Abstract
Itraconazole (ICZ)‡ is commonly used for the treatment of fungal infections, particularly in immunocompromised patients. In addition, ICZ has been recently found to have anti-angiogenic effects and is currently being tested as a new chemotherapeutic agent in several cancer clinical trials. We have previously shown that ICZ impaired complex N-linked glycosylation processing, leading to the accumulation of high-mannose glycoproteins on the surface of macrophages. This investigation was directed at determining the effects of ICZ on phagocytosis as a major function of macrophages. We found a significant decrease in the phagocytosis of opsonized bacterial particles in ICZ-treated murine macrophages in comparison with non-treated macrophages. Furthermore, the impairment of phagocytosis was associated with a decrease in cell surface expression of Fcγ receptors (FcγR) as well as alteration of their glycosylation pattern. Concomitantly, a reduction in all three isoforms of the FcγR family (i.e., Fcgr1, Fcgr2 and Fcgr3) mRNA levels was observed after incubation with ICZ. The effect of ICZ on phagocytosis and FcγR expression was reversed by addition of LDL. These studies indicate that ICZ treatment certainly has a dramatic effect on macrophage function, which could result in a potential impairment of the immune system's ability to respond to pathogens and may lead to elevated incidence of infections.
Keywords: Macrophages, phagocytosis, Fcgamma receptors, innate immunity, bacteria, cancer
Introduction
Itraconazole (ICZ) is a systemic triazole antifungal with a broad spectrum of activity commonly used for the treatment of Candida spp. and Aspergillus spp. infections, the most frequent fungal pathogens (1, 2). Since ICZ is very well tolerated and has a favorable safety profile, it is frequently used in chemoprophylaxis of systemic fungal infections, which are usually difficult to diagnose and often treated empirically (1, 2). ICZ prevents fungal cell growth by inhibiting lanosterol 14α-demethylase, which belongs to the cytochrome P450 (CYP450) oxidase family, blocking the synthesis of ergosterol, a key component of fungal cell membranes. In mammals, ICZ also interacts with this enzyme, but with much lower affinity (1, 3), resulting in the accumulation of lanosterol and inhibition of the cholesterol biosynthesis pathway.
ICZ has also been described as an anti-angiogenic agent, predominantly by inhibiting the binding of vascular endothelial growth factor to its receptor (4, 5). Preclinical studies have suggested that ICZ impairs proliferation, migration and tube formation of endothelial cells (6). Due to the fact that ICZ is a Food and Drug Administration (FDA)-approved antifungal with a long-standing record of safe use and relatively high plasma levels, it has been identified as a potential chemotherapeutic agent (5, 6). Indeed, there are several clinical trials currently underway (e.g., NCT00798135, NCT00887458, NCT01409018, NCT00769600) with the aim to establish the efficacy of ICZ against various types of cancer. Furthermore, the empirical use of ICZ in immunosuppressed patients (NCT00002370) or in patients undergoing bone marrow or stem cell transplantation is being evaluated (NCT00003883, NCT00079222).
We have previously demonstrated that ICZ causes an impairment of N-linked glycosylation processing leading to the accumulation of high mannose glycoproteins in macrophages (Møs). Despite this alteration in glycosylation, glycoproteins were still delivered to the plasma membrane (7). The present study was aimed at determining whether this alteration in glycosylation affects Mø function, at the level of phagocytosis. Phagocytosis is initiated with the recognition of foreign particles by specific receptors on the surface of Møs, followed by particle engulfment and destruction inside phagolysosomes. In particular, immunoglobulin G (IgG) opsonized particles are recognized by Fcγ receptors (FcγR) (8, 9), which specifically recognize the constant fragment (Fc) of IgG molecules (10). In addition, these receptors have been shown to be involved in other processes of the immune system, including cell degranulation, cytokine/chemokine release, and antibody-dependent cellular cytotoxicity. In fact, FcγR have been postulated to be the link between the high specificity of the adaptive immune system and the effector cells of the innate immune system (10).
Here we report a dramatic decrease in the rate of phagocytosis of fluorescently labeled opsonized E. coli bacterial particles both in a murine Mø cell line (J774.1 cells) as well as in isolated naïve mouse peritoneal Møs treated with ICZ. Furthermore, we establish the mechanism of this effect as being mediated by a significant reduction in the expression of all FcγR family subtypes.
Materials and Methods
Cell culture and reagents
J774.1 murine Møs were obtained from the American Type Culture Collection (ATCC) and maintained in RPMI 1640 media containing 10% fetal bovine serum (FBS) and penicillin (50 IU/mL)/streptomycin (50 μg/mL) (Cellgro, Manassas, VA). Cells were plated in non-tissue-culture, 12-well plates 24 h prior to experiments at a concentration of 1 × 106 cells/well. ICZ, dimethyl sulfoxide (DMSO), low-density lipoprotein (LDL) from human plasma and IgG from murine serum were obtained from Sigma (St. Louis, MO). Bioparticles – Opsonized E. coli fluorescein labeled particles were obtained from Molecular Probes – Life Technologies (Carlsbad, CA) and prepared following the manufacturer's instructions. Purified rat anti-mouse CD16/CD32 (clone 2.4G2), FITC-labeled anti-CD16/CD32 (clone 2.4G2) and FITC Rat IgG2b, κ isotype control (clone A95-1) antibodies were obtained from BD Pharmingen (San Jose, CA). APC anti-mouse CD64 (clone X54-5/7.1) and APC mouse IgG1, κ isotype control (clone MOPC-21) antibodies were obtained from Biolegend (San Diego, CA). APC anti-mouse F4/80 (clone BM8) antibody was obtained from eBioscience (San Diego, CA). Rabbit anti-rat IgG horseradish peroxidase (HRP) polyclonal antibody was from Enzo life sciences (Farmingdale, NY). Bovine serum albumin (BSA) was obtained from Sigma. Alexa Fluor 488 conjugated Concanavalin A was from Life Technologies. Concanavalin A from Canavalia ensiformis (Jack bean) horseradish peroxidase conjugate and α-Methyl mannoside were obtained from Sigma and Vector laboratories (Burlingame, CA), respectively. trypan blue solution was from Cellgro (Manasas, VA) and propidium iodide solution from Molecular Probes – Life Technologies (Carlsbad, CA).
