Summary
Dendritic spines receive most excitatory connections in pyramidal cells and many other principal neurons. But why do neurons use spines, when they could accommodate excitatory contacts directly on their dendritic shafts? One suggestion is that spines serve to connect with passing axons, and increasing the connectivity of the dendrites. Another hypothesis is that spines are biochemical compartments that enable input-specific synaptic plasticity. A third possibility is that spines have an electrical role, filtering synaptic potentials and electrically isolating inputs from each other.
In this essay, I argue that, when viewed from the perspective of the circuit function, these three functions dovetail with one another to achieve a single overarching goal: to implement a distributed circuit with widespread connectivity. Spines would endow these circuits with non-saturating, linear integration and input-specific learning rules, which would enable them to function as neural networks, with emergent encoding and processing of information.
Keywords: Neural networks, helixes, plasticity, LTP
Introduction: the spine problem
Even a neophyte who has never before looked at a Golgi stain of cortical samples can distinguish two basic structural features: dendritic trees covered with spines, and axons coursing straight through the neuropil (Figure 1). In this essay I argue that these two simple observations can point to a general model for how neurons integrate inputs and how neural circuits may function.
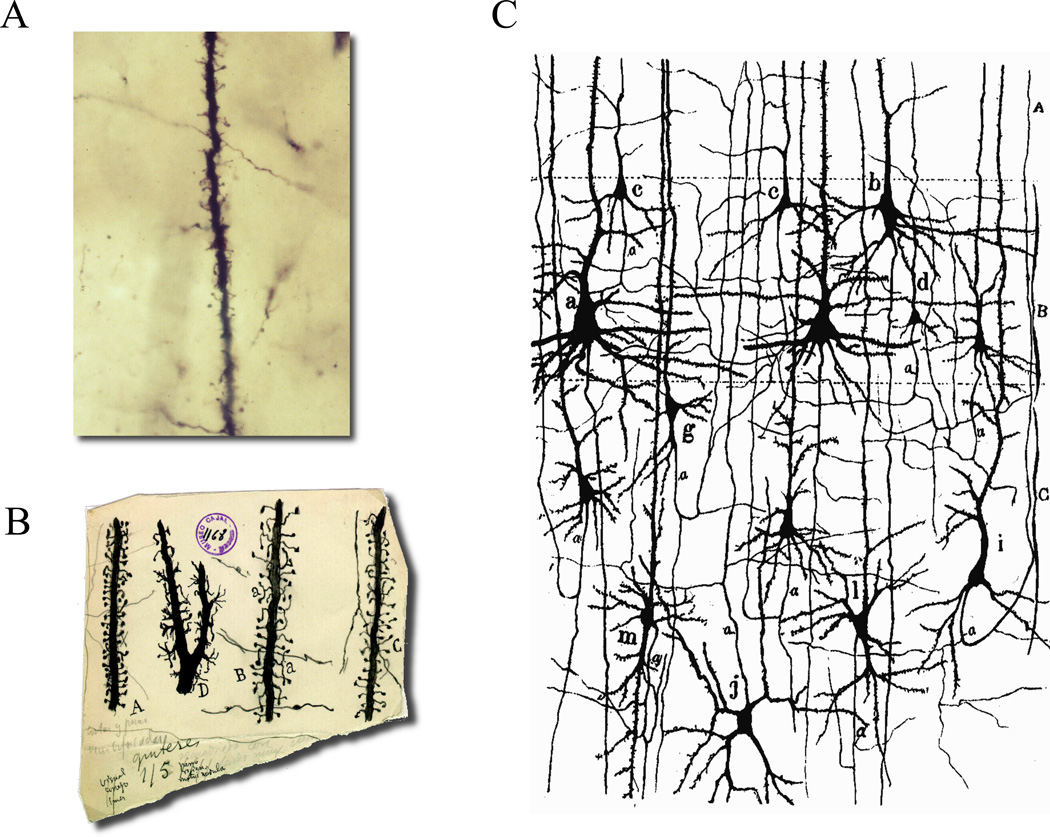
Figure 1. Golgi stains reveal spines and straight axon.
(A) Photomicrograph of an original Golgi preparation from Cajal. The image shows a segment of a dendrite from pyramidal neuron with abundant spines. In the background there are some stained axons crossing transversally. Note how the axonal trajectories are straight. (B) Cajal drawings of different types of spines. Note how spines protrude to cover the neighboring volume. Some axons are also drawn, with straight trajectories. (C) Cajal’s drawing of cellular elements of cerebral cortex. Note how axons have straight, vertical trajectories and basal dendrites are well positioned to intercept them. Reproduced with permission from “Herederos de Santiago Ramón y Cajal.”.
Spines cover the dendritic tree of most neurons in the forebrain (Ramón y Cajal, 1888), and it has been known for over five decades that they receive input from excitatory axons (Gray, 1959). What is less appreciated is that, while essentially every spine has a synapse (Arellano et al., 2007b), the dendritic shaft is normally devoid of excitatory input. So why do excitatory axons choose to contact neurons on spines, rather than on dendritic shafts? Why do neurons make tens of thousands of spines to receive excitatory inputs, when they have plenty of available membrane to accommodate them on their dendritic shafts in the first place (Braitenberg and Schüz, 1998; Schuz and Dortenmann, 1987)? This is what I define as the “spine problem”: what exactly do spines contribute to the neuron? Spines cannot be an accidental design feature: their large numbers and the fact that they mediate essentially all excitation in many brain regions suggests that they must play a key role in the function of the CNS. In fact, given the prevalence of spines throughout the brain, one might even go so far as to say that their role is likely to be so prominent that one may not be able to understand the function of brain circuits without solving the spine problem first.
Starting with Cajal’s idea that spines increase the surface area of dendrites (Ramón y Cajal, 1899), there have been many different proposals that have aimed to explain the specific raison d’être of spines (Shepherd, 1996). These ideas can be grouped into three different hypotheses: (i) that spines serve to enhance synaptic connectivity, (ii) that spines are electrical compartments that modify synaptic potentials and (iii) that spines are biochemical compartments that implement input-specific synaptic plasticity. In this essay, I review these three hypotheses and argue that all three proposals are likely correct, and that, moreover, when viewed from a circuit perspective, they are not contradictory with each other but actually fit nicely into a single function: to build circuits that are distributed, linearly integrating and plastic (Yuste, 2010).
