comparison with FibroScan M and XL probes and FibroTest in patients with chronic liver disease. Radiology. 2013;269:283–92. doi: 10.1148/radiol.13122208. [DOI] [PubMed] [Google Scholar]
- 26.Craxi A. EASL Clinical practice guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55:245–64. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–9. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 29.EASL clinical practical guidelines: Management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal E, Poiree M, Pradier C, et al. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study) AIDS. 2003;17:1803–9. doi: 10.1097/00002030-200308150-00009. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Ordonez MA, Colmenero JD, Jimenez-Onate F, Martos F, Martinez J, Juarez C. Diagnostic usefulness of percutaneous liver biopsy in HIV-infected patients with fever of unknown origin. J Infect. 1999;38:94–98. doi: 10.1016/s0163-4453(99)90075-0. [DOI] [PubMed] [Google Scholar]
- 32.Myles A, Mugford GJ, Zhao J, Krahn M, Wang PP. Physicians’ attitudes and practice toward treating injection drug users with hepatitis C: Results from a national specialist survey in Canada. Can J Gastroenterol. 2011;25:135–9. doi: 10.1155/2011/810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bain VG, Wong WW, Greig PD, Yoshida EM. Hepatology and the Canadian gastroenterologist: Interest, attitudes and patterns of practice: Results of a national survey from the Canadian Association of Gastroenterology. Can J Gastroenterol. 2003;17:25–9. doi: 10.1155/2003/709303. [DOI] [PubMed] [Google Scholar]
- 34.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: The smaller the sample, the milder the disease. J Hepatol. 2003;39:239–44. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 35.Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C. A cost-effectiveness analysis. Ann Intern Med. 2000;133:665–75. doi: 10.7326/0003-4819-133-9-200011070-00008. [DOI] [PubMed] [Google Scholar]
- 36.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: A population-based study including 4275 biopsies. Liver Int. 2008;28:705–12. doi: 10.1111/j.1478-3231.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 37.Cohen JA, Kaplan MM. The SGOT/SGPT ratio – an indicator of alcoholic liver disease. Dig Dis Sci. 1979;24:835–8. doi: 10.1007/BF01324898. [DOI] [PubMed] [Google Scholar]
- 38.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: Potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–22. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- 39.Shiha G, Sarin SK, Ibrahim AE, et al. Liver fibrosis: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) Hepatol Int. 2009;3:323–33. doi: 10.1007/s12072-008-9114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonny C, Rayssiguier R, Ughetto S, et al. [Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region] Gastroenterol Clin Biol. 2003;27:1021–5. [PubMed] [Google Scholar]
- 41.Muir AJ, Trotter JF. A survey of current liver biopsy practice patterns. J Clin Gastroenterol. 2002;35:86–8. doi: 10.1097/00004836-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Castera L, Denis J, Babany G, Roudot-Thoraval F. Evolving practices of non-invasive markers of liver fibrosis in patients with chronic hepatitis C in France: Time for new guidelines? J Hepatol. 2007;46:528–529. doi: 10.1016/j.jhep.2006.12.002. author reply 529–530. [DOI] [PubMed] [Google Scholar]
- 43.Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57:376–83. doi: 10.1016/j.jhep.2012.03.019. [DOI] [PubMed] [Google Scholar]