Mice
Male CD-1 mice (8–10 weeks old) were obtained from Charles River Laboratory (San Diego, CA) and housed in the University of California San Diego School of Medicine vivarium. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California San Diego and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Isolation of mouse peritoneal macrophages
Resident naïve peritoneal Møs (PMøs) were isolated by peritoneal cavity lavage as described previously (11). Male CD-1 mice (8–10 weeks old) were euthanized by cervical dislocation under anesthesia with isoflurane via a vaporizer at 1.5–2.5 minimum alveolar concentrations (MAC). Under sterile conditions, a 16-gauge catheter was inserted into the peritoneum and used to introduce ice-cold RPMI 1640 medium (5 mL) supplemented with penicillin (50 IU/mL)/streptomycin (50 μg/mL). The abdomen was gently massaged, and the lavage was withdrawn through the catheter. The peritoneal lavage was centrifuged at 360 × g for 10 min at room temperature. The pellet containing the cells was resuspended in 1 mL of complete media – RPMI 1640 medium supplemented with 10% FBS and penicillin (50 IU/mL)/streptomycin (50 μg/mL). Approximately 1 × 106 PMøs were isolated per mouse. The cell suspension was seeded on a 12-well plate at a concentration of 5 × 105 cells per well. Cells were allowed to attach for 1 h at 37°C in a CO2 incubator. Non-adherent cells were removed, and attached cells were washed 3× with complete medium. Cells were incubated for an additional 24 h and then treated with either ICZ (1 μM) or vehicle control (DMSO) for 16 h. A spare well from each treatment group was used to determine cellular viability and total cell count using the trypan blue exclusion test (90% viability was considered acceptable). The purity of PMøs was determined by staining with F4/80 Ab. A 90–95% population of Møs was considered acceptable. At the end of the treatment period, cells were evaluated for their phagocytic capacity and expression of cell surface FcγR by flow cytometry as described below.
Phagocytosis assay
The phagocytic capacity of J774.1 Møs and PMøs, was determined using opsonized E. coli fluorescein-labeled bioparticles (Molecular Probes – Life Technologies, Carlsbad, CA). Møs were treated with ICZ (0.5, 1, 2 or 10 μM) or vehicle control (DMSO) for various time periods as described in the figure legends. After ICZ incubation, a spare well from each treatment group was used to determine the total cell count. Bioparticles were applied at a ratio of 10:1 (bioparticles:cells) for 15, 30 and 60 min at 37°C. At the end of each incubation period, cells were harvested, transferred to polystyrene tubes, washed with ice-cold FACS staining buffer (FSB–DPBS supplemented with 0.5% [w/v] BSA) by centrifuging at 360 × g for 8 min at 4°C and fixed with 1% paraformaldehyde in FSB. Cell surface fluorescence was quenched using trypan blue (0.2%) in the fixing solution. Phagocytosis was analyzed by flow cytometry.
IgG inhibition experiments
To demonstrate the specificity of FcγR-mediated phagocytosis, J774.1 Møs were submitted to the phagocytosis assay as described above in the presence of purified mouse IgG isolated from serum (Sigma). After incubation with ICZ (1 μM) or DMSO for 16 h, the phagocytic capacity of J774.1 Møs was determined using opsonized E. coli fluorescein-labeled bioparticles at a ratio of 10:1 (particles:cells) applied concomitantly with purified mouse IgG (10 μg/mL) for 60 min. Møs were then washed and fixed. Cell surface fluorescence was quenched with trypan blue in the fixing solution and phagocytosis evaluated by flow cytometry.
Assessment of non-FcγR-mediated phagocytosis
To determine non-Fc-mediated phagocytosis, fluorescent latex beads (FluoSpheres – carboxylate-modified microspheres 0.5 μm – Life Technologies, Carlsbad, CA) were used following the same protocol as bioparticles at a ratio of 10:1 (beads:cells).
Immunoblot analysis
Following treatments, J774.1 Møs (∼ 1 × 106 cells) were collected and centrifuged at 300 × g for 8 min at 22°C, the supernatant was discarded, and cells were resuspended and washed once in ice-cold PBS. Cells were centrifuged again using the same settings and resuspended in 100 μL of ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) containing the complete mini EDTA-free protease inhibitor mixture from Roche (Indianapolis,IN). Protein concentration was determined using the BCA protein assay kit from Pierce (Rockford, IL). Cell lysates were mixed with 4× SDS-PAGE loading buffer, and 50 μg of total protein was separated by SDS-PAGE using NuPAGE 4 – 12% Bis-Tris gels (Life Technologies, Carlsbad, CA). The separated proteins were transferred to nitrocellulose membranes and blocked in 5% BSA diluted in Tris-buffered saline (TBS) for 1 h at room temperature. Blots were probed with the rat anti-mouse CD16/CD32 antibody (1:500) in TBS supplemented with 5% bovine serum albumin and 0.1% Tween 20 (5% BSA-TBST) at 4°C overnight followed by three 15 min washes in TBS supplemented with 0.1% Tween 20 (TBST) at room temperature. Blots were then incubated with HRP-conjugated rabbit anti-rat IgG antibodies (1:10,000) in 5% BSA-TBST for 2 h at room temperature. After three 15 min washes in TBST, bands were detected by chemiluminescence using SuperSignal reagents from Pierce.
Flow cytometry
Cells (J774.1 Møs and PMøs) were treated with ICZ (0.5, 1, 2 or 10 μM) or vehicle control (DMSO) for various time periods as described in the figure legends. At the end of each incubation time, cells were transferred to 5 mL polystyrene round-bottom tubes and washed with ice-cold FACS staining buffer (FSB – DPBS supplemented with 0.5% [w/v] BSA) by centrifuging at 360×g for 8 min at 4°C. Cells were incubated with APC-labeled anti-CD64 and/or FITC-labeled anti-CD16/CD32 antibodies (1 μg/million cells in 100 μL at 4°C for 30 min). At the end of the incubation period, cells were washed twice in ice-cold FSB. Cells were then fixed in 1% paraformaldehyde in PBS and examined by flow cytometry. To determine total protein expression (cell surface and intracellular) at the end of the ICZ treatment, cells were transferred into 5 mL polystyrene round-bottom tubes, washed with ice-cold FSB and subsequently fixed and permeabilized in 100 μL of BD Cytofix/Cytoperm solution (BD Biosciences) for 15 min at 4°C. Møs were then incubated with APC-labeled anti-CD64 and/or FITC anti-CD16/CD32 as mentioned above and analyzed by flow cytometry (FACSCantoII – BD Biosciences, San Jose, CA). Data were acquired by BD FACSDiva software (BD Biosciences, San Jose, CA), analyzed, and presented using FlowJo (TreeStar, Ashland, OR).