Solutions to the spine problem
A- Spines enhance synaptic connectivity
Let’s begin with a Golgi stain of neocortical tissue (Figure 1). In the background of fields of stained neurons, labeled axons course through the neuropil. These are mostly excitatory axons from pyramidal cells, with trajectories that are essentially straight over short distances. This is peculiar, given that straight lines are not particularly common in nature. Why are most axons straight? Cajal argued that straight trajectories shorten the wire length and therefore speed the transfer of neuronal communication by reducing the time it takes for electrical signals to travel (Chklovskii, 2004; Ramón y Cajal, 1899). But there is a structural interpretation to the straight trajectories of axons: from the point of view of the circuit connectivity, straight axons, by not hovering around any particular zone, move to new parts of the neuropil, thus making contact with as many postsynaptic neurons as possible (Figure 1A – C). So pyramidal neurons (and similarly other excitatory cells) apparently aim to distribute their output as widely as possible, particularly if “double-hits” with the same dendrites are avoided ((Wen et al., 2009); see below). A corollary of this design is that the influence of any given axon on any given cell is minimized: excitatory inputs, particularly in the neocortex, are indeed especially weak (Abeles, 1991; Braitenberg and Schüzt, 1991).
How do these straight axons connect with dendrites? Returning to a Golgi preparation, one can see how dendrites branch out in space, as if aimed at catching passing axons (Figure 1C). Looking at high magnification, one notices that spines resemble small branches, as if they were attempting to better sample the neuropil (Figure 1B). This idea has been pointed out many times, from Cajal on: spines could help to connect with axons, by sampling a cylindrical volume around the dendrite, as a “virtual dendrite” (Ramón y Cajal, 1899; Stepanyants et al., 2002; Swindale, 1981; Ziv and Smith, 1996). In fact, the recent discovery of spine and filopodial motility (Dunaevsky et al., 1999; Fischer et al., 1998;Ziv and Smith, 1996) makes this idea quite tenable: motility peaks during periods of synaptogenesis (Dunaevsky et al., 1999; Konur and Yuste, 2004a), and spines can elongate and physically interact with nearby axonal terminals (Konur and Yuste, 2004b); see for example: http://www.pnas.org/content/suppl/1999/11/08/96.23.13438.DC1/3833movie3.mov. This type of motility is exactly what one would expect to see if spines played an active role in connecting with passing axons.
Another hint of this connectivity function can be found in the patterns in which spines are positioned along some dendrites. In Purkinje cells, spines are arranged in helical patterns, positioned regularly along the dendrite with constant spacing and angular displacement between them (Figure 2; (O'Brien and Unwin, 2006). Helixes are a common structural design principle in nature (for example, in DNA, viral capsides, protein polymers, and leaf patterns on trees) and are an efficient strategy to systematically sample or fill a linear volume, because they maximize the distance in three dimensions between points (Nisoli et al., 2009). Spines could be arranged in helixes to minimize the number of spines used to sample a given volume of neuropil while maximizing their chances of contacting passing axons. The helical topology of spines would thus reduce the probability of connecting several spines from the same dendrite with the same axon, minimizing “double-hits”, and maximize the numbers of connections with different axons, as if the circuit were trying to maximize the richness of inputs that each neuron receives and thus completely fill the connectivity matrix. Consistent with this idea, geometrical arguments show that, by using spines, neurons increase their “potential connectivity”, i.e., the diversity of presynaptic partners (Chklovskii et al., 2002).
Figure 2. Helical distribution of spines.
Regular features along the dendritic shaft of Purkinje cells from electric fish. (A) Spine necks forming regular linear arrays over the shaft surface, revealed in confocal sections. (B) Periodic linear arrays of spines (e.g., circles) in a dendrite. Scale bars = 1 um. (C) Diffraction pattern of B, showing two pairs of peaks arranged with mirror vertical symmetry; the distance of these peaks from the equator indicates that the periodicities repeat every 1.25 um. (D) Filtered images revealing the paths traced by lines of spines on the near sides of the dendrite, made by including only terms associated with the N pair of peaks within the masks. Reprinted with permission from O'Brien and Unwin, (2006). Organization of spines on the dendrites of Purkinje cells. Proc Natl Acad Sci U S A 103, 1575-1580. Copyright (2006) National Academy of Sciences, U.S.A. (E). Schematic rendering of a helical pattern of spines along a dendrite.
These structural features, straight axons and helical spines, reveal a consistent logic of the connectivity of spiny circuits. Excitatory axons distribute information to as many neurons as possible, and spiny neurons make contacts with as many different axons as possible. This creates a distributed topology with large fan-out and fan-in factors and could explain why the excitatory axons connect to spines, rather than to dendritic shafts: the circuit is trying to maximize the distribution and reception of information. For the cerebellar granule-Purkinje cells projection, this strategy may have been optimized to the physical limit, with the parallel fibers running at right angles to the Purkinje cell dendrites (Wen and Chklovskii, 2008). Each granule cell may make just a single contact with each Purkinje cell, which may use helixes to perform this strategy as efficiently as possible (Palay and Chan-Palay, 1974). A similar strategy, although perhaps not so evident, might be present in cortical pyramidal neurons or striatal spiny cells (Wen et al., 2009).
B-Spines enable linear integration of inputs
Distributed circuits generate a significant necessity: postsynaptic neurons now receive many inputs that need to be integrated in a manner in which their individual contributions are functionally incorporated without interfering with each other. In this discussion, although it is simple to imagine integration of inputs arriving simultaneously to the dendritic tree, it is important to note that integration in time is also important. But regardless of when the inputs arrive, unless the activity of each input is independently registered by the postsynaptic cell, it seems pointless to generate a distributed circuit in the first place, since the advantages of receiving inputs from many neurons would be lost if they interfere with each other. The postsynaptic neurons that receive distributed inputs thus need to implement a “synaptic democracy”, i.e., an integrating circuit where every single input is tallied and can jointly contribute to the firing of the cell. Like in an electorate poll, the neuron may not need to keep track of which input has been activated, or identify the individual contribution of each of them, but simply avoid interference between them and sum them up, ideally using a linear integration function (Cash and Yuste, 1999).
Unfortunately, the biophysical constraints of the membrane create a significant interference problem when integrating many inputs. Active synapses open membrane conductances, lowering the membrane resistance, and making the neuron less excitable. When many inputs are activated simultaneously, this electrical shunting could become a serious problem, since their added conductances could short-circuit the membrane, rendering the neuron refractory to simulation.
