Abstract
Objective
Pancreatic islets have fewer antioxidant enzymes than other tissues and thus are vulnerable to oxidative stress. In the present study, the effects of nine specifically selected Iranian medical plants on the mitochondria function and survival of isolated rat islets were examined.
Materials and Methods
In this experimental study, following laparotomy, pancreases of rats were removed and the islets isolated and incubated in vitro for 24 hours. Logarithmic doses of plant materials were added to the islets and incubated for an additional 24 hours after which the viability of the cells and production of reactive oxygen species (ROS) were measured. Levels of insulin production in relation to static and stimulated glucose concen- trations were also determined.
Results
The tested compounds markedly increased survival of the islet cells, their mi- tochondrial activity, and insulin levels at the same time as reducing production of ROS. Greatest effects were observed in the following order: Peganum harmala, Glycyrrhiza glabra, Satureja hortensis, Rosmarinus officinalis, Teucrium scordium, Aloe vera, Zingiber officinale, Silybum marianum, and Hypericum perforatum at doses of 10, 103, 104, 10, 102, 102, 10-1, 10 and 103μgmL-1, respectively.
Conclusion
Based on these results, we suggest that pretreatment with these select- ed Iranian medical plants can improve the outcomes of pancreas transplants and grafts through the control of oxidative stress damage.
Keywords: Islets of Langerhans, Oxidative Stress, Medicine, Iranian Traditional, In Vitro
Introduction
Diabetes mellitus (DM), which affects many organs in the body, is one of the most prevalent metabolic disorders in all parts of the world. Insulin and oral natural or synthetic hypoglycemic agents are used to treat DM but these drugs treat rather than cure the disease and have noticeable side effects (1).
Cell-based therapy for DM using activated pancreatic islet cells is theoretically possible as a cure for DM patients who don’t respond to current drugs (2, 3). A major drawback of this technique is that pancreatic islet cells face excessive oxidative stress during the isolation and transplantation procedures (4).
Oxidative stress is the result of an imbalance between antioxidants and free radicals involved in cellular damage (5). For instance, increasing reactive oxygen species (ROS) in pancreatic beta cells reduces insulin gene expression and insulin release and also damages the islets (6).
Nowadays, several plants are known for their antioxidant properties (7). Lessening oxidative stress through the use of herbal sources may improve survival of isolated rat pancreatic islets by scavenging harmful free radicals (3). Use of herbal medicine is not new. Herbal extracts have been used traditionally to cure diseases in every culture over the last 1000 years (8) and scientists today continue to investigate their potential to provide reliable therapies (9).
The present study aimed to screen the potential of selected herbs with strong anti-oxidative stress properties in relation to the viability and functionality of rat pancreatic islets. These herbs were identified from Persian folk medicine as listed in table 1.
Materials and Methods
Chemicals
All chemicals in this study were supplied from Sigma-Aldrich Co. (Gmbh, Munich, Germany). Rat specific insulin ELISA kit was obtained from Mercodia (Sweden). All other chemicals which were used in the study were obtained from standard commercial suppliers.
Animals
Male adult Wistar rats (2-3 months), with a weight of approximately ~250 ± 25 g, from the animal house of the Faculty of Pharmacy, Tehran University of Medical Science (TUMS) were used. All animals were treated according to guidelines, which are approved by the TUMS Ethics Committee under the code number 90- 03-33-15668.
Experimental protocols for islet isolation and culture
After the accommodation of the rats to the lab environment, the pancreas was removed and washed with Krebs buffer (8 NACL, 0.27 KCL, 0.42 NaH2PO4, 0.05 MgCL2, 2.38 HEPES, 0.22 CaCL2.2H2O, 0.5 glucose. 1H2O, in grams per liter, at pH=7.4) in order to remove lymph nodes, fat and blood vessels. The pancreas was then cut into small pieces on ice and washed two times by centrifuging at 3000 g for 60 seconds. Collagenase enzyme was added to the falcon tissue culture dishes to digest tissues surrounding the islets at 37˚C. Digestion was stopped by adding bovine serum albumin (BSA) and the pieces were again centrifuged twice at 3000 g for 60 seconds.
Islets approximately 100-150 μm in size were isolated by use of a sampler under a stereomicroscope. Standard RPMI 1640 culture medium containing fetal bovine serum (FBS), penicillin streptomycin (Pen Strep) and 8.3 mmol/l glucose was added to the fresh islets. They were then kept for 24 hours at 37˚C with 0.5% CO2 for experimental use (4).
Plant materials
Nine medicinal herbs were obtained from the Research Institute of Medicinal Plants- ACECR in June 2009 and air-dried at room temperature. Samples were authenticated by a botanist (Y. Ajani), and verified specimens were preserved in the central herbarium of medicinal plants (ACECR). The scientific names and tested parts of the plant materials are detailed in table 2.
Table 2.
Details of plant extractions.
| S. No | Scientific name | Plant part used | Extraction yield (mgg-1) | Doses (µgmL-1) |
|---|---|---|---|---|
| 1 | Aloe vera | Gel | 4.87 | 10, 102, 103, 104 |
| 2 | Glycyrrhiza glabra | Root | 129.52 | 1, 10, 102, 103 |
| 3 | Hypericum perforatum | Aerial parts | 100.58 | 1, 10, 102, 103 |
| 4 | Peganum harmala | Seed | 169.25 | 10-1, 1, 10, 102 |
| 5 | Rosmarinus officinalis | Aerial parts | 236.51 | 10-1, 1, 10, 102 |
| 6 | Satureja hortensis | Aerial parts | 134 | 10, 102, 103, 104 |
| 7 | Silybum marianum | Seed | 123.49 | 1, 10, 102, 103 |
| 8 | Teucrium scordium | Aerial parts | 205 | 10, 102, 103, 104 |
| 9 | Zingiber officinale | Rhizome | 140.57 | 10-3, 10-2, 10-1, 1 |
Preparation of plant extracts and specific doses
Dried plant powder (40 g) was extracted by methanol using a percolation method at room temperature. Solvents were completely removed by drying under reduced pressure at 40˚C in a rotary evaporator. The samples were stored at 4˚C until used. Specifically for Aloe vera, the leaves (1000 g) were washed in a suitable bactericide (chlorhexidine). Sections were ground to a liquid, and the pulp was removed by filtering. The resultant gel was then freeze dried.
Various logarithmic concentrations from each plant extraction were made in RPMI medium culture and exposed to the islets for 24 hours at 37˚C. Doses of the plant materials are listed in table 2. To make sure of the range of doses, a pilot study was undertaken using doses from the literature.
Measurement of metabolism in the islets
Mitochondrial metabolism in the islets was tested using dimethylthiazole-2-y1-2, 5-diphenyltetrazolium bromide (MTT) in order to investigate the effect of plant extracts on cell viability. After in vitro treatment with various doses of extracts for incubation periods of 24 hours, islets were washed twice with Krebs- HEPES and incubated with 20 μl of MTT (0.5 mg/mL) at 37˚C. This yellow solution was reduced and became purple in the living cells. After 3 hours, dimethyl sulfoxide (DMSO) was added and shaken for 30 minutes, then the absorbance of the samples was measured at 590 nm using a microplate reader (6).
Measurement of insulin secretion in the islets
The level of insulin secretion in a glucose static enviroment was measured for investigating the function of the islets. Isolated islets which were cultured at 37˚C in a humidified atmosphere of 5% CO2 in air in RPMI-1640 medium were washed twice with Krebs-HEPES buffer. Wells which contained 10 islets were then incubated in 2.8 mM glucose in buffer for 30 minutes. After that, half of the islet groups were treated with 2.8 mM glucose (basal dose) and the others with 16.7 mM glucose (stimulant dose) for 30 minutes at 37˚C. At the end, insulin was measured in the medium using a rat insulin ELISA kit. Results are reported as insulin released in the medium.
Measurements ROS production in the islets
2’, 7’-dichlorodihydrofluorescin diacetate (DCFHDA) was used to measure production of ROS and the oxidation of this compound was measured using an ELISA reader.
Each of the ten islets, which were collected in separate microtubes, was homogenized using extraction buffer and then centrifuged at 2375 g for 15 minutes. After that, 10 μl 2’,7’-dichlorodihydrofluorescin (DCFH) and 162 μl assay buffer was mixed together and 50 μl supernatant of the islet extraction was added. These solutions were incubated at 37˚C for 15 minutes. At the end, the absorbance of samples was measured every 10 minutes up to 60 minutes using a microplate reader. Remaining islets were collected for measurement of protein concentration, which was used to normalize the concentrations.
Protein assay
Total protein level was measured by adding Bradford reagent dye to diluted samples and using BSA as the standard. After 5 minutes, the absorbance was read at 595 nm using a microplate reader.
Statistical analysis
Data were expressed as the mean ± SEM of 3 different experiments, each read in duplicate. Oneway ANOVA followed by Tukey tests were used to analyze the data. Differences between groups were considered significant if the p value was lower or equal to 0.05. StatsDirect version 2.7.9 was used to analyze data.