The presence of high-mannose glycoproteins on the cell surface of Møs was measured using the lectin Concanavalin A. Møs (∼1 × 106) were treated with ICZ (1 μM) or vehicle control (DMSO) for 16 h. At the end of this incubation time, cells were transferred to 5 mL polystyrene round-bottom tubes and washed with ice-cold FSB and subsequently incubated with 100 μg/ml Alexa Fluor 488-conjugated ConA for 30 min at 4°C followed by 3 washes in ice-cold FSB. Cells were then fixed in 1% paraformaldehyde in FSB and examined by flow cytometry as described above.
LDL supplementation experiments
Møs (J774.1 cells ∼1 × 106) were incubated for 16 h at 37°C with ICZ (1 μM) or DMSO in regular cell culture medium or culture medium supplemented with low-density lipoprotein (LDL – 100 μg/mL). FcγR-mediated phagocytosis was evaluated as described above. Briefly, after 16 h incubation with ICZ or DMSO, both in the presence or absence of LDL supplementation, Møs were challenged with opsonized E. coli fluorescein-labeled bioparticles at a ratio of 10:1 (particles:cells) for 30 min. Møs were then washed and fixed. Cell surface fluorescence was quenched with trypan blue in the fixing solution and phagocytosis evaluated by flow cytometry. In addition, cell surface and total cellular expression of Fcγ III/II receptors as well as ConA binding were determined as described above
Lectin Blotting
After incubation with either ICZ (1 μM) or vehicle control (DMSO), J774.1 cells were washed twice with ice-cold PBS and scraped in ice-cold lysis buffer containing complete mini EDTA-free protease inhibitor cocktail from Roche (as described above). Protein concentration was determined using the BCA protein assay kit from Pierce. Cell lysates were mixed with 4× SDS-PAGE loading buffer, and 50 μg of total protein was separated by SDS-PAGE using NuPAGE 4–12% Bis-Tris gels (Life Technologies). The separated proteins were transferred onto nitrocellulose membranes and blocked in 5% BSA-TBST for 30 min at room temperature. Blots were then incubated with 1 μg/mL horseradish peroxidase-conjugated Concanavalin A (HRP-ConA) overnight at 4°C on a rocking platform. At the end of the incubation period, blots were washed 3× for 15 min each time in TBST. Bands were detected by chemiluminescence using SuperSignal reagents from Pierce.
RNA isolation and quantitative reverse transcriptase-PCR
To determine gene expression, mRNA levels were measured using quantitative real time reverse transcriptase-PCR (qRT-PCR). Total RNA was isolated from J774.1 Møs using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA was then treated with DNase I (DNA-free kit, Ambion, Carlsbad, CA) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit from Applied Biosystems (Carlsbad, CA). cDNA was then diluted and stored at -20°C until further use. The relative quantities of Fcgr1, Fcgr2b and Fcgr3 mRNA were quantified by qRT-PCR using validated Qiagen primer sets (QuantiTect Primer Assays - Fcgr1: QT00097013, Fcgr2b: QT00198891 and Fcgr3: QT00117803) and the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA) in a 7500 fast real time PCR system (Applied Biosystems). All samples were run in triplicate and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH – QT01658692, Qiagen, Valencia, CA) mRNA levels. Data were analyzed using the comparative CT method and confirmed by the standard curve method.
Statistical analysis
All data were analyzed using GraphPad Prism software (GraphPad Prism Software, La Jolla, CA). Significance was determined by t-test or one-way ANOVA followed by Newman-Keuls multiple comparisons test. A p value of < 0.05 was considered statistically significant.
Results
ICZ decreased phagocytosis of opsonized bacterial particles
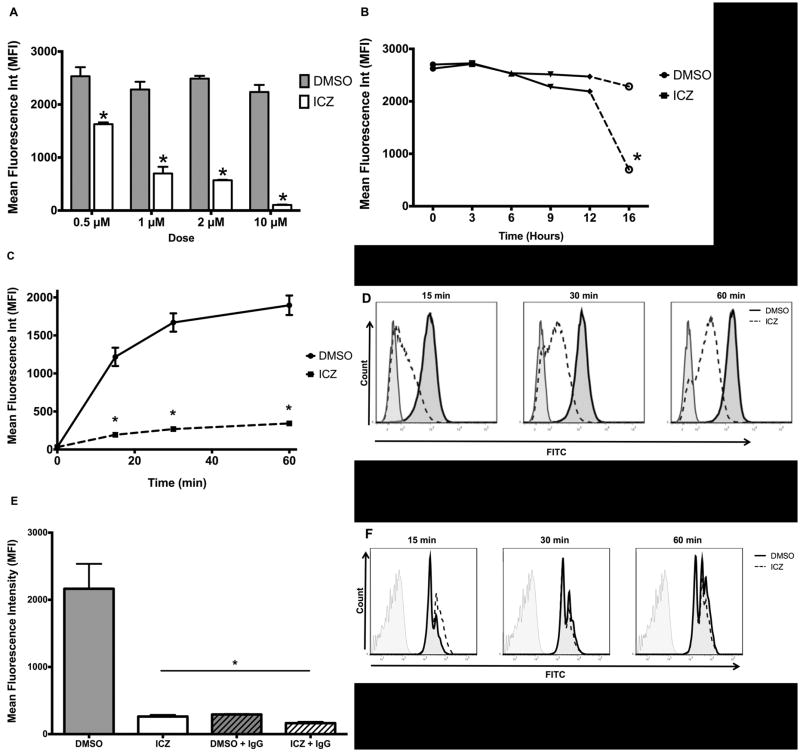
Previous studies from our laboratory demonstrated that ICZ induces the accumulation of high mannose glycoproteins on the surface of Møs (7). However, this change in the glycosylation pattern of cell surface molecules has not been correlated with modifications of major cellular functions. Therefore, we investigated whether or not treatment of Møs with ICZ affects their phagocytic capacity. Murine Møs (J774.1 cells) were treated with ICZ at various doses, and incubation times and phagocytosis was evaluated by incubation with fluorescently labeled E. coli particles. We found that a decrease in phagocytosis was observed between 0.5 and 10 μM (Fig. 1A). The higher dose (10 μM) resulted in toxicity as evidenced by decreased cell viability determined by trypan blue exclusion and propidium iodide binding assays, whereas no toxicity was observed at lower concentrations of ICZ. For the following experiments, the clinically relevant dose of 1 μM was used. We also observed that the maximum effect of ICZ (1 μM) was observed within 16 h of treatment (Fig. 1B), as previously described (7). Therefore, cells were treated with ICZ (1 μM) or vehicle (DMSO) for 16 h for the rest of the study. After treatment with ICZ (1μM) or vehicle (DMSO), cells were challenged with fluorescently labeled E. coli particles at a ratio of 10:1 (bioparticles:Møs) for 15, 30 and 60 min at 37°C. Phagocytosis of fluorescently labeled particles was determined by flow cytometry. A significant decrease in the rate of phagocytosis was observed as early as 15 min of the incubation period with bioparticles in the ICZ-treated cells as opposed to non-treated Møs, which was maintained until 60 min of the challenge (Fig. 1C). Representative flow cytometry graphs are presented in Fig. 1D.