One solution to avoid this shunting is to electrically isolate the synapses, separate them as much as possible in the dendritic tree. This strategy could work as long as neighboring synapses are not activated simultaneously, particularly if axons are avoind “double-hits” on the same dendrite. But if the circuit is very active, or receive synchronous inputs, the saturation problem would remain. Another, more general solution is to achieve the electrical isolation of the synapses by placing them behind a barrier that protects the dendrite from their open conductances. For this to work, the synapse needs to inject current into the dendrite to generate a significant depolarization, while minimizing at the same time the changes its open receptors generate in the cell’s input resistance. These ideal synapses would become current injecting devices, rather than conductance shunts (Llinás and Hillman, 1969).
The spine neck, if it had a high electrical resistance, could act as such barrier, as pointed out many times (Chang, 1952; Jack et al., 1975; Llinás and Hillman, 1969; Rall, 1974b; Rall and Rinzel, 1971). In fact, many of these proposals highlight how this could help to linearize input summation and avoid saturation. Indeed, numerical simulations indicate that an increased neck resistance generates a linear integration of inputs (Grunditz et al., 2008). In addition, if the spine neck had a significant electrical resistance, besides preventing conductance shunting between active inputs, it could also diminish the interference between inputs by simply reducing the amplitude of the depolarization they generate, as it arrives to the dendrite or soma. Although to do so it may seem simpler to just reduce the number of synaptic receptors, a small receptor number might make the synaptic response too variable from one presynaptic spike to the next. A high neck resistance, on the other hand, could preserve the reliability of the synaptic signal and yet allow for a low effective synaptic conductance without excessive variability.
But this electrical isolation would only make sense for excitatory inputs, because inhibitory inputs, which aim at preventing the neuron from firing, could take advantage of the generated shunt and decreases in the neuron’s input resistance to silence the cell. Interestingly, inhibitory inputs indeed generally contact dendritic shafts, and they also activate significant conductances, higher than excitatory inputs. This indicates that for the neuron it is not important to maintain the independent integration of different inhibitory inputs, as if the information they each carried were similar. Consistent with this, the connectivity profiles of inhibitory circuits show that inhibitory neurons connect promiscuously to all local pyramidal cells, passing to each of them the same exact functional signals (Fino and Yuste, 2011; Packer and Yuste, 2011).
The resistance of the spine neck is still unknown. For its direct measurement, one needs to inject a current into the head of the spine and record it at its base, a difficult proposition experimentally. Indirect estimates of the spine neck resistance, based on cable models or on calculations from diffusional fluxes, vary greatly. While some argue that the spine neck resistance is too low to significantly affect electrical properties of synaptic potentials (Koch and Zador, 1993; Svoboda et al., 1996), others calculate that it could be high enough to filter synaptic potentials (Araya et al., 2006b; Bloodgood and Sabatini, 2005). Although direct measurements of spine neck resistance are still missing, there is recent evidence that, at least in some regimes, a spine can experience a significantly different electrical potential from its parent dendrite, acting as partly isolated electrical compartments. A first hint of this came from calcium imaging experiments that revealed that spine NMDARs flux significant amounts of calcium under minimal quantal synaptic stimulation (Koester and Sakmann, 1998; Kovalchuk et al., 2000; Yuste et al., 1999), where the somatic depolarization is very small (<1mV). These calcium accumulations are unexpected if the NMDARs at resting voltages are mostly blocked by Mg2+. While it is possible that there are some unblocked NMDARs at rest, another interpretation is that the actual voltage experience by the NMDAR at the spine could be significantly larger than that measured at the soma. Consistent with this, voltage-gated conductances can be differentially activated in the spine and the dendritic shaft, something that should not occur if both compartments are isopotential (Araya et al., 2007; Bloodgood et al., 2009). Also, under synaptic stimulation, some spines can sustain substantially higher voltages than their neighboring dendritic shafts (Palmer and Stuart, 2009).
These results indicate that the spine may not be isopotential with its parent dendrite. The simplest explanation for this is that the spine neck resistance must be high enough to filter membrane potential and cause this electrical compartmentalization. Indeed, uncaging glutamate experiments, activating one spine at a time, reveal an inverse relation between the spine neck length and the amplitude of the uncaging potential, when measured at the soma (Araya et al., 2006b). A similar relation has been found with quantal synaptic events (Araya and Yuste, unpublished observations). These results indicate that the spine neck could significantly attenuate the membrane potential as it passes to the dendritic shaft. The exact mechanisms behind this filtering, whether it is due to passive features of the electrical structure of the spine neck (like physical constrictions, clogging by small organelles or abnormal flow of current), or to active conductances, such as potassium channels, in the spine neck membrane, remain unknown.
Attenuating a synaptic potential makes little functional sense: why would a neuron diminish the amplitude of a synaptic signal when it has worked so hard to be able to generate?. As suggested, above, filtering synaptic potentials would electrically isolate inputs form one another, preventing their interaction and preserving their independent integration. This would occur by reducing the average effective conductance of each input and by making synapses current-injecting devices. Both mechanisms could help generate a linear input integration regime (Jack et al., 1975; Llinás and Hillman, 1969; Rall, 1974b; Rall and Rinzel, 1971). If this were the case, linear integration must be so important that a neuron is willing to pay the price of reducing synaptic voltages to maintain it.
But is input integration actually linear? Indeed, when several excitatory inputs, or several dendritic spines, are stimulated simultaneously, one observes a linear summation of their potentials, even when inputs are in close proximity to each other (Araya et al., 2006a; Cash and Yuste, 1998, 1999). Similar results have been reported among inputs from connected pairs of excitatory neurons (Reyes and Sakmann, 1996). In experiments when inputs were activated with various delays, linear integration in time was also found (Cash and Yuste, 1999). Moreover, when glutamate is uncaged onto neighboring positions on the dendritic shaft, the resulting potentials shunt each other greatly (up to 40% for two simultaneous inputs), confirming the major biophysical limitation associated by placing synaptic inputs directly on the dendrite (Araya et al., 2007). Interestingly, inhibitory inputs mostly contact the dendritic shaft, and one observes sublinear summation when neighboring inhibitory inputs are integrated by pyramidal neurons, or when neighboring excitatory inputs are received by aspiny neurons (Tamas et al., 2003).