Results
Peganum harmala (PH)
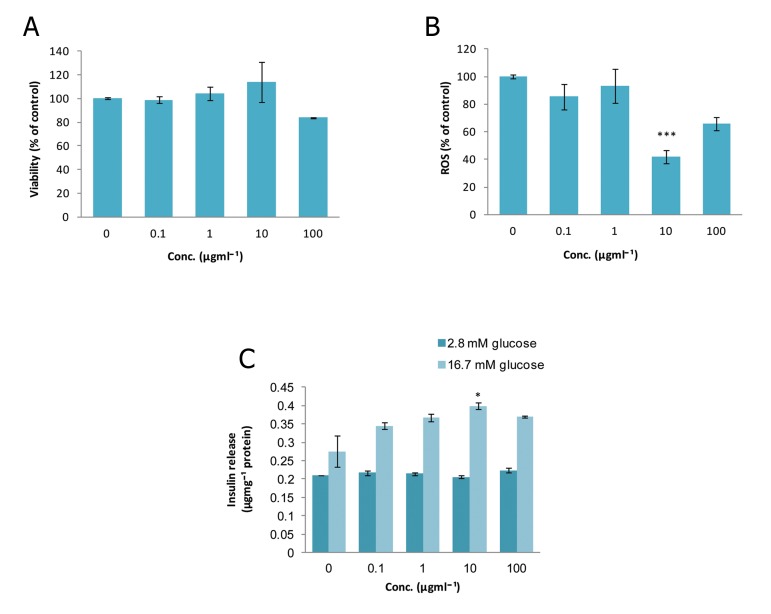
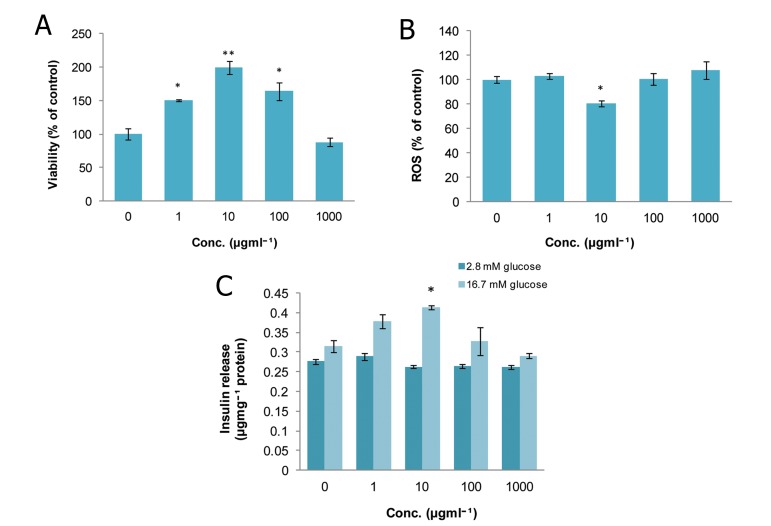
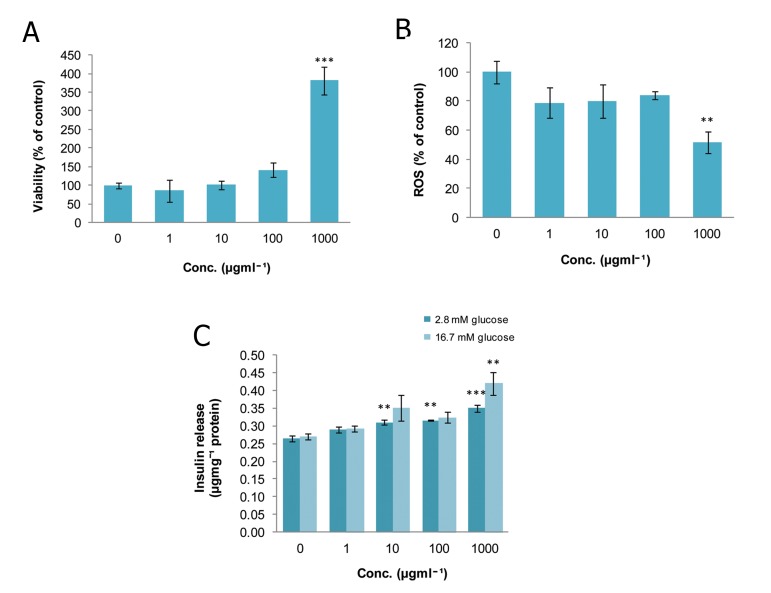
As shown in figure 1A, there was no significant change in the viability of islets at any concentration of PH compared to the control group. However, at a dose of 10 μgmL-1, a remarkable reduction (p<0.001) was observed in ROS production which decreased to 58% of that of the control (Fig 1B). Also, this concentration caused a significant increase (p<0.05) in insulin release in comparison to control group in the stimulated condition (16.7 mM glucose).
Fig 1.
Effect of different concentrations of Peganum harmala on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001 and *; p<0.05.
Satureja hortensis (SH)
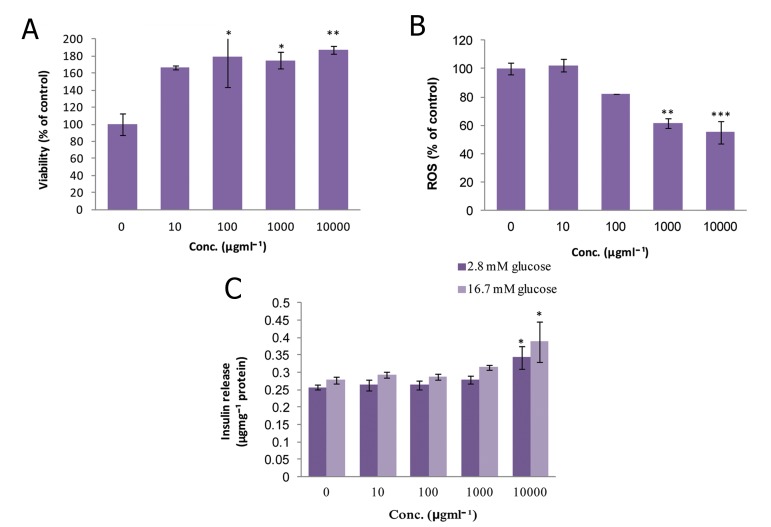
The viability of islets at doses of 102, 103, and 104 μgmL-1 of SH were considerably increased (p<0.05, p<0.05, and p<0.01 respectively) in comparison to the control group. Only the 10 μgmL-1 dose had no significant effect on cell viability (Fig 2A). As indicated in figure 2B, ROS production dropped significantly to 38.49% and 44.85% of that of the control group on exposure to doses of 103 and 104 μgmL-1 of SH (p<0.01 and p<0.001, respectively). Administration of SH at a concentration of 104 μgmL-1 significantly increased (p<0.05) insulin secretion in the static (2.8 mM glucose) and stimulated (16.7 mM glucose) environment in comparison to the matched controls (Fig 2C).
Fig 2.
Effect of different concentrations of Satureja hortensison on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001, **; p<0.01 and *; p<0.05.
Rosmarinus officinalis (RO)
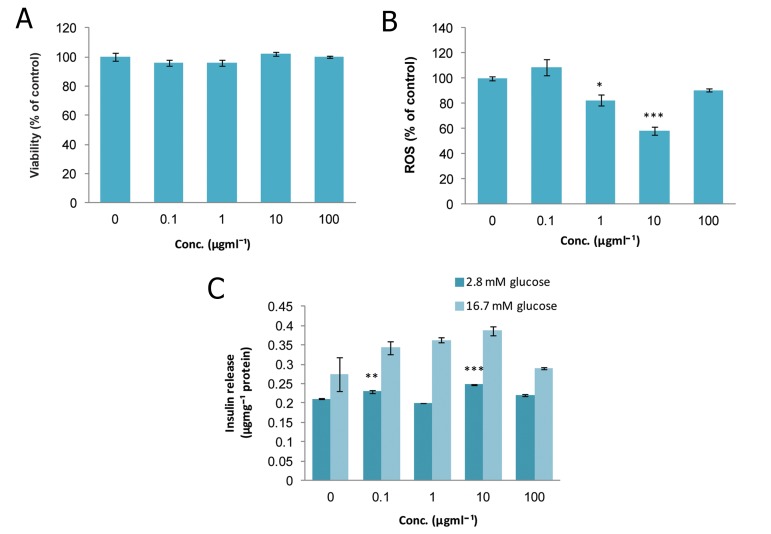
As shown in figure 3A, there was no significant change in the viability of islets at any concentration of RO compared to the control group. Results in figure 3B show that ROS production dropped significantly to 17.7% and 42% of that of the controls on exposure to doses of 1 and 10 μgmL-1 (p<0.05 and p<0.001, respectively). As indicated in Fig 3C, treatment of incubated islets in the basal state (2.8 mM glucose) with doses of 0.1 and 10 μgmL-1 meaningfully increased insulin secretion (p<0.01 and p<0.001, respectively), whereas in the stimulated state (16.7 mM glucose), only treatment with a dose of 10 μgmL-1 resulted in a significant relative increase in insulin secretion (p<0.05).
Fig 3.
Effect of different concentrations of Rosmarinus officinalis on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001, **; p<0.01 and *; p<0.05.
Zingiber officinale (ZO)
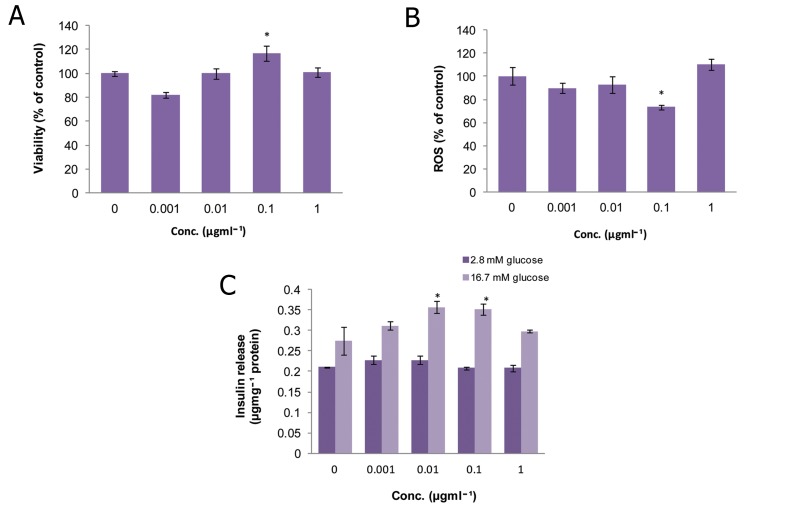
As seen in figure 4A, the metabolic activity of islets in 10-3, 10-2, and 1 μgmL-1 of ZO did not significantly change but at 10-1 μgmL-1, the viability increased significantly (p<0.05) when compared with controls. Additionally, this concentration caused a significant decrease (p<0.05) in ROS production compared to the control group (Fig 4B) and reduced ROS to 26.6% of that seen in the controls. Administration of ZO at concentrations of 10-2 and 10-1 μgmL-1 significantly increased (p<0.05) the release of insulin in the stimulated state (16.7 mM glucose). However, there was no significant change in the secretion of insulin in the basal state (2.8 mM glucose) when compared to the matched control (Fig 4C).
Fig 4.
Effect of different concentrations of Zingiber officinale on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at *; p<0.05.
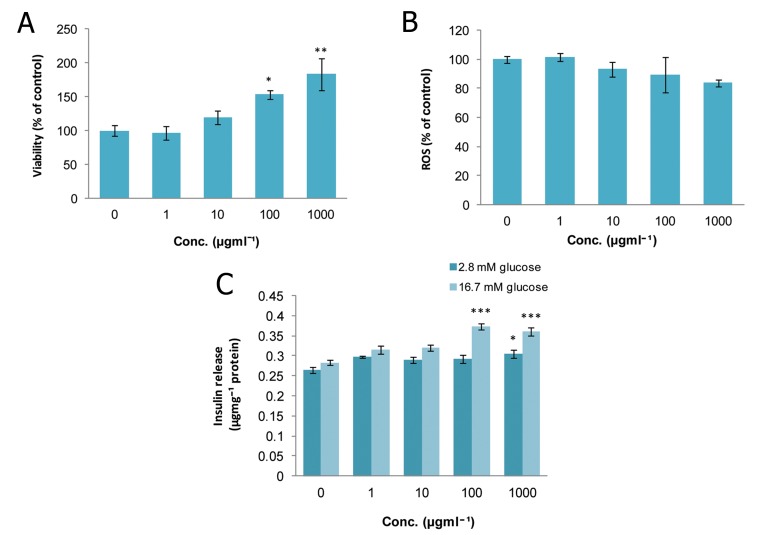
Sylibum marianum (SM)
As seen in figure 5A, SM significantly increased the viability of the islets at concentrations of 1, 10 and 102 μgmL-1, with the maximum change observed at 10 μgmL-1. This concentration also resulted in a significantly greater release of insulin (p<0.05) in comparison to the control group in the stimulated phase (16.7 mM glucose), and decreased ROS production to 19.25% of that seen in the control (Fig 5B).
Fig 5.
Effect of different concentrations of Sylibum marianum on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at **; p<0.01 and *; p<0.05.