Figure 1. ICZ decreased phagocytosis of opsonized bacterial particles.
A, Phagocytosis dose response curve. J774.1 cells (∼1 × 106) were treated with ICZ at various doses (0.5, 1, 2 or 10 μM) for 16 h at 37°C or equivalent concentrations of vehicle (DMSO) then challenged with opsonized fluorescent bacterial particles at a ratio of 10:1 (bioparticles:Møs) for 60 min. Phagocytosis was determined by flow cytometry. The phagocytic rate was calculated for each treatment dose from the mean fluorescence intensity (MFI) after quenching cell surface fluorescence using trypan blue. Results from three independent experiments performed in triplicate for each sample are presented. B, Phagocytosis time response curve. The phagocytic rate of opsonized fluorescent bioparticles (ratio of 10:1 bioparticles:Møs, 60 min challenge) was determined (as described above) after incubation of J774.1 cells (∼1 × 106) with ICZ (1 μM) or vehicle (DMSO) for 0, 3, 6, 9, 12 or 16 h. C, Phagocytic rate after ICZ treatment. J774.1 cells (∼1 × 106) were treated for 16 h at 37°C with ICZ (1 μM) or vehicle (DMSO), then challenged with opsonized fluorescent bacterial particles (ratio of 10:1 bioparticles:Møs) for 15, 30 and 60 min. Results from three independent experiments performed in triplicate for each sample are presented. D, Representative flow cytometry plots at 15, 30 and 60 min of bacterial challenge (as described in C) are depicted: ICZ, dotted lines, DMSO, solid lines, non-stained control, grey lines. E, Phagocytosis of opsonized bacterial particles is Fcγ-receptor-mediated. The specificity of Fcγ-receptor-mediated phagocytosis of opsonized bacterial particles was determined by competition with purified mouse IgG applied concomitantly to bacterial particles. J774.1 Møs (∼1 × 106) were treated with ICZ (1 μM) or vehicle (DMSO) for 16 h and then challenged with opsonized bacterial particles as described above for 60 min in the presence or absence of purified mouse IgG (10 μg/mL). F, Non FcγR-mediated phagocytosis is unaffected by ICZ. A global effect on phagocytosis was evaluated using fluorescent latex beads. After incubation with ICZ (1 μM) or vehicle (DMSO) for 16 h, J774.1 cells (∼1 × 106) were exposed to fluorescent latex beads at a ratio of 10:1 (beads:Møs) for 15, 30 and 60 min at 37°C. Representative flow cytometry plots are depicted: ICZ, dotted lines, DMSO, solid lines, non-stained control, grey lines. Statistical analysis was performed using ANOVA. * P ≤ 0.05 compared to Control (DMSO).
Since phagocytosis of opsonized particles is mainly mediated by FcγR, we investigated whether or not the decrease in phagocytosis upon ICZ treatment was, indeed, mediated by these types of receptors by competition with purified mouse IgG. After ICZ treatment, cells were co-incubated with mouse IgG and fluorescently labeled E. coli particles. Certainly, mouse IgG effectively inhibited the uptake of fluorescent bioparticles, demonstrating that the process was, indeed, FcγR-dependent (Fig. 1E). Furthermore, we wanted to establish if the ICZ effect was a global alteration of phagocytosis; therefore, we determined non-FcγR-mediated phagocytosis by evaluating the internalization rate of fluorescent latex beads by flow cytometry. ICZ did not have an effect on the uptake of latex beads (Fig. 1F), providing further evidence to the specificity of the effect of ICZ on FcγR mediated phagocytosis.
ICZ decreased the expression of FcγR
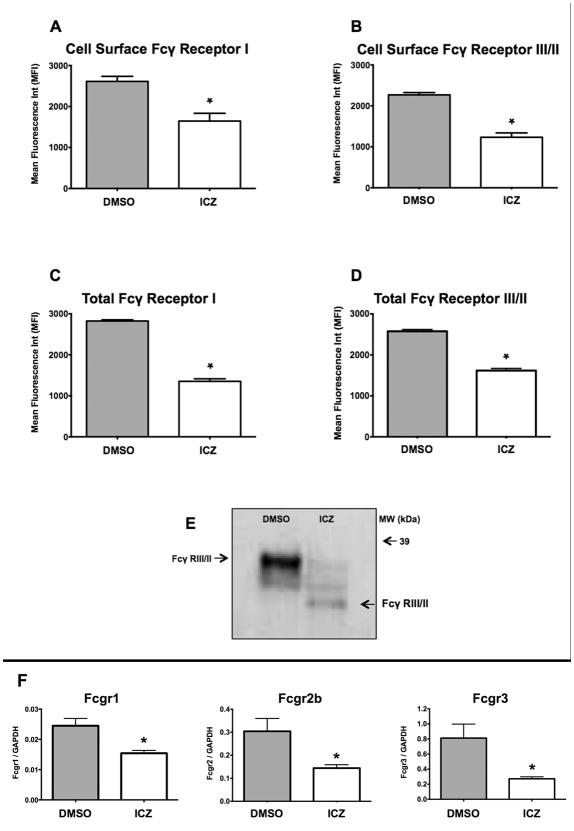
Due to the substantial decrease in the phagocytic rate observed in the ICZ-treated cells, we hypothesized that this drug could affect the expression of FcγR. There are three classes of FcγR, i.e., FcγRI: Fcgr1 (CD64), FcγRII: Fcgr2b (CD32) and FcγRIII: Fcgr3 (CD16). First, we measured the cell surface levels of the FcγRI and FcγRIII/II receptors using flow cytometry. A significant decrease (FcγRI: ∼40% and FcγRIII/II: ∼50%) of the surface levels of these receptors was observed in ICZ-treated cells (Figs. 2A and 2B), which was mirrored by the total cellular levels measured in permeabilized cells (Figs. 2C and 2D). In addition, we observed a change in the electrophoretic mobility of Fcγ RIII/II upon incubation with ICZ as visualized by Western blotting, which is consistent with a change in the glycosylation pattern (Fig. 2E). To further document the effect of ICZ on N-linked glycosylation we evaluated the presence of high-mannose glycoproteins by Concanavalin A (ConA) binding. The lectin binding blot analysis confirmed our prior report (7) that, indeed, ICZ treatment resulted in increased ConA binding of total glycoproteins as compared to vehicle control (Supplementary Figure 1). ConA binding was demonstrated to be specific for mannose moieties by coincubation with α-methyl mannoside, which precluded the binding of the aforementioned lectin.
