Abstract
Background
Visual impairment is a common health-related disability in the U.S. The association between clinical measurements of age-related eye diseases and visual impairment in data from a national survey has not been reported.
Purpose
To examine common eye conditions and other correlates associated with visual impairment in the U.S.
Methods
Data from the 2005–2008 National Health and Nutrition Examination Survey of 5222 Americans aged ≥40 years were analyzed in 2012 for visual impairment (presenting distance visual acuity worse than 20/40 in the better-seeing eye), and visual impairment not due to refractive error (distance visual acuity worse than 20/40 after refraction). Diabetic retinopathy (DR) and age-related macular degeneration (AMD) were assessed from retinal fundus images; glaucoma was assessed from two successive frequency-doubling tests and a cup-to-disc ratio measurement.
Results
Prevalence of visual impairment and of visual impairment not due to refractive error was 7.5% (95% CI=6.9%, 8.1%) and 2.0% (1.7%, 2.3%), respectively. The prevalence of visual impairment not due to refractive error was significantly higher among people with AMD (2.2%) compared to those without AMD (0.8%), or with DR (3.5%) compared to those without DR (1.2%). Independent predictive factors of visual impairment not due to refractive error were AMD (OR=4.52, 95% CI=2.50, 8.17); increasing age (OR=1.09 per year, 95% CI=1.06, 1.13); and less than a high school education (OR=2.99, 95% CI=1.18, 7.55).
Conclusions
Visual impairment is a public health problem in the U.S. Visual impairment in two thirds of adults could be eliminated with refractive correction. Screening of the older population may identify adults at increased risk of visual impairment due to eye diseases.
Introduction
Visual impairment, a reduction in the clarity with which a person can see objects, is a major public health problem. Visual impairment is associated with diminished quality of life because people with visual impairment often report difficulty performing everyday activities such as reading, meal preparation, and driving a car.1–4 Moreover, visual impairment is associated with an increased risk of falls, fall-related injuries, depression, social isolation, and poorer overall health. Vision problems create a substantial burden on individuals and society. The estimated total annual cost of visual impairment and blindness among individuals aged ≥40 years in the U.S. is $5.5 billion.7–9
Extrapolating from data pooled from a collection of previously completed population-based studies, the Eye Diseases Prevalence Research Group estimated approximately 3.4 million U.S. adults aged ≥40 years had visual impairment. This figure was projected to reach 5.5 million by 2020.10 An analysis of visual acuity data collected from the National Health and Nutrition Examination Survey (NHANES) in 1999–2002 reported uncorrected refractive error as the primary cause of visual impairment defined as visual acuity of worse than 20/40 in the eye with the better vision.11
Given the aging population, increased prevalence of chronic diseases such as diabetes, and the percentage of the population using electronic devices such as computers and smartphones, it is possible that the prevalence of visual impairment may have changed since the earlier reports.12–15 Further, it has been estimated that at least half of all cases of legal blindness could be prevented by early detection and timely treatment of associated conditions.16 From 2005 to 2008, the NHANES collected visual acuity data, supplementing earlier data collection with fundus photography to assess retinal diseases and frequency-doubling technology (FDT) to evaluate visual field loss. The present study evaluates, for the first time in a national survey, the common eye conditions and other correlates associated with visual impairment, using the most recent data available from NHANES.
Methods
Data and Study Population
Data were obtained from the 2005–2008 NHANES, a complex, multistage probability survey of a sample of the U.S. civilian, non-institutionalized population, conducted by the National Center for Health Statistics (NCHS) of the CDC. A detailed description of the design and data collection of the NHANES survey has been published elsewhere.17
Participants selected for the survey were interviewed in their homes, where self-reported demographic, socioeconomic, and some health-related data were collected. Participants were then offered a physical examination, to be conducted in a specially designed and equipped Mobile Examination Center (MEC). The MEC examination included visual acuity testing and autorefraction for participants aged ≥12 years and FDT and retinal photography for participants aged ≥40 years, as described elsewhere.10,18,19
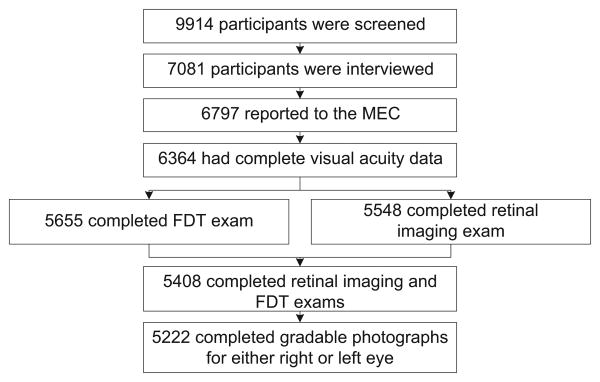
The response rate for participants aged ≥40 years was 69.0% for the NHANES sample invited to the MEC in 2005–2008.20 Of the 7081 participants aged ≥40 years who participated in NHANES, 6797 reported to the MEC for a health examination. All NHANES physical exam participants were eligible for the vision examination except those who were blind (had no light perception); had an eye infection; or wore eye patches on both eyes (n=6). Among the participants who had the exam, complete visual acuity data were obtained for 6364 (93.6%); of these, 5408 (85.0%) received both the retinal imaging and FDT exam components. Complete gradable photographs for either their right or left eye were available from 5222 survey participants (Figure 1).