Spiny dendrites can also integrate inputs in a non-linear regime. Local dendritic spikes (also known as “calcium spikes” “calcium plateaus” or “NMDA spikes”) are generated by focal stimulation of a dendrite (Holthoff et al., 2004; Polsky et al., 2009; Schiller et al., 2000; Yuste et al., 1994). With two-photon uncaging, linear summation is observed when up to 30 neighboring spines are stimulated, although, if more inputs are stimulated, local spikes are triggered (Losonczy and Magee, 2006). A dendritic spike is a non-linear phenomenon which bypasses the “synaptic democracy” and prevents the integration of additional inputs. But dendritic spikes could also significantly enrich the computational repertoire of the neuron, enabling the functional association of local inputs (Mel, 1994). Also, local dendritic spikes, like the ones that occur in the distal apical dendrite of neocortical pyramidal neurons, could enable the amplification of distant inputs that would otherwise not be transmitted to the soma (Larkum et al., 2009; Yuste et al., 1994). Other functions of these local spikes could be to generate either intrinsic firing patterns (Elaagouby and Yuste, 1999) or persistent activity by the neuron (Major et al., 2008). Finally, local dendritic spikes can generate a strong form of LTD (Holthoff et al., 2004) that could be used as a “punishing signal” to prevent input association and, paradoxically, help preserve linear integration.
But regardless of the presence or absence of local dendritic spikes, the neuron still has to solve the conductance shunting problem that arises with simultaneous activation of inputs. Given that, in vivo, dendrites are probably bombarded with hundreds or perhaps even thousands of active inputs at any given time, if excitatory inputs were located on dendritic shafts, dendrites could be essentially short-circuited all the time, making it impossible for voltage signals, including local dendritic spikes, to propagate along. The neuron will also be more reliable if its dendritic integration and signaling were constant under different conditions of synaptic inputs. For all of these reasons, it appears advantageous for the neuron to protect itself from the large conductance changes associated with synaptic transmission, and electrically isolating excitatory inputs into spines could be a solution to this problem. Spines could use neck filtering to ensure a non-saturating regime of integration and fully exploit the benefits of a distributed input connectivity and in addition, make dendritic integration more reliable and less dependent on the amount of synaptic activity present. This linear integration can provide a reason for the apparent counter-intuitive strategy of filtering synaptic potentials. Also, it could explain the mystery of why excitatory inputs terminate on spines and not on shafts, or why inhibitory inputs terminate on shafts. Finally, the neck filtering could help could explain why spines are not much longer, which, for example, could enable the sampling of even more axons and making the connectivity matrix even more distributed. The increasing filtering created by the additional spine neck resistance might eventually render them functionally useless.
C- Spines enable and regulate input-specific plasticity
The discussion about the potential function of the spines so far has proceeded from pointing out their contribution to generate a distributed excitatory connections to the realization that this only makes sense if those inputs can be integrated in a linear regime, without saturation. But even a perfectly wired and perfectly integrating circuit would be completely useless for an animal unless it could change. These distributed connections need to be plastic for the circuit to learn or adapt to novel situations, and it could be argued that the entire purpose of having a nervous system is to be able to adapt a motor program to future circumstances (Llinás, 2002).
A circuit could change its function either by altering its connections or their strength. Indeed, in neocortex there is a significant pruning of connections during early postnatal development (Katz and Shatz, 1996; Rakic et al., 1986). But once the basic circuit is laid out, the creation of new connections might be problematic, for example, if one needs to rewire the circuit everytime a new computation needs to happen, or a new memory needs to be stored. Given the structural constraints of the mature neuropil, where thousands of axons are coursing through a packed wiring, it may be physically impossible to connect specific sets of neurons after the developmental period has terminated. The topological problem associated with rewiring the adult brain could thus be unworkable.
Because of this, for the mature circuit to change its function, it would be easier to alter the synaptic strengths of already existing connections. In fact, a most effective solution would be to wire up all elements together as much as possible and then make all connections plastic. So one needs to make this synaptic plasticity input-specific, again, to take advantage of the functional individuality of each of the inputs and preserve the full computational power associated with a distributed matrix of connectivity.
By implementing the biochemical isolation necessary for input-specific changes in synaptic strength, spines could contribute to making distributed circuits plastic. Indeed, spines compartmentalize calcium: calcium enters into an individual spine during synaptic stimulation while the calcium concentration of neighboring spines, or of the parent dendritic shaft, is unaffected (Koester and Sakmann, 1998; Kovalchuk et al., 2000; Yuste and Denk, 1995). This it is mostly due to the strong calcium extrusion mechanisms present in spine heads, although is probably also aided by the diffusional bottleneck created by the spine neck (Holthoff et al., 2002; Majewska et al., 2000a; Sabatini et al., 2002).
Calcium compartmentalization by spines could allow long-term synaptic plasticity at individual synaptic sites (Holmes, 1990; Koch and Zador, 1993; Malenka et al., 1988). Indeed, very high spine calcium accumulations are triggered by stimulation protocols that generate LTP (Koester and Sakmann, 1998; Yuste et al., 1999). Moreover, the increase in synaptic strength after LTP is accompanied by a corresponding increase in the volume of the spine head (Matsuzaki et al., 2004), and this volume is proportional to the size of the PSD and the number of glutamate receptors in it (Arellano et al., 2007a; Harris et al., 1992; Schikorski and Stevens, 1999). All of these separate pieces of evidence are consistent with a model by which the stimulation of an individual spine, when paired with backpropagating action potentials, triggers a calcium influx specific to the activated spine and elicits LTP by inserting glutamate receptors into that synapse, without affecting the neighboring synapses.
Besides this biochemical compartmentalization, there is an additional mechanism by which spines could enable input-specific alterations in synaptic strength. If the spine neck has a significant resistance, as discussed above, changes in its length or width, or in its electrical properties that may not be morphologically detectable, could alter synaptic strength. This idea, first proposed by Rall (Rall, 1974a, 1995), has become more tenable through the realization that spines are not rigid structures but can dynamically alter their shape and length, in a matter of seconds (Dunaevsky et al., 1999; Fischer et al., 1998). In fact, significant alterations in the dimensions of the spine neck occur spontaneously (Dunaevsky et al., 1999; Majewska et al., 2000b; Parnass et al., 2000) and changes in spine neck diffusion occurs in response to synaptic activity (Bloodgood and Sabatini, 2005). Moreover, electron microscopic reconstructions indicate that the spine neck becomes shorter and wider after LTP (Fifkova and Anderson, 1981;Fifkova and Van Harrefeld, 1977), potentially explaining the increase of synaptic strength. These neck-based changes in synaptic strength could be fast and would not require altering the number of synaptic receptors, but merely alter the synapse strength by modifying its electrical coupling to the dendrite.
Finally, there is a third mechanism by which spines provide enhanced synaptic plasticity. As mentioned above, by specifically enabling connections with a larger variety of axons, spines could allow rewiring which would be much more extensive than if synapses were on dendritic shaft and were to contact only a limited assortment of axons (Chklovskii et al., 2004; Chklovskii et al., 2002).
