Abstract
Although genetics determines endocrine phenotypes, it cannot fully explain the great variability and reversibility of the system in response to environmental changes. Evidence now suggests that epigenetics, i.e. heritable but reversible changes in gene function without changes in nucleotide sequence, links genetics and environment in shaping endocrine function. Epigenetic mechanisms, including DNA methylation, histone modification, and microRNA, partition the genome into active and inactive domains based on endogenous and exogenous environmental changes and developmental stages, creating phenotype plasticity that can explain interindividual and population endocrine variability. We will review the current understanding of epigenetics in endocrinology, specifically, the regulation by epigenetics of the three levels of hormone action (synthesis and release, circulating and target tissue levels, and target-organ responsiveness) and the epigenetic action of endocrine disruptors. We will also discuss the impacts of hormones on epigenetics. We propose a three-dimensional model (genetics, environment, and developmental stage) to explain the phenomena related to progressive changes in endocrine functions with age, the early origin of endocrine disorders, phenotype discordance between monozygotic twins, rapid shifts in disease patterns among populations experiencing major lifestyle changes such as immigration, and the many endocrine disruptions in contemporary life. We emphasize that the key for understanding epigenetics in endocrinology is the identification, through advanced high-throughput screening technologies, of plasticity genes or loci that respond directly to a specific environmental stimulus. Investigations to determine whether epigenetic changes induced by today's lifestyles or environmental `exposures' can be inherited and are reversible should open doors for applying epigenetics to the prevention and treatment of endocrine disorders.
Introduction and background
The most simplistic view of an endocrine axis is one that involves the release of a hormone from an endocrine gland, in response to a stimulus, into the circulation. The hormones reach all body cells but elicit changes only in target organs that express cognate receptors, and hormone–receptor interactions transduce the message encoded in the hormone to cellular responses. Thus, at least three levels of regulation govern the normal functioning of an endocrine axis: the proper synthesis and timely release of the hormone; the maintenance of an effective hormone level in the circulation; and the expression of appropriate levels of functional receptors in target organs. Positive- or negative-feedback loops operate to accentuate or mitigate respectively the action of a hormone by modulating its release and/or end-organ responsiveness. The malfunctioning or dysregulation at any of these three levels of control or the disruption of a key feedback loop could result in the initiation or progression of endocrine disease.
In this review, we describe current advances and understanding of epigenetics in endocrinology by searching PubMed, GeneCards, and Entrez Gene databases for current state of knowledge of epigenetics and endocrinology. This article addresses the topic from the cellular and the organismal level. Specifically, it will review how epigenetics regulates the three levels of hormone action (synthesis and release, circulating and target tissue hormone levels, and target-organ responsiveness) and serves as a key mediator of endocrine disruption, and will provide insights into why epigenetics is the missing link between genetics, the environment, and endocrine functions.
Genetics as a key determinant of endocrine function
Genetics is traditionally viewed as the sole factor controlling the differentiation and function of various endocrine axes. As such, mutations in key hormone-synthesis genes cause endocrine disorders (Lobato et al. 1999, Scully & Rosenfeld 2002, Davis et al. 2009, Montanelli & Tonacchera 2010). However, mutational events are rare and cannot explain the high interindividual and interpopulation variability observed in the endocrine system. Genetic polymorphisms are present in the population at a higher frequency than mutation and are considered as normal variations in the genome. They are responsible for many of the normal differences in endocrine function and susceptibility to disorders observed among individuals or populations (Correa et al. 1999, Franceschi et al. 2005, Rubello et al. 2005, Lee et al. 2008). A genome-wide association study recently identified and confirmed multiple susceptibility variants in at least ten loci for type 2 diabetes (Scott et al. 2007). Thus, the normal development and function of the endocrine system and its variability among people are, without a doubt, highly dependent on genetic control. However, genetic mutations and variability either are inherited from a parent or are acquired during one's lifetime. The inherited variability is static and does not change in response to the environment. The acquired variability can be caused by an environmental factor such as u.v. radiation from the sun (exogenous) or reactive oxygen species generated during metabolism (endogenous). But once acquired these effects are permanent and irreversible. Thus, inherited and acquired variability, either alone or in concert, cannot fully explain the high degree of variability and the reversibility of the endocrine system in response to the environment.
Phenotype plasticity and developmental plasticity: bases of genotype by environment interaction
The environment, endogenous or exogenous, plays a highly significant role in determining the function and variability of an endocrine axis. Most cells or organs have various degrees of phenotypic plasticity, whereby the phenotype expressed by a genotype is dependent on environmental influences (Feinberg 2007). This is best illustrated by studies in discordant phenotypes in monozygotic (MZ) twins (Fraga et al. 2005) showing that the genetic contribution to endocrine disorders such as type 1 diabetes and Graves' disease (Gale et al. 2001) is 30–50%, whereas acquired obesity – a change in endogenous environment – contributes over 50% to the risk of insulin resistance in MZ twin pairs (Pietilainen et al. 2008). Collectively, these findings indicate that nongenetic factors, including the environment, are important determinants of variability in endocrine function and risk of disorders. Endocrine glands and their target organs, because they function to maintain homeostasis in the body, must be highly responsive to environmental changes.
In addition to changing transcriptional programs in response to an environmental stimulus, endocrine tissues can use developmental plasticity (Bateson et al. 2004) to establish adaptive phenotypes that have a more long-lasting impact. Such responses are long-term adjustments of an endocrine axis, which are based on present guesses about the probable demands in later life. Plastic responses that evolved to confer benefits in later life are more likely to be established during critical developmental periods such as in utero, during puberty, and during pregnancy, times of great tissue differentiation (Kuzawa & Quinn 2009). These adaptive traits are usually beneficial to the health of the individual. However, exceptions arise when an individual who is developmentally adapted to one environment is exposed to a contradictory environment. A high degree of mismatch between the adaptive trait and the future environment, which includes aging, changes in lifestyle, or the introduction of new chemicals, pathogens, and pollutants, may increase the risk of developing disease. Prime examples are the strong correlations observed between hyponutrition and/or low birth weight with many endocrine disorders related to thyroid function, calcium balance, utilization of glucose, insulin sensitivity, and adrenal gland function (Vaag & Poulsen 2007, Hyman et al. 2009, Latini et al. 2009).
The mechanisms underlying the interactions of genetics and the environment, which produce an adaptive phenotype in an endocrine axis, remain elusive. However, a growing body of literature suggests that the missing connection resides in epigenetics, a pivotal mechanism of interactions between genes and the environment (Jaenisch & Bird 2003, Cook et al. 2005, Jirtle & Skinner 2007, Tang & Ho 2007, Vaag & Poulsen 2007, Ling & Groop 2009; Fig. 1).
Figure 1.
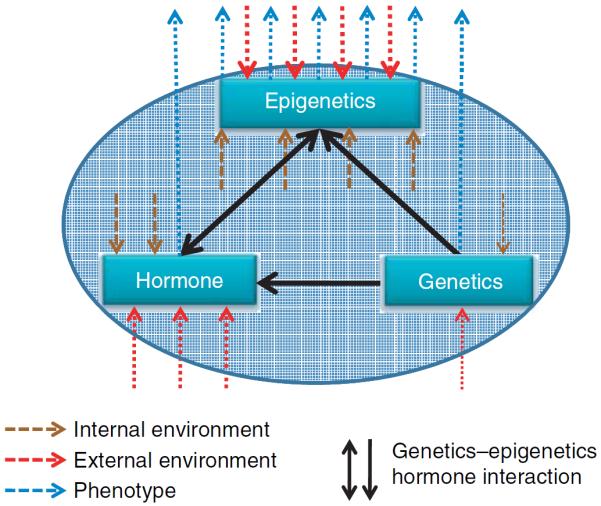
Epigenetics links genetics with the environment in endocrine function. Hormone levels vary in response to internal and external environmental changes. Epigenetics, in response to exogenous and endogenous environmental cues, defines active and repressed domains of the genome. These responses explain the high phenotypic plasticity observed in the endocrine system, in which different genetic programs are executed from the same genome based on changes in the environment. The endocrine system is more plastic during certain developmental periods such as in utero, during puberty, and with aging. A dysregulation in epigenetic and/or genetic control of endocrine function is frequently the cause of disease pathogenesis. On the other hand, the effects of the environment or the hormonal milieu on genetics are limited, with nucleotide or chromosomal changes induced by radiation as an example.
Epigenetic mechanisms that landscape the genome
Epigenetic modifications defined as heritable changes in gene function that occur without a change in the nucleotide sequence (Bird 2007, Goldberg et al. 2007, Berger et al. 2009). They are mitotically and transgenerationally inheritable (Rakyan et al. 2002, 2003, Hitchins et al. 2007) and potentially reversible (Bannister & Kouzarides 2005, Weaver et al. 2005). The most studied mechanisms known to affect the epigenome are DNA methylation, histone modification, and aberrant expression of microRNAs (miRNAs; Esteller 2005). These processes along with other epigenetic events determine when and whether various sets of genes are expressed in a tissue or cell. They therefore play crucial roles in determining the transcriptional programs of the endocrine glands and their target organs (Crews & McLachlan 2006, Deladoey et al. 2007, Musri et al. 2007, Tang & Ho 2007, Rampersaud et al. 2008, Ling & Groop 2009, Blaustein 2010). Inherited or sporadic epimutations (Holliday 1991) or dysregulation of the epigenome in an endocrine gland or its target organs could lead to disease development. In principle, epigenetic changes are reversible because no primary DNA sequences or chromosomal changes are involved. This unique property provides an explanation for the versatility of the endocrine system and affords great opportunities for devising intervention strategies (e.g. lifestyle changes) and epigenetic therapies (Ganesan et al. 2009).
DNA methylation involves the addition of a methyl group to the 5′ position of the cytosine pyrimidine ring and targets primarily the cytosine residues in CpG dinucleotides (Ooi et al. 2009). CpG dinucleotides are underrepresented in the mammalian genome (1–2%) but tend to cluster as CpG islands in gene promoter regions. Hypermethylation of promoter CpG island is commonly associated with transcriptional silencing, but exactly how this is achieved is less clear. It has been proposed that the methylated promoter has reduced affinity for transcriptional factors and that it tends to associate with methylated DNA-binding proteins (e.g. MeCP2, MBD1, MBD2, MBD3, and MBD4; Bogdanovic & Veenstra 2009), which further recruit histone deacetyltransferases and other corepressors. Methylated promoters are also associated with unique repressive histone markers (see below, Tiwari et al. 2008). DNA methylation requires the activity of DNA methyltransferases (DNMTs). DNMT1 is responsible for reproducing the DNA methylation pattern of the parent cell in its daughter cells (maintenance methylation), whereas DNMT3a and DNMT3b (de novo methylation) can generate new methylation patterns in quiescent cells (Hermann et al. 2004, Siedlecki & Zielenkiewicz 2006). The mechanism of DNA demethylation is less well understood. Loss of binding to methylated DNA-binding proteins may allow the promoter to enter into a state of transcription. However, the association of methylated DNA with MBD2 or MBD4 has been proposed to induce active DNA demethylation, a hypothesis that is still under serious debate (Lal & Bromberg 2009, Patra & Bettuzzi 2009).
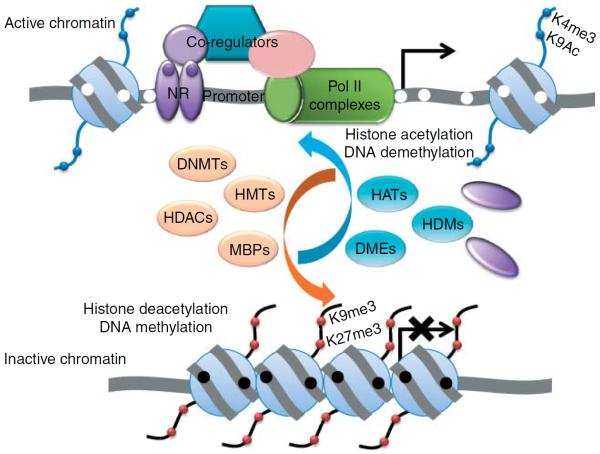
Histones are special proteins that facilitate the packaging of the DNA into nucleosomes, the basic building block of the chromatin. Posttranslational modifications such as acetylation, methylation, phosphorylation, sumoylation, and ubiquitination occur at specific residues in histones N-terminal tails (Cosgrove et al. 2004). These modifications determine whether the DNA wrapped around histones is accessible to the transcriptional machinery. Specific histone modifications are associated with gene activation and silencing and also regulate other chromatin remodeling events that control transcription, replication, recombination, and higher-order chromosomal organization (Clapier & Cairns 2009). Chromatin structure is now recognized as a primary target of signal transduction (Cheung et al. 2000) whereby extracellular signals, including those encoded in hormones such as IGF1, are transduced to genomic events via specific histone modifications (Sun & D'Ercole 2006). Histones are modified by specific enzymes that include histone acetyltransferases, histone deacetylases, and histone methyltransferases (Miremadi et al. 2007). In most instances, histone modifications work hand-in-hand with DNA methylation to achieve short- and long-term changes in transcriptional programs through transient or permanent reorganization of the chromatin architecture (Kondo 2009; Fig. 2).
Figure 2.