Figure 1.
Flow diagram of participants aged ≥40 years in National Health and Nutrition Examination Survey, 2005–2008, included in analysis
FDT, frequency-doubling technology; MEC, Mobile Examination Center
Visual Acuity Measures
Presenting visual acuity with the participant wearing usual distance vision correction (i.e., eyeglasses or contact lenses), if any, was measured in a dimmed room using an ARK-760 automated refractometer. After presenting visual acuity was measured, the participant removed any vision correction, and the autorefractor was used to measure the objective refraction (sphere, cylinder, and axis) for each eye. In eyes with presenting visual acuity of worse than 20/40, visual acuity was further assessed as aided with autorefraction results. Visual impairment was defined as presenting distance visual acuity in the better-seeing eye of worse than 20/40. Visual impairment not due to refractive error was defined as visual acuity of worse than 20/40 while aided with autorefraction.11
Ocular Condition Measures
An FDT examination and a retinal imaging examination were performed to assess visual field loss and retinal lesions and optic disc characteristics, respectively. FDT was performed twice on each eye using the Humphrey Matrix Visual Field Instrument and the N-30-5 FDT screening protocol, a 19-point supra-threshold screening test.19 Retinal images were taken using the Canon CR6-45NM Ophthalmic Digital Imaging System and the Canon EOS 10D digital camera.
The procedures for image capture and their assessment have been described in detail elsewhere.21,22 In brief, two 45° nonmydriatic digital retinal images were taken per eye, one centered on the optic nerve (Field 1), and one centered on the fovea (Field 2). These were then graded in a masked fashion using a modification of the Wisconsin Age-Related Maculopathy Grading System for age-related macular degeneration (AMD) and Airlie House Classification scheme for diabetic retinopathy (DR).22
Participants were considered to have AMD based on the presence of lesions defining early AMD (e.g., large retinal soft drusen or any drusen with retinal pigmentary abnormalities) or late AMD (e.g., exudative AMD or geographic atrophy).23 An eye was defined as having DR if the participant had both (1) diabetes (defined by self-report of a previous diagnosis made by a physician [excluding gestational diabetes]) or hemoglobin A1c of 6.5% or greater and (2) one or more retinal microaneurysms or retinal blot hemorrhages with or without more-severe retinal lesions.16 Visual field loss was defined for each eye using the 2-2-1 algorithm: having at least two abnormal field results (p < 1% for two or more of the 19 tested locations) in each of the two frequency-doubling exams, with one common abnormal field.19 Glaucoma was defined as visual field loss combined with a cup-to-disc ratio of ≥0.7.24 A person was determined to have glaucoma if at least one eye had glaucoma.
Demographics and Health Conditions Measures
For the descriptive analysis, age was classified into five categories: 40–49 years, 50–59 years, 60–69 years, 70–79 years, and ≥80 years. Age in years was utilized as a continuous variable to compute age-adjusted rates and for the multivariate logistic regression analyses. Race/ethnicity was categorized as non-Hispanic white; non-Hispanic black; Mexican American; or Other. The latter group included other Hispanics, multiracial people, and other races/ethnicities not included in the preceding categories. Mexican American and other Hispanic ethnicities were separated in accordance with the recommendations of the survey sponsor, the NCHS, given changes in sampling methodology in 2007.25
Educational attainment was classified into three categories: less than a high school education, high school diploma, or more than a high school education.26 Insurance coverage was defined as insured (including health insurance obtained through employment or through government programs, such as Medicare and Medicaid) or uninsured. Smoking status was classified as never smoker, past smoker, and current smoker. BMI was derived from height and weight measurements collected during the NHANES physical examination. A BMI of <25.0 was classified as normal, 25.0–29.9 as overweight, and ≥30.0 as obese.27 Hypertension was defined by either systolic blood pressure of ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or taking antihypertensive medication. History of cardiovascular disease was defined by a self-report of having had a heart attack, congestive heart failure, or stroke.