Figure 2. Itraconazole decreased expression and altered the electrophoretic mobility of Fcγ receptors.
J774.1 Cells (∼1 × 106) were treated for 16 h at 37°C with ICZ (1 μM) or vehicle control (DMSO). A and B, cell surface and C and D, total cellular expression of FcγI and FcγIII/II receptors was determined by flow cytometry using APC-labeled anti-mouse CD64 and FITC-labeled rat anti-mouse CD16/CD32 antibodies. E, ICZ altered the electrophoretic mobility of FcγIII/II receptors. At the end of the incubation period with either ICZ or DMSO, cells were lysed, equal amounts of protein for each sample (50 μg) were resolved by SDS-PAGE, and the presence of FcγRIII/II was determined by Western blotting. F, mRNA levels of FcγRI: Fcgr1 (CD64), FcγRII: Fcgr2b (CD32), FcγRIII: Fcgr3 (CD16) were determined using qRT-PCR. Values were determined using a standard curve and expressed as copy number. All values were normalized to GAPDH mRNA levels. Results from three independent experiments performed in triplicate for each sample were used to calculate the mean and standard error depicted. Statistical analysis was performed using ANOVA. * P ≤ 0.05 compared to Control (DMSO).
Finally, we investigated whether or not incubation with ICZ altered FcγR expression at the mRNA level, measured by qRT-PCR. Indeed, mRNA levels of Fcgr1 (CD64), Fcgr2b (CD32) and Fcgr3 (CD16) were all significantly reduced after ICZ incubation in comparison with non-treated cells (Fig. 2F). In summary, ICZ decreases cellular levels of FcγR and alters the expression of the mature form of the protein, therefore causing a drastic reduction in the ability of Møs to phagocytize opsonized bacterial particles.
Itraconazole exerts similar effects on isolated naïve peritoneal macrophages
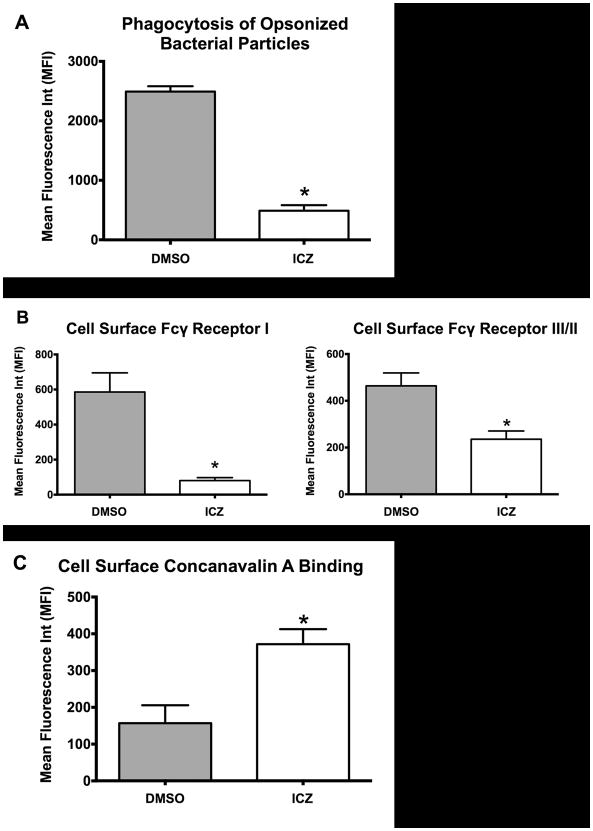
In order to further validate our results with the murine Mø cell line J774.1, we treated resident naïve peritoneal macrophages (PMøs) isolated by peritoneal lavage of CD1 mice (8–10 weeks old). Isolated PMøs were allowed to recover for 24 h and subsequently incubated with ICZ (1 μM) or vehicle control (DMSO) for 16 h. At the end of the incubation time, the phagocytic rate (Fig. 3A) and cell surface FcγR levels (Fig. 3B) were determined by flow cytometry as described above. We found that ICZ (1 μM for 16 h) indeed exerted the same effect on PMøs as on J774.1 Møs, decreasing both phagocytosis of opsonized bacterial particles and FcγR cell surface expression levels (Fig. 3A and 3B, respectively). Furthermore, we evaluated the effect of ICZ on N-linked glycosylation by quantifying binding of Alexa Fluor 488-conjugated ConA to cell surface glycoproteins using flow cytometry as described above. As previously reported (7), ICZ lead to an accumulation of high-mannose glycoproteins in the PMø cell surface as evidenced by the increased binding of Alexa Fluor 488-conjugated ConA (Fig. 3C).
Figure 3. Itraconazole exerted similar effects on isolated mouse naïve peritoneal macrophages.
Resident naïve peritoneal Møs (PMøs ∼1 × 106) were isolated by peritoneal cavity lavage from CD1 mice (8–10 weeks old) and treated for 16 h at 37°C with ICZ (1 μM) or vehicle control (DMSO). At the end of the ICZ incubation period, the phagocytic rate (A) and FcγRI and FcγRIII/II cell surface expression levels (B) were determined. A, Phagocytosis of opsonized bioparticles (ratio of 10:1, 60 min exposure) was evaluated by flow cytometry. B, Cell surface expression of Fcγ I and Fcγ III/II receptors was determined by flow cytometry using APC-labeled anti-mouse CD64 and FITC-labeled rat anti-mouse CD16/CD32 antibodies. C, ICZ treatment resulted in increased Alexa Fluor 488-ConA binding to PMø cell surface glycoproteins as quantified by flow cytometry. Results from three independent experiments performed in triplicate for each sample were used to calculate the mean and standard error depicted. Statistical analysis was performed using ANOVA. * P ≤ 0.05 compared to Control (DMSO).