DNA methylation and histone modification are two major epigenetic mechanisms that corroborate in regulating endocrine-related gene expression. Packaging genes into active or inactive chromatin determines whether they are transcriptionally accessible or not. The N-termini of histones have specific amino acids that are sensitive to posttranslational modifications, which contribute to chromatin status. Moreover, hypermethylation of promoter is associated with transcriptional silencing due, in part, to the loss of affinity for transcriptional factors such as the nuclear receptor (NR) and accessibility by the transcriptional machinery as represented by RNA Pol II complexes. The inactive chromatin has increased affinity for methylated DNA-binding proteins (MBPs), which further recruit histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and histone methyltransferases (HMTs), and other corepressors. Methylated promoters are associated with unique repressive histone markers, which classically include trimethylation of histone 3 (H3), lysine (K) 9, and H3–K27. In contrast, unmethylated promoters are associated with gene transcription. They have increased affinity for histone acetylases (HATs), histone demethylases (HDMs), DNA demethylases (DMEs; e.g. DNA N-glycosylase), and histone marks associated with active chromatin, including acetylated H3–K9 and trimethylated H3–K4. Nucleosome remodeling such as repositioning and ejection in promoter results in gene transcription (bent arrow). Me, histone methylation; Ac, histone acetylation; black filled circle, methylated CpG dinucleotides; white filled circle, unmethylated CpG dinucleotides; filled purple circle, hormone or endocrine disruptors that bind to NR.
miRNAs function as posttranscriptional regulators of cognate target gene expression (Rodriguez et al. 2004). They are a class of small noncoding RNAs produced from either their own genes or introns/exons of other genes. They bind to target mRNAs with complete or incomplete complementarities and/or degrade/modify target mRNAs to suppress protein translation (Cannell et al. 2008). Therefore, one miRNA may target multiple mRNAs and one mRNA may be regulated by different miRNAs. Because of the high false-positive rate of current prediction programs for miRNA targets, the validation process of miRNA targets is laborious and time consuming (Wang & Wang 2006). Thus, although the field is still in an early stage of development, it holds great potential to reveal a new level of epigenetic regulation.
With the rapid advances in technologies for high-throughput global analysis of DNA methylation, histone modification, and miRNA profiling (Kong et al. 2009) and the advent of next-generation sequencing technology (Metzker 2010), we expect an explosive phase of growth in genome and epigenome science. Along with these advances will be a significant deepening of our understanding of the interplay of genetics and epigenetics with the environment in sculpting the endocrine system at the individual and population level and of the etiology of endocrine disorders.
Epigenetic regulation of the action of steroid hormones, thyroid hormones, retinoic acid, and calcitriol
A recent review has discussed certain aspects of epigenetic regulation of the expression of genes involved in steroid biosynthesis and action (Martinez-Arguelles & Papadopoulos 2010). In this review, we have presented additional insights into this topic.
Steroid hormones, thyroid hormone (triiodothyronine and thyroxine), retinoic acid (vitamin A), and calcitriol belong to a family of hormones that signal through the nuclear receptors that act as transcriptional factors (Evans 1988). Calcitriol (Huhtakangas et al. 2004) is derived from vitamin D from dietary sources or manufactured in the skin from sun exposure; steroid hormones are synthesized by the adrenal glands and the gonads; and thyroid hormones are synthesized in the thyroid gland. All of these hormones are small lipophilic molecules that can pass through the cell membrane and even reach the chromatin directly. Traditionally, these hormones have been thought to interact with their cognate nuclear receptors, which form a superfamily comprising two broad classes (Evans 1988, Novac & Heinzel 2004). Type I nuclear receptors reside in the cytosol through interaction with heat–shock proteins. When these hormones bind to type I receptors, the receptors are activated, released from the heat–shock complex, and translocated to the cell nucleus, where they bind to DNA sequences known as hormone-responsive elements and initiate transcription. Type II receptors reside in the nucleus even in the absence of ligand and bind to cis-elements as heterodimers.
Both the circulating levels of steroid hormones and the levels of active hormones in target organs are dependent on the rate of biosynthesis, the levels of steroid hormone-binding proteins in circulation, and the balance between metabolic activation and degradation in the target tissues. Ultimately, the levels of free steroid present in the target organs determine the degree of hormonal stimulation. Genes encoding key enzymes in steroid biogenesis/metabolic activation–degradation are the members of the cytochrome P450 (CYP) superfamily (Miller 1988). CYPs in subfamilies 1–3 are involved primarily in the metabolism of xenobiotic compounds, whereas the other subfamilies are responsible for the metabolism of endogenous compounds, including steroid hormones (Rodriguez-Antona et al. 2010). In addition to the CYPs, the steroidogenic acute regulatory or StAR protein that regulates cholesterol entry to the mitochondria and ultimately cholesterol synethesis is considered a gate-keeping molecule for steroidogenesis (Miller 2007). In our survey of the literature for reports of possible epigenetic regulation of a total of 16 genes encoding StAR and enzymes central to the biosynthesis or degradation of steroid hormones and responsible for calcitriol synthesis, we found only 5 that have been studied for DNA methylation (Table 1).
Table 1.
Epigenetic regulation of steroidogenesis and degradation genes
| Key enzyme |
Gene |
Methylation control |
miRNA control |
Related tissue/cell |
References |
|---|---|---|---|---|---|
| StAR | STAR | U | U | ||
| P450scc | CYP11A1 | Yes | U | Ovarian follicles | Vanselow et al. (2010) |
| 3β-Hydroxysteroid dehydrogenase | HSD3B1 | Yes | U | Ovarian follicles | Vanselow et al. (2010) |
| HSD3B2 | U | U | |||
| 21α-Hydroxylase | CYP21A2 | U | U | ||
| 11β-Hydroxylase | CYP11B1 | U | U | ||
| Aldosterone synthase | CYP11B2 | U | U | ||
| 17α-Hydroxylase | CYP17A1 | Yes | U | Hepatoma cells; adrenal cortex | Dannenberg & Edenberg (2006) and Missaghian et al. (2009) |
| 17β-Hydroxysteroid dehydrogenases | HSD17B3 | U | U | ||
| Aromatase | CYP19A1 | Yes | U | Ovarian follicles, endometrial, and endometriotic stromal cells; breast adipose fibroblasts; hepatoma cells | Dannenberg & Edenberg (2006), Demura & Bulun (2008), Izawa et al. (2008), Vanselow et al. (2008) and Knower et al. (2010) |
| 5α-Reductase | SRD5A1 | U | U | ||
| SRD5A2 | U | U | |||
| Vitamin D synthesis | CYP27A1 | U | U | ||
| CYP27B1 | Yes | U | Mouse kidney proximal tubule-derived MCT cells and human embryonic kidney-derived 293F | Kim et al. (2009) | |
| Steroid degradation/bile acid synthesis | CYP3A4 | U | U | ||
| CYP7A1 | U | U |
As a general reference, the substrate(s) and product(s) of the enzymes can be found at http://en.wikipedia.org/wiki/Steroid (accessed 1 September 2010).
U, unknown or unclear, denotes no or very few papers reported as of 1 August 2010.
A study of bovine follicles designed to study cell-type-specific methylation investigated three key steroido-genesis enzymes (CYP11A1, HSD3B1, and CYP19A) and found that individual but not islands of CpG dinucleo-tides located proximal from the transcriptional start sites of these genes were differentially methylated (Vanselow et al. 2010). DNA methylation was also shown to be involved in the permanent silencing of promoter 2-directed CYP19A1 expression in lutein cells. The epigenetic shutdown of CYP19A1, the enzyme responsible for the conversion of androgens to estrogens, is physiologically relevant to luteinization. Other studies also found a role of DNA methylation in the CYP19A1 regulation in endometrial and endometriotic stromal cells (Feinberg 2007, Izawa et al. 2008), breast adipose fibroblasts, breast cancer cells (Demura & Bulun 2008, Knower et al. 2010), and human hepatoma cells (Dannenberg & Edenberg 2006). Similarly, DNA methylation was found to regulate the expression of CYP17A1, which encodes a key steroidogenesis enzyme in a human placental cell line and the rodent adrenal glands (Missaghian et al. 2009). Collectively, these studies demonstrated the important roles DNA methylation had in regulating the key enzymes of the hormone biosynthesis. However, the expression of the STAR gene was found to be epigenetically regulated by histone modifications (Hiroi et al. 2004).
Epigenetics also plays important roles in regulating thyroid hormone and retinoic acid metabolism. For example, the expression of the sodium iodide symporter (SLC5A5), which is responsible for the uptake of iodine in the thyroid, was shown to be regulated by cytosine methylation of its promoter (Venkataraman et al. 1999, Smith et al. 2007). Similarly, the transcriptional response of CYP26A1, a specific CYP hydrolase involved in retinoic acid catabolism, is under epigenetic regulation through DNA methylation and histone modification (Pozzi et al. 2006, Kashyap & Gudas 2010).
The final step in the bioactivation of vitamin D takes place in the kidney through the action of 1α hydroxylase encoded by CYP27B1 (Omdahl et al. 2002). A recent study showed that DNA methylation regulates CYP27B1 expression (Kim et al. 2009). However, the most important contribution of the study was the identification of the mechanism underlying the demethylation of this gene. While vitamin D represses CYP27B1 via the vitamin D receptor-interacting repressor complex at the transcriptional level (Kim et al. 2007), parathyroid hormone (PTH) stimulates expression of the gene via induction of demethylation of the CYP27B1 promoter. Further investigation uncovered the mechanism underlying the demethylation process. It involved PTH-induced phosphorylation of the MBD4 glycosylase to exert active DNA demethylation through the base-excision-repair pathway. These findings illustrate the complex interplay between hormone and epigenetic factors in the regulation of vitamin D biosynthesis via a dietary-hormonal feedback loop and provide a mechanism by which a peptide hormone can induce epigenetic changes via activation of chromatin remodeling enzymes.
Inappropriate expression of a steroidogenesis enzyme is now considered an important etiological factor for endocrine-related cancer and its progression from dependency on gonadal or adrenal steroids to an independent state. One example is provided by the reactivation of permanently silenced steroidogenesis genes in prostate cancer. Intratumoral testosterone levels were found to be higher in metastases removed from castrated men than in primary prostate cancer from untreated eugonadal men. The ability of the cancer to survive and progress to a higher grade in the absence of testicular androgens can be attributed to the overexpression of various steroidogenesis enzyme genes (CYP17A1, HSD3B1, HSD17B3, and CYP19A1) in the metastases tissues (Montgomery et al. 2008, Chun et al. 2009, Knudsen & Penning 2010). One mechanism for the aberrant reactivation could be a relaxation in epigenetic silencing. Indeed, promoter methylation contributes to local downregulation of CYP7B1 in prostate tissues, resulting in the accumulation of 5α-androstane-3β,17β-diol, a purported estrogen receptor-β agonist (Olsson et al. 2007). The question of whether the re-expression of steroidogenesis enzyme genes is mediated by epigenetic changes will be an important future area of investigation.
Target-organ/cell sensitivity and responsiveness to these lipophilic hormones is mediated in part by the levels of their receptors. We surveyed 14 common type I or type II nuclear receptor genes for evidence of epigenetic regulation. Most type I receptor genes have been found to be regulated by DNA methylation and to a lesser extent by miRNA (Table 2). Generally speaking, DNA methylation must work in concert with histone deacetylation and histone methylation (most commonly methylation of histone 3 at lysine 9) to create a repressed chromatin state for gene silencing (Fuks 2005). Thus, we were surprised to find very little information on specific activation or repression histone marks (Peterson & Laniel 2004) associated with the regulation of these nuclear receptors. Owing to this data void, we have not included histone modifications in our tables.
Table 2.
Epigenetic regulation of type I and type II nuclear receptors
| Type |
Major receptor |
Gene |
Methylation control |
miRNA control |
Related disorders |
References |
|---|---|---|---|---|---|---|
| I | Androgen receptor | AR | Yes | U | Prostate; endometrial | Kinoshita et al. (2000) and Sasaki et al. (2000) |
| Estrogen receptor 1 | ESR1 | Yes | miR-206 | Breast | Yoshida et al. (2000), Archey et al. (2002), Adams et al. (2007), Champagne & Curley (2008) and Kondo et al. (2008) | |
| Estrogen receptor 2 | ESR2 | Yes | U | Prostate; breast; ovary | Zhao et al. (2003), Zhu et al. (2004), Zhang et al. (2007) and Zama & Uzumcu (2009) | |
| Progesterone receptor | PGR | Yes | U | Endometrial; prostate | Sasaki et al. (2003) | |
| Glucocorticoid receptor | NR3C1 | Yes | miR-124a | Brain disorder; obesity | Vreugdenhil et al. (2009) and Stevens et al. (2010) | |
| Mineralocorticoid receptor | NR3C2 | Yes | miR-124 and -135a | Testes | Martinez-Arguelles et al. (2009) and Sober et al. (2010) | |
| II | Retinoic acid receptor-α | RARA | Yes | U | Leukemia | Chim et al. (2005) |
| Retinoic acid receptor-β | RARB | Yes | U | Gastric and esophageal cancer | Hayashi et al. (2001) and Wang et al. (2003) | |
| Retinoic acid receptor-γ | RARG | U | miR-182 | Senescence | Li et al. (2009a) | |
| Retinoid X receptor-α | RXRA | U | miR-27a and -27b | Liver fibrosis | Ji et al. (2009) | |
| Retinoid X receptor-β | RXRB | U | U | |||
| Retinoid X receptor-γ | RXRG | U | U | |||
| Thyroid hormone receptor-α | THRA | U | U | |||
| Retinoic acid receptor-β | THRB | U | U |
U, unknown or unclear, denotes no or very few papers reported as of 1 August 2010.