Therefore, because of their local calcium compartmentalization, their electrical filtering by the spine neck and their enhanced neuropil sampling, spines are ideally suited to enhance circuit plasticity and regulate synaptic strength in an input-specific fashion. Spines could turn a distributed synaptic matrix into one in which each of the synaptic inputs can be modified individually.
A synthesis: the distributed circuit model
Summarizing so far, one could argue that spines help neural circuits achieve three goals. The first one is to make the circuit connectivity matrix more distributed. The second is to make excitatory input integration non-saturating and linear. And the third is to make these connections independently plastic. But when considering them together, it becomes apparent that these three functions go hand in hand and are, in reality, part of the same plan: to create a distributed circuit and exploit the advantages of their design. In distributed circuits, information is widely dispersed and collected, and each neuron linearly tallies its inputs and fires if it reaches action potential threshold (Figure 3). From this point of view, the key computation that spiny neurons achieve is the integration of as many inputs as possible. This explains why EPSPs, particularly when NMDAR-mediated, are especially slow (since to integrate with low noise it is convenient to have a long time window of integration), why excitatory inputs are functionally so small (to be able to integrate as many of them as possible), why spines may form helixes (to enhance the connectivity) and why excitatory inputs generally impinge on spines, rather than on dendritic shaft (to ensure they are independently integrated).
Figure 3. Distributed circuit model.
Excitatory neurons are connected in a distributed topology, by which each cell contacts many other neurons, but makes few (or one) contact with each of them and postsynaptic cells receive inputs from many presynaptic neurons. Active neurons and inputs are black, silent cells white. Active inputs are integrated linearly and those neurons whose arithmetic input sum reaches threshold (three simultaneous inputs in this case), fire an action potential (cells 1 and 5). Meanwhile, neurons that receive a smaller number of active inputs (neurons 2, 3 and 4), fail to do so.
In such a distributed and integrating network the operation of the circuit is simplified, in the sense that the role of each of a cells is merely to add its inputs arithmetically until threshold is reached. Although deceivingly innocent, circuits built with such simple elements have great computational power, as demonstrated by the neural network literature (Hopfield, 1982; McCulloch and Pitts, 1943). For these integrating neurons, as long as every input is tallied, the exact position where the input arrives is irrelevant, and the dendritic tree becomes a mere recipient of as many inputs as possible, without any additional functional reason in its design. Neurons would be essentially summing up inputs, and differences in synaptic strength would prime some inputs over others, depending on the past history of the activity of the network. But, why is the neuron, and the dendritic tree in particular, full of non-linear mechanisms (Stuart et al., 1999; Yuste and Tank, 1996)? Like in electronic circuits, perhaps the role of non-linearities is precisely to keep the transfer function of the system non-saturating and linear over a large input operating range (Mead, 1989). Indeed, the linear integration of pyramidal neurons in vitro arises from a precise balance of non-linear mechanisms, as if the neurons were using nonlinearities to compensate for one another (Cash and Yuste, 1998, 1999).
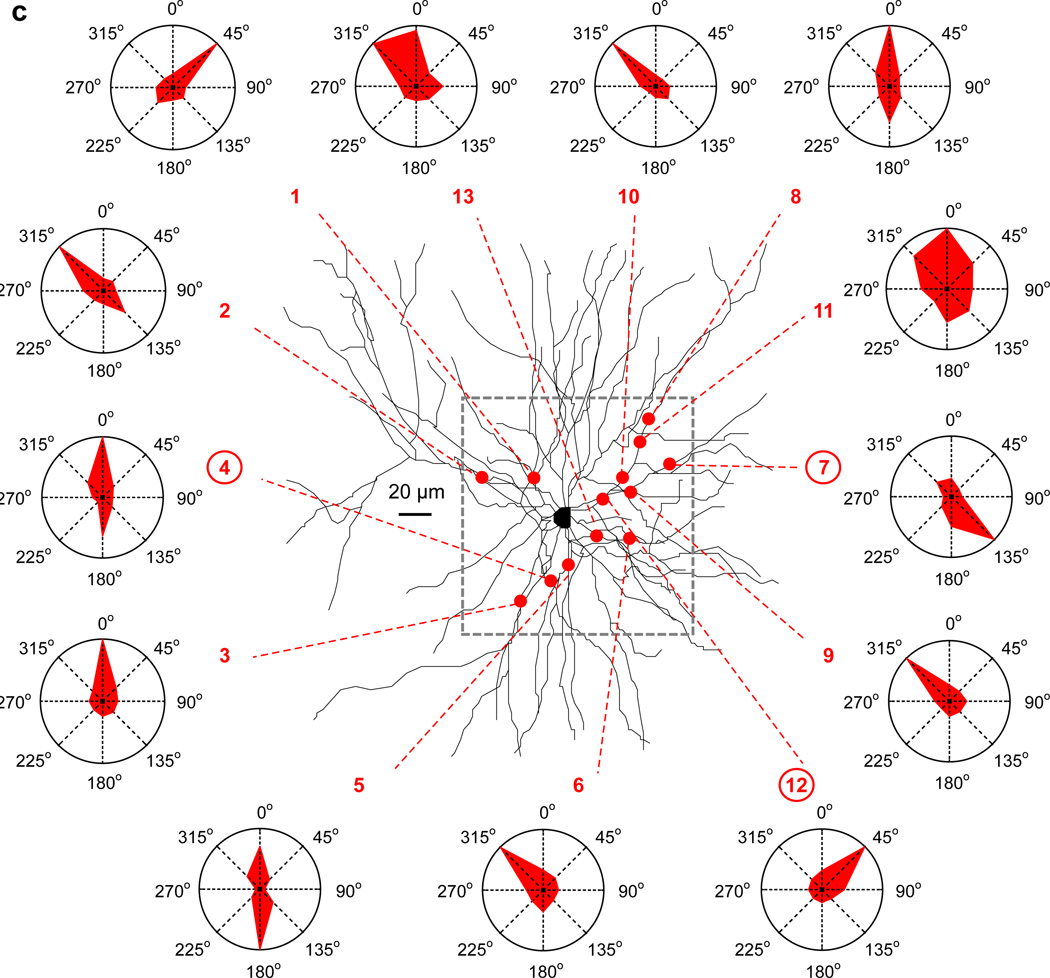
How do spiny neurons integrate in neural circuits in vivo? Two recent studies have examined this. In the first one, the authors performed calcium imaging of spiny dendrites from pyramidal neurons in visual cortex (Jia et al., 2010). Stimulation with visual patterns of different orientations generated local dendritic calcium accumulations (“hotspots”), with dimensions consistent with the activation of individual dendritic spines. Individual neurons had a preference to respond to particular orientations, and this selectivity matched the average selectivity of the sum of their individual hotspots, distributed throughout their dendritic trees. There was no evidence of dendritic spikes or of clustering of active inputs with the same (Figure 4). To a first approximation, the selectivity of the neuron reflected the average orientation selectivity of its dendritic tree, as if inputs were summed linearly (Jia et al., 2010). These results were extended by a second study in auditory cortex, that demonstrates that hotspots were indeed activated dendritic spines (Chen et al., 2011). Spines tuned for different frequencies were highly interspersed on the same dendrites: even neighboring spines were mostly tuned to different frequencies.