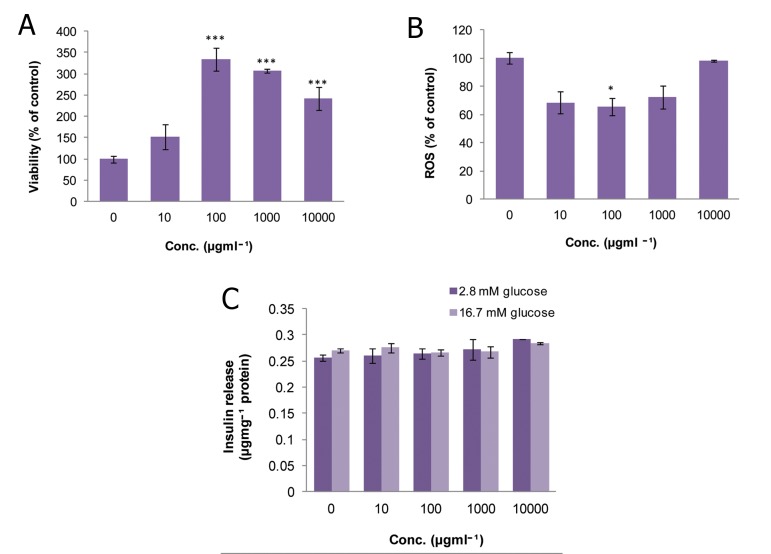
Aloe vera (AV)
The viability of islets at AV doses of 102, 103 and 104 μgmL-1 was significantly higher (p<0.001) than in the control group. Only the dose of 10 μg mL-1 showed no significant effect on cell viability (Fig 6A). As indicated in figure 6B, there was no significant change in ROS production at doses of 10, 103 and 104 μgmL-1 but at a dose of 102 μgmL-1 a significant reduction (p<0.05) was observed and ROS production decreased to 34.28% of that of the control. Results in figure 6C also show that there was no significant change in insulin release at any concentration of AV when compared with the matched control.
Fig 6.
Effect of different concentrations of Aloe vera on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001 and *; p<0.05.
Hypericum perforatum (HP)
As seen in figure 7A, HP significantly increased the metabolic activity of islets at doses of 102 and 103 μgmL-1. Despite the lack of significant differences in viability of islets at doses of 1 and 10 μgmL-1, the observed changes in the concentrations studied were dose-dependent. Results in figure 7B showed that ROS production in the islets did not significantly change in comparison to the control group. As indicated in figure 7C, in the basal state (2.8 mM glucose) only treatment of the incubated islets with a dose of 103 μgmL-1 HP increased insulin secretion significantly (p<0.05), whereas in the stimulated state (16.7 mM glucose) treatment of the incubated islets with doses of 102 and 103 μgmL-1 significantly increased insulin secretion (p<0.001).
Fig 7.
Effect of different concentrations of Hypericum perforatum on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001, **; p<0.01 and *; p<0.05.
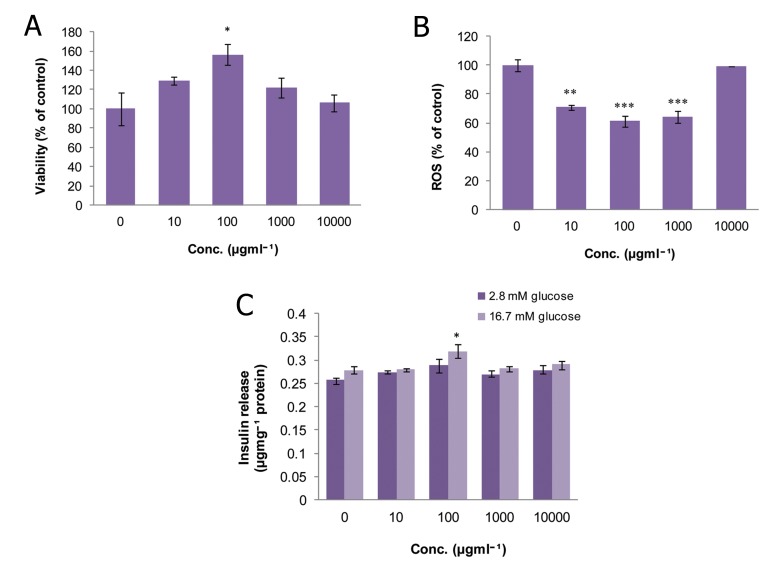
Teucrium scordium (TS)
As seen in figure 8A, the metabolic activity of islets at concentrations of 10, 103, 104 μgmL-1 TS did not change significantly, but at 102 μgmL-1 the viability increased in comparison to the control group. In addition, this concentration caused a significant increase (p<0.05) in insulin release in comparison to the control group in the stimulated phase (16.7 mM glucose). However, administration of TS at other concentrations had no significant effect on insulin secretion (Fig 8C). TS also significantly decreased ROS production at concentrations of 10, 102 and 103 μgmL-1, with maximum change observed at 102 μgmL-1. The dose of 102 μgmL-1 decreased ROS production to 38.55% of that for the control (Fig 8B).
Fig 8.
Effect of different concentrations of Teucrium scordium on A. viability, B. level of reactive oxygen species ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001, **; p<0.01 and *; p<0.05.
Glycyrrhiza glabra (GG)
As seen in figure 9A, the metabolic activity of islets in 1, 10, 102 μgmL-1 GG did not significantly change but at 103 μgmL-1, viability increased significantly (p<0.001). Additionally, this concentration resulted in a significant decrease (p<0.01) in ROS production (Fig 9B), reducing ROS to 48.5% of that of the control. As indicated in figure 9C, insulin secretion in the basal state was significantly increased at doses of 10, 102, and 103 μgmL-1 GG (p<0.01, p<0.01, and p<0.001, respectively). Only a concentration of 1 μgmL-1 was observed to have no significant effect on insulin secretion. Furthermore, administration of a 103 μgmL-1 dose of GG significantly increased (p<0.01) insulin secretion in the stimulated (16.7 mM glucose) state when compared with the matched control.
Fig 9.
Effect of different concentrations of Glycyrrhiza glabra on A. viability, B. level of ROS and C. released insulin from isolated rat pancreatic islets. Data are expressed as Mean ± SEM of 3 different experiments performed in duplicate. Significantly different from control group at ***; p<0.001 and **; p<0.01.
Discussion
Pancreatic islets have fewer antioxidant enzymes than other tissues and are thus more vulnerable to oxidative stress. Augmenting antioxidant protection mechanisms in pancreatic islets helps them to fight oxidative stress, especially during the implant process. The present findings showed a significant improvement in the antioxidant status of isolated islets exposed to extracts of several famed Iranian plants; PH, GG, SH, RO, TS, AV, ZO, SM, and HP. These plant extracts reduced ROS production to 58, 48.5, 44.85, 42, 38.55, 34.28, 26.6, 19.25, and 16.14% of that of the controls at their effective doses; 10 μgmL-1, 103 μgmL-1, 104 μgmL-1, 10 μgmL-1, 102 μgmL-1, 102 μgmL-1, 10-1 μgmL-1, 10 μgmL-1 and 103 μgmL-1 respectively. Interestingly, not only a reduction in ROS production but also an increase in released insulin was observed with the use of all the extracts except Aloe vera. Moreover, at the selected doses used no trace of toxicity was seen for any of the plant extracts.
In this report, we have indicated that extract obtained from the seeds of PH is associated with the greatest improvement in the antioxidant defense of isolated pancreatic islets. PH decreased ROS to 58% of that for the controls in parallel with an increased release of insulin. The antioxidant activity of the principal compounds in PH has been indicated previously in numerous studies. Also, it has been reported that PH contains flavonoids. These are known to scavenge free radicals (24, 25); modulate the expression of genes encoding antioxidant enzymes, such as glutathione reductase, glutathione peroxidase, glutathione-S-reductase, and quinone reductase (26); and enhance the expression of other intracellular endogenous antioxidants, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) (27). In addition, Kim et al. (28) have investigated the protective effects of PH alkaloids against the damage induced by oxidative stress and showed that β-carbolines depress the loss of viability in PC- 12 cells through a scavenging action on ROS and inhibition of the oxidation of thiols. Furthermore, Berrougui et al. (24) have shown that alkaloids from the seeds of pH exert an antioxidant effect on the in vitro oxidation of low-density lipoproteins (LDLs) by decreasing conjugated diene and monoamine oxidase (MDA) formation. The active ingredient in this plant is mostly harmaline and its activity is thought to be mediated by chelating Cu2+, scavenging free radicals and thus inhibiting the oxidation of human LDLs. The change of β-carboline to dihydro-β-carboline (i.e. harmine to harmaline) seems to increase the potency of the antioxidant activity of the alkaloids (24). In the present study, we have observed that 10 μgmL-1 of pH is the effective dose in relation to oxidative stress and antioxidant status in isolated islets.
It has been observed that both the ethanolic extract and the essential oil of SH reverse the oxidative damage to rat lymphocytes induced by hydrogen peroxide (29). Another study has indicated that SH has a high phenolic and flavonoid content (128 mg gallic acid equivalents/g dried extract and 88.5 mg catechin equivalents/g dried extract, respectively). The results of this study clearly showed that SH has the ability to scavenge ABTS and DPPH radicals as well as reduce the ability of ferric ions in a dose-dependent manner due to the phenolic content of the extract. Additionally, this study suggests that SH is a natural, rich source of antioxidants for probable pharmaceutical application in the prevention of many free radical-associated diseases (30). Based on our data, it can be concluded that SH improves the antioxidant status of isolated islets in a dose-dependent manner and its effective dose decreases ROS to 44.85% as well as increasing insulin release.
Previous studies on RO have shown that the antioxidant activity of its extracts is mainly ascribable to phenolic compounds classified into three groups including phenolic diterpenes possessing an abietic acid framework, flavonoids, and phenolic acids (31, 32). The principle relevant constituents are composed of vast numbers of polyphenolics including carnosic acid, carnosol, rosemarinic acid, ursolic acid, etc. (33). Among these, carnosic acid and carnosol, abietane-type diterpenes, caffeic acid and its derivative, and rosmarinic acid are the main antioxidant compounds present in RO (34, 35). In one study, the diterpenes and genkwanin (a flavonoid component of RO) showed membrane-rigidifying effects, which may contribute to their antioxidant capacity through hindering diffusion of free radicals (36). In another study, it was demonstrated that aqueous extract of RO can be efficient against the oxidative stress secondary to experimental diabetes (37). In addition, due to its potent antioxidant properties, the RO extract exerts a remarkable antidiabetogenic effect (38). The antioxidant effects of RO shown here are in agreement with previous reports and our data show that the extract obtained from aerial parts of RO improves antioxidant status in isolated islets and produces its greatest effects, including significant enhancement of insulin release at 10 μg mL-1.