Data Analysis
Statistical analyses were conducted using SAS version 9.2 and SUDAAN version 10.1 software to calculate national estimates of visual impairment and their SEs, while accounting for the complex survey sampling methods. The prevalences of visual impairment and visual impairment not due to refractive error were estimated by age. Using the 2000 Census population, the age-standardized prevalence of visual impairment, and of visual impairment not due to refractive error, was estimated by AMD, glaucoma, DR, gender, race/ethnicity, educational attainment, insurance coverage, BMI, smoking status, hypertension, and cardiovascular disease. Chi-square tests were used to identify associations between each of these factors and visual impairment.
Multivariate logistic regression models were used to evaluate the relationship of visual impairment not due to refractive error with AMD, DR, and glaucoma, while adjusting for age (Model 1). Then a second logistic model (Model 2) was run by adding possible confounders such as demographic variables (gender, race/ethnicity, education, and insurance coverage) and health-related conditions (BMI, smoking status, hypertension, and cardiovascular disease).10,16,21,28 ORs and their 95% CIs were calculated. In the logistic models, terms for age and age2 were included to account for possible nonlinearity. The squared term (age2) was dropped because it was not significant. Associations were considered significant if p<0.05. All analyses were conducted in 2012.
Results
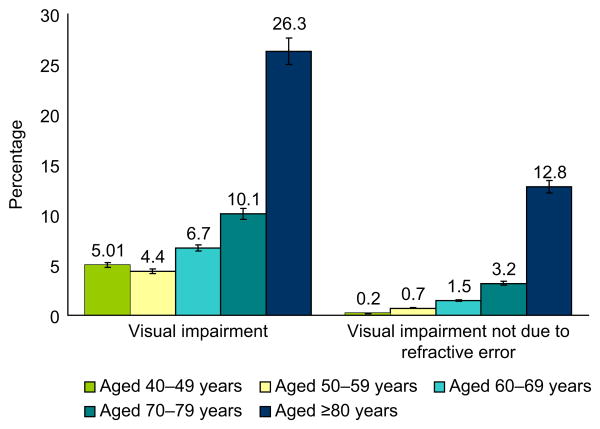
The overall age-standardized prevalence of visual impairment was 7.5% (95% CI=6.9%, 8.1%) and visual impairment not due to refractive error was 2.0% (95% CI=1.7%, 2.3%) (Appendix A, available online at www.ajpmonline.org). The prevalence of visual impairment was associated with increasing age, with the highest rates found in people aged ≥80 years (26.3%, 95% CI=23.4%, 29.4%; Figure 2). After standardizing for age, the results showed a higher prevalence of visual impairment among people with DR (14.5%, 95% CI=10.4%, 19.8%) than those without DR (6.3%, 95% CI=5.6%, 7.0%). Almost three quarters of participants with visual impairment had refractive error as the cause of their inability to see with a visual acuity of 20/40 or better.
Figure 2.
Prevalence of presenting visual impairment, and visual impairment that is not due to refractive error, by age group among those aged ≥40 years
Note: p < 0.001 for association of increasing age with visual impairment and with visual impairment that is not due to refractive error. Error bars indicate 95% CI. Data source: National Health and Nutrition Examination Survey, 2005–2008
The remainder had visual impairment due to other nonrefractive conditions. The age-standardized prevalence of visual impairment not due to refractive error was almost thrice as high in those with AMD (2.2%, 95% CI=1.5%, 3.2%) compared with those without AMD (0.8%, 95% CI=0.6%, 1.1%). In addition, the prevalence of visual impairment not due to refractive error was also higher in people with DR than in those without DR (Appendix A, available online at www.ajpmonline.org). The presence of AMD, glaucoma, or DR or combination of these eye diseases was identified in a half of all participants whose visual impairment was not attributed to refractive error (Appendix B, available online at www.ajpmonline.org).