Figure 4. Input integration in vivo.
Distribution of activated dendritic inputs in pyramidal cells from mouse visual cortex. Red dots indicate hotspots of local dendritic calcium signaling, evoked by drifting gratings of different orientations, superimposed on the Z-projection of the reconstructed dendritic tree. Red dashed lines point to the polar plot obtained for the corresponding local calcium signals. The frame (grey dashed line) indicates the area of imaging. Note the salt-and-pepper distribution of the orientation-tuned hot spots. The neuron was tuned for the vertical orientation, the orientation that is more represented in this sample. Reprinted by permission from Macmillan Publishers Ltd: [Nature] (Jia et al., Dendritic organization of sensory input to cortical neurons in vivo), copyright (2010).
Although more extensive experimental probing of physiological input integration is necessary, these results agree well with a distributed circuit model of linear integration (Figure 3).
From distributed circuits to random connectivity and emergent computations
If spiny neurons are indeed building circuits with distributed inputs and outputs and input specific plasticity, it is interesting to speculate what other structural or functional features these circuits can sustain. At the physical limit, in a distributed circuit, every neuron would be connected to every other neuron by a single synapse, and it would itself receive inputs from all the other neurons. Although these maximally distributed circuits may seem unrealistic for real brains, a mathematically analogous circuit is one where the connectivity may not complete, but is a random assortment of the synaptic matrix elements. The term “random” is used here to denote the idea that each synaptic connection is chosen by chance, independently from others. In fact, random networks could preserve some basic properties characteristics of completely connected ones, such as the existence of self-sustained activity and dynamical attractors (Hopfield and Tank, 1986). The possibility that in many parts of the brain the microcircuitry (i.e. the local connectivity in a small region, such as for example, within a neocortical layer) is essentially random has been suggested based on anatomical reconstructions (Braitenberg and Schüz, 1998), forming the basis of Peters’ Rule (i.e, that axons contact target neurons in the same proportion as they encounter them in the neuropil) (Peters et al., 1976). Consistent with this, excitatory axons from the olfactory bulb activate an apparent random assortment of neurons in the olfactory cortex (Miyamichi et al., 2010; Sosulski et al., 2011; Stettler and Axel, 2009). Given that most neurons in olfactory cortex are spiny (Shepherd, 1990), one may expect that these (excitatory) axons would preferentially contact spines.
If the local connectivity is indeed random, the functional micro-topography of the circuit should reflect this randomness. With two-photon calcium imaging, one can able to measure, for the first time, the functional properties of larger territories of cortex, while maintaining single cell resolution (Ohki et al., 2005; Stosiek et al., 2003). Indeed, in rodent cortex, neighboring neurons have very different functional properties, as if they reflected a non-ordered input connectivity (Figure 5). On the other hand, in cat cortex, neighboring neurons are endowed with similar, and spatially ordered, functional properties (Ohki et al., 2005). Nevertheless, perhaps the larger size of the cat visual cortex could make randomness in micro-connectivity difficult to discern, since neighboring neurons could be exposed to homogeneous populations of axons.
Figure 5. Disordered functional circuit organization in visual cortex.
Functional maps of selective responses in rat visual cortex. a, In vivo images of cortical cells stained with a calcium indicator, OGB-1 AM. The top panel shows a volume of stained cells; the bottom panel is a cell-based orientation map in which hue is determined by the best orientation overlaid with the anatomical image. Visually responsive cells are colored according to their preferred orientation (green=vertical; red=horizontal; blue and yellow=oblique). Note the apparently random spatial arrangement of orientation specificity. Scale bar, 100 um. Reprinted by permission from Macmillan Publishers Ltd: [Nature] (Ohki et al., Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex), copyright (2005).
A distributed circuit, if it follows Peter’s rule, would greatly simplify the developmental problem of building the connectivity diagram, arguably the most significant problem that the developing nervous system needs to solve. There would be no need to developmentally specify a detailed connectivity matrix, where each neuron would need to meet a precisely determined synaptic partner. To build a specific connectivity matrix could be a task of formidable complexity in circuits such as the neocortex, if one considers the large diversity of neuronal cell types and the high density and apparently disordered packing of the neuropil. The strategy for distributed circuits, rather, is simple: allow for connections to be as promiscuous as possible, with a secondary step where activity-driven learning rules could first prune and later, alter the synaptic weight matrix, adapting it to the computational task at hand. This modification of connections would be most powerful if these circuits were endowed with input-specific plasticity, such as the one that spines enable.
In fact, a distributed circuit would allow a higher degree of plasticity than a specifically built one, since due to the complete or random connectivity matrix, any two neurons could potentially be linked together dynamically, either directly or indirectly. This circuit-level plasticity could explain the success of some optogenetic experiments, where the activation of unspecifically transfected sets of neurons generate significant behavioral changes (Deisseroth, 2011). If circuits were specifically wired, it should be difficult to elicit coordinated behavioral responses from the stimulation of a random assortment of cells. For the same reasons, a random, plastic circuit could also explain the success of experiments where the activity of a random assortment of cortical cells is used to successfully predict the behavior of the animal, or to train external devices (such as computer cursors) using simple linear algorithms (see for example (Wessberg and Nicolelis, 2004)).
Finally, a distributed circuit model also has clear implications for the nature of neural coding. In such circuits, the role of any given neuron becomes irrelevant, since the wider the connectivity matrix, the less importance that each neuron has. Therefore, describing the feature selectivity of a neuron is meaningless because the coding becomes an emergent property, based on the multidimensional space generated by the activity of the entire network. The idea of emergent codes and functional states, such as dynamical attractors, is a cornerstone of the neural network literature ((Buonomano, 2009; Hopfield, 1982; Maass et al., 2003; Sussillo and Abbott, 2009), and is a major departure from the traditional view of using receptive field responses of individual cells to characterize the functional properties of a circuit.