Previous work has shown that the active ingredients of ZO roots, such as zingerone, zingiberofficinalediol, bisabolene, gingerols, zingibrene, shogaols and paradol produce antioxidant activity (39). It has been shown to contain vitamin B, C and minerals such as calcium, magnesium, potassium, phosphorus and linoleic acid (40). Although data on ZO are very scant and depend mostly on in vitro data, it can be said that ZO has the potential to inhibit ROS in human erythrocytes (41), and prevent malathion-induced ROS-mediated toxicity through its curcumin and zingerone constituents (42). The 6-gingerol is another effective constituent of ZO that scavenges ROS (43, 44). In addition, other findings have shown that ginger extract reduces blood glucose in a dose dependent manner. Action may be via stimulation of insulin production from the pancreatic islets or by increasing peripheral utilization or inhibition of the proximal tubular reabsorption mechanism for glucose in the kidney (45). Our results are in agreement with the earlier reports indicating that ZO is a strong activator of insulin release and antioxidant in isolated islets at an effective dose of 10-1 μgmL-1.
SM is also a plant that contains lots of phenolic compounds such as flavonolignans, flavonoid taxifolin, and some other minor compounds. Silymarin, a standardized extract from the seeds of SM contains mainly silibinin and a minority of other phenolic compounds (46, 47). Silymarin or silybin acts through the scavenging of free radicals (48), modulations of antioxidant and inflammatory enzymes (49, 50), inhibition of mitogenic and cell survival signaling or modulations of cell cycle regulators (51). Silymarin is considered as one of the most effective drugs used in type II diabetes mellitus (52) and may be therapeutically beneficial for type I diabetes too (53). Moreover, it has been reported that silymarin causes significant reduction in blood free fatty acids and triglyceride (54). Previous findings showed that silymarin improves β-cell function in obese rats (55). In the present study, we tried to determine the effective dose of SM extract for treatment of isolated rat pancreatic islets. A concentration of 10 μgmL-1 not only decreased ROS to 19.25% of the level in controls, but also enhanced insulin release.
Aloe vera (Aloe barbadensis) is one of the medicinal plants, which is a traditionally well approved in the management of diabetes. Many studies report that the high contents of phenolic compounds, glycosides (aloins), 1, 8-dihydroxyanthraquinone derivatives, β-1, 4 acetylated mannan, mannosephosphate, alprogenglucoprotein, inorganic compounds such as Zn, Cr, Mn, vitamin E, and vitamin C in AV are responsible for its biological action (56, 57). Among these components, it has been shown that the total phenols, flavonoids, vitamin C and vitamin E in AV gel extract directly scavenge reactive oxidants; they are assumed to organize a vital endogenous defense against oxidative cell and tissue injury (58). In addition, the increased levels of plasma insulin observed in previous studies suggest that the antihyperglycemic activity of AV could be due to insulinogenic activity of the gel extract (59). Our results are in consonance with earlier reports of the antioxidant activity of AV as a significant reducer of ROS. Nevertheless, there was no change in insulin release from the isolated islets when they treated with our selected dose of AV (10-104 μgmL-1).
Recently, it has been shown that HP extracts are highly effective in scavenging ROS and inhibiting ascorbate/iron-induced lipid peroxidation in rat cortical synaptosomes (60). HP has also been reported for its potent antihyperglycemic activity in diabetic rats. It seems that, HP stimulates insulin action and increases the pancreatic secretion of insulin (61). HP contains several phytochemical constituents such as rutin, flavonoids including quercetin and isoquercetin (62). For example, rutin has been reported to enhance insulin release and decrease blood glucose in diabetic animals (63). Our results are consistent with earlier reports of a marked increase in insulin secretion from isolated islets in a dose-dependent manner.
Previous findings have demonstrated the presence of diterpenes, flavonoids, saponnins, tannins and volatile oil in the aerial parts of TS. It has been suggested that this plant is a source of natural b-caryophyllene and (E)-b-farnesene and caryophyllene oxide (64-66). Additionally, other major compounds were β-farnesene, menthofuran, 1,8 cineole and α-humulene (67). Moreover, Kovacevic et al. (66) reported that TS oil was rich in alpha-pinene and beta-pinene (68). Although a survey of the literature produced no report on the antioxidant activity of this plant, our data showed that the extract obtained from aerial parts of TS improves antioxidant status in isolated islets and produces its greatest effects at a dose of 102 μgmL-1; a dose which simultaneously enhanced insulin release.