After adjusting for age, participants with AMD had greater odds of having visual impairment not due to refractive error than those without AMD (OR=4.03, 95% CI=2.23, 7.29; Table 1). Individuals with glaucoma or DR also had elevated odds of having visual impairment not due to refractive error compared with those without the respective condition, although the risk of visual impairment associated with either glaucoma or DR was not significant (OR=3.18, 95% CI=0.88, 11.47, and OR=4.25, 95% CI=0.98, 14.43, respectively). In Model 2, after accounting for age, gender, race/ethnicity, and education, insurance coverage, BMI, smoking status, history of cardiovascular disease, and hypertension, the odds of having visual impairment among individuals with AMD increased to almost a fivefold higher risk, whereas the odds associated with glaucoma or DR were reduced.
Table 1. Odds of visual impairment not due to refractive error among those aged ≥40 years, OR (95% CI).
| Model 1 | Model 2 | |
|---|---|---|
| Age-related macular degeneration | ||
| No | 1 | 1 |
| Yes | 4.03 (2.23, 7.29) | 4.52 (2.50, 8.17) |
| Glaucoma | ||
| No | 1 | 1 |
| Yes | 3.18 (0.88, 11.47) | 2.46 (0.70, 8.67) |
| Diabetic retinopathy | ||
| No | 1 | 1 |
| Yes | 4.25 (0.98, 14.43) | 2.55 (0.68, 9.51) |
| Age, per 1-year increase at ages >40 years | 1.09 (1.06, 1.12) | 1.09 (1.06, 1.13) |
| Gender | ||
| Female | 1 | |
| Male | 1.60 (0.90, 2.86) | |
| Race/ethnicity | ||
| Non-Hispanic white | 1 | |
| Non-Hispanic black | 2.10 (0.83, 5.34) | |
| Mexican-American | 1.30 (0.40, 4.44) | |
| Other | 0.63 (0.18, 2.18) | |
| Education | ||
| >High school | 1 | |
| High school | 1.70 (0.57, 5.11) | |
| <High school | 2.99 (1.18, 7.55) | |
| Insurance coveragea | ||
| Uninsured | 0.49 (0.16, 1.58) | |
| Insured | 1 | |
| BMIb | ||
| Normal | 1 | |
| Overweight | 0.71 (0.34, 1.48) | |
| Obese | 1.27 (0.57, 2.82) | |
| Smoking status | ||
| Never smoker | 1 | |
| Past smoker | 0.71 (0.34, 1.48) | |
| Current smoker | 1.02 (0.35, 3.01) | |
| Cardiovascular diseasec | ||
| No | 1 | |
| Yes | 1.48 (0.63, 3.46) | |
| Hypertensiond | ||
| No | 1 | |
| Yes | 1.73 (0.74, 4.09) |
Note: Data source: National Health and Nutrition and Examination Survey (NHANES), 2005–2008. Visual impairment not due to refractive error is defined as visual impairment after autorefractive correction.
Insurance coverage included health insurance obtained through employment or provided through government programs such as Medicare and Medicaid.
BMI was classified as <25.0 for normal, 25.0–29.9 for overweight, and ≥30.0 for obese.
Cardiovascular disease was defined as having self-reported heart attack, heart failure, or stroke.
Hypertension was defined as having systolic blood pressure ≥140, diastolic blood pressure ≥90, or being on hypertension medication.
Discussion
This study examined the age-related eye diseases and other correlates of visual impairment among U.S. adults aged ≥40 years participating in a national survey. It also determined how much visual impairment could be attributed to refractive error and how much to at least one of three major eye diseases that the study protocol allowed the current study to detect. Further, it found that nearly one in 13 adults (9.0 million) had visual impairment, of which an estimated 6.8 million cases were simply the result of not having refractive correction in the form of eyeglasses, contact lenses, or refractive surgery. The remaining 2.2 million people had visual impairment due to the nonrefractive causes such as AMD, glaucoma, or DR, which this study could detect, or other likely causes such as cataract, ocular injury, or eye diseases that, because of the study protocol used in NHANES, the current study was not able to detect. Hence, the present study's findings confirm earlier reports documenting uncorrected refractive error as the major cause of visual impairment in the U.S.11,29
In this study, the estimate of the prevalence of visual impairment among U. S. individuals aged ≥40 years in 2005–2008 is higher than the estimate for adults of comparable age reported from the earlier NHANES survey conducted in 1999–2002.11 The reasons for the increase are unclear, although underutilization of eye care services is one likely explanation for the increase in prevalence of visual impairment. A study has shown that the main reasons for not seeking eye care were cost concerns, lack of health insurance, and the perception of not having a need for it.30
The present study, consistent with other studies, found visual impairment to be more prevalent in non-Hispanic blacks, Mexican Americans, those with less than a high school education, and those without health insurance.31– 35 The majority of the increase in visual impairment can be attributed to an increase in the rate of uncorrected refractive error. This estimate in this study that 2.2 million adults in the U.S. have visual impairment due to an eye disease is only two thirds of the estimate obtained from the meta-analysis performed in 2004 and is also less than the prevalence estimated from the 1999–2002 NHANES. Although it is possible that early detection of eye disease and improvements in therapeutic intervention of eye disease may explain, in part, the reduction in estimates of visual impairment attributable to eye disease over time, the authors cannot speculate about the extent to which this may be the case.