The structure of the connectivity diagram of mammalian circuits, and how exactly these neurons integrate their inputs, are open and key questions. It is intriguing to think, however, that underlying the apparently daunting functional and structural complexity of neuronal circuits, there could be relatively simple principles that apply widely. These principles might be obscured by layers of additional mechanisms necessary to keep the circuit operational. I would argue that spines are the anatomical signatures of distributed neural networks, and that understanding their structure and function might provide us with deep insight into the logic of neural circuits. There could be an underlying simplicity in the design of many brain circuits, and even a lowly Golgi stain, with its spine-laden dendrites and straight axons, might reveal some of these fundamental principles.
Acknowledgements
The author thanks M. Dar for help and L. Abbott, P. Adams, R. Araya, J. DeFelipe, S. Golob and anonymous reviewers for their comments. Supported by the Kavli Institute for Brain Science and the National Eye Institute.
References
- Abeles M. Corticonics. Cambridge, England: Cambrdige University Press; 1991. [Google Scholar]
- Araya R, Eisenthal KB, Yuste R. Dendritic spines linearize the summation of excitatory potentials. Proc Natl Acad Sci USA. 2006a;103:18779–18804. doi: 10.1073/pnas.0609225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci USA. 2006b;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Nikolenko V, Eisenthal KB, Yuste R. Sodium channels amplify spine potentials. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12347–12352. doi: 10.1073/pnas.0705282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, DeFelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies Frontiers. Neuroscience. 2007a;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Espinosa A, Fairâen A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007b;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Giessel AJ, Sabatini BL. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS biology. 2009;7:e1000190. doi: 10.1371/journal.pbio.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Schüz A. Anatomy of the cortex. Second Edition edn. Berlin: Springer; 1998. [Google Scholar]
- Braitenberg V, Schüzt A. Anatomy of the cortex. Berlin: Springer; 1991. [Google Scholar]
- Buonomano DV. Harnessing chaos in recurrent neural networks. Neuron. 2009;63:423–425. doi: 10.1016/j.neuron.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Yuste R. Input summation by cultured pyramidal neurons is linear and position-independent. J Neurosci. 1998;18:10–15. doi: 10.1523/JNEUROSCI.18-01-00010.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron. 1999;22:383–394. doi: 10.1016/s0896-6273(00)81098-3. [DOI] [PubMed] [Google Scholar]
- Chang HT. Cortical neurons with particular reference to the apical dendrite. Cold Spring Harbor Symp Quant Biol. 1952;17:189–202. doi: 10.1101/sqb.1952.017.01.019. [DOI] [PubMed] [Google Scholar]
- Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A. Functional mapping of single spines in cortical neurons in vivo. Nature. 2011 doi: 10.1038/nature10193. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB. Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron. 2004;43:609–617. doi: 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Schikorski T, Stevens CF. Wiring optimization in cortical circuits. Neuron. 2002;34:341–347. doi: 10.1016/s0896-6273(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaagouby A, Yuste R. Role of calcium electrogenesis in apical dendrites: generation of intrinsic oscillations by an axial current. Journal of computational neuroscience. 1999;7:41–53. doi: 10.1023/a:1008915510264. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Anderson CL. Stimulation-induced changes in dimensions of stalks of dendritic spines in the dentate molecular layer. Exp Neurol. 1981;74:621–627. doi: 10.1016/0014-4886(81)90197-7. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Van Harrefeld A. Long-lasting morphological chnages in dendritic spines of dentate granular cells followeing stimualtion of the netorhinal area. J Neurocyt. 1977;6:211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spine. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of synaptic contacts on dendritic spines of the cerebral cortex. Nature. 1959;183:1592–1594. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- Grunditz A, Holbro N, Tian L, Zuo Y, Oertner TG. Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13457–13466. doi: 10.1523/JNEUROSCI.2702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. Is the function of dendritic spines to concentrate calcium? Brain Res. 1990;519:338–342. doi: 10.1016/0006-8993(90)90098-v. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Kovalchuk Y, Yuste R, Konnerth A. Single-shock LTD by local dendritic spikes in pyramidal neurons of mouse visual cortex. The Journal of physiology. 2004;560:27–36. doi: 10.1113/jphysiol.2004.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff K, Tsay D, Yuste R. Calcium dynamics of spines depend on their dendritic location. Neuron. 2002;33:425–437. doi: 10.1016/s0896-6273(02)00576-7. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ, Tank DW. Computing with neural circuits: A model. Science. 1986;233:625–633. doi: 10.1126/science.3755256. [DOI] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electric current flow in excitable cells. London: Oxford University Press; 1975. [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274 doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Koch C, Zador A. The Function of Dendritic Spines - Devices Subserving Biochemical Rather Than Electrical Compartmentalization. J Neuroscience. 1993;13:413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of□ □back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci U S A. 1998;95:9596–9601. doi: 10.1073/pnas.95.16.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Yuste R. Developmental regulation of spine and filopodial motility in primary visual cortex: reduced effects of activity and sensory deprivation. Journal of neurobiology. 2004a;59:236–246. doi: 10.1002/neu.10306. [DOI] [PubMed] [Google Scholar]
- Konur S, Yuste R. Imaging the motility of dendritic protrusions and axon terminals: roles in axon sampling and synaptic competition. Molecular and cellular neurosciences. 2004b;27:427–440. doi: 10.1016/j.mcn.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA Receptor-Mediated Subthreshold Ca(2+) Signals in Spines of Hippocampal Neurons. J Neurosci. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science(New York, NY) 2009;325:756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Llinás R. I of the vortex: From neurons to self. Cambridge, Mass: MIT Press; 2002. [Google Scholar]
- Llinás R, Hillman DE. Physiological and morphological organization of the cerebellar circuits in various vertebrates. In: Llinas R, editor. Neurobiology of Cerebellar Evolution and Development. Chicago: American Medical Association Education□ and Research Foundation; 1969. pp. 43–73. [Google Scholar]