Addition of LDL prevents the effects of ICZ
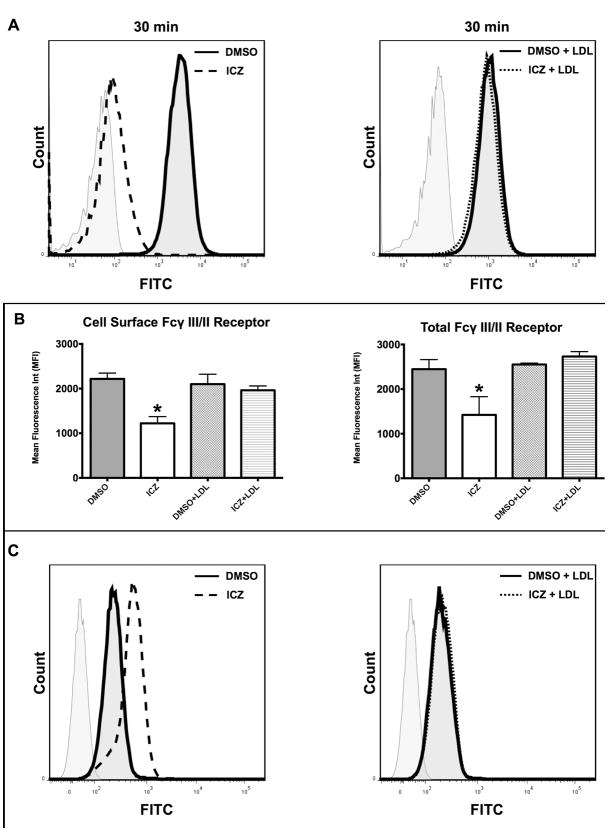
Silverstein et al. (12), previously reported that agents directed at blocking the cholesterol biosynthesis pathway, such as statins (e.g., lovastatin and simvastatin), reduced phagocytosis of opsonized particles. They found that the reduction in phagocytosis could be prevented by co-incubation of statins with LDL. Considering the fact that both ICZ and statins inhibit the cholesterol biosynthesis pathway, but at different levels, we tested whether or not the reduction of phagocytosis after treatment with ICZ could be reverted with the addition of LDL. Møs (J774.1 cells) were incubated with ICZ (1μM) or DMSO for 16 h in the presence of LDL (100 μg/mL), and phagocytosis was evaluated. We found that the addition of LDL reversed the effect of ICZ alone on phagocytosis (Fig. 4A). We also observed that the level of FcγR was restored to initial levels before ICZ treatment (Fig. 4B).
Figure 4. LDL prevented the effect of ICZ.
J774.1 Cells (∼1 × 106) were treated for 16 h at 37°C with ICZ (1μM) or DMSO in the presence or absence of LDL (100 μg/mL). A, LDL inhibits the ICZ effect on phagocytosis. At the end of the incubation period, cells were challenged with opsonized fluorescent bacterial particles at a ratio of 10:1 (bioparticles:Møs) for 30 min. Cells were then washed and fixed. Cell surface fluorescence was quenched with trypan blue in the fixing solution and phagocytosis determined by flow cytometry. Representative flow cytometry plots are depicted. B, LDL inhibits the ICZ effect on FcγR levels. Cell surface and total cellular expression of Fcγ III/II receptors was determined by flow cytometry using FITC-labeled rat anti-mouse CD16/CD32 antibodies: ICZ, dotted lines, DMSO, solid lines, non-stained control, grey lines C, LDL reverses ICZ effect on cell surface glycoproteins, as evidenced by decreased fluorescent ConA binding. Representative flow cytometry plots. Statistical analysis was performed using ANOVA. * P ≤ 0.05 compared to vehicle control (DMSO).
The reversal of the ICZ-induced effect on phagocytosis and FcγR levels prompted us to evaluate the potential effect of LDL on the N-linked glycosylation pattern in Møs treated with ICZ. Therefore, we used ConA binding as a measure of the presence of high mannose glycoproteins on the cell surface. We found that Møs treated concomitantly with ICZ and LDL had the same levels of ConA binding when compared to the control groups (DMSO and DMSO+LDL), which were lower than in cells treated with ICZ alone (Fig. 4C), indicating that the ICZ-induced alteration in glycoconjugate processing could also be reverted by co-incubation with LDL. In summary, LDL can abrogate the ICZ-induced effect on phagocytosis and FcγR expression and alteration in glycoprotein processing. These results suggest that ICZ may inhibit FcγR-dependent phagocytosis by altering Møs cholesterol pools or by affecting the normal trafficking of cholesterol within the cell.
Discussion
Previous studies from our laboratory have demonstrated that ICZ induces an alteration in the processing of N-linked glycosylation resulting in the accumulation of high mannose glycans (7). However, this change in the glycosylation pattern of cell surface molecules has not been correlated with modifications of cellular functions. Therefore, we set out to investigate whether or not treatment of Møs with ICZ affects their phagocytic capacity. Phagocytosis plays a fundamental role in the immune system response to invading microorganisms and pathogens. In addition, this cellular process is critical in tissue homeostasis and remodeling as well as in the removal of cellular debris and apoptotic cells (13).
We found that treatment of Møs with ICZ impaired FcγR-mediated phagocytosis of opsonized particles. The mechanism of this decrease in phagocytosis was associated with a reduction of FcγR, both at the protein and mRNA levels. Furthermore, we showed that FcγR displayed an alteration in the glycosylation pattern, which appeared to affect their function. Indeed, the decrease in the phagocytic rate after ICZ treatment (85%) was greater than the reduction in cell surface FcγR levels (40–50%). Other studies have shown an alteration in glycosylation of FcγR in isolated monocytes and neutrophils from patients undergoing gram-negative infection (14). Since phagocytosis plays a central role in the clearance of pathogens, apoptotic cells and tumor cells (15-17), our results suggest a potential risk for the use of ICZ, particularly in vulnerable populations such as those who are receiving chemotherapy and are already immunocompromised.
Our studies demonstrated that ICZ exerted its effects at the level of FcγR gene expression, because mRNA levels of all three main subtypes of this receptor (Fcgr1, Fcgr2 and Fcgr3) were reduced. This finding is in contrast to the effect of ICZ on the major lipopolysaccharide (LPS) binding protein CD14, which we reported to be upregulated (7). The effect of ICZ on FcγR gene expression could be explained by either alteration of transcription or changes in mRNA stability. Transcription of FcγR is regulated by several factors, such as interferon γ (IFN-γ) (18, 19), Interleukin 10 (20), 6 (21), 1β (22) and granulocyte-colony stimulating factor (19, 23). In addition, Interleukin 4 has been shown to reduce the expression of all three FcγR (20). Modulation of FcγRI in particular has been shown to be susceptible to treatment with glucocorticoids, which oppose the effect of IFN-γ (24). Furthermore, neither IFN-γ nor dexamethasone affects mRNA stability of FcγR (24, 25). At the molecular level, transcriptional regulation of FcγRI has been shown to be dependent upon two main factors: PU.1 and Stat1. PU.1 is required for basal expression and for IFN-γ induced FcγRI promoter activation, while Stat1 alone is not able to initiate transcription (26).