Of the three eye diseases NHANES measured, AMD had the largest impact on visual impairment among those with visual impairment not due to uncorrected refractive error. AMD has been reported to be the primary cause of visual impairment in older U.S., European, and Australian populations.29,36 However, nearly half of all people with visual impairment that is not due to refractive error in this sample had eye diseases that the NHANES protocol was not designed to detect because logistic limitations in time and scope precluded a complete dilated-eye examination. Specifically, neither lens opacity nor corneal disease was assessed. It is plausible that a sizable proportion of those with visual impairment that is not due to refractive error may have had cataracts.
Consistent with previous studies, the current study found that individuals with AMD were more likely to have visual impairment that is not due to refractive error than those without AMD.10,37 Not surprisingly, age remained strongly related both to visual impairment and to visual impairment not due to refractive error.32,38,39 Low education attainment also was associated with visual impairment regardless of underlying condition. Thus, the results from the current study corroborate previous studies reporting that individuals with visual impairment, regardless of age, were likely to be less educated.40–42 Taken together, these studies suggest that targeted screening of the older population may help to identify those at increased risk of visual impairment so that refractive correction can be provided to those who would benefit and others with underlying eye conditions can be medically managed to prevent further loss of vision.
Limitations
This study has several limitations in addition to those stated above. NHANES, by design, does not include people who are institutionalized and as a result, the prevalence of visual impairment likely was underestimated. Because individuals without light perception were excluded from visual acuity testing, the current study was unable to generate estimates of blindness, and the estimates of visual impairment are artificially low. However, because cases of total blindness are relatively rare, the effects on statistical estimates are minimal. In addition, definition of glaucoma used was visual field loss combined with a cup-to-disc ratio ≥0.7; this does not allow for as precise a diagnosis of glaucoma as would be possible from a thorough clinical exam.19 Finally, visual impairment can be produced by visual field constriction as well as visual acuity decrement. Because NHANES did not include data on visual field constriction, the current study could not account for this aspect. Thus, the prevalence of visual impairment might be underestimated.
Conclusion
Extrapolating from NHANES data to the entire U.S population, the present study estimates that nearly 9 million people aged ≥40 years are visually impaired. Of the ocular conditions assessed in NHANES, the condition most likely to result in visual impairment is uncorrected refractive error. Three of every four people could have their visual impairment alleviated solely with refractive correction, leaving only one in four people with visual impairment due to eye disease. The ocular disease most associated with visual impairment in the NHANES sample was AMD. Increasing age and low educational attainment were predictors of visual impairment. Screening of the elderly may identify adults at increased risk of visual impairment due to eye diseases. Increasing public awareness of vision health and the availability and affordability of eye healthcare services could help to reduce avoidable visual impairment and better address the needs of those with underlying eye disease who might benefit from timely therapeutic intervention.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the thoughtful editorial assistance of Tony Pearson-Clarke, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, CDC, Atlanta, Georgia. The authors also thank the NHANES participants, without whom this study would not be possible.
This study was supported by the National Center for Health Statistics (NCHS), CDC. Funding for the National Health and Nutrition Examination Survey (NHANES) retinal and frequency-doubling technology component was provided by the Intra Agency Agreement 05FED47304 from the Division of Diabetes Translation, CDC. Funding for the vision component was provided by the National Eye Institute, NIH, Intramural Research Program award ZIAEY000402.