As a general observation, epigenetic dysregulation of the expression of type I receptor genes is closely linked to endocrine-related disorders including cancers of the breast, prostate, testis, and endometrium. DNA methylation dysregulates androgen receptor expression in prostate and endometrial cancer (Kinoshita et al. 2000, Sasaki et al. 2000), estrogen receptor-α in breast cancer (Yoshida et al. 2000, Archey et al. 2002, Adams et al. 2007, Champagne & Curley 2008), estrogen receptor-β in ovarian, prostate, and breast cancer (Zhao et al. 2003, Zhu et al. 2004, Zhang et al. 2007, Zama & Uzumcu 2009), and progesterone receptor in endometrial cancer (Sasaki et al. 2003). Besides cancer, the expression of mineralocorticoid receptor in the rat testis was found to be dysregulated by di-(2-ethylhexyl) phthalate (Martinez-Arguelles et al. 2009). Meanwhile, acetylation of histone H3K9 and hypomethylation in the glucocorticoid receptor (GR) promoter region were found to increase hypothalamic GR expression in fetuses of undernourished ewes that develop obesity in later life (Stevens et al. 2010). Of late, an increasing number of miRNAs have been identified, which regulate the expression of type I nuclear receptor. For example, miR-206 is reported to decrease the levels of estrogen receptor-α mRNA and protein in human MCF-7 breast cancer cells (Kondo et al. 2008). miR-124a reduces levels of GR protein and GR-mediated responses during the stress hyporesponsive period of early brain development (Vreugdenhil et al. 2009).
In contrast, fewer type II receptor genes have been reported to be epigenetically regulated, perhaps because fewer studies have been conducted on type II than type I nuclear receptors. Our in silico analyses using CpG island predicting programs (Li & Dahiya 2002, Wolfsberg 2010), however, reveal that type II receptors have CpG islands in their promoters and could be potential targets of DNA methylation-mediated transcriptional regulation. Apropos to type II receptors, aberrant promoter methylation was observed for the retinoic acid receptor-α in leukemia (Chim et al. 2005) and for retinoic acid receptor-β in gastric and esophageal cancer (Hayashi et al. 2001, Wang et al. 2003). Retinoic acid receptor-γ (RARG), on the other hand, was found to be a target of miR-182, which plays an important role in downregulating RARG expression during stress-induced premature senescence in primary cultures of human diploid fibroblast and human trabecular meshwork cells (Li et al. 2009a). Furthermore, expression of retinoid X receptor-α was shown to be regulated by miR-27a and miR-27b in liver sclerosis (Ji et al. 2009). Thus, type II nuclear receptors appear to be as susceptible as type I receptors to tissue-specific epigenetic regulation of expression, a key determinant of hormone responsiveness in target tissues.
Collectively, epigenetics appears to be a common mechanism for regulating the expression of the nuclear receptors in hormone-sensitive organs. An unscheduled or deviant expression of regulatory miRNA or promoter methylation of the receptor gene can greatly affect end-organ sensitivity for an endocrine axis. DNA methylation represents the most well-studied mechanism, but studies of miRNA-mediated regulation are rapidly gaining ground. Information on activation or repressive histone modifications associated with receptor regulation or hormone biosynthesis is clearly lacking and should attract growing interest in the future.
Epigenetic regulation of peptide hormone action
Peptide hormones are another major class of hormones, which have a broad spectrum of action, including regulation of energy metabolism (e.g. insulin), adiposity (e.g. leptin), growth (e.g. GH), and differentiation (e.g. FSH). These peptides are secreted by endocrine glands/cells with high tissue specificity. The hypothalamus, pituitary, gastrointestinal tract, and nonendocrine tissues such as adipocytes and neurons are the major sources of peptide hormones.
Peptide hormones, similar to other proteins, are encoded by one or two genes for different subunits (Pearson et al. 1993). They often are produced as pro-hormones, then turned into the active forms through multiple steps of intracellular processing and posttranslational modification, and released into the circulation as mature hormones. The release can be episodic (pulsatile) or may follow the circadian rhythm (Veldhuis & Bowers 2003, Maywood et al. 2007). Response is mediated through the interaction with a cell membrane receptor, which triggers a cascade of events within the target cells, which leads to peptide hormone action. These cascades usually involve a second messenger (e.g. cAMP, cGMP, and calcium) and multiple steps of phosphorylation events to activate the final targets. The action of peptide hormones is normally faster than that of steroid hormones at the target cells because the immediate effects of peptide hormones are mediated by enzymatic processes in the cytoplasm without involving gene transcription and the synthesis of new proteins (Jansen 1984).
Genes encoding peptide hormones or their receptors are potential epigenetic targets in the large scheme of peptide hormone action. A total of 18 representative genes encoding 18 peptide hormones/subunits that secret from different endocrine glands or nonendocrine tissues were selected for analysis (Table 3). Although promoter CpG islands have been identified in most peptide hormone genes, only a few of these genes were found to be regulated by DNA methylation (somatostatin, vasopressin, melanocyte-stimulating hormone, secretin, insulin, and leptin). Only the gene encoding insulin (INS) was also reported to be regulated by miR-30d (Tang et al. 2009). Table 4 lists the corresponding receptors for the peptide hormones listed in Table 3. With the exception of INSR, GHR, PRLR, and leptin receptor (LEPR), all are G-protein-coupled receptors, a superfamily of receptors that signal via the cAMP signaling pathway or the phosphatidylinosital signaling cascade (Gilman 1987). Again, few of the genes encoding these receptors have been found to be under the control of DNA methylation for gene expression, and no miRNAs affecting their synthesis have been identified. Disruption of the synthesis of peptide hormones or their cognate receptors by epigenetic events often leads to metabolic changes (e.g. obesity and metabolic syndrome; Plagemann et al. 2009) and abnormalities in neuropsychological behavior (e.g. autism and alcohol dependence; Gregory et al. 2009, Hillemacher et al. 2009), as opposed to cancer, the predominant disorder for epigenetic dysregulation of steroid hormones and their receptors (Widschwendter et al. 2004).
Table 3.
Epigenetic regulation of peptide hormone genes
| Gland/tissue |
Hormone |
Gene |
Methylation control |
miRNA control |
Related tissue/cell |
References |
|---|---|---|---|---|---|---|
| Hypothalamus | GnRH | GNRH1 | U | U | ||
| TRH | TRH | U | U | |||
| Somatostatin | SST | Yes | U | Esophageal carcinogenesis; colon cancer | Mori et al. (2006) and Jin et al. (2008a) | |
| Vasopressin | VAP | Yes | U | Alcohol dependence | Hillemacher et al. (2009) | |
| Oxytocin | OXT | U | U | |||
| Pituitary | FSH | FSHB | No | U | Whitfield & Kourides (1985) | |
| LH | LHB | No | U | Whitfield & Kourides (1985) | ||
| TSH | TSHB | No | U | Whitfield & Kourides (1985) | ||
| MSH | POMC | Yes | U | Anorexia nervosa; ectopic ACTH syndrome; Cushing's syndrome | Newell-Price (2003), Ye et al. (2005) and Ehrlich et al. (2010) | |
| GH | GH1 | U | U | |||
| Prolactin | PRL | U | U | |||
| Gastrointestine | Gastrin | GAST | U | U | ||
| Cholecystokinin | CCK | U | U | |||
| Secretin | SCT | Yes | U | Cell lines | Lee et al. (2004) | |
| Insulin | INS | Yes | miR-30d | Pancreatic β-cell and β-cell line | Kuroda et al. (2009) and Tang et al. (2009) | |
| Glucagon | GCG | U | U | |||
| Adipocyte | Leptin | LEP/OB | Yes | U | Osteoarthritic chondrocytes; adipose and leukocytes; preadipocytes maturation | Melzner et al. (2002), Stoger (2006) and Iliopoulos et al. (2007) |
| Adiponectin | ADIPOQ | U | U |
U, unknown or unclear, denotes no or very few papers reported as of 1 August 2010.
Table 4.
Epigenetic regulation of peptide hormone receptor genes
| Hormone |
Receptor gene |
Methylation control |
miRNA control |
Related tissue/cell |
References |
|---|---|---|---|---|---|
| GnRH | GNRHR | U | U | ||
| TRH | TRHR | U | U | ||
| Somatostatin | SSTR1 | U | U | ||
| Vasopressin | AVPR1A | U | U | ||
| Oxytocin | OXTR | Yes | U | Temporal cortex in autism | Gregory et al. (2009) |
| FSH | FSHR | Yes | U | Male gonad | Griswold & Kim (2001) |
| LH | LHCGR | U | U | ||
| TSH | TSHR | Yes | U | Thyroid tumors | Xing et al. (2003) |
| MSH | MC1R | U | U | ||
| GH | GHR | U | U | ||
| Prolactin | PRLR | U | U | ||
| Gastrin | CCKBR | U | U | ||
| Cholecystokinin | CCKAR | U | U | ||
| Secretin | SCTR | U | U | ||
| Insulin | INSR | U | U | ||
| IGF2 | IGF1R/IGF2R | U/yes | miR-223/hsa-miR-657 | Neutrophils/HEK 293 and Hep G2 cells | Stoger et al. (1993), Johnnidis et al. (2008), Lv et al. (2008) and Schayek et al. (2010) |
| Glucagon | GCGR | U | U | ||
| Leptin | LEPR | No | U | Adipose tissue | Noer et al. (2006) |
| Adiponectin | ADIPOR1/ADIPOR2 | U | U |
U, unknown or unclear, denotes no or very few papers reported as of 1 August 2010.
Notably, epigenetic regulation of genes encoding peptide hormones or their receptors is largely related to developmental stage- and tissue-specific function or the development of a metabolic or neural disorder. For example, in cultures of mouse embryonic stem cells, the hypermethylated promoter of the insulin gene undergoes demethylation as these cells differentiate into hormone-producing cells; and in both the mouse and human insulin gene promoters, the CpG sites are demethylated in insulin-producing pancreatic β-cells but not in other tissues without insulin expression (Kuroda et al. 2009). Leptin is an adipocyte hormone regulating energy homeostasis. The hormone is not expressed in human preadipocytes because the leptin promoter is hypermethylated and the gene is silenced (Melzner et al. 2002). But as humans mature, the gene is switched on through promoter demethylation. Both long-distance and promoter CpG methylation are associated with regulation of gene expression (Stoger 2006). Disruption of these epigenetic programs leads to a range of metabolic diseases and their related disorders, including osteoarthritis (Iliopoulos et al. 2007). The promoter of LEPR is also under epigenetic regulation through DNA methylation, as reported in a study with mesenchymal stem cells of adipose tissue (Noer et al. 2006).
In a recent study of autism-spectrum disorders, hypermethylation of the gene promoter encoding the oxytocin receptor was found to be associated with a reduced level of mRNA expression and was significantly associated with autism (Gregory et al. 2009). In another report, significant alterations of the mRNA expression and promoter-related DNA methylation of vasopressin were reported in patients with alcohol dependence (Hillemacher et al. 2009). Both vasopressin and oxytocin are now known as `social neuropetides', regulating a multitude of prosocial behaviors (Rossignol 2009, Stein 2009). Although further investigations and stronger evidence are required, these reports open new avenues for research in epigenetic regulation of human behavior (Gurrieri & Neri 2009).
Endocrine regulation of key epigenetic-modifying enzymes
DNMTs, methyl-CpG (MeC)-binding proteins, and histone-modifying enzymes (see the section `Introduction and background') are key epigenetic modifiers of the genome. These enzymes belong to multiple enzyme families and are often part of multi-subunit protein complexes that determine their binding preferences and catalytic activity (Dekker & Haisma 2009). Studies have shown that cross talk exists between the two epigenetic mechanisms of DNA methylation and histone modifications through the formation of different permutations of these protein complexes (Kondo 2009).
Epigenetic regulation of genes encoding these enzymes or the MeC-binding proteins by hormones provides a reciprocal means of interplay between hormones and epigenetics. As shown in Table 5, hormones have a profound impact on the expression of these epigenetic-modifying genes. As discussed above, DNMT1 is a maintenance DNMT and DNMT3A and DNMT3B function in de novo methylation. Studies of transgenic mice lacking Dnmt3a and/or Dnmt3b and of human carriers of DNMT3B mutations have demonstrated that both of these enzymes are crucial for mammalian development (Hansen et al. 1999, Okano et al. 1999, Xu et al. 1999). In humans, DNMT3B mutation results in immunodeficiency, centromere instability, and facial anomalies (ICF) syndrome, which is associated with a wide range of altered histone modifications (including acetylation and methylation) and aberrant expression of genes regulating development, neuro-genesis, and immune function (Jin et al. 2008b). Of significant interest to us is the observation that estrogen is the hormone most commonly found to exert epigenetic influences on these epigenetic modifiers. Evolutionary studies have suggested that estrogen is the most ancient form of hormone and the one most widely distributed among animals (Thornton 2001, Jin et al. 2008b). An estrogen receptor ortholog has been identified in the mollusk Aplysia californica (Thornton et al. 2003). The antiquity of estrogen may explain its importance in the epigenetic programming of these multi-families of proteins.
Table 5.