We also observed that the effect of ICZ on phagocytosis was reverted by co-incubation with LDL. This observation echoed prior reports indicating that LDL affected FcγR-mediated phagocytosis by regulating the expression of the high affinity FcγR, namely FcγRI (27, 28). Moreover, it was reported that phagocytosis was more sensitive to lipid depletion than reduction in the expression of FcγRI (28). Also, our findings could be interpreted in light of a recent report in which ICZ was found to have an inhibitory effect on the mTORC pathway by altering cholesterol trafficking (29). The authors demonstrated that ICZ blocks cholesterol egress from endosomal/lysosomal compartments to the plasma membrane, which leads to inhibition of both mTORC1 and mTORC2, which highlights a possible role of ICZ in cholesterol trafficking (29). Indeed, ICZ-treated cells exhibited a rearrangement of cholesterol distribution in a pattern reminiscent of Niemann-Pick disease type C (NPC) (29). In this lysosomal storage disease, accumulation of unesterified cholesterol, sphingolipids and other lipids is due to mutations in either of the genes encoding the NPC1 or the NPC2 proteins, which are essential for normal intracellular cholesterol trafficking (30, 31). Similar to NPC, ICZ leads to accumulation of cholesterol in late endosomes and lysosomes with a concomitant depletion at the plasma membrane (29, 30).
The effects of ICZ on Mø function could be further interpreted by considering the effect of this drug on glycoprotein processing. Alteration of N-linked glycosylation could have a profound effect on a wide array of key proteins, including NPC1, which is a late endolysosomal glycoprotein with 14 potential N-glycosylation sites (32). This protein plays a critical role in the normal shuttling of cholesterol within the lysosome (32). Indeed, wild type NPC1 is resistant to endoglycosydase H treatment, whereas the most prevalent NPC mutation (NPC1I1061T) is endoglycosydase H-sensitive (33), which is consistent with the effect of ICZ on glycoprotein processing. Human fibroblasts homozygous for the NPC1I1061T mutation displayed a marked impairment of LDL-stimulated cholesterol esterification and accumulation of unesterified cholesterol in lysosomes (33). Therefore, alteration of the NPC1 glycosylation pattern after treatment with ICZ may lead to a deficient NPC1 protein unable to shuttle cholesterol within the late endosome/lysosome and causing the accumulation of unesterified cholesterol as previously reported (33). These findings underscore the role of proper glycosylation and the potential link between cholesterol trafficking and the expression of cell surface receptors. Therefore, a change in the glycosylation pattern of FcγR could also contribute to the reduction of phagocytosis induced by ICZ treatment.
Renewed interest in the use of ICZ has stemmed from recent findings suggesting its potential application as a chemotherapeutic agent against cancer. ICZ has been shown to have anti-tumor properties, including inhibition of angiogenesis (34), the Hedgehog pathway (35) and the mTOR pathway (29). Taking advantage of the fact that ICZ is an FDA-approved agent, clinical trials have swiftly been initiated to determine its efficacy. Currently, clinical trials aimed at establishing the use of ICZ in malignancies of the breast (NCT00798135), prostate (NCT00887458) and lung (NCT007769600) are underway. But as suggested by Ringshausen et al. (36) and our studies, there are potential issues that need to be addressed before considering a widespread use of ICZ as a chemotherapeutic agent.
In addition, ICZ has been reported to have anti-inflammatory effects and lead to reduced systemic immune activation by decreasing IgG and IgE levels (37). Moreover, ICZ has been recently reported to interfere with rituximab immunotherapy in patients suffering from B-cell lymphoma (36). In summary, we present evidence that the use of ICZ in vulnerable populations such as those immunosuppressed patients or those receiving targeted immunotherapy should be approached with caution and under strict surveillance to prevent detrimental side effects.
Supplementary Material
Acknowledgments
The authors thank Molly Wofford for her editorial assistance and technical support.
Footnotes
This work was supported by NIH grants F31 GM090681 and RO1 GM083275.
The abbreviations used in this article include the following: ICZ, itraconazole; Møs, macrophages; ANOVA, analysis of variance; DMSO, dimethyl sulfoxide; LDL, low-density lipoprotein
References
- 1.De Beule K, Van Gestel J. Pharmacology of itraconazole. Drugs. 2001;61(Suppl 1):27–37. doi: 10.2165/00003495-200161001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Meis JF, Verweij PE. Current management of fungal infections. Drugs. 2001;61(Suppl 1):13–25. doi: 10.2165/00003495-200161001-00002. [DOI] [PubMed] [Google Scholar]
- 3.Zonios DI, Bennett JE. Update on azole antifungals. Semin Respir Crit Care Med. 2008;29(2):198–210. doi: 10.1055/s-2008-1063858. [DOI] [PubMed] [Google Scholar]
- 4.Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Jr, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2(4):263–70. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 5.Nacev BA, Grassi P, Dell A, Haslam SM, Liu JO. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J Biol Chem. 286(51):44045–56. doi: 10.1074/jbc.M111.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudin CM, Brahmer JR, Juergens RA, Hann CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P, Liu JO. Phase 2 Study of Pemetrexed and Itraconazole as Second-Line Therapy for Metastatic Nonsquamous Non-Small-Cell Lung Cancer. Journal of Thoracic Oncology. 2013;8(5):619–623. doi: 10.1097/JTO.0b013e31828c3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey T, De Maio A. The antifungal agent itraconazole induces the accumulation of high mannose glycoproteins in macrophages. J Biol Chem. 2009;284(25):16882–90. doi: 10.1074/jbc.M109.007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukushima K, Takasaki S. Processing inhibition of N-linked sugar chains associated with induction of Fc receptor-mediated phagocytosis in the mouse monocytoid cells. Glycobiology. 1993;3(1):15–22. doi: 10.1093/glycob/3.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Bilyy RO, Shkandina T, Tomin A, Munoz LE, Franz S, Antonyuk V, Kit YY, Zirngibl M, Furnrohr BG, Janko C, Lauber K, Schiller M, Schett G, Stoika RS, Herrmann M. Macrophages discriminate glycosylation patterns of apoptotic cell-derived microparticles. J Biol Chem. 287(1):496–503. doi: 10.1074/jbc.M111.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–25. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 11.Vega VL, De Cabo R, De Maio A. Age and caloric restriction diets are confounding factors that modify the response to lipopolysaccharide by peritoneal macrophages in C57BL/6 mice. Shock. 2004;22(3):248–53. doi: 10.1097/01.shk.0000133590.09659.a1. [DOI] [PubMed] [Google Scholar]
