Abstract
The amygdala is a central target of emotion regulation. It is overactive and dysregulated in affective and anxiety disorders and amygdala activity normalizes with successful therapy of the symptoms. However, a considerable percentage of patients do not reach remission within acceptable duration of treatment. The amygdala could therefore represent a promising target for real-time functional magnetic resonance imaging (rtfMRI) neuro-feedback. rtfMRI neurofeedback directly improves the voluntary regulation of localized brain activity. At present, most rtfMRI neurofeedback studies have trained participants to increase activity of a target, i.e. up-regulation. However, in the case of the amygdala, down-regulation is supposedly more clinically relevant. Therefore, we developed a task that trained participants to down-regulate activity of the right amygdala while being confronted with amygdala stimulation, i.e. negative emotional faces. The activity in the functionally-defined region was used as online visual feedback in six healthy subjects instructed to minimize this signal using reality checking as emotion regulation strategy. Over a period of four training sessions, participants significantly increased down-regulation of the right amygdala compared to a passive viewing condition to control for habilitation effects. This result supports the concept of using rtfMRI neurofeedback training to control brain activity during relevant stimulation, specifically in the case of emotion, and has implications towards clinical treatment of emotional disorders.
Keywords: Real-time fMRI, Neurofeedback, Amygdala, MPFC, Emotion regulation, Affective disorders, Anxiety, Faces
Introduction
The amygdala is activated by negative and positive emotional stimuli (Sergerie et al. 2008), and it is a central target of emotion regulation (Ochsner et al. 2012). Cognitive strategies such as reappraisal and reality checking can reduce activity of the amygdala and related emotion propagating brain regions in emotionally arousing situations (Ochsner et al. 2012; Diekhof et al. 2011; Buhle et al. 2013) particularly through top-down control of (dorso)medial prefrontal cortex [(D)MPFC, Herwig et al. 2007; Hartley and Phelps 2010; Maren and Quirk 2004; Kalisch 2009; Delgado et al. 2008]. In affective and emotion regulation disorders, the amygdala is often hyperactive (Hamilton et al. 2012; Etkin and Wager 2007; Schmahl et al. 2006) and normalizes with successful treatment (Quide et al. 2012). It has been suggested that voluntary control of amygdala activity could represent a method to strengthen emotion regulation and to treat affective and emotion regulation disorders (Schmahl et al. 2006).
Real-time functional magnetic resonance imaging (rtfMRI) can provide direct feedback information from the activity of circumscribed brain regions, networks (Sitaram et al. 2011) or from other physiological measures such as connectivity (Lee et al. 2012; Koush et al. 2013). Subjects can then use this information to learn to control the given signal and in this way to regulate the underlying neural activity (Cox et al. 1995; Goebel 2001; Sulzer et al. 2013a). Studies using rtfMRI neurofeedback have shown that it is possible to voluntarily self-regulate the activity of various cortical and subcortical brain regions and subregions (for review: Ruiz et al. 2013).
Voluntary up-regulation of amygdala activity has been the target of multiple neurofeedback studies, despite evidence that reducing activation may be more clinically relevant. For instance, two studies focused solely on amygdala for the purpose of up-regulation, using cognitive strategies such as inducing a sad mood (Posse et al. 2003), or contemplating positive autobiographical memories (Zotev et al. 2011). More clinically-oriented research has included amygdala up-regulation in the broader context of the emotional network, for instance in healthy participants (Johnston et al. 2010, 2011) and depressed patients (Linden et al. 2012). Both studies in healthy subjects revealed increased activity in the amygdaloid area due to neurofeedback, with a pronounced effect in the ventral striatum in the studies using targets defined by the reaction to positive stimuli (Johnston et al. 2011; Linden et al. 2012). Participants in the above named studies trained amygdala regulation in the absence of any stimuli. However, in everyday life, many problems in mood and anxiety disorders occur when patients anticipate or perceive emotional stimuli, and this emotional experience is associated with an increased activity and dysregulation of the amygdala. Therefore, the voluntary down-regulation of the amygdala during emotional stimulation might be a realistic model for training emotion regulation and a potential novel path to treat affective and related conditions. Similar approaches have just recently been applied in smokers (Li et al. 2012; Hanlon et al. 2013), when inducing craving by presenting smoking-associated cues to the participants and then training to reduce craving assisted by neurofeedback of the anterior cingulate cortex. Informed by research on affective disorders, we focused on the regulation of the amygdala during emotional stimulation.