The isolation of various chemical constituents from GG has been reported previously (Table 1). Among these constituents, the isoflavone derivatives of GG have a protective role against oxidative stress (68,69). In addition, liquorice polyphenol extract prevents ROS-induced injury to cells by permeating cell membranes and increasing intracellular antioxidant activity (70). On the other hand, previous studies have shown that glycyrrhizin is quite effective against hepatotoxicity, hyperglycemia, hyperlipidemia and associated oxidative stress, and thus a useful treatment for diabetes (71, 72). In addition, the results of a recent study indicated that glycyrrhizic acid may be a good candidate for islet transplantation procedures to maintain islets in a viable and functional state (6). In another study, Kalaiarasi and Pugalendi (73) have shown that glycyrrhetinic acid enhances plasma insulin and reduces the gluconeogenic enzymes in the liver and kidney of diabetic rats. Our results are in consistent with earlier reports and prove a marked insulin secretory effect in isolated islets in a dose-dependent manner. The effective dose of GG decreased ROS up to 48.5%.
Table 1.
List of Iranian medicinal plants and their usages
| S. No. | Scientific name | Family | Antioxidant properties or medicinal use | Active compounds | Main component | References |
|---|---|---|---|---|---|---|
| 1 | Aloe vera | Liliaceae | Gastrointestinal disorders including peptic ulcer, antibacterial, anticancer, antifungal, antioxidant, anti-inflammatory and improves wound healing in diabetes | Phenolic compounds,glycosides(aloins), 8-C-ß-D-(2-O-(E)-coumaroyl)glucopyranosyl-2-(2-hydroxy)-propyl-7-methoxy-5-methylchromoone,acemannan,1,8-dihydroxyanthraquinone derivatives,β-1,4 acetylated mannan, mannosephosphate,alprogenglucoprotein, inorganiccompounds as Zn, Cr, Se, Mn, vitamins (A(ß-carotene), C and E) | Aloin (C21H22O9)
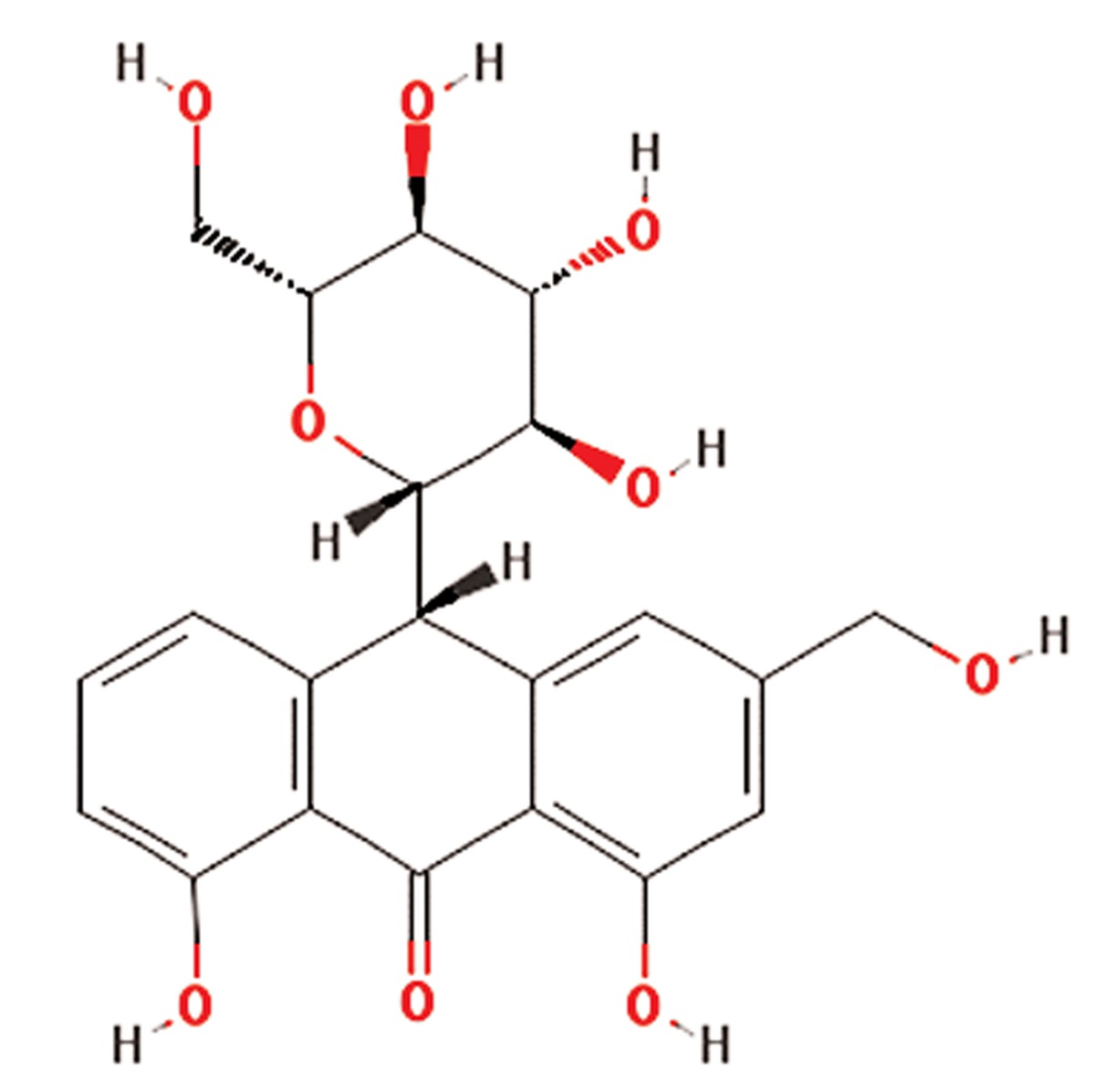
|
(10, 11) |
| 2 | Glycyrrhiza glabra | Fabaceae | Antioxidative, anti-inflammatory, anti-viral, treatment of fever, diarrhea and rheumatism | Glycyrrhizin, glycyrrhizinic acid, glabridin,glabrene, glabrol, licoflavonol, glycyrol, licoricone,formononetin, phaseollinisoflavan,hispaglabridin A&B, 3-hydroxy glabrol,3-methoxy glabridin, glabranin isomer,narigenin, and lupiwightenone | Glycyrrhizin (C42H62O16)
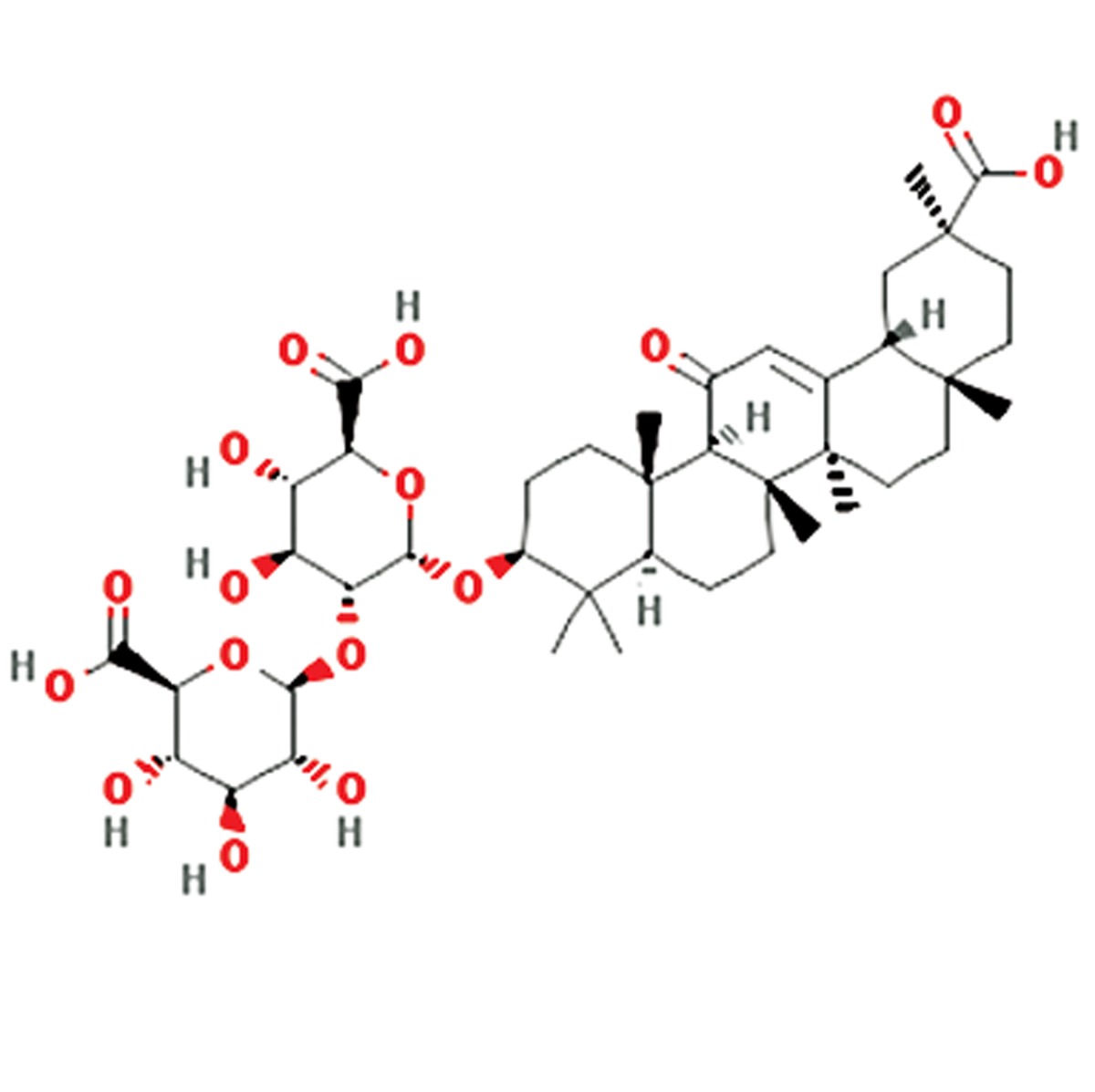
|
(6, 12) |
| 3 | Hypericum perforatum | Hypericaceae | Antibiotic, antiviral, antioxidant, anti-stress, anticancer, anti-depression | Hyperforin, flavonoids, phloroglucinols,naphthodiathrones, hypericin, hyperoside,quercetin, isoquercetin, quercitrin, rutin,campherol, luteolin, 13-118-biapigenin,1,3,6,7-tetra-hydroxyxanthone andprocyanidines | Hyperforin (C35H52O4)
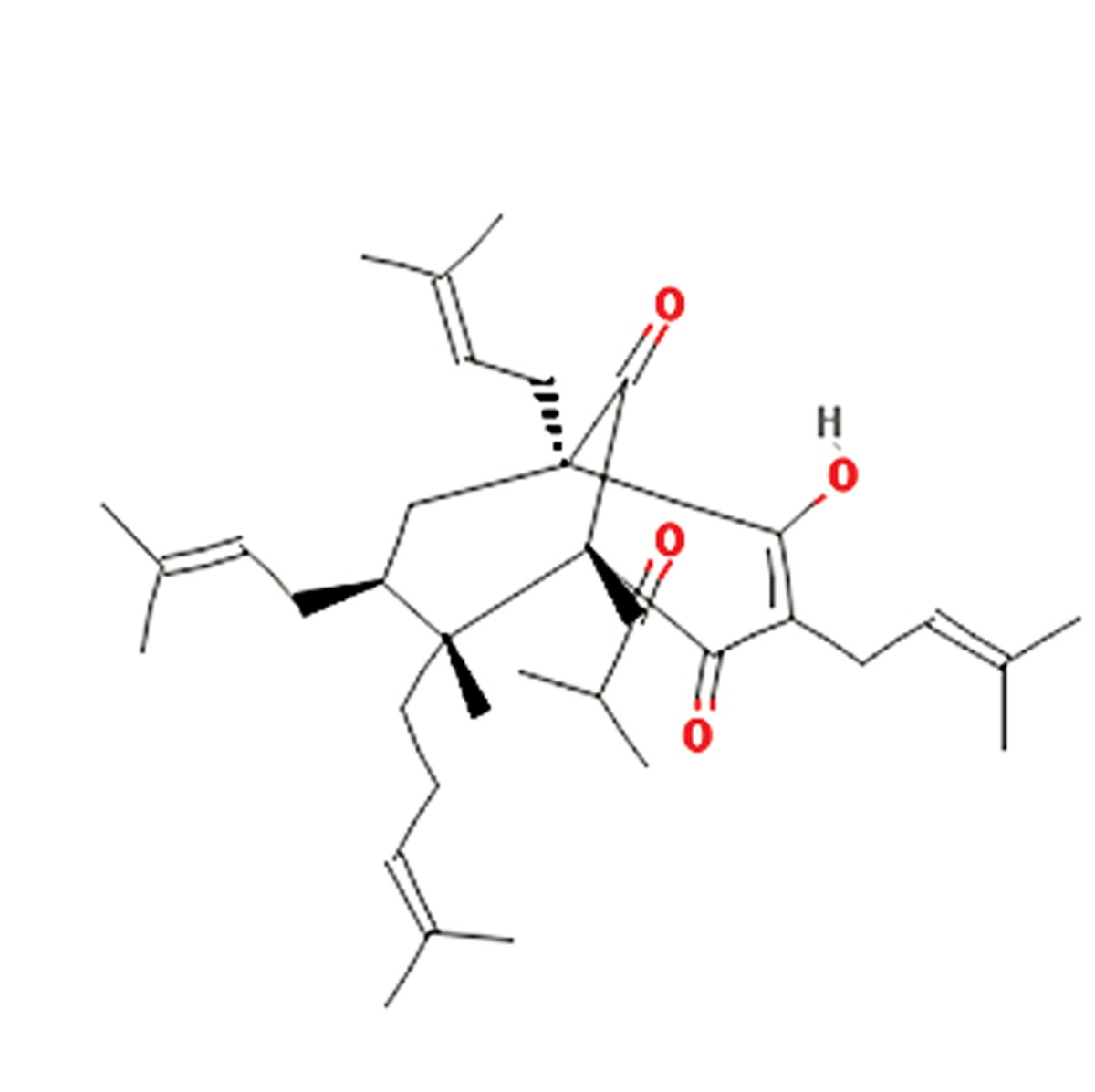
|
(13) |
| 4 | Peganum harmala | Nitrariaceae | Antibacterial, antiviral, antifungal, anticancer, antihistaminic, hypoglycemic effects | β-Carboline alkaloids such as harmaline,harmine, harmalol, harman, tetrahydroharmine,quinazoline derivatives, vasicine,vasicinon, anthroquinons and fixed oils | Harmaline (C13H14N2O)
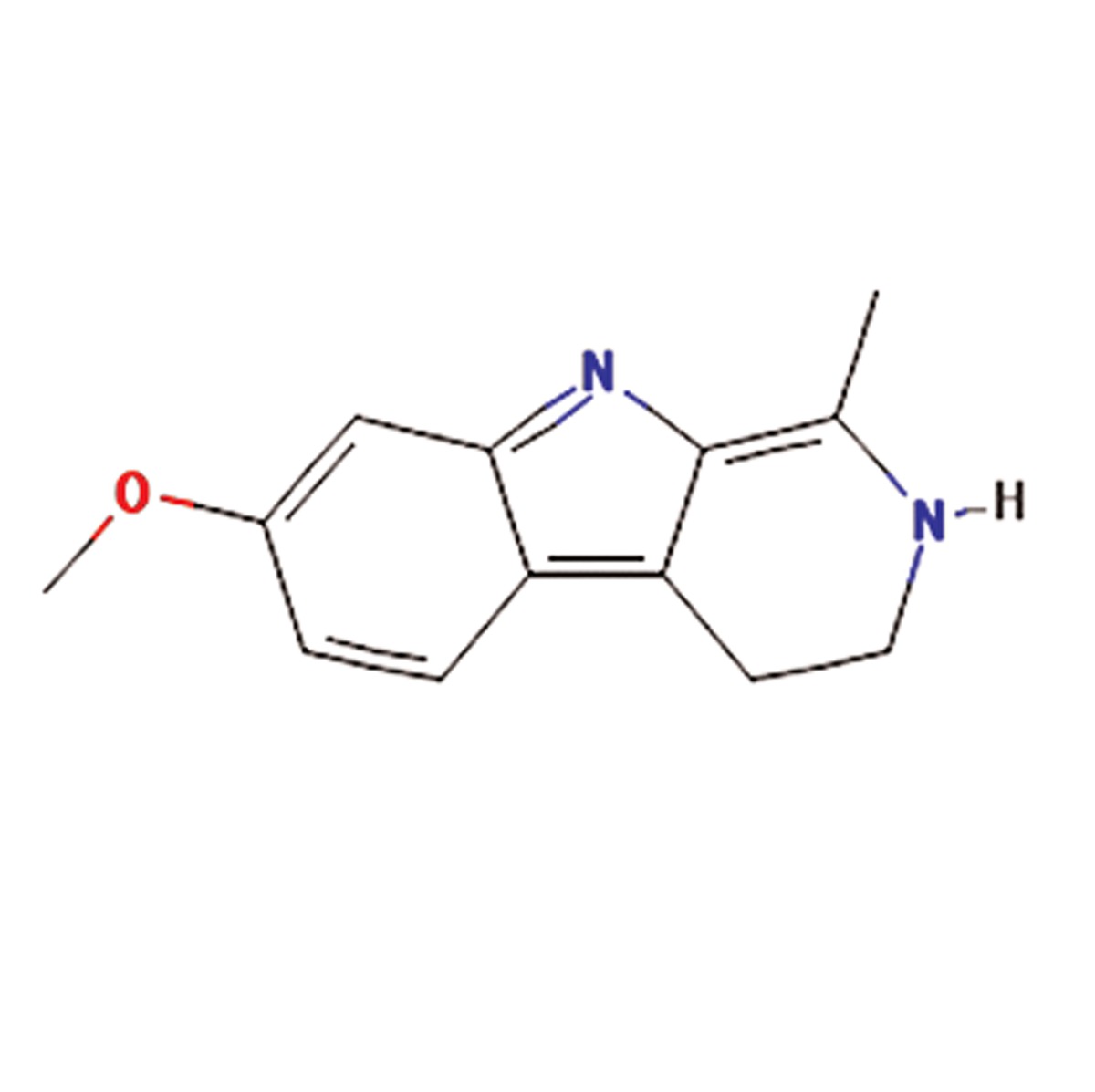
|
(14, 15) |
| 5 | Rosmarinus officinalis | Lamiaceae | Relaxing smooth muscles, hepatoprotective, anti-inflammatory, anti tumergenic activity | Polyphenolics, including rosemarinicacid, carnosic acid, carnosol, ursolic acid,abietane-type diterpenes, caffeic acid, flavonoides,triterpenes, and phenolic acids | Rosemaric acid (C18H16O8)
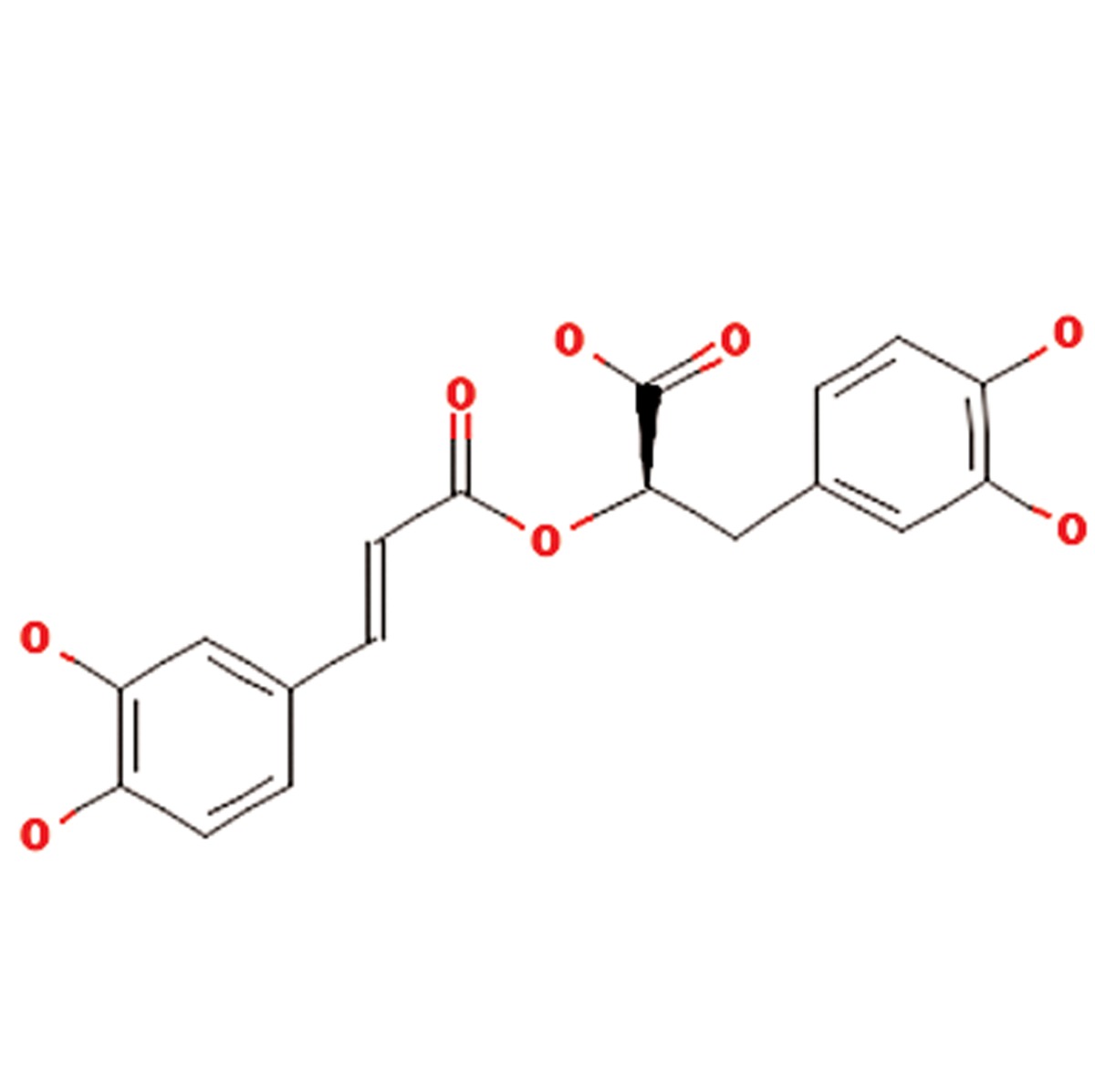
|
(7) |
| 6 | Satureja hortensis | Lamiaceae | Anti-diarrheal, antispasmodic, anti-inflammatory, antioxidant, antibacterial, antifungal, muscle pains, nausea, indigestion, diarrhea and infectious diseases | Carvacrol, thymol, , γ-terpinene, p-cymeneand linalool | Carvacrol (C10H14O))
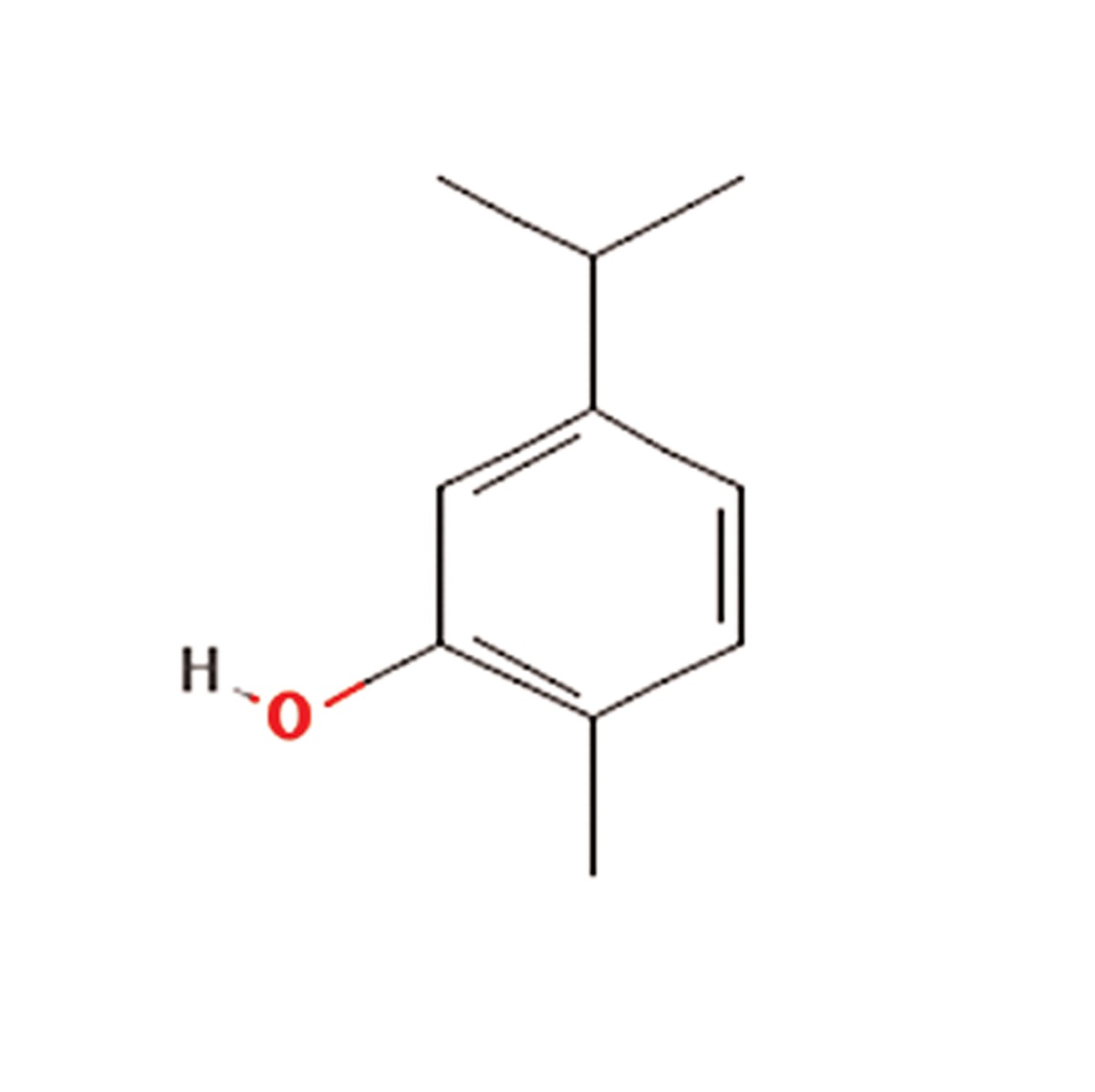
|
(16, 17) |
| 7 | Silybum marianum | Asteraceae | Silymarin or silybin (C25H22O10) | Silymarin flavonolignans (silibinin, alsocalled silybin, and its diastereoisomersisosilybin,silydianin, silychristin anddehydrosilybin), flavonoid taxifolin, betaine,trimethylglycine and essential fatty acids | Silymarin or silybin (C25H22O10
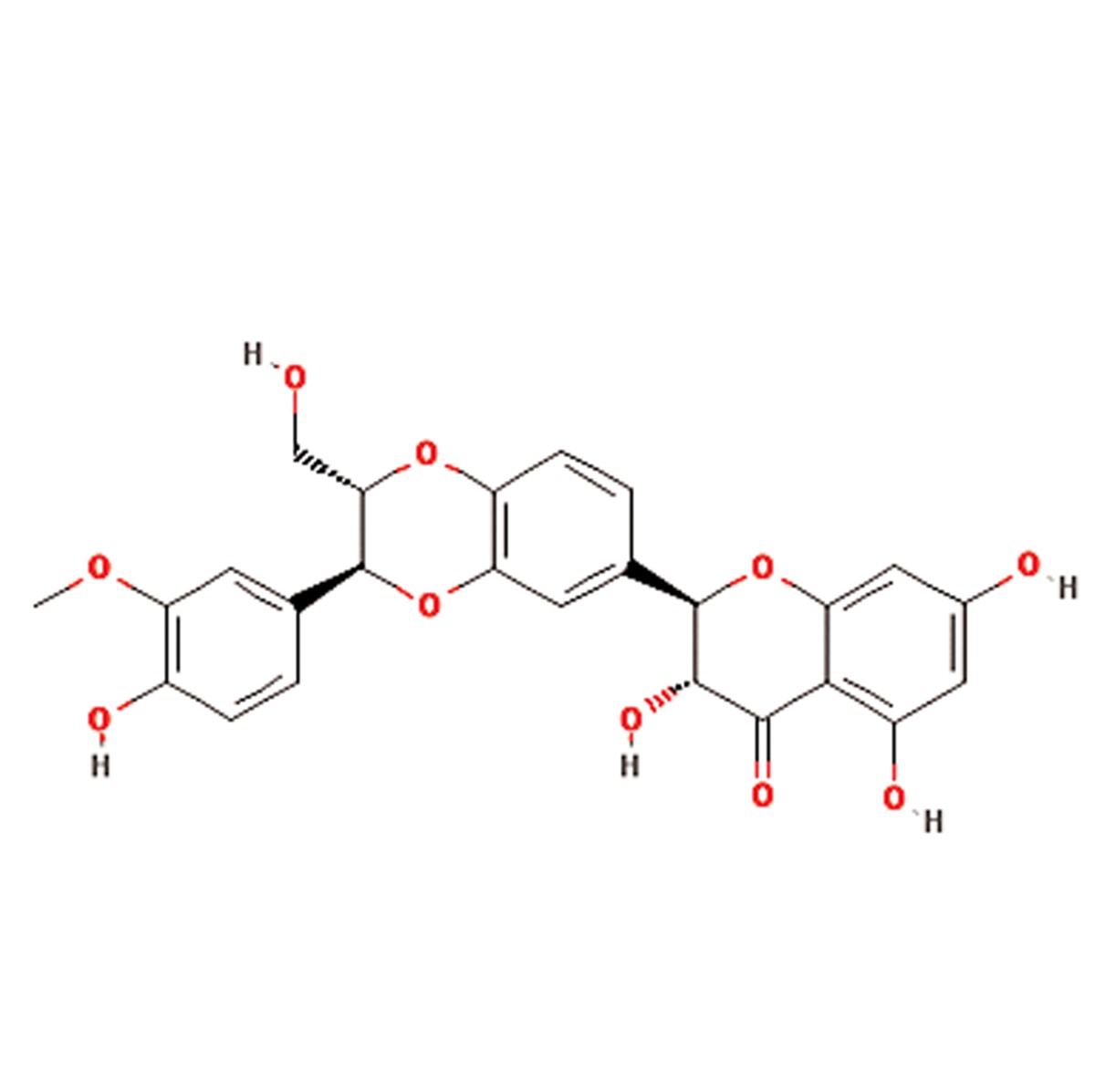
|
(18, 19) |
| 8 | Teucrium scordium | Lamiaceae | Antiinflammatory, antipyretic, antiseptic,astringent, diuretic, laxative, vermifuge,festering and inflamedwounds, bronchialailments, diarrhea, fever, hemorrhoids, andintestinal parasites | β-Caryophyllene, 6-AcetylteucjaponinB, (E)-b-farnesene, caryophyllene oxide,alpha-pinene and beta-pinene, β-farnesene,menthofuran, 1,8 cineole, β-eudesmol,α-humulene, diterpenes, flavonoids,saponnins, furanoid, pulegone, tannins andvolatile oil | β-Caryophyllene (C15H24O)
|
(20-22) |
| 9 | Zingiber officinale | Zingiberaceae | Antiinflammatory, antitumor, antilipedimic,antioxidant pharmacologic effects,Inhibited the induced hyperglycemia | Gingerols, zingerone, zingiberofficinalediol,zingibrene, shogaols, paradol and vanilloids | Gingerol (C17H26O4)
|
(23) |
Conclusion
Taken together, the present findings show extracts from several Iranian plants; PH, GG, SH, RO, TS, AV, ZO, SM, and HP produce a significant improvement in the markers involved in the successful transplantation of islets, including viability of cells, ROS and insulin secretion. Based on these results, concentrations of 10 μgmL-1, 103 μgmL-1, 104 μgmL-1, 10 μgmL-1, 102 μgmL-1, 102 μgmL-1, 10-1 μgmL-1, 10 μgmL-1 and 103 μgmL-1 are respectively the effective doses of these plants for isolated islets. Their effects on insulin release are especially promising in the treatment of diabetes. On the basis of these findings, the authors suggest that the next step is to test combinations of some of the most effective of these plants with the hope of producing a more efficient cell-based therapy for diabetes through the prevention of oxidative stress in isolated islets and improving mitochondrial function during the implant process. Although safety issues should not be forgotten, the efficacy of these extracts in the clinic should be studied according to guidelines to see whether these alone or in combination can control diabetes or maintain vitality of cells during the transplantation pancreatic islets. The present findings are very promising and pave the way to success in further studies.