The NCHS was involved in the design and conduct of the NHANES study and in data collection but not in the analysis or interpretation of the study results or in the preparation of the article. The Division of Diabetes Translation provided funding support for the retinal component and was involved in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of this article before submission. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the CDC or the National Eye Institute.
Footnotes
No financial disclosures were reported by the authors of this paper.
Appendix: Supplementary data: Supplementary data associated with this article can be found, in the online version at http://dx.doi.org/10.1016/j.amepre.2013.02.018.
Contributor Information
Chiu-Fang Chou, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, CDC, Atlanta, Georgia.
Mary Frances Cotch, Division of Epidemiology and Clinical Applications, National Eye Institute, NIH, Bethesda.
Susan Vitale, Division of Epidemiology and Clinical Applications, National Eye Institute, NIH, Bethesda.
Xinzhi Zhang, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, CDC, Atlanta, Georgia.
Ronald Klein, Department of Ophthalmology and Visual Sciences, University of Wisconsin, School of Medicine and Public Health, Madison, Wisconsin.
David S. Friedman, Wilmer Eye Institute, Johns Hopkins School of Medicine, Baltimore, Maryland.
Barbara E.K. Klein, Department of Ophthalmology and Visual Sciences, University of Wisconsin, School of Medicine and Public Health, Madison, Wisconsin.
Jinan B. Saaddine, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, CDC, Atlanta, Georgia.
References
- 1.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP Los Angeles Latino Eye Study Group. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143(6):1013–23. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R Los Angeles Latino Eye Study Group. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941–8. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldzweig CL, Rowe S, Wenger NS, et al. Preventing and managing visual disability in primary care: clinical applications. JAMA. 2004;291(12):1497–502. doi: 10.1001/jama.291.12.1497. [DOI] [PubMed] [Google Scholar]
- 4.Vu HT, Keeffe JE, McCarty CA, Taylor HR. Impact of unilateral and bilateral vision loss on quality of life. Br J Ophthalmol. 2005;89(3):360–3. doi: 10.1136/bjo.2004.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health. 2004;94(5):823–9. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood JM, Lacherez P, Black AA, Cole MH, Boon MY, Kerr GK. Risk of falls, injurious falls, and other injuries resulting from visual impairment among older adults with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(8):5088–92. doi: 10.1167/iovs.10-6644. [DOI] [PubMed] [Google Scholar]
- 7.Prevent Blindness America. The economic impact of vision problems. www.preventblindness.org/sites/default/files/national/documents/EI_introduction.pdf.
- 8.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the U.S. Arch Ophthalmol. 2006;124:1754–60. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 9.Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the U.S. Arch Ophthalmol. 2007;125(4):544–50. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- 10.The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the U.S. Arch Ophthalmol. 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 11.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the U.S. JAMA. 2006;295(18):2158–63. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz A. The prevalence and consequences of vision impairment in later life. Top Geriatr Rehabil. 2004;20(3):185–95. [Google Scholar]
- 13.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dame Eye Study. Ophthalmology. 2007;114(2):253–62. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE. Are individuals with diabetes seeing better? A long-term epidemiological perspective. Diabetes. 2010;59(8):1853–60. doi: 10.2337/db09-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW. Computer vision syndrome: a review. 2005;50(3):253–62. doi: 10.1016/j.survophthal.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in East Baltimore. N Engl J Med. 1991;325(20):1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 17.CDC, National Center for Health Statistics (NCHS) NHANES— survey questionnaire and exam components, 2007-2008. www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm.
- 18.Friedman DS, Wolfs RC, O'Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the U.S. Arch Ophthalmol. 2004;122(4):532–8. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry AL, Paulose-Ram R, Tilert T, et al. The methodology of visual field testing with frequency doubling technology in the National Health and Nutrition Examination Survey, 2005–2006. Ophthalmic Epidemiol. 2010;17(6):411–21. doi: 10.3109/09286586.2010.528575. [DOI] [PubMed] [Google Scholar]
- 20.CDC; National Center for Health Statistics. National Health and Nutrition Examination Surveys: response rates and CPS population totals. www.cdc.gov/nchs/nhanes/response_rates_CPS.htm.
- 21.National Center for Health Statistics. National Health and Nutrition and Examination Survey (NHANES) Ophthalmology Procedures Manual. 2005 Sep; www.cdc.gov/nchs/data/nhanes/nhanes_05_06/OP.pdf.