Endocrine regulation of epigenetic-modifymg gene expression
| Enzyme |
Gene |
Gene transcriptiona |
Global regulation by hormoneb |
References |
|---|---|---|---|---|
| DNA methyl-transferases | De novo methylation (DNMT3A/3B); methylates hemimethylated DNA (DNMT1) | Repression | DNMT1, DNMT3A, and DNMT3B expressions are downregulated by progesterone and estrogen in human endometrium; estrogen upregulates the expression of DNMT3B in Ishikawa endometrial adenocarcinoma cells | Cui et al. (2009) and Yamagata et al. (2009) |
| Methyl-CpG-binding proteins | MECP2, MBD1, MBD2, and MBD4 | Repression | A direct regulation of MeCP2 mRNA via the estrogen receptor pathway in MCF-7 cells | Muller et al. (2003) |
| Histone-modifying enzymes | Histone acetyltransferase (HATs, a large family) | Activation | RAR α and RXR α negatively regulate CLOCK gene expression in vascular cells; androgen deprivation increases EP300 expression in prostate cancer cells | McNamara et al. (2001) and Heemers et al. (2007) |
| Histone deacetylase (HDACs, a large family) | Repression | MCF-7 treated with estradiol showed increased expression of HDAC6 | Saji et al. (2005) | |
| Histone methyltransferases (HMTs, a large family) | Activation/repression | U |
U, unknown or unclear, denotes no or very few papers reported as of 1 August 2010.
General description, may have exception. For example, MeCP2 is found bound to promoters that are actively expressed (Yasui et al. 2007).
Other factors such as signaling pathways, transcription factors, and posttranslational modifications may also be involved in the gene regulation.
We noticed that histone-modifying enzymes belong to a larger family of epigenetic modifiers than do the proteins involved in DNA methylation. However, relatively few genes encoding histone-modifying enzymes have been identified as epigenetically regulated by hormones, perhaps because many of these histone-modifying enzymes have been identified only recently and not enough time has passed for studies to be published. An analogous argument can be made for the relatively few reports on the influence of hormones on miRNA expression (not shown in Table 5). Recent reviews have covered the regulation of miRNA expression by estrogen (Klinge 2009) and androgen (Shi et al. 2008a,b); therefore, we will not expand on this topic in this review. However, the influence of hormones on miRNAs will obviously be a rapidly developing field.
Multi-dimensional interaction shapes the epigenome landscape at the organismal level: a possible explanation for the etiology of endocrine disorders
At the organismal level, the functioning of an endocrine axis involves multiple endocrine organs: for example, the hypothalamo–pituitary-gonadal axis comprising at least three hormone-producing tissues and many target tissues. The coordination of the entire axis, representing the first dimension of regulation controlled by genetic programs, is complex and meticulously well controlled. The interaction of these programs with the environment produces variable epigenomes, greatly amplifying the complexity of interaction and outcomes. These interactions can be viewed as the second dimension of influence. In this section, we will use a few examples to highlight the complexity of these interactions and demonstrate how disorders arise when the coordination breaks down or when early-life adaptive traits are in conflict with later-life demands. Finally, we will emphasize the effects of lifespan events that have strong modifying influences on epigenetics and pay special attention to windows of susceptibility during human development from conception to death. This will be the third dimension of interactions. Collectively, these multi-dimensional interactions provide insights into the challenges involved in maintaining normal endocrine function and why endocrine regulation can go astray, leading to disorders at the organismal level. Below, we will provide examples that apply to several thematic topics of interest to illustrate these various dimensions (Fig. 3).
Figure 3.
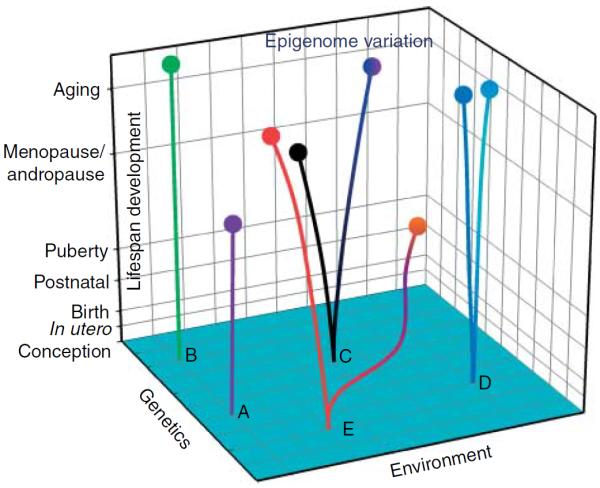
Genetics, environment, and stages of lifespan development interact in a three-dimensional space to create discordant endocrine phenotypes (epigenomes) from an identical genetic background (a single genome). Here, we use five pairs of monozygotic twins, in a schematic representation, to illustrate our understanding of this model. We applied the concept of principal component analysis to generate this diagram. The traditional view that an individual's phenotype is controlled solely by genetics (x-axis) is represented by two twin pairs A and B. According to this gene-centric view, the two twin pairs will have identical phenotypes despite continuous changes in their environment (z-axis) and over developmental time (y-axis). The more contemporary view argues that the interactions among genetics, the environment, and the developmental stages during the lifespan produce two different epigenomes, hence phenotypes, over developmental time in the twin pairs, albeit their identical genome at conception. The divergence of the phenotypes (epigenomes) of the twin pairs varies depending on the degree of environmental variations. Phenotype discordance is greatest in the E twin pair as compared with the C and D twin pairs (smallest variation), in agreement with their environmental variation. With advance in age (developmental time), their divergence in phenotype also expands. This model gives the various stages of lifespan development different weights to reflect their susceptibility to epigenetic modifications.
A widely studied area of epigenetics–environment– lifespan interactions is the relationship between birth weight and disease in later life. Animal studies have demonstrated that retardation of intrauterine growth results in progressive loss of β-cell function and the eventual development of type 2 diabetes in the adult. This association directly links chromatin remodeling with suppression of gene transcription (Simmons 2009). Studies of the effects of war-time famine revealed persistent differences in methylation of the leptin gene and imprinted IGF2 gene associated with prenatal exposure to famine that were both timing- and sex-specific (Gluckman et al. 2009, Tobi et al. 2009). A review of human studies also indicated an inverse relationship between birth weight and susceptibility to endocrine metabolic disorders such as insulin resistance, type 2 diabetes, hyperlipidemia, and obesity (Godfrey 2006). Studies in Pima Indian populations showed an association between maternal diabetes and increased adult obesity in offspring (Pettitt et al. 1983), which may have a transgenerational effect (Pettitt et al. 1991). Moreover, a review of birth weight and the etiology of breast cancer indicated that improper imprinting of IGF2 may affect intrauterine environment leading to higher birth weight and premenopausal breast cancer risk in daughters (Michels & Xue 2006). The relationship between birth weight and prostate cancer is less clear (Platz et al. 1998, Ekbom et al. 2000, Cnattingius et al. 2009).
Discordance in phenotypes of monozygous twins provides a unique opportunity to study these three levels of interaction. Monozygous twins share a common genotype but have different epigenomes, especially in later life (Fraga et al. 2005, Kaminsky et al. 2009), thus providing an excellent model for studying epigenetic-mediated endocrine disorders. Most MZ twin pairs are not identical in their susceptibility to disease and anthropomorphic features. Studies of type 1 and 2 diabetes in a population-based cohort of twins suggested a significant role of both genetic and environmental effects (Kaprio et al. 1992). Additional support is provided by the work of Fraga et al. (2005), which demonstrated an age-dependent increase in epigenome disparity and in differences in disease susceptibility between two MZ twins albeit the fact that they have the same genome. These data support the current view that multiple epigenomes can evolve over time from one genome and are dependent on a multitude of environmental inputs (Fig. 3).
To understand early-life impacts on the endocrine system one must consider events upstream of in utero development and the obvious concern about the fidelity of the sperm epigenome (Oakes et al. 2007, Carrell & Hammoud 2010). Recent studies have indicated sperm-specific DNA methylation and revealed the unique nature of the sperm epigenome (Trasler 2009). Although genetic abnormalities play a role in male infertility, in most cases the cause of the infertility is unknown (Stouffs et al. 2009). Attention is now focused on epigenetic changes. The first report on such changes, which used the global DNA methylation analysis approach, showed that a broad spectrum of epigenetic defects is associated with abnormal semen parameters (Houshdaran et al. 2007). Methylation was elevated at numerous sequences in DNA from poor quality sperm. Thus, male infertility may perhaps be added to the growing list of adult disorders with a fetal origin.
Epigenetics has an impact on the endocrine system throughout life. However, the sensitivity of the epigenome to the environment is likely to decrease as the rate of growth slows (Gluckman et al. 2009). Timing is an important factor in the effect of epigenetics on the endocrine system during the lifespan. Early development, from conception at least to birth (Gluckman et al. 2009), is considered the window of epigenetic reprogramming that predisposes aging (Thompson & Einstein 2010) and later-life diseases such as metabolic disorders (Simmons 2009) and cancer (Ho et al. 2006). Others have proposed that time points in utero, during the neonatal period, and during puberty are critical developmental windows sensitive to epigenetic modification (Prins 2008). In a rat study, neonatal treatments with a low-dose bisphenol A (BPA) or estradiol induced persistent hypomethylation of the phosphodiesterase Pde4d4 promoter and concordant gene overexpression in the prostates of adult animals (Ho et al. 2006). Furthermore, these epigenetic gene expression changes were associated with a higher risk of developing premalignant lesions in the adult prostate. In a human study, transplacental exposure to traffic-related polycyclic aromatic hydrocarbons (PAHs) was shown to increase childhood risk of asthma that was linked to aberrant methylation of the ACSL3 gene (Perera et al. 2009). As epigenetic alternation is inheritable, early-life epigenetic reprogramming may have transgenerational effects, as shown in a DES daughter and granddaughter study (Prins 2008).
Insulin resistance is another example of epigenetic dysregulation resulting in the loss of function in an endocrine axis over time when it is constantly challenged by environmental changes such as specific dietary deficits. It is the condition in which normal amounts of insulin are insufficient to produce a normal insulin response from insulin-sensitive organs/tissues such as the liver, muscle, and adipose tissue, which all play an important role in the etiology and clinical course of patients with type 2 diabetes, high blood pressure, or coronary heart disease (Reaven 1993). Recent studies of the effects of restricting the supply of specific B vitamins and methionine in the periconceptional diet of mature female sheep found altered methylation status in 4% of the CpG islands in the genome in the fetal liver. These diet-induced epigenetic changes in the liver of the affected offspring were found to be associated with increases in body weight and adiposity, hypersensitivity to allergens, insulin resistance, and hypertension when compared with the offspring of ewes fed with a nonrestricted diet (Sinclair et al. 2007). The data provide the first evidence that a deficiency in specific dietary elements during early life can modify the disease susceptibility of offspring during adulthood through epigenetic reprogramming (Sinclair et al. 2007). This has moved the vulnerability window of early origin of disease close to orat the time offertilization when paternal and maternal genetic information are re-shuffled and segregated.
Puberty is initiated by increased pulsatile release of GnRH from the hypothalamus to the gonads, resulting in sexual maturation. This process requires the participation of multiple sets of genes within functionally connected networks. An optimal timing of sexual maturation is critical for an individual to best benefit from the environment. A recent genome-wide analysis of hypothalamic DNA methylation sequences revealed profound changes in methylation patterns associated with the onset of female puberty (Ojeda et al. 2010). These findings indicated that epigenetic mechanisms might provide the coordination and transcriptional plasticity in the control of the onset of puberty. In addition, reelin (RELN), which plays a pivotal role in neural development, was found to undergo pubertyinduced hypermethylation in its promoter and a decrease in gene expression in temporocortical tissues (Lintas & Persico 2010). The surge in reproductive hormones during puberty is believed to have triggered methylation of the RELN promoter. These findings link reproductive hormones to the onset of puberty through epigenetic alteration of the RELN promoter. The alteration may also explain why both the onset of schizophrenia and the worsening of autism, both associated with loss of RELN expression, typically occur at puberty (Lintas & Persico 2010). Figure 3 presents a three-dimensional model to illustrate the same or different genetic background, and the interaction between epigenetics and the environment over the lifespan of an individual to produce hormone-induced/dependent phenotypes linked to critical windows of developmental susceptibility.
Epigenetics in endocrine disruptors
Endocrine disruptors are environmental chemicals that mimic hormone or antihormone activities in the endocrine system and disrupt the physiologic function of endogenous hormones. Plants are the sources of some of these chemicals, such as phytoestrogens, and others are natural substances such as heavy metals or synthetic compounds or drugs. Food is a major source of exposure to endocrine disruptors. Other routes include water and air. Notably, many synthetic compounds such as dichlorodiphenyltrichloroethane are hydrophobic and can be accumulated from the environment into the fat tissue in the food chain (Clarkson 1995). Although endocrine disruptors generally function as steroid hormones and produce estrogenic, androgenic, and antiandrogenic actions, less is known about their disruption of the signaling pathways controlled by other hormones, especially peptide hormones. Table 6 summarizes endocrine disruptors with known epigenetic effects.
Table 6.