Acknowledgments
This study was partly supported by TUMS and INSF. Authors declare no conflict of interest.
References
- 1.Zhang HN, He JH, Yuan L, Lin ZB. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 2003;73(18):2307–2319. doi: 10.1016/s0024-3205(03)00594-0. [DOI] [PubMed] [Google Scholar]
- 2.Mohseni Salehi Monfared SS, Larijani B, Abdollahi M. Islet transplantation and antioxidant management: a comprehensive review. World J Gastroenterol. 2009;15(10):1153–1161. doi: 10.3748/wjg.15.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larijani B, Salimi M, Pourkhalili N, Mohammadirad A, Baeeri M, Nili-Ahmadabadi A, et al. Positive response of isolated rat pancreatic islets to IMOD; hopes for better transplant outcome and graft function. Asian J Anim Vet Adv. 2011;6(10):1019–1025. [Google Scholar]
- 4.Pourkhalili N, Hosseini A, Nili-Ahmadabadi A, Rahimifard M, Navaei-Nigjeh M, Hassani S, et al. Improvement of isolated rat pancreatic islets function by combination of cerium oxide nanoparticles/sodium selenite through reduction of oxidative stress. Toxicol Mech Methods. 2012;22(6):476–482. doi: 10.3109/15376516.2012.673093. [DOI] [PubMed] [Google Scholar]
- 5.Ghasemzadeh A, Azarifar M, Soroodi O, Jaafar HZE. Flavonoid compounds and their antioxidant activity in extract of some tropical plants. J Med Plants Res. 2012;6(13):2639–2643. [Google Scholar]
- 6.Rahimifard M, Navaii-Nigjeh M, Nilli-Ahmadabadi A, Pourkhalili N, Baeeri M, Mohammadirad A, et al. On the benefit of pure glycyrrhizic acid on the function and metabolic activity of isolated pancreatic Langerhans islets in vitro. Asian J Anim Vet Adv. 2012;7(11):1212–1218. [Google Scholar]
- 7.Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets. 2009;8(1):2–10. doi: 10.2174/187152809787582561. [DOI] [PubMed] [Google Scholar]
- 8.Hasani-Ranjbar S, Nayebi N, Moradi L, Mehri A, Larijani B, Abdollahi M. The efficacy and safety of herbal medicines used in the treatment of hyperlipidemia; a systematic review. Curr Pharm Des. 2010;16(26):2935–2947. doi: 10.2174/138161210793176464. [DOI] [PubMed] [Google Scholar]
- 9.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A novel management of diabetes by means of strong antioxidants’ combination. J Med Hypotheses Ideas. 2013;7(1):25–30. [Google Scholar]
- 10.Patil B. In vitro study of cytoprotection by aloe vera leaf gel against Apap (aap) mediated oxidative stress in rat liver slices. Int J Adv Biotech. 2010;1(2):73–86. [Google Scholar]
- 11.Irshad S, Butt M. Younus H.In-Vitro antibacterial activity of Aloe Barbadensis Miller (Aloe Vera) Intl R J of Pharmaceuticals. 2011;1(4):59–64. [Google Scholar]
- 12.Chan HT, Chan C, Ho JW. Inhibition of glycyrrhizic acid on aflatoxin B1-induced cytotoxicity in hepatoma cells. Toxicology. 2003;188(2-3):211–217. doi: 10.1016/s0300-483x(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 13.Feizi A, Dadian F. Evaluating the effect of Hypericum perforatum on antibody titers obtained from B1 and La Sota vaccines in broiler chicks with HI test. J Cell Anim Biol. 2012;6(10):160–163. [Google Scholar]
- 14.Asgarpanah J, Ramezanloo F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr J Pharm Pharmacol. 2012;6(22):1573–1580. [Google Scholar]
- 15.Monsef HR, Ghobadi A, Iranshahi M, Abdollahi M. Antinociceptive effects of peganum harmala L.alkaloid extract on mouse formalin test. J Pharm Pharmaceut Sci. 2004;7(1):65–69. [PubMed] [Google Scholar]
- 16.Momtaz S, Abdollahi M. An update on pharmacology of satureja species; from antioxidant, antimicrobial, antidiabetes and ati-hyperlipidemic to reproductive stimulation. Int J Pharmacol. 2010;6(4):454–461. [Google Scholar]
- 17.Momtaz S, Abdollahi M. A systematic review of the biological activities of Satureja L. Species Pharmacologyonline. 2008;2:34–54. [Google Scholar]
- 18.Ozturk M, Akdogan M, Keskin I, Kisioglu AN, Oztas S, Yildiz K. Effect of Silybum marianum on acute hepatic damage caused by carbon tetrachloride in rats. Biomedical Research. 2012;23(2):268–274. [Google Scholar]
- 19.Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Cent Eur J Biol. 2009;4(2):204–213. [Google Scholar]
- 20.Morteza-semnani K, saeedi M, akbarzadeh M. Essential oil composition of Teucrium scordium L. Acta Pharm. 2007;57:499–504. doi: 10.2478/v10007-007-0040-6. [DOI] [PubMed] [Google Scholar]
- 21.Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of teucrium species; from anti-oxidant to anti-diabetic effects. Int J Pharmacol. 2010;6(4):315–325. [Google Scholar]
- 22.Abdolghaffari AH, Baghaei A, Moayer F, Esmaily H, Baeeri M, Monsef-Esfahani HR, et al. On the benefit of Teucrium in murine colitis through improvement of toxic inflammatory mediators. Hum Exp Toxicol. 2010;29(4):287–295. doi: 10.1177/0960327110361754. [DOI] [PubMed] [Google Scholar]
- 23.Al-Azhary, DB. Ginger enhances antioxidant activity and attenuates atherogenesis in diabetic cholesterol-fed rats. Aust J Basic Appl Sci. 2011;5(12):2150–2158. [Google Scholar]