- 22.National Center for Health Statistics. National Health and Nutrition and Examination Survey (NHANES) Digital Grading Protocol. 2005 Jan 15; www.cdc.gov/nchs/data/nhanes/nhanes_05_06/NHANES_ophthamology_digital_grading_protocol.pdf.
- 23.Klein R, Chou CF, Klein B, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the U.S. population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 24.Crowston JG, Hopley CR, Healey PR, Lee A, Mitchell P. The effect of optic disc diameter on vertical cup to disc ratio percentiles in a population based cohort: the Blue Mountains Eye Study. Br J Ophthalmol. 2004;88(6):766–70. doi: 10.1136/bjo.2003.028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC, National for Health Statistics Center (NCHS) Analytic note regarding 2007-2010 survey design changes and combing data across other survey cycles. www.cdc.gov/nchs/data/nhanes/analyticnote_2007-2010.pdf.
- 26.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the U.S., 2005–2008. JAMA. 2010;304(6):649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the U.S. Am J Clin Nutr. 2000;72(5):1074–81. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 28.Torpy JM, Lynm C, Glass RM. Causes of visual impairment. JAMA. 2003;290(15):2088. doi: 10.1001/jama.290.15.2088. [DOI] [PubMed] [Google Scholar]
- 29.Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Elevation Study. Arch Ophthalmol. 2000;118(6):819–25. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 30.Chou CF, Sherrod CE, Zhang X, et al. Reasons for not seeking eye care among adults aged ≥40 years with moderate-to-severe visual impairment—21 states, 2006-2009. MMWR Morb Mortal Wkly Rep. 2011;60(19):610–3. [PubMed] [Google Scholar]
- 31.Lam BL, Lee DJ, Gomez-Marin O. Prevalence of usual-corrected binocular distance visual acuity impairment in Hispanic and non-Hispanic adults. Ophthalmic Epidemiol. 2000;7(1):73–83. [PubMed] [Google Scholar]
- 32.Resnikoff S, Pascolini D, Mariotti SP, Pokharel G. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull WHO. 2008;86(1):63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livingston PM, McCarty CA, Taylor HR. Visual impairment and socioeconomic factors. Br J Ophthalmol. 1997;81(7):574–7. doi: 10.1136/bjo.81.7.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varma R, Chung J, Foong AW, et al. Los Angeles Latino Eye Study Group. Four-year incidence and progression of visual impairment in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):713–27. doi: 10.1016/j.ajo.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varma R, Wang M, Ying-Lai M, Donofrio J, Azen SP. Prevalence and risk indicators of visual impairment and blindness in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(6):1132–40. doi: 10.1016/j.ophtha.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Foran S, Wang JJ, Mitchell P. Cause of visual impairment in two older population cross-sections: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10(4):215–25. doi: 10.1076/opep.10.4.215.15906. [DOI] [PubMed] [Google Scholar]
- 37.Varma R, Wang MY, Ying-Lai M, Donofrio J, Azen SP. The prevalence and risk indicators of uncorrected refractive error and unmet refractive need in Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2008;49(12):5264–73. doi: 10.1167/iovs.08-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiagalingam S, Cumming RG, Mitchell P. Factors associated with undercorrected refractive errors in an older population: the Blue Mountains Eye Study. Br J Ophthalmol. 2002;86(9):1041–5. doi: 10.1136/bjo.86.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liou H, McCarty CA, Jin CL, et al. Prevalence and predictors of undercorrected refractive errors in the Victorian population. Am J Ophthalmol. 1999;127(5):590–6. doi: 10.1016/s0002-9394(98)00449-8. [DOI] [PubMed] [Google Scholar]
- 40.Tielsch JM, Sommer A, Katz J, Quigley H, Ezrine S. Socioeconomic status and visual impairment among urban Americans. Arch Ophthalmol. 1991;109(5):637–41. doi: 10.1001/archopht.1991.01080050051027. [DOI] [PubMed] [Google Scholar]
- 41.Dandona R, Dandona L. Socioeconomic status and blindness. Br J Ophthalmol. 2001;85(12):1484–8. doi: 10.1136/bjo.85.12.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein R, Klein BEK, Jensen SC, et al. The relation of socioeconomic factors to age-related cataract, maculopathy, and impaired vision: the Beaver Dam Eye Disease Study. Ophthalmology. 1994;101(12):1969–79. doi: 10.1016/s0161-6420(13)31077-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.