Epigenetic effect of endocrine disruptors
| Endocrine disruptora |
Routes of exposure |
Major hormonal effectb |
Epigenetic effectc |
References |
|---|---|---|---|---|
| Phytoestrogens such as genistein | Soybean | Estrogenic; anti-estrogenic | Hypomethylation of Nsbp1 in uterine; hypomethylation of TERT promoter and chromatin remodeling in MCF-7 cells; BTG3 promoter demethylation and histone modification in prostate cancer | Tang et al. (2008), Li et al. (2009b) and Majid et al. (2010) |
| Diethylstilbestrol (DES) | Drug | Estrogenic | Hypermethylation and chromatin repression in miR-9-3 promoter in breast; hypermethylation of the HOXA10 in endometrial cells | Prins (2008), Bromer et al. (2009) and Hsu et al. (2009) |
| Bisphenol A (BPA) | Food/plastic | Estrogenic | Hypomethylation of Pde4d in prostate; maternal exposure decreased CpG methylation in an intracisternal A particle retrotransposon upstream of the Agouti gene in offspring | Ho et al. (2006) and Dolinoy et al. (2007) |
| Polybrominated diphenyl ethers (PBDEs) | House dust/flame retardants | Estrogen; thyroid hormone imbalance | Decrease global gene DNA methylation in hippocampal neurons | Siddiqi et al. (2003), Ceccatelli et al. (2006) and Chen et al. (2010) |
| Dioxins such as TCDD | Food, air, water/combustion | Estrogenic; anti-estrogenic | Exposure at the preimplantation stage increased methylation at H19/Igf2 imprint control region at embryonic day 14; miRNAs are unlikely to play a significant role in dioxin toxicity in adult rodent liver | Safe & Wormke (2003), Wu et al. (2004), Boverhof et al. (2006) and Moffat et al. (2007) |
| Polychlorinated biphenyls (PCBs) | Food/coolants and lubricants | Estrogenic; anti-androgenic; thyroid hormone homeostasis | U | Sikka & Wang (2008) and Salay & Garabrant (2009) |
| Perfluorooctanoic acid (PFOA) | Surfactant | Estrogenic | U | Tilton et al. (2008) |
| Heavy metals such as cadmium | Industry, cigarette, food, soil | Estrogenic | Hypermethylation of RASSF1A and p16 promoter in prostate cells; increased genomic DNA methylation in embryo lung fibroblast cells | Benbrahim-Tallaa et al. (2007), Jiang et al. (2008) and Denier et al. (2009) |
| Polycyclic aromatic hydrocarbons (PAHs) | Air, food | Steroid metabolism | Hypermethylation of ACSL3 in asthmatic children | Yang et al. (1961), Rocha Monteiro et al. (2000) and Perera et al. (2009) |
| Dichlorodiphenyltrichloroethane (DDT) | Food/pesticide | Anti-androgenic | Inverse correlation with global methylation levels in human; DNA methylation in the hypothalamus of young male rats | Kelce et al. (1995), Rusiecki et al. (2008) and Shutoh et al. (2009) |
| Vinclozolin | Fungicide | Anti-androgenic | Altered DNA methylation patterns related to transgenerational male infertility | Anway et al. (2005) |
U, unknown or unclear, denotes no or very few papers reported as of 1 August 2010.
These compounds may also have other effects. Phytoestrogens exhibit both risks and benefits to health, whereas most other endocrine disruptors are risk factors to health.
Including direct, indirect, or metabolites effect.
The effect of endocrine disruptors on miRNA expression and histone modification are less reported.
As discussed previously, estrogens, including xenoestrogens and phytoestrogens, are the most primitive hormones and also the most widely distributed among organisms. Our literature showed that this class of endocrine disruptor has epigenetic effects on a wide variety of cellular and physiological functions and may also disrupt many different animal species.
Phytoestrogens are a diverse group of compounds produced by plants as a part of a defense system believed to provide protection against insects (Rochester & Millam 2009) and to act as modulators of herbivore fertility (Hughes 1988, Rochester & Millam 2009). Because of their structural similarity with the natural estrogens estradiol-17β, estrone, or estriol, phytoestrogens may impose both risks and beneficial effects on health, depending on the types, concentrations, and target organs (Adlercreutz 2002). Epidemiologic studies have demonstrated that genistein, a phytoestrogen abundant in soy products, is linked to a low occurrence of prostate cancer, with epigenetics playing an important role (Molinie & Georgel 2009). On the other hand, the same compound may have either a protective effect or the potential for tumor growth promotion, depending on its concentration (Moiseeva & Manson 2009). We recently demonstrated that neonatal exposure of mice to genistein or DES induced unscheduled expression of nucleosome-binding protein 1 (Nsbp1), which plays a role in nucleosome positioning in the mouse uterus via hypomethylation of its promoter (Tang et al. 2008). Phytoestrogens also cause hypomethylation of the promoter of telomerase reverse transcriptase (TERT), chromatin remodeling in MCF-7 breast cancer cells, and demethylation of the promoter of tumor suppressor gene B-cell translocation gene 3 (BTG3), a gene encoding a putative tumor suppressor in prostate cancer (Majid et al. 2010).
Another type of endocrine disruptor, which is becoming more prevalent, is xenobiotic endocrine disruptor, including xenoestrogens. Many of them have been linked to epigenetic modifications of chromatin and aberrant activation or inactivation of specific genes. Most synthetic compounds have not been present in our biosphere until very recently in human and vertebrate evolutionary history. Therefore, biological evolution has not had enough time to evolve mechanisms against the adverse effects of the disruption caused by these chemicals. The worst scenario is that many of them exert significant epigenetic action; thus, the adverse effects may persist and even be transgenerational. The latter effect should truly raise serious concern about the safe use of such synthetic products in our modern society and their contamination to our environment.
One excellent example of such a compound is vinclozolin, a common fungicide used in vineyards and other agricultural settings. It has been shown to cause transgenerational transmission of induced epigenetic changes transmitted through the sperm (Anway et al. 2005). Several generations of offspring display disorders such as adult-onset male infertility, accelerated aging, abnormal behavior, and increased frequency of prostate disease in aged males. Similarly, the xenoestrogen DES, once used for the treatment of prostate cancer or habitual miscarriages, exhibits chronic toxicity in a manner that can pass its effects to subsequent generations via epigenetic memories (Prins 2008).
Recently, DES has been shown to induce hyper-methylation and chromatin repression in miR-9-3 promoter in breast epithelial progeny derived from mammospheres. The action of the xenoestrogen can be explained mechanistically by the observation that repressive chromatin marks were recruited to the miR-9-3 locus along with DNMT1 (Hsu et al. 2009). DES also silences the homeobox gene, HOXA10, in endometrial cells of animals that have been exposed to DES in utero (Bromer et al. 2009).
BPA is another epigenetically active xenoestrogen. It is widely used in the manufacture of polycarbonated plastics and the epoxy lining of canned food. BPA was found in the urine of 92.6% of American men and women (Calafat et al. 2008). Neonatal exposure of rats to environmentally relevant doses of BPA induced hypermethylation of the promoter of a cAMP-regulating gene Pde4d and overexpression of the gene in the prostate. These aberrant changes were associated with an increase in the risk of developing prostate lesions in the adult rats (Ho et al. 2006). Notably, a recent study conducted by Lamartiniere et al. demonstrate that oral prenatal exposure to BPA increases mammary cancer susceptibility in offspring and shifts the window of susceptibility for dimethylbenzanthracene-induced tumorigenesis in the rat mammary gland (Betancourt et al. 2010). Moreover, maternal exposure of Agouti mice to BPA was shown to decrease CpG methylation in an intracisternal A particle retrotransposon upstream of the Agouti gene in offspring, a change that could be reversed by maternal dietary supplementation with either methyl donors or a phytoestrogen (Dolinoy et al. 2007). Finally, it is still controversial as to whether early-life exposure would increase risks of obesity and diabetes in later life (Ryan et al. 2010, Sharpe & Drake 2010), an important topic that warrants further investigation. In March 2010, the US Environmental Protection Agency (www.epa.gov) formally listed BPA as a `chemical of concern'.
Other estrogenic epigenetic pollutants include polybrominated diphenyl ethers, fire retardants, that reduce global DNA methylation in hippocampal neurons (Siddiqi et al. 2003, Ceccatelli et al. 2006, Chen et al. 2010); dioxins that disrupt H19/IGF2 imprinting in the preimplantation embryo and induce a host of miRNAs in the liver of adult rats (Safe & Wormke 2003, Wu et al. 2004, Boverhof et al. 2006, Moffat et al. 2007); and heavy metals, such as cadmium, that transactivate via estrogen receptor-α and promote the growth of cancer cells by inducing hypermethylation of Ras association domain-containing protein 1 (RASSF1A) and the p16 promoter (Benbrahim-Tallaa et al. 2007, Jiang et al. 2008, Denier et al. 2009).
The list provided in Table 6 is by no means complete. We expect that many more untested chemicals or pollutants will be found to disrupt endocrine function through epigenetic mechanisms. Unfortunately, the effects of endocrine disruptors are not readily discerned and often are ignored. Their long-lasting adverse effects on human health and wildlife are distressing. The fact that many of their effects are observed at very low doses in a nonlinear manner makes it difficult for regulatory agencies to set guidelines. Future research must continue to focus on revealing their actions through the use of high-throughput, unbiased technologies.
Summary and perspectives
It has become apparent that genetics alone is insufficient to explain the dynamic and complex interdependent relationships between the endocrine system and endogenous and exogenous environmental changes. Genetics alone also fails to address issues related to the progressive changes in endocrine functions over an individual's lifespan, the early origin of endocrine disorders, phenotype discordance between MZ twins, and rapid shifts in disease patterns among populations experiencing major changes in lifestyle, such as immigration. Mounting evidence now suggests that epigenetics is the missing link between genetics, the environment, and endocrine function. In this regard, genetics provides a basis for epigenetic modifications and a blueprint for hormone action. However, the great variability in endocrine function and susceptibility to endocrine-related diseases among individuals or populations is clearly determined by epigenetics. Epigenetics serves as a mechanism mediating the continuous `editing' of the genome or epigenetic marks laid down in early life by exposures and experiences during later life. This paradigm has expanded the static and gene-centric view of phenotypic attributes to a more plastic and adaptive view molded by epigenetics. To fully understand the impacts of epigenetics on endocrine function and vice versa, we need a genome-wide search for plasticity genes or loci directly responsive to a specific environmental stimulus. To achieve this goal, current research is applying high-throughput investigative technologies to uncover global changes in the methylome(s), miRNA signatures, and the histone codes defining the interplay and advanced informatics to produce biologically meaningful data and conclusions. To advance these investigations, our focus should be placed on two commonly raised questions: 1) whether epigenetic changes induced by environmental exposures or lifestyle choices in one generation can be passed to the next and 2) whether these `inherited' changes can be reversed upon removal of the exposures or through lifestyle modifications. Answers to the first question are of paramount importance to the primary prevention of endocrine disorders such as obesity, and answers to the second would open doors to the use of epigenetic drugs or interventions for the reversal of endocrine disorders with a strong epigenetic etiology. The opportunities of applying epigenetics to the prevention and treatment of endocrine disorders are limitless and certainly will emerge rapidly in the near future.
Acknowledgements
We thank Nancy K Voynow for her editorial assistance, and Yuk Yin Cheung and Amy Fullenkamp for their assistance in preparing the manuscript.
Funding This work was supported by the NIH grants 2P30ES006096, 5R01ES015584, 1RC2ES018758, 1RC2ES018789, 5R01CA112532, and 5R01CA015776.
Footnotes
Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review reported.