- 24.Berrougui H, Isabelle M, Cloutier M, Hmamouchi M, Khalil A. Protective effects of Peganum harmala L.extract, harmine and harmaline against human low-density lipoprotein oxidation. J Pharm Pharmacol. 2006;58(7):967–974. doi: 10.1211/jpp.58.7.0012. [DOI] [PubMed] [Google Scholar]
- 25.Sharaf M, el-Ansari MA, Matlin SA, Saleh NA. Four flavonoid glycosides from Peganum harmala. Phytochemistry. 1997;44(3):533–536. doi: 10.1016/s0031-9422(96)00531-6. [DOI] [PubMed] [Google Scholar]
- 26.Viña J, Gomez-Cabrera MC, Borras C. Fostering antioxidant defences: up-regulation of antioxidant genes or antioxidant supplementation? Br J Nutr. 2007;98(Suppl 1):S36–40. doi: 10.1017/S0007114507839596. [DOI] [PubMed] [Google Scholar]
- 27.Vina J, Borras C, Gomez-Cabrera MC, Orr WC. Part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression.Role of reactive oxygen species and (phyto) oestrogens in the modulation of adaptive response to stress. Free Radic Res. 2006;40(2):111–119. doi: 10.1080/10715760500405778. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine- induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur J Neurosci. 2001;13(10):1861–1872. doi: 10.1046/j.0953-816x.2001.01563.x. [DOI] [PubMed] [Google Scholar]
- 29.Mosaffa F, Behravan J, Karimi G, Iranshahi M. Antigenotoxic effects of Satureja hortensis L.on rat lymphocytes exposed to oxidative stress. Arch Pharm Res. 2006;29(2):159–164. doi: 10.1007/BF02974278. [DOI] [PubMed] [Google Scholar]
- 30.Bahramikia S, Yazdanparast R, Nosrati N. A comparision of antioxidant capacities of ethanol extracts of satureja hortensis and artemisiadracunculus leaves. Pharmacologyonline. 2008;2:694–704. [Google Scholar]
- 31.Cronin H, Draelos ZD. Top 10 botanical ingredients in 2010 anti-aging creams. J Cosmet Dermatol. 2010;9:218–225. doi: 10.1111/j.1473-2165.2010.00516.x. [DOI] [PubMed] [Google Scholar]
- 32.Zeng HH, Tu PF, Zhou K, Wang H, Wang BH, Lu JF. Antioxidant properties of phenolic diterpenes from Rosmari nus officinalis. Acta Pharmacol Sin. 2001;22(12):1094–1098. [PubMed] [Google Scholar]
- 33.Señoráns FJ, Ibañez E, Cavero S, Tabera J, Reglero G. Liquid chromatographic-mass spectrometric analysis of supercritical-fluid extracts of rosemary plants. J Chromatogr A. 2000;870(1-2):491–499. doi: 10.1016/s0021-9673(99)00941-3. [DOI] [PubMed] [Google Scholar]
- 34.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40(2):223–231. doi: 10.1080/10715760500473834. [DOI] [PubMed] [Google Scholar]
- 35.Yesil-Celiktas O, Nartop P, Gurel A, Bedir E, Vardar-Sukan F. Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis' calli. J Plant Physiol. 2007;164(11):1536–1542. doi: 10.1016/j.jplph.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Fons L, Aranda FJ, Guillén J, Villalaín J, Micol V. Rosemary (Rosmarinus officinalis) diterpenes affect lipid polymorphism and fluidity in phospholipid membranes. Arch Biochem Biophys. 2006;453(2):224–236. doi: 10.1016/j.abb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Silva AM, Andrade-Wartha ER, Carvalho EB, Lima A, Novoa AV, Mancini- Filhoovoa J. Effect of aqueous rosemary extract (Rosmarinus officinalis L.) on the oxidative stress of diabetic rats. Rev Nutr. 2011;24(1):121–130. [Google Scholar]
- 38.Bakirel T, Bakirel U, Keleş OU, Ulgen SG, Yardibi H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J Ethnopharmacol. 2008;116(1):64–73. doi: 10.1016/j.jep.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Zancan KC, Marques MO, Petenate AJ, Meireles MA. Extraction of Zingiber officinale oleoresins with CO and co-solvents: A study of the 2 antioxidant action of the extracts. J Supercri Fluids. 2002;24(1):57–67. [Google Scholar]
- 40.Bhattacharjee SK. Handbook of Aromatic plants. India: Pointer Publisher; 2000. pp. 473–447. [Google Scholar]
- 41.Sujatha R, Srinivas L. Modulation of lipid peroxidation by dietary components. Toxicol In Vitro. 1995;9(3):231–236. doi: 10.1016/0887-2333(95)00005-s. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed RS, Seth V, Pasha ST, Banerjee BD. Influence of dietary ginger (Zingiber officinales Rosc) on oxidative stress induced by malathion in rats. Food Chem Toxicol. 2000;38(5):443–450. doi: 10.1016/s0278-6915(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 43.Aeschbach R, Löliger J, Scott BC, Murcia A, Butler J, Halliwell B, et al. Antioxidant actions of thymol, carvacol, 6-gingerol, zingerone and hydroxytyrosol. Food ChemToxiol. 1994;32(1):31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- 44.Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9(7):526–533. [PubMed] [Google Scholar]
- 45.Ozougwu JC, Eyo JE. Evaluation of the activity of zingiber officinale (ginger) aqueous extracts on alloxaninduced diabetic rats. Pharmacologyonline. 2011;1:258–269. [Google Scholar]
- 46.Skottová N, Vecera R, Urbánek K, Vána P, Walterová D, Cvak L. Effects of polyphenolic fraction of silymarin on lipoprotein profile in rats fed cholesterol-rich diets. Pharmacol Res. 2003;47(1):17–26. doi: 10.1016/s1043-6618(02)00252-9. [DOI] [PubMed] [Google Scholar]
- 47.Baluchnejadmojarad T, Roghani M. Chronic oral silybum marianum aqueous extract attenuates streptozotocin-diabetic neuropathy. IJDLD. 2009;8:65–76. [Google Scholar]
- 48.Rahimi HR, Gholami M, Khorram-Khorshid HR, Gharibdoost F, Abdollahi M. On the protective effects of IMOD and silymarin combination in a rat model of acute hepatic failure through anti oxidative stress mechanisms. Asian J Anim Vet Adv. 2012;7(1):38–45. [Google Scholar]