- Losonczy A, Magee J. Integrative Properties of Radial Oblique Dendrites in Hippocampal CA1 Pyramidal Neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Maass W, Natschlager T, Markram H. Real-time computing without stable states: a new framework for neural computation based on perturbations. Neural Computation. 2003;14:2531–2560. doi: 10.1162/089976602760407955. [DOI] [PubMed] [Google Scholar]
- Majewska A, Brown E, Ross J, Yuste R. Mechanisms of Calcium Decay Kinetics in Hippocampal Spines: Role of Spine Calcium Pumps and Calcium Diffusion through the Spine Neck in Biochemical Compartmentalization. J Neurosci. 2000a;20:1722–1734. doi: 10.1523/JNEUROSCI.20-05-01722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska A, Tashiro A, Yuste R. Regulation of spine calcium dynamics by rapid spine motility. J Neurosci. 2000b;20:8262–8268. doi: 10.1523/JNEUROSCI.20-22-08262.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Polsky A, Denk W, Schiller J, Tank DW. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. Journal of neurophysiology. 2008;99:2584–2601. doi: 10.1152/jn.00011.2008. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal slice transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bulletin of mathematical biology. 1943;52:99–115. discussion 173–197. [PubMed] [Google Scholar]
- Mead C. Analog VLSI and neural systems. Reading, MA: Addison-Wesley; 1989. [Google Scholar]
- Mel BW. Information Processing in Dendritic Trees. Neural Computation. 1994;6:1031–1085. [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2010 doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli C, Gabor NM, Lammert PE, Maynard JD, Crespi VH. Static and dynamical phyllotaxis in a magnetic cactus. Physical review. letters. 2009;102:186103. doi: 10.1103/PhysRevLett.102.186103. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Unwin N. Organization of spines on the dendrites of Purkinje cells. Proc Natl Acad Sci U S A. 2006;103:1575–1580. doi: 10.1073/pnas.0507884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011 doi: 10.1523/JNEUROSCI.3131-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. New York: 1974. [Google Scholar]
- Palmer LM, Stuart GJ. Membrane potential changes in dendritic spines during action potentials and synaptic input. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6897–6903. doi: 10.1523/JNEUROSCI.5847-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnass Z, Tashiro A, Yuste R. Analysis of spine morphological plasticity in developing hippocampal pyramidal neurons [In Process Citation] Hippocampus. 2000;10:561–568. doi: 10.1002/1098-1063(2000)10:5<561::AID-HIPO6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Peters A, Paley SL, Webster HdF. The Fine Structure of the Nervous System. Philadelphia: Saunders; 1976. [Google Scholar]
- Polsky A, Mel B, Schiller J. Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11891–11903. doi: 10.1523/JNEUROSCI.5250-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rall W. Dendritic spines and synaptic potency. In: Porter R, editor. In Studies in Neurophysiology. Cambridge University Press: Cambridge; 1974a. pp. 203–209. [Google Scholar]
- Rall W. Dendritic spines, synaptic potency and neuronal plasticity. In: Woody CD, Brown kA, Crow TJ, Knispel JD, editors. In Cellular mechanisms subserving changes in neuronal activity. Brain Information Services: Los Angeles; 1974b. pp. 13–21. [Google Scholar]
- Rall W. The theoretical foundation of dendritic function. Cambridge, Mass: MIT Press; 1995. [Google Scholar]
- Rall W, Rinzel J. Dendritic spine function and synaptic attenuation calculations. Soc Neurosci Abst. 1971;1:64. [Google Scholar]
- Ramón y, Cajal S. Estructura de los centros nerviosos de las aves. Rev Trim Histol Norm Pat. 1888;1:1–10. [Google Scholar]
- Ramón y, Cajal S. La Textura del Sistema Nerviosa del Hombre y los Vertebrados. Madrid Moya: Primera Edicion; 1899. [Google Scholar]
- Reyes AD, Sakmann B. Summation of synaptic potentials in layer V pyramidal neu- rons. Soc Neurosci Abstr. 1996;22:792. [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative fine-structural analysis of olfactory cortical synapses. Proc Natl Acad Sci U S A. 1999;96:4107–4112. doi: 10.1073/pnas.96.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Schuz A, Dortenmann M. Synaptic density on non-spiny dendrites in the cerebral cortex of the house mouse. A phosphotungstic acid study. J Hirnforsch. 1987;28:633–639. [PubMed] [Google Scholar]
- Shepherd G. The dendritic spine: a multifunctional integrative unit. J Neurophysiol. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The Synaptic Organization of the Brain. Oxford: Oxford University Press; 1990. [Google Scholar]
- Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyants A, Hof PR, Chklovskii DB. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Hausser M. Dendrites. Oxford: Oxford University Press; 1999. [Google Scholar]
- Sussillo D, Abbott LF. Generating coherent patterns of activity from chaotic neural networks. Neuron. 2009;63:544–557. doi: 10.1016/j.neuron.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Tank DW, Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- Swindale NV. Dendritic spines only connect. TINS. 1981;4:240–241. [Google Scholar]
- Tamas G, Szabadics J, Somogyi P. Cell type- and subcellular position-dependent summation of unitary postsynaptic potentials in neocortical neurons. J Neurosci. 2003;22:740–747. doi: 10.1523/JNEUROSCI.22-03-00740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, Chklovskii DB. A cost-benefit analysis of neuronal morphology. Journal of neurophysiology. 2008;99:2320–2328. doi: 10.1152/jn.00280.2007. [DOI] [PubMed] [Google Scholar]
- Wen Q, Stepanyants A, Elston GN, Grosberg AY, Chklovskii DB. Maximization of the connectivity repertoire as a statistical principle governing the shapes of dendritic arbors. Proc Natl Acad Sci U S A. 2009;106:12536–12541. doi: 10.1073/pnas.0901530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J, Nicolelis M. Optimizing a linear algorithm for real-time robotic control using chronic cortical ensemble recordings in monkeys. J Cogn Neurosci. 2004;16:1022–1035. doi: 10.1162/0898929041502652. [DOI] [PubMed] [Google Scholar]
- Yuste R. Dendritic Spines. Cambridge: Mass., MIT Press; 2010. [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Yuste R, Gutnick MJ, Saar D, Delaney KR, Tank DW. Ca2+ accumulations in dendrites of neocortical pyramidal neurons: an apical band and evidence for two functional compartments. Neuron. 1994;13:23–43. doi: 10.1016/0896-6273(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Yuste R, Majewska A, Cash S, Denk W. Mechanisms of calcium influx into spines: Heterogeinity among spines, coincidence detection by NMDA receptors and optical quantal analysis. J Neurosci. 1999;19:1976–1987. doi: 10.1523/JNEUROSCI.19-06-01976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Tank DW. Dendritic Integration in Mammalian Neurons, a Century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Ziv N, Smith S. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]