References
- Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Molecular Endocrinology. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. doi:10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Phytoestrogens and breast cancer. Journal of Steroid Biochemistry and Molecular Biology. 2002;83:113–118. doi: 10.1016/s0960-0760(02)00273-x. doi:10.1016/S0960-0760(02)00273-X. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. doi:10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archey WB, McEachern KA, Robson M, Offit K, Vaziri SA, Casey G, Borg A, Arrick BA. Increased CpG methylation of the estrogen receptor gene in BRCA1-linked estrogen receptor-negative breast cancers. Oncogene. 2002;21:7034–7041. doi: 10.1038/sj.onc.1205844. doi:10.1038/sj.onc.1205844. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. doi:10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Dill AL, Webber MM, Waalkes MP. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environmental Health Perspectives. 2007;115:1454–1459. doi: 10.1289/ehp.10207. doi:10.1289/ehp.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes and Development. 2009;23:781–783. doi: 10.1101/gad.1787609. doi:10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environmental Health Perspectives. 2010;118:1614–1619. doi: 10.1289/ehp.1002148. doi:10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. doi:10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. The year in neuroendocrinology. Molecular Endocrinology. 2010;24:252–260. doi: 10.1210/me.2009-0350. doi:10.1210/me.2009-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic O, Veenstra GJ. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118:549–565. doi: 10.1007/s00412-009-0221-9. doi:10.1007/s00412-009-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boverhof DR, Kwekel JC, Humes DG, Burgoon LD, Zacharewski TR. Dioxin induces an estrogen-like, estrogen receptor-dependent gene expression response in the murine uterus. Molecular Pharmacology. 2006;69:1599–1606. doi: 10.1124/mol.105.019638. doi:10.1124/mol.105.019638. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. doi: 10.1210/en.2009-0071. doi:10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. doi:10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochemical Society Transactions. 2008;36:1224–1231. doi: 10.1042/BST0361224. doi:10.1042/BST0361224. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Hammoud SS. The human sperm epigenome and its potential role in embryonic development. Molecular Human Reproduction. 2010;16:37–47. doi: 10.1093/molehr/gap090. doi:10.1093/molehr/gap090. [DOI] [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220:104–116. doi: 10.1016/j.tox.2005.12.004. doi:10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Maternal regulation of estrogen receptor alpha methylation. Current Opinion in Pharmacology. 2008;8:735–739. doi: 10.1016/j.coph.2008.06.018. doi:10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liufu C, Sun W, Sun X, Chen D. Assessment of the neurotoxic mechanisms of decabrominated diphenyl ether (PBDE-209) in primary cultured neonatal rat hippocampal neurons includes alterations in second messenger signaling and oxidative stress. Toxicology Letters. 2010;192:431–439. doi: 10.1016/j.toxlet.2009.11.020. doi:10.1016/j.toxlet.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. doi:10.1016/S0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Chim CS, Wong SY, Pang A, Chu P, Lau JS, Wong KF, Kwong YL. Aberrant promoter methylation of the retinoic acid receptor alpha gene in acute promyelocytic leukemia. Leukemia. 2005;19:2241–2246. doi: 10.1038/sj.leu.2403937. doi:10.1038/sj.leu.2403937. [DOI] [PubMed] [Google Scholar]
- Chun JY, Nadiminty N, Dutt S, Lou W, Yang JC, Kung HJ, Evans CP, Gao AC. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clinical Cancer Research. 2009;15:4815–4822. doi: 10.1158/1078-0432.CCR-09-0640. doi:10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annual Review of Biochemistry. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. doi:10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. Environmental contaminants in the food chain. American Journal of Clinical Nutrition. 1995;61:682S–686S. doi: 10.1093/ajcn/61.3.682S. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Lundberg F, Sandin S, Gronberg H, Iliadou A. Birth characteristics and risk of prostate cancer: the contribution of genetic factors. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:2422–2426. doi: 10.1158/1055-9965.EPI-09-0366. doi:10.1158/1055-9965.EPI-09-0366. [DOI] [PubMed] [Google Scholar]
- Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. PNAS. 2005;102:8644–8649. doi: 10.1073/pnas.0503218102. doi:10.1073/pnas.0503218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P, Rastad J, Schwarz P, Westin G, Kindmark A, Lundgren E, Akerstrom G, Carling T. The vitamin D receptor (VDR) start codon polymorphism in primary hyperparathyroidism and parathyroid VDR messenger ribonucleic acid levels. Journal of Clinical Endocrinology and Metabolism. 1999;84:1690–1694. doi: 10.1210/jcem.84.5.5707. doi:10.1210/jc.84.5.1690. [DOI] [PubMed] [Google Scholar]
- Cosgrove MS, Boeke JD, Wolberger C. Regulated nucleosome mobility and the histone code. Nature Structural and Molecular Biology. 2004;11:1037–1043. doi: 10.1038/nsmb851. doi:10.1038/nsmb851. [DOI] [PubMed] [Google Scholar]
- Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–S10. doi: 10.1210/en.2005-1122. doi:10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- Cui M, Wen Z, Yang Z, Chen J, Wang F. Estrogen regulates DNA methyltransferase 3B expression in Ishikawa endometrial adenocarcinoma cells. Molecular Biology Reports. 2009;36:2201–2207. doi: 10.1007/s11033-008-9435-9. doi:10.1007/s11033-008-9435-9. [DOI] [PubMed] [Google Scholar]
- Dannenberg LO, Edenberg HJ. Epigenetics of gene expression in human hepatoma cells: expression profiling the response to inhibition of DNA methylation and histone deacetylation. BMC Genomics. 2006;7:181. doi: 10.1186/1471-2164-7-181. doi:10.1186/1471-2164-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, et al. Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Molecular and Cellular Endocrinology. 2009;323:4–19. doi: 10.1016/j.mce.2009.12.012. doi:10.1016/j.mce.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker FJ, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discovery Today. 2009;14:942–948. doi: 10.1016/j.drudis.2009.06.008. doi:10.1016/j.drudis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Deladoey J, Vassart G, Van VG. Possible non-Mendelian mechanisms of thyroid dysgenesis. Endocrine Development. 2007;10:29–42. doi: 10.1159/000106818. doi:10.1159/000106818. [DOI] [PubMed] [Google Scholar]
- Demura M, Bulun SE. CpG dinucleotide methylation of the CYP19 I.3/II promoter modulates cAMP-stimulated aromatase activity. Molecular and Cellular Endocrinology. 2008;283:127–132. doi: 10.1016/j.mce.2007.12.003. doi:10.1016/j.mce.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Denier X, Hill EM, Rotchell J, Minier C. Estrogenic activity of cadmium, copper and zinc in the yeast estrogen screen. Toxicology in Vitro. 2009;23:569–573. doi: 10.1016/j.tiv.2009.01.006. doi:10.1016/j.tiv.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. PNAS. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. doi:10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Weiss D, Burghardt R, Infante-Duarte C, Brockhaus S, Muschler MA, Bleich S, Lehmkuhl U, Frieling H. Promoter specific DNA methylation and gene expression of POMC in acutely underweight and recovered patients with anorexia nervosa. Journal of Psychiatric Research. 2010;44:827–833. doi: 10.1016/j.jpsychires.2010.01.011. doi:10.1016/j.jpsychires.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Wuu J, Adami HO, Lu CM, Lagiou P, Trichopoulos D, Hsieh C. Duration of gestation and prostate cancer risk in offspring. Cancer Epidemiology, Biomarkers and Prevention. 2000;9:221–223. [PubMed] [Google Scholar]
- Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annual Review of Pharmacology and Toxicology. 2005;45:629–656. doi: 10.1146/annurev.pharmtox.45.120403.095832. doi:10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor super-family. Science. 1988;240:889–895. doi: 10.1126/science.3283939. doi:10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. doi:10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, et al. Epigenetic differences arise during the lifetime of monozygotic twins. PNAS. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. doi:10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, Salvioli S, Valensin S, De Benedictis G, Di Iorio A, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mechanisms of Ageing and Development. 2005;126:351–361. doi: 10.1016/j.mad.2004.08.028. doi:10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Current Opinion in Genetics and Development. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. doi:10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gale EA, Bingley PJ, Eisenbarth GS, Redondo MJ, Kyvik KO, Petersen JS. Reanalysis of twin studies suggests that diabetes is mainly genetic. BMJ. 2001;323:997–998. doi:10.1136/bmj.323.7319.997a. [PMC free article] [PubMed] [Google Scholar]
- Ganesan A, Nolan L, Crabb SJ, Packham G. Epigenetic therapy: histone acetylation, DNA methylation and anti-cancer drug discovery. Current Cancer Drug Targets. 2009;9:963–981. doi: 10.2174/156800909790192428. doi:10.2174/156800909790192428. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annual Review of Biochemistry. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. doi:10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nature Reviews. Endocrinology. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. doi:10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- Godfrey K. The `developmental origins' hypothesis: epidemiology. In: Gluckman PD, Hanson MA, editors. Developmental Origins of Health and Disease. Cambridge University Press; Cambridge: 2006. pp. 6–32. [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. doi:10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Medicine. 2009;7:62. doi: 10.1186/1741-7015-7-62. doi:10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD, Kim JS. Site-specific methylation of the promoter alters deoxyribonucleic acid–protein interactions and prevents follicle-stimulating hormone receptor gene transcription. Biology of Reproduction. 2001;64:602–610. doi: 10.1095/biolreprod64.2.602. doi:10.1095/biolreprod64.2.602. [DOI] [PubMed] [Google Scholar]
- Gurrieri F, Neri G. Defective oxytocin function: a clue to understanding the cause of autism? BMC Medicine. 2009;7:63. doi: 10.1186/1741-7015-7-63. doi:10.1186/1741-7015-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, Gartler SM. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. PNAS. 1999;96:14412–14417. doi: 10.1073/pnas.96.25.14412. doi:10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yokozaki H, Goodison S, Oue N, Suzuki T, Lotan R, Yasui W, Tahara E. Inactivation of retinoic acid receptor beta by promoter CpG hypermethylation in gastric cancer. Differentiation. 2001;68:13–21. doi: 10.1046/j.1432-0436.2001.068001013.x. doi:10.1046/j.1432-0436.2001.068001013.x. [DOI] [PubMed] [Google Scholar]
- Heemers HV, Sebo TJ, Debes JD, Regan KM, Raclaw KA, Murphy LM, Hobisch A, Culig Z, Tindall DJ. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Research. 2007;67:3422–3430. doi: 10.1158/0008-5472.CAN-06-2836. doi:10.1158/0008-5472.CAN-06-2836. [DOI] [PubMed] [Google Scholar]
- Hermann A, Gowher H, Jeltsch A. Biochemistry and biology of mammalian DNA methyltransferases. Cellular and Molecular Life Sciences. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. doi:10.1007/s00018-004-4201-1. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Luber K, Yazici A, Muschler MA, Lenz B, Wilhelm J, Kornhuber J, Bleich S. Epigenetic regulation and gene expression of vasopressin and atrial natriuretic peptide in alcohol withdrawal. Psychoneuroendocrinology. 2009;34:555–560. doi: 10.1016/j.psyneuen.2008.10.019. doi:10.1016/j.psyneuen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi H, Christenson LK, Strauss JF., III Regulation of transcription of the steroidogenic acute regulatory protein (StAR) gene: temporal and spatial changes in transcription factor binding and histone modification. Molecular and Cellular Endocrinology. 2004;215:119–126. doi: 10.1016/j.mce.2003.11.014. doi:10.1016/j.mce.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Hitchins MP, Wong JJ, Suthers G, Suter CM, Martin DI, Hawkins NJ, Ward RL. Inheritance of a cancer-associated MLH1 germ-line epimutation. New England Journal of Medicine. 2007;356:697–705. doi: 10.1056/NEJMoa064522. doi:10.1056/NEJMoa064522. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Research. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. doi:10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Mutations and epimutations in mammalian cells. Mutation Research. 1991;250:351–363. doi: 10.1016/0027-5107(91)90192-q. doi:10.1016/0027-5107(91)90192-Q. [DOI] [PubMed] [Google Scholar]
- Houshdaran S, Cortessis VK, Siegmund K, Yang A, Laird PW, Sokol RZ. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE. 2007;2:e1289. doi: 10.1371/journal.pone.0001289. doi:10.1371/journal.pone.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PY, Deatherage DE, Rodriguez BA, Liyanarachchi S, Weng YI, Zuo T, Liu J, Cheng AS, Huang TH. Xenoestrogen-induced epigenetic repression of microRNA-9-3 in breast epithelial cells. Cancer Research. 2009;69:5936–5945. doi: 10.1158/0008-5472.CAN-08-4914. doi:10.1158/0008-5472.CAN-08-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CL., Jr Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environmental Health Perspectives. 1988;78:171–174. doi: 10.1289/ehp.8878171. doi:10.2307/3430517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Molecular Endocrinology. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. doi:10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Hyman SJ, Novoa Y, Holzman I. Perinatal endocrinology: common endocrine disorders in the sick and premature newborn. Endocrinology and Metabolism Clinics of North America. 2009;38:509–524. doi: 10.1016/j.ecl.2009.06.005. doi:10.1016/j.ecl.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Malizos KN, Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Annals of the Rheumatic Diseases. 2007;66:1616–1621. doi: 10.1136/ard.2007.069377. doi:10.1136/ard.2007.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa M, Harada T, Taniguchi F, Ohama Y, Takenaka Y, Terakawa N. An epigenetic disorder may cause aberrant expression of aromatase gene in endometriotic stromal cells. Fertility and Sterility. 2008;89:1390–1396. doi: 10.1016/j.fertnstert.2007.03.078. doi:10.1016/j.fertnstert.2007.03.078. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33(Supplement):245–254. doi: 10.1038/ng1089. doi:10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Endocrine response in the fallopian tube. Endocrine Reviews. 1984;5:525–551. doi: 10.1210/edrv-5-4-525. doi:10.1210/edrv-5-4-525. [DOI] [PubMed] [Google Scholar]
- Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Letters. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. doi:10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Jiang G, Xu L, Song S, Zhu C, Wu Q, Zhang L, Wu L. Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicology. 2008;244:49–55. doi: 10.1016/j.tox.2007.10.028. doi:10.1016/j.tox.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Jin Z, Mori Y, Hamilton JP, Olaru A, Sato F, Yang J, Ito T, Kan T, Agarwal R, Meltzer SJ. Hypermethylation of the somatostatin promoter is a common, early event in human esophageal carcinogenesis. Cancer. 2008a;112:43–49. doi: 10.1002/cncr.23135. doi:10.1002/cncr.23135. [DOI] [PubMed] [Google Scholar]
- Jin B, Tao Q, Peng J, Soo HM, Wu W, Ying J, Fields CR, Delmas AL, Liu X, Qiu J, et al. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Human Molecular Genetics. 2008b;17:690–709. doi: 10.1093/hmg/ddm341. doi:10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews. Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. doi:10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. doi:10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, et al. DNA methylation profiles in monozygotic and dizygotic twins. Nature Genetics. 2009;41:240–245. doi: 10.1038/ng.286. doi:10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, Stengard J, Kesaniemi YA. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35:1060–1067. doi: 10.1007/BF02221682. doi:10.1007/BF02221682. [DOI] [PubMed] [Google Scholar]
- Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. Journal of Biological Chemistry. 2010;285:14534–14548. doi: 10.1074/jbc.M110.115345. doi:10.1074/jbc.M110.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. doi:10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kim MS, Fujiki R, Kitagawa H, Kato S. 1α,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Molecular and Cellular Endocrinology. 2007;265–266:168–173. doi: 10.1016/j.mce.2006.12.014. doi:10.1016/j.mce.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kondo T, Takada I, Youn MY, Yamamoto Y, Takahashi S, Matsumoto T, Fujiyama S, Shirode Y, Yamaoka I, et al. DNA demethylation in hormone-induced transcriptional derepression. Nature. 2009;461:1007–1012. doi: 10.1038/nature08456. doi:10.1038/nature08456. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Research. 2000;60:3623–3630. [PubMed] [Google Scholar]
- Klinge CM. Estrogen regulation of microRNA expression. Current Genomics. 2009;10:169–183. doi: 10.2174/138920209788185289. doi:10.2174/138920209788185289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knower KC, To SQ, Simpson ER, Clyne CD. Epigenetic mechanisms regulating CYP19 transcription in human breast adipose fibroblasts. Molecular and Cellular Endocrinology. 2010;321:123–130. doi: 10.1016/j.mce.2010.02.035. doi:10.1016/j.mce.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends in Endocrinology and Metabolism. 2010;21:315–324. doi: 10.1016/j.tem.2010.01.002. doi:10.1016/j.tem.2010. 01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Medical Journal. 2009;50:455–463. doi: 10.3349/ymj.2009.50.4.455. doi:10.3349/ymj.2009.50.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. miR-206 Expression is down-regulated in estrogen receptor alpha-positive human breast cancer. Cancer Research. 2008;68:5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. doi:10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- Kong W, Zhao JJ, He L, Cheng JQ. Strategies for profiling microRNA expression. Journal of Cellular Physiology. 2009;218:22–25. doi: 10.1002/jcp.21577. doi:10.1002/jcp.21577. [DOI] [PubMed] [Google Scholar]
- Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, Mullen Y, Pfeifer GP, Ferreri K. Insulin gene expression is regulated by DNA methylation. PLoS ONE. 2009;4:e6953. doi: 10.1371/journal.pone.0006953. doi:10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Quinn EA. Developmental origins of adult function and health: evolutionary hypotheses. Annual Review of Anthropology. 2009;38:131–147. doi:10.1146/annurev-anthro-091908-164350. [Google Scholar]
- Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–3735. doi: 10.1182/blood-2009-05-219584. doi:10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, Marcovecchio ML, Del VA, Gallo F, Bertino E, Chiarelli F. Influence of environment on insulin sensitivity. Environment International. 2009;35:987–993. doi: 10.1016/j.envint.2009.03.008. doi:10.1016/j.envint.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Lee LT, Tan-Un KC, Pang RT, Lam DT, Chow BK. Regulation of the human secretin gene is controlled by the combined effects of CpG methylation, Sp1/Sp3 ratio, and the E-box element. Molecular Endocrinology. 2004;18:1740–1755. doi: 10.1210/me.2003-0461. doi:10.1210/me.2003-0461. [DOI] [PubMed] [Google Scholar]
- Lee DO, Jee BC, Ku SY, Suh CS, Kim SH, Choi YM, Moon SY, Kim JG. Relationships between the insulin-like growth factor I (IGF-I) receptor gene G3174A polymorphism, serum IGF-I levels, and bone mineral density in postmenopausal Korean women. Journal of Bone and Mineral Metabolism. 2008;26:42–46. doi: 10.1007/s00774-007-0795-3. doi:10.1007/s00774-007-0795-3. [DOI] [PubMed] [Google Scholar]
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. doi:10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mechanisms of Ageing and Development. 2009a;130:731–741. doi: 10.1016/j.mad.2009.09.002. doi:10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Andrews LG, Tollefsbol TO. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. International Journal of Cancer. 2009b;125:286–296. doi: 10.1002/ijc.24398. doi:10.1002/ijc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–2725. doi: 10.2337/db09-1003. doi:10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas C, Persico AM. Neocortical RELN promoter methylation increases significantly after puberty. Neuroreport. 2010;21:114–118. doi: 10.1097/WNR.0b013e328334b343. doi:10. 1097/WNR.0b013e328334b343. [DOI] [PubMed] [Google Scholar]
- Lobato MN, Ordonez-Sanchez ML, Tusie-Luna MT, Meseguer A. Mutation analysis in patients with congenital adrenal hyperplasia in the Spanish population: identification of putative novel steroid 21-hydroxylase deficiency alleles associated with the classic form of the disease. Human Heredity. 1999;49:169–175. doi: 10.1159/000022866. doi:10.1159/000022866. [DOI] [PubMed] [Google Scholar]
- Lv K, Guo Y, Zhang Y, Wang K, Jia Y, Sun S. Allele-specific targeting of hsa-miR-657 to human IGF2R creates a potential mechanism underlying the association of ACAA-insertion/deletion polymorphism with type 2 diabetes. Biochemical and Biophysical Research Communications. 2008;374:101–105. doi: 10.1016/j.bbrc.2008.06.102. doi:10.1016/j.bbrc.2008.06.102. [DOI] [PubMed] [Google Scholar]
- Majid S, Dar AA, Shahryari V, Hirata H, Ahmad A, Saini S, Tanaka Y, Dahiya AV, Dahiya R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer. 2010;116:66–76. doi: 10.1002/cncr.24662. doi:10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Papadopoulos V. Epigenetic regulation of the expression of genes involved in steroid hormone biosynthesis and action. Steroids. 2010;75:467–476. doi: 10.1016/j.steroids.2010.02.004. doi:10.1016/j.steroids.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Culty M, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate decreases mineralocorticoid receptor expression in the adult testis. Endocrinology. 2009;150:5575–5585. doi: 10.1210/en.2009-0847. doi:10.1210/en.2009-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, O'Neill JS, Reddy AB, Chesham JE, Prosser HM, Kyriacou CP, Godinho SI, Nolan PM, Hastings MH. Genetic and molecular analysis of the central and peripheral circadian clockwork of mice. Cold Spring Harbor Symposia on Quantitative Biology. 2007;72:85–94. doi: 10.1101/sqb.2007.72.005. doi:10.1101/sqb.2007.72.005. [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. doi:10.1016/S0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Melzner I, Scott V, Dorsch K, Fischer P, Wabitsch M, Bruderlein S, Hasel C, Moller P. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. Journal of Biological Chemistry. 2002;277:45420–45427. doi: 10.1074/jbc.M208511200. doi:10.1074/jbc.M208511200. [DOI] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies – the next generation. Nature Reviews. Genetics. 2010;11:31–46. doi: 10.1038/nrg2626. doi:10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. International Journal of Cancer. 2006;119:2007–2025. doi: 10.1002/ijc.22004. doi:10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- Miller WL. Molecular biology of steroid hormone synthesis. Endocrine Reviews. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. doi:10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta. 2007;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. doi:10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Miremadi A, Oestergaard MZ, Pharoah PD, Caldas C. Cancer genetics of epigenetic genes. Human Molecular Genetics. 2007;16:R28–R49. doi: 10.1093/hmg/ddm021. doi:10.1093/hmg/ddm021. [DOI] [PubMed] [Google Scholar]
- Missaghian E, Kempna P, Dick B, Hirsch A, Alikhani-Koupaei R, Jegou B, Mullis PE, Frey BM, Fluck CE. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. Journal of Endocrinology. 2009;202:99–109. doi: 10.1677/JOE-08-0353. doi:10.1677/JOE-08-0353. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Boutros PC, Celius T, Linden J, Pohjanvirta R, Okey AB. microRNAs in adult rodent liver are refractory to dioxin treatment. Toxicological Sciences. 2007;99:470–487. doi: 10.1093/toxsci/kfm189. doi:10.1093/toxsci/kfm189. [DOI] [PubMed] [Google Scholar]
- Moiseeva EP, Manson MM. Dietary chemopreventive phytochemicals: too little or too much? Cancer Prevention Research. 2009;2:611–616. doi: 10.1158/1940-6207.CAPR-08-0102. doi:10.1158/1940-6207.CAPR-08-0102. [DOI] [PubMed] [Google Scholar]
- Molinie B, Georgel P. Genetic and epigenetic regulations of prostate cancer by genistein. Drug News and Perspectives. 2009;22:247–254. doi: 10.1358/dnp.2009.22.5.1378633. doi:10.1358/dnp.2009.22.5.1378633. [DOI] [PubMed] [Google Scholar]
- Montanelli L, Tonacchera M. Genetics and phenomics of hypothyroidism and thyroid dys- and agenesis due to PAX8 and TTF1 mutations. Molecular and Cellular Endocrinology. 2010;322:64–71. doi: 10.1016/j.mce.2010.03.009. doi:10.1016/j.mce.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Research. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. doi:10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Cai K, Cheng Y, Wang S, Paun B, Hamilton JP, Jin Z, Sato F, Berki AT, Kan T, et al. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology. 2006;131:797–808. doi: 10.1053/j.gastro.2006.06.006. doi:10.1053/j.gastro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Muller HM, Fiegl H, Goebel G, Hubalek MM, Widschwendter A, Muller-Holzner E, Marth C, Widschwendter M. MeCP2 and MBD2 expression in human neoplastic and non-neoplastic breast tissue and its association with oestrogen receptor status. British Journal of Cancer. 2003;89:1934–1939. doi: 10.1038/sj.bjc.6601392. doi:10.1038/sj.bjc.6601392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musri MM, Gomis R, Parrizas M. Chromatin and chromatin-modifying proteins in adipogenesis. Biochemistry and Cell Biology. 2007;85:397–410. doi: 10.1139/O07-068. doi:10.1139/O07-068. [DOI] [PubMed] [Google Scholar]
- Newell-Price J. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing's syndrome and beyond. Journal of Endocrinology. 2003;177:365–372. doi: 10.1677/joe.0.1770365. doi:10.1677/joe.0.1770365. [DOI] [PubMed] [Google Scholar]
- Noer A, Sorensen AL, Boquest AC, Collas P. Stable CpG hypomethylation of adipogenic promoters in freshly isolated, cultured, and differentiated mesenchymal stem cells from adipose tissue. Molecular Biology of the Cell. 2006;17:3543–3556. doi: 10.1091/mbc.E06-04-0322. doi:10.1091/mbc.E06-04-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novac N, Heinzel T. Nuclear receptors: overview and classification. Current Drug Targets. Inflammation and Allergy. 2004;3:335–346. doi: 10.2174/1568010042634541. doi:10.2174/1568010042634541. [DOI] [PubMed] [Google Scholar]
- Oakes CC, La SS, Smiraglia DJ, Robaire B, Trasler JM. Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells. Developmental Biology. 2007;307:368–379. doi: 10.1016/j.ydbio.2007.05.002. doi:10.1016/j.ydbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau U, Matagne V. New concepts on the control of the onset of puberty. Endocrine Development. 2010;17:44–51. doi: 10.1159/000262527. doi:10.1159/000262527. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. doi:10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Olsson M, Gustafsson O, Skogastierna C, Tolf A, Rietz BD, Morfin R, Rane A, Ekstrom L. Regulation and expression of human CYP7B1 in prostate: overexpression of CYP7B1 during progression of prostatic adenocarcinoma. Prostate. 2007;67:1439–1446. doi: 10.1002/pros.20630. doi:10.1002/pros.20630. [DOI] [PubMed] [Google Scholar]
- Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annual Review of Nutrition. 2002;22:139–166. doi: 10.1146/annurev.nutr.22.120501.150216. doi:10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- Ooi SK, O'Donnell AH, Bestor TH. Mammalian cytosine methylation at a glance. Journal of Cell Science. 2009;122:2787–2791. doi: 10.1242/jcs.015123. doi:10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra SK, Bettuzzi S. Epigenetic DNA-(cytosine-5-carbon) modifications: 5-aza-2′-deoxycytidine and DNA-demethylation. Biochemistry. 2009;74:613–619. doi: 10.1134/s0006297909060042. doi:10.1134/S0006297909060042. [DOI] [PubMed] [Google Scholar]
- Pearson RK, Anderson B, Dixon JE. Molecular biology of the peptide hormone families. Endocrinology and Metabolism Clinics of North America. 1993;22:753–774. [PubMed] [Google Scholar]
- Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS ONE. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. doi:10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Current Biology. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. doi:10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. New England Journal of Medicine. 1983;308:242–245. doi: 10.1056/NEJM198302033080502. doi:10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes. 1991;40(Supplement 2):126–130. doi: 10.2337/diab.40.2.s126. [DOI] [PubMed] [Google Scholar]
- Pietilainen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keranen H, Suomalainen A, Gotz A, Suortti T, Yki-Jarvinen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Medicine. 2008;5:e51. doi: 10.1371/journal.pmed.0050051. doi:10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, Ziska T, Schellong K, Rodekamp E, Melchior K, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. Journal of Physiology. 2009;587:4963–4976. doi: 10.1113/jphysiol.2009.176156. doi:10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Giovannucci E, Rimm EB, Curhan GC, Spiegelman D, Colditz GA, Willett WC. Retrospective analysis of birth weight and prostate cancer in the Health Professionals Follow-up Study. American Journal of Epidemiology. 1998;147:1140–1144. doi: 10.1093/oxfordjournals.aje.a009412. [DOI] [PubMed] [Google Scholar]
- Pozzi S, Rossetti S, Bistulfi G, Sacchi N. RAR-mediated epigenetic control of the cytochrome P450 Cyp26a1 in embryocarcinoma cells. Oncogene. 2006;25:1400–1407. doi: 10.1038/sj.onc.1209173. doi:10.1038/sj.onc.1209173. [DOI] [PubMed] [Google Scholar]