- 49.Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods. 2011;21(3):200–208. doi: 10.3109/15376516.2010.547887. [DOI] [PubMed] [Google Scholar]
- 50.Lettéron P, Labbe G, Degott C, Berson A, Fromenty B, Delaforge M, et al. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice.Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem Pharmacol. 1990;39(12):2027–2034. doi: 10.1016/0006-2952(90)90625-u. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad N, Gali H, Javed S, Agarwal R. Skin cancer chemopreventive effects of a flavonoid antioxidant silymarin are mediated via impairment of receptor tyrosine kinase signaling and perturbation in cell cycle progression. Biochem Biophys Res Commun. 1998;247(2):294–301. doi: 10.1006/bbrc.1998.8748. [DOI] [PubMed] [Google Scholar]
- 52.Malihi F, Hosseini-Tabatabaei A, Esmaily H, Khorasani R, Baeeri M, Abdollahi M. Improvement of inflammatory and toxic stress biomarkers by silymarin in a murine model of type one diabetes mellitus. Cent Eur J Biol. 2009;4(3):369–380. [Google Scholar]
- 53.Matsuda T, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, et al. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005;146(1):175–185. doi: 10.1210/en.2004-0850. [DOI] [PubMed] [Google Scholar]
- 54.Skottová N, Kazdová L, Oliyarnyk O, Vecera R, Sobolová L, Ulrichová J. Phenolics-rich extracts from Silybum marianum and Prunella vulgaris reduce a high-sucrose diet induced oxidative stress in hereditary hypertriglyceridemic rats. Pharmacol Res. 2004;50(2):123–130. doi: 10.1016/j.phrs.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Elhadi GM, Ghareib SA, Mohamed MA. Protective effect of Ecliptaalba extract, silymarin and their combination against obesity induce insulin resistance and hyperglycemia in rats. N Y Sci J. 2011;4(9):86–97. [Google Scholar]
- 56.Ramachandraiahgari R, Somesula S, Adi P, Mannur I, Enamalaa M, Matcha B. Protective role of ethanolic extract of aloe vera antioxidant properties on liver and kidney of streptozotocin-induced diabetic rats. Dig J Nanomater Bios. 2012;7(1):175–184. [Google Scholar]
- 57.Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13(8):1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohamed E. Antidiabetic, antihypercholestermic and antioxidative effect of Aloe vera gel extract in alloxan induced diabetic rats. Aust J Basic Appl Sci. 2011;5(11):1321–1327. [Google Scholar]
- 59.Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocininduced diabetes in experimental rats. J Med Food. 2004;7(1):61–66. doi: 10.1089/109662004322984725. [DOI] [PubMed] [Google Scholar]
- 60.Silva BA, Ferreres F, Malva JO, Dias ACP. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005;90(1-2):157–167. [Google Scholar]
- 61.Arokiyaraj S, Balamurugan R, Augustian P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1(5):386–390. doi: 10.1016/S2221-1691(11)60085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Can OD, Öztürk Y, Öztürk N, Sagratini G, Ricciutelli M, Vittori S, et al. Effects of treatment with St.John’s Wort on blood glucose levels and pain perceptions of streptozotocin- diabetic rats. Fitoterapia. 2011;82(4):576–584. doi: 10.1016/j.fitote.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006;98(1):97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- 64.Hasani P, Yasa N, Vosough-Ghanbari S, Mohammadirad A, Dehghan G, Abdollahi M. In vivo antioxidant potential of Teucrium polium, as compared to alpha-tocopherol. Acta Pharm. 2007;57(1):123–129. doi: 10.2478/v10007-007-0010-z. [DOI] [PubMed] [Google Scholar]
- 65.Kundakovic T, Milenkovic M, Topic A, Stanojkovic T, Juranic Z, Lakusic B. Cytotoxicity and antimicrobial activity of Teucrium scordium L.(Lamiaceae) extracts. Afr J Microbiol Res. 2011;5(18):2692–2696. [Google Scholar]
- 66.Kovacevic NN, Lakusic BS, Ristic MS. Composition of the essential oils of seven Teucrium species from serbia and montenegro. J Essent Oil Res. 2001;13(3):163–165. [Google Scholar]
- 67.Sharififar F, Mahdavi Z, Mirtajaldini M, Purhematy A. Volatile constituents of aerial parts of Teucrium scordium L.from Iran. J Essent Oil Res. 2010;22(3):202–204. [Google Scholar]
- 68.Sultana S, Haque A, Hamid K, Urmi KF, Roy S. Antimicrobial, cytotoxic and antioxidant activity of methanolic extract of Glycyrrhiza glabra. Agric Biol J N Am. 2010;1(5):957–960. [Google Scholar]
- 69.Haraguchi H, Yoshida N, Ishikawa H, Tamura Y, Mizutani K, Kinoshita T. Protection of mitochondrial functions against oxidative stresses by isoflavans from Glycyrrhiza glabra. J Pharm Pharmacol. 2000;52(2):219–223. doi: 10.1211/0022357001773724. [DOI] [PubMed] [Google Scholar]
- 70.D'Angelo S, Morana A, Salvatore A, Zappia V, Galletti P. Protective effect of polyphenols from Glycyrrhiza glabra against oxidative stress in Caco-2 cells. J Med Food. 2009;12(6):1326–1333. doi: 10.1089/jmf.2008.0285. [DOI] [PubMed] [Google Scholar]
- 71.Dehpour AR, Zahedi H, Amini Sh, Akhgari M, Abdollahi M. Effects of Glycyrrhiza derivatives against acetaminopheninduced hepatotoxicity. Iran J Med Sci. 1999;24:26–31. [Google Scholar]
- 72.Sen S, Roy M, Chakraborti AS. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J Pharm Pharmacol. 2011;63(2):287–296. doi: 10.1111/j.2042-7158.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- 73.Kalaiarasi P, Pugalendi KV. Antihyperglycemic effect of 18 beta-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozotocin- diabetic rats. Eur J Pharmacol. 2009;606(1-3):269–273. doi: 10.1016/j.ejphar.2008.12.057. [DOI] [PubMed] [Google Scholar]