Since the amygdala is a bilateral structure, lateralization of specific functions of the region, and thus self-regulation of the putative unilateral area may be appropriate, but such organized laterality is controversial. Meta-analyses on emotion processing resulted in mixed findings, with some showing stronger activations of the amygdala in one hemisphere (Fusar-Poli et al. 2009), whereas others found no clear general laterality effects (e.g. Sergerie et al. 2008; Kober et al. 2008; Sabatinelli et al. 2011). Some studies point to a preference of right amygdala to an early, rapid and possibly more automatic detection of emotional stimuli with less habituation and eventually a preferential reaction to negative stimuli (Dyck et al. 2011; Baeken et al. 2010; Sergerie et al. 2008), whereas the left amygdala is supposed to be involved in more elaborate stimulus evaluation and, for instance, more complex cognitive stimuli such as semantic stimuli (Dyck et al. 2011; Sergerie et al. 2008). In patients suffering from affective and anxiety disorders, several meta-analyses have shown similarly mixed results (stronger activity on the right side: Groenewold et al. 2013; Fitzgerald et al. 2008; Hattingh et al. 2013; Etkin and Wager 2007, left side: Sacher et al. 2012, bilateral: Hamilton et al. 2012). Due to our focus on regulation of early, less elaborate reactions to ‘hard-wired’ stimuli as well as the potential future transfer to patients with affective disorders, we selected the right amygdala as our target region.
As such, our goal was to develop and examine the feasibility of using online neurofeedback to assist participants in self-reduction of amygdala activity. In addition to neurofeedback, six healthy participants were exposed to negative faces as emotional stimulation, a robust technique for eliciting amygdala activation (Breiter et al. 1996; Whalen et al. 1998, meta-analysis: Sabatinelli et al. 2011), with a supposed “hard-wired” evolutionary basis (Liddell et al. 2005; Emery 2000; Adolphs 2008). We used color-based instead of motion-based feedback typical in rtfMRI studies (Sulzer et al. 2013a), since it may interfere with attention to the most salient aspects of emotional facial stimuli (i.e. eyes and mouth) for amygdala activation (e.g. Ellis 1975; Morris et al. 2002; Adolphs et al. 2005). As this study aimed at proving the principle of rtfMRI neurofeedback-assisted training during emotional stimulation, we examined the effects of repeated training sessions on the individual ability to down-regulate the amygdala in contrast to a “view” condition without regulation. We hypothesized enhanced downregulation of right amygdala activity in the “regulate” compared to the “view” condition over four rtfMRI neurofeedback training sessions.
Materials and Methods
Participants
We examined six healthy participants (4 female, 2 male, mean age 26 years, standard deviation 3.8 years). The participants were recruited via personal contact and email-lists. All participants were healthy, as was assessed with semi-structured interviews and checklists [abbreviated version of the mini neuropsychiatric interview (MINI, Sheehan et al. 1998)] performed by an experienced psychiatrist (ABB). Exclusion criteria were prior and current neurological and psychiatric illnesses; pregnancy; intake of any medication (except for oral contraceptives) or psychotropic drugs including excessive consumption of alcohol (regular intake of >7 units/week), cigarettes (>1 pack/ day) and caffeine (>5 cups/day) and general contraindications against MRI examinations. After each feedback run, subjects were asked via microphone regarding drowsiness and tiredness. We further interviewed the participants after each completed session in a structured interview on drowsiness and tiredness, general feelings, specific experiences and the strategies used for regulation. Each subject completed four sessions. The mean period between sessions was 6.8 days. The study was approved by the ethics committee of the canton of Zürich and conducted in compliance with the declaration of Helsinki (World Medical Association 2008). All participants gave written informed consent and received financial compensation.
Experimental Task