- Prins GS. Estrogen imprinting: when your epigenetic memories come back to haunt you. Endocrinology. 2008;149:5919–5921. doi: 10.1210/en.2008-1266. doi:10.1210/en.2008-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan VK, Blewitt ME, Druker R, Preis JI, Whitelaw E. Metastable epialleles in mammals. Trends in Genetics. 2002;18:348–351. doi: 10.1016/s0168-9525(02)02709-9. doi:10.1016/S0168-9525(02)02709-9. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. PNAS. 2003;100:2538–2543. doi: 10.1073/pnas.0436776100. doi:10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud E, Mitchell BD, Naj AC, Pollin TI. Investigating parent of origin effects in studies of type 2 diabetes and obesity. Current Diabetes Reviews. 2008;4:329–339. doi: 10.2174/157339908786241179. doi:10.2174/157339908786241179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annual Review of Medicine. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. doi:10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- Rocha Monteiro PR, Reis-Henriques MA, Coimbra J. Polycyclic aromatic hydrocarbons inhibit in vitro ovarian steroidogenesis in the flounder (Platichthys flesus L.) Aquatic Toxicology. 2000;48:549–559. doi: 10.1016/s0166-445x(99)00055-7. doi:10.1016/S0166-445X(99)00055-7. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Millam JR. Phytoestrogens and avian reproduction: exploring the evolution and function of phytoestrogens and possible role of plant compounds in the breeding ecology of wild birds. Comparative Biochemistry and Physiology. Part A, Molecular and Integrative Physiology. 2009;154:279–288. doi: 10.1016/j.cbpa.2009.06.017. doi:10.1016/j.cbpa.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Research. 2004;14:1902–1910. doi: 10.1101/gr.2722704. doi:10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Antona C, Gomez A, Karlgren M, Sim SC, Ingelman-Sundberg M. Molecular genetics and epigenetics of the cytochrome P450 gene family and its relevance for cancer risk and treatment. Human Genetics. 2010;127:1–17. doi: 10.1007/s00439-009-0748-0. doi:10.1007/s00439-009-0748-0. [DOI] [PubMed] [Google Scholar]
- Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Annals of Clinical Psychiatry. 2009;21:213–236. [PubMed] [Google Scholar]
- Rubello D, Giannini S, D'Angelo A, Nobile M, Carraio G, Rigotti P, Marchini F, Zaninotto M, Dalle CL, Sartori L, et al. Secondary hyperparathyroidism is associated with vitamin D receptor polymorphism and bone density after renal transplantation. Biomedicine and Pharmacotherapy. 2005;59:402–407. doi: 10.1016/j.biopha.2004.09.015. doi:10.1016/j.biopha.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environmental Health Perspectives. 2008;116:1547–1552. doi: 10.1289/ehp.11338. doi:10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151:2603–2612. doi: 10.1210/en.2009-1218. doi:10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chemical Research in Toxicology. 2003;16:807–816. doi: 10.1021/tx034036r. doi:10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, Itoh A, Funata N, Schreiber SL, Yoshida M, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24:4531–4539. doi: 10.1038/sj.onc.1208646. doi:10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- Salay E, Garabrant D. Polychlorinated biphenyls and thyroid hormones in adults: a systematic review appraisal of epidemiological studies. Chemosphere. 2009;74:1413–1419. doi: 10.1016/j.chemosphere.2008.11.031. doi:10.1016/j.chemo-sphere.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Oh BR, Dharia A, Fujimoto S, Dahiya R. Inactivation of the human androgen receptor gene is associated with CpG hypermethylation in uterine endometrial cancer. Molecular Carcinogenesis. 2000;29:59–66. doi: 10.1002/1098-2744(200010)29:2<59::aid-mc2>3.0.co;2-6. doi:10.1002/1098-2744(200010)29:2!59∷AIDMC2O3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Kaneuchi M, Fujimoto S, Tanaka Y, Dahiya R. Hypermethylation can selectively silence multiple promoters of steroid receptors in cancers. Molecular and Cellular Endocrinology. 2003;202:201–207. doi: 10.1016/s0303-7207(03)00084-4. doi:10.1016/S0303-7207(03)00084-4. [DOI] [PubMed] [Google Scholar]
- Schayek H, Bentov I, Sun S, Plymate SR, Werner H. Progression to metastatic stage in a cellular model of prostate cancer is associated with methylation of the androgen receptor gene and transcriptional suppression of the insulin-like growth factor-I receptor gene. Experimental Cell Research. 2010;316:1479–1488. doi: 10.1016/j.yexcr.2010.03.007. doi:10.1016/j.yexcr.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. doi:10.1126/science.142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. doi:10.126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Drake AJ. Bisphenol A and metabolic syndrome. Endocrinology. 2010;151:2404–2407. doi: 10.1210/en.2010-0445. doi:10.1210/en.2010-0445. [DOI] [PubMed] [Google Scholar]
- Shi XB, Tepper CG, deVere White RW. Cancerous miRNAs and their regulation. Cell Cycle. 2008a;7:1529–1538. doi: 10.4161/cc.7.11.5977. [DOI] [PubMed] [Google Scholar]
- Shi XB, Tepper CG, White RW. MicroRNAs and prostate cancer. Journal of Cellular and Molecular Medicine. 2008b;12:1456–1465. doi: 10.1111/j.1582-4934.2008.00420.x. doi:10.1111/j.1582-4934.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutoh Y, Takeda M, Ohtsuka R, Haishima A, Yamaguchi S, Fujie H, Komatsu Y, Maita K, Harada T. Low dose effects of dichlorodiphenyltrichloroethane (DDT) on gene transcription and DNA methylation in the hypothalamus of young male rats: implication of hormesis-like effects. Journal of Toxicological Sciences. 2009;34:469–482. doi: 10.2131/jts.34.469. [DOI] [PubMed] [Google Scholar]
- Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clinical Medicine and Research. 2003;1:281–290. doi: 10.3121/cmr.1.4.281. doi:10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlecki P, Zielenkiewicz P. Mammalian DNA methyltransferases. Acta Biochimica Polonica. 2006;53:245–256. [PubMed] [Google Scholar]
- Sikka SC, Wang R. Endocrine disruptors and estrogenic effects on male reproductive axis. Asian Journal of Andrology. 2008;10:134–145. doi: 10.1111/j.1745-7262.2008.00370.x. doi:10.1111/j.1745-7262.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of adult disease. Pediatric Clinics of North America. 2009;56:449–466. doi: 10.1016/j.pcl.2009.03.004. doi:10.1016/j.pcl.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. PNAS. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. doi:10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Fan CY, Zou C, Bodenner D, Kokoska MS. Methylation status of genes in papillary thyroid carcinoma. Archives of Otolaryngology – Head and Neck Surgery. 2007;133:1006–1011. doi: 10.1001/archotol.133.10.1006. doi:10.1001/archotol.133.10.1006. [DOI] [PubMed] [Google Scholar]
- Sober S, Laan M, Annilo T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochemical and Biophysical Research Communications. 2010;391:727–732. doi: 10.1016/j.bbrc.2009.11.128. doi:10.1016/j.bbrc.2009.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ. Oxytocin and vasopressin: social neuropeptides. CNS Spectrums. 2009;14:602–606. doi: 10.1017/s1092852900023841. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151:3652–3664. doi: 10.1210/en.2010-0094. doi:10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- Stoger R. In vivo methylation patterns of the leptin promoter in human and mouse. Epigenetics. 2006;1:155–162. doi: 10.4161/epi.1.4.3400. doi:10.4161/epi.1.4.3400. [DOI] [PubMed] [Google Scholar]
- Stoger R, Kubicka P, Liu CG, Kafri T, Razin A, Cedar H, Barlow DP. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71. doi: 10.1016/0092-8674(93)90160-r. doi:10.1016/0092-8674(93)90160-R. [DOI] [PubMed] [Google Scholar]
- Stouffs K, Vandermaelen D, Tournaye H, Liebaers I, Van SA, Lissens W. Genetics and male infertility. Verhandelingen – Koninklijke Academie Voor Geneeskunde Van Belgie. 2009;71:115–139. [PubMed] [Google Scholar]
- Sun LY, D'Ercole AJ. Insulin-like growth factor-I stimulates histone H3 and H4 acetylation in the brain in vivo. Endocrinology. 2006;147:5480–5490. doi: 10.1210/en.2006-0586. doi:10.1210/en.2006-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Reviews in Endocrine and Metabolic Disorders. 2007;8:173–182. doi: 10.1007/s11154-007-9042-4. doi:10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. doi:10. 1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription. RNA. 2009;15:287–293. doi: 10.1261/rna.1211209. doi:10.1261/rna.1211209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Einstein FH. Epigenetic basis for fetal origins of age-related disease. Journal of Women's Health. 2010;19:581–587. doi: 10.1089/jwh.2009.1408. doi:10.1089/jwh.2009.1408. [DOI] [PubMed] [Google Scholar]
- Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. PNAS. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. doi:10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. doi:10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Tilton SC, Orner GA, Benninghoff AD, Carpenter HM, Hendricks JD, Pereira CB, Williams DE. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environmental Health Perspectives. 2008;116:1047–1055. doi: 10.1289/ehp.11190. doi:10.1289/ehp.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari VK, McGarvey KM, Licchesi JD, Ohm JE, Herman JG, Schubeler D, Baylin SB. PcG proteins, DNA methylation, and gene repression by chromatin looping. PLoS Biology. 2008;6:2911–2927. doi: 10.1371/journal.pbio.0060306. doi:10. 1371/journal.pbio.0060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Human Molecular Genetics. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. doi:10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasler JM. Epigenetics in spermatogenesis. Molecular and Cellular Endocrinology. 2009;306:33–36. doi: 10.1016/j.mce.2008.12.018. doi:10.1016/j.mce.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Vaag A, Poulsen P. Twins in metabolic and diabetes research: what do they tell us? Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10:591–596. doi: 10.1097/MCO.0b013e3282ab9ea6. doi:10.1097/MCO.0b013e3282ab9ea6. [DOI] [PubMed] [Google Scholar]
- Vanselow J, Selimyan R, Furbass R. DNA methylation of placenta-specific Cyp19 promoters of cattle and sheep. Experimental and Clinical Endocrinology and Diabetes. 2008;116:437–442. doi: 10.1055/s-2008-1058083. doi:10.1055/s-2008-1058083. [DOI] [PubMed] [Google Scholar]
- Vanselow J, Spitschak M, Nimz M, Furbass R. DNA methylation is not involved in preovulatory down-regulation of CYP11A1, HSD3B1, and CYP19A1 in bovine follicles but may have a role in permanent silencing of CYP19A1 in large granulosa lutein cells. Biology of Reproduction. 2010;82:289–298. doi: 10.1095/biolreprod.109.079251. doi:10.1095/biolreprod.109.079251. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Bowers CY. Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen. Journal of Pediatric Endocrinology and Metabolism. 2003;16(Supplement 3):587–605. [PubMed] [Google Scholar]
- Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodide uptake in dedifferentiated thyroid carcinoma: relationship to human Na+/I– symporter gene methylation status. Journal of Clinical Endocrinology and Metabolism. 1999;84:2449–2457. doi: 10.1210/jcem.84.7.5815. doi:10.1210/jc.84.7.2449. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T, Meijer OC, de Kloet , et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. doi:10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang X. Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Research. 2006;34:1646–1652. doi: 10.1093/nar/gkl068. doi:10.1093/nar/gkl068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fang MZ, Liao J, Yang GY, Nie Y, Song Y, So C, Xu X, Wang LD, Yang CS. Hypermethylation-associated inactivation of retinoic acid receptor beta in human esophageal squamous cell carcinoma. Clinical Cancer Research. 2003;9:5257–5263. [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. Journal of Neuroscience. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. doi:10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield GK, Kourides IA. Expression of chorionic gonadotropin alpha- and beta-genes in normal and neoplastic human tissues: relationship to deoxyribonucleic acid structure. Endocrinology. 1985;117:231–236. doi: 10.1210/endo-117-1-231. doi:10.1210/endo-117-1-231. [DOI] [PubMed] [Google Scholar]
- Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Research. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. doi:10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- Wolfsberg TG. Using the NCBI map viewer to browse genomic sequence data. Current Protocols in Bioinformatics. 2010;Chapter 1(Unit 1.5):1–25. doi: 10.1002/0471250953.bi0105s16. doi:10.1002/0471250953.bi0105s29. [DOI] [PubMed] [Google Scholar]
- Wu Q, Ohsako S, Ishimura R, Suzuki JS, Tohyama C. Exposure of mouse preimplantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters the methylation status of imprinted genes H19 and Igf2. Biology of Reproduction. 2004;70:1790–1797. doi: 10.1095/biolreprod.103.025387. doi:10.1095/biolreprod.103.025387. [DOI] [PubMed] [Google Scholar]
- Xing M, Usadel H, Cohen Y, Tokumaru Y, Guo Z, Westra WB, Tong BC, Tallini G, Udelsman R, Califano JA, et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Research. 2003;63:2316–2321. [PubMed] [Google Scholar]
- Xu GL, Bestor TH, Bourc'his D, Hsieh CL, Tommerup N, Bugge M, Hulten M, Qu X, Russo JJ, Viegas-Pequignot E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–191. doi: 10.1038/46052. doi:10.1038/46214. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, Taketani T, Matsuoka A, Tamura H, Sugino N. DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Human Reproduction. 2009;24:1126–1132. doi: 10.1093/humrep/dep015. doi:10.1093/humrep/dep015. [DOI] [PubMed] [Google Scholar]
- Yang NC, Castro AJ, Lewis M, Wong TW. Polynuclear aromatic hydrocarbons, steroids and carcinogenesis. Science. 1961;134:386–387. doi: 10.1126/science.134.3476.386. doi:10.1126/science.134.3476.386. [DOI] [PubMed] [Google Scholar]
- Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. PNAS. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. doi:10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Li X, Kong X, Wang W, Bi Y, Hu L, Cui B, Li X, Ning G. Hypomethylation in the promoter region of POMC gene correlates with ectopic overexpression in thymic carcinoids. Journal of Endocrinology. 2005;185:337–343. doi: 10.1677/joe.1.05963. doi:10.1677/joe.1.05963. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Eguchi H, Nakachi K, Tanimoto K, Higashi Y, Suemasu K, Iino Y, Morishita Y, Hayashi S. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: methylation of the gene and alteration of trans-acting factors. Carcinogenesis. 2000;21:2193–2201. doi: 10.1093/carcin/21.12.2193. doi:10.1093/carcin/21.12.2193. [DOI] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150:4681–4691. doi: 10.1210/en.2009-0499. doi:10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26:7346–7354. doi: 10.1038/sj.onc.1210537. doi:10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–7606. doi: 10.1038/sj.onc.1207100. doi:10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. American Journal of Pathology. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]