- 12.Loike JD, Shabtai DY, Neuhut R, Malitzky S, Lu E, Husemann J, Goldberg IJ, Silverstein SC. Statin inhibition of Fc receptor-mediated phagocytosis by macrophages is modulated by cell activation and cholesterol. Arterioscler Thromb Vasc Biol. 2004;24(11):2051–6. doi: 10.1161/01.ATV.0000143858.15909.29. [DOI] [PubMed] [Google Scholar]
- 13.Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 14.Chimaolera M, Launay P, Montenegro V, Rivero MC, Velasco IT, Monteiro RC. Enhanced expression of Fc alpha receptor I on blood phagocytes of patients with gram-negative bacteremia is associated with tyrosine phosphorylation of the FcR-gamma subunit. Shock. 2001;16:344–8. doi: 10.1097/00024382-200116050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 16.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 10(5):317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcgammaRI. J Immunol. 186(5):2699–704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- 18.Bovolenta C, Gasperini S, Cassatella MA. Granulocyte colony-stimulating factor induces the binding of STAT1 and STAT3 to the IFNgamma response region within the promoter of the Fc(gamma)RI/CD64 gene in human neutrophils. FEBS Lett. 1996;386(2-3):239–42. doi: 10.1016/0014-5793(96)00453-x. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Borja F, Santiago E, Weiss-Steider B. Fc gamma receptors in health and disease. Rev Invest Clin. 1998;50(6):529–40. [PubMed] [Google Scholar]
- 20.Capsoni F, Minonzio F, Ongari AM, Carbonelli V, Galli A, Zanussi C. IL-10 up-regulates human monocyte phagocytosis in the presence of IL-4 and IFN-gamma. J Leukoc Biol. 1995;58(3):351–8. doi: 10.1002/jlb.58.3.351. [DOI] [PubMed] [Google Scholar]
- 21.Ruhl S, Pluznik DH. Dissociation of early and late markers of murine myeloid differentiation by interferon-gamma and interleukin-6. J Cell Physiol. 1993;155(1):130–8. doi: 10.1002/jcp.1041550117. [DOI] [PubMed] [Google Scholar]
- 22.Santiago E, Mendoza JF, Corona TM, Lopez R, Sanchez L, Mora LM, Flores F, Valencia E, Weiss-Steider B. Induction of Fc receptors on murine macrophages and leukemic cells by interleukin-1 beta. Eur Cytokine Netw. 1993;4(3):223–8. [PubMed] [Google Scholar]
- 23.Kerst JM, van de Winkel JG, Evans AH, de Haas M, Slaper-Cortenbach IC, de Wit TP, von dem Borne AE, van der Schoot CE, van Oers RH. Granulocyte colony-stimulating factor induces hFc gamma RI (CD64 antigen)-positive neutrophils via an effect on myeloid precursor cells. Blood. 1993;81(6):1457–64. [PubMed] [Google Scholar]
- 24.Comber PG, Lentz V, Schreiber AD. Modulation of the transcriptional rate of Fc gamma receptor mRNA in human mononuclear phagocytes. Cell Immunol. 1992;145(2):324–38. doi: 10.1016/0008-8749(92)90335-m. [DOI] [PubMed] [Google Scholar]
- 25.Comber PG, Rossman MD, Rappaport EF, Chien P, Hogarth PM, Schreiber AD. Modulation of human mononuclear phagocyte Fc gamma RII mRNA and protein. Cell Immunol. 1989;124(2):292–307. doi: 10.1016/0008-8749(89)90132-9. [DOI] [PubMed] [Google Scholar]
- 26.Aittomaki S, Yang J, Scott EW, Simon MC, Silvennoinen O. Molecular basis of Stat1 and PU.1 cooperation in cytokine-induced Fcgamma receptor I promoter activation. Int Immunol. 2004;16(2):265–74. doi: 10.1093/intimm/dxh037. [DOI] [PubMed] [Google Scholar]
- 27.Bigler RD, Brown HM, Guyre PM, Lund-Katz S, Scerbo L, Esfahani M. Effect of low-density lipoprotein on the expression of high affinity Fc gamma receptors. Biochim Biophys Acta. 1989;1011(2-3):102–9. doi: 10.1016/0167-4889(89)90195-x. [DOI] [PubMed] [Google Scholar]
- 28.Bigler RD, Khoo M, Lund-Katz S, Scerbo L, Esfahani M. Identification of low density lipoprotein as a regulator of Fc receptor-mediated phagocytosis. Proc Natl Acad Sci U S A. 1990;87(13):4981–5. doi: 10.1073/pnas.87.13.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc Natl Acad Sci U S A. 107(10):4764–9. doi: 10.1073/pnas.0910872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liscum L. Niemann-Pick type C mutations cause lipid traffic jam. Traffic. 2000;1(3):218–25. doi: 10.1034/j.1600-0854.2000.010304.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang TY, Reid PC, Sugii S, Ohgami N, Cruz JC, Chang CC. Niemann-Pick type C disease and intracellular cholesterol trafficking. J Biol Chem. 2005;280(22):20917–20. doi: 10.1074/jbc.R400040200. [DOI] [PubMed] [Google Scholar]
- 32.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–31. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 33.Gelsthorpe ME, Baumann N, Millard E, Gale SE, Langmade SJ, Schaffer JE, Ory DS. Niemann-Pick type C1 I1061T mutant encodes a functional protein that is selected for endoplasmic reticulum-associated degradation due to protein misfolding. J Biol Chem. 2008;283(13):8229–36. doi: 10.1074/jbc.M708735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aftab BT, Dobromilskaya I, Liu JO, Rudin CM. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 71(21):6764–72. doi: 10.1158/0008-5472.CAN-11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Tang JY, Gong R, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, Beachy PA. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 17(4):388–99. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringshausen I, Feuerstacke Y, Krainz P, den Hollander J, Hermann K, Buck A, Peschel C, Meyer Zum Bueschenfelde C. Antifungal therapy with itraconazole impairs the anti-lymphoma effects of rituximab by inhibiting recruitment of CD20 to cell surface lipid rafts. Cancer Res. 70(11):4292–6. doi: 10.1158/0008-5472.CAN-10-0259. [DOI] [PubMed] [Google Scholar]
- 37.Wark PA, Hensley MJ, Saltos N, Boyle MJ, Toneguzzi RC, Epid GD, Simpson JL, McElduff P, Gibson PG. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol. 2003;111(5):952–7. doi: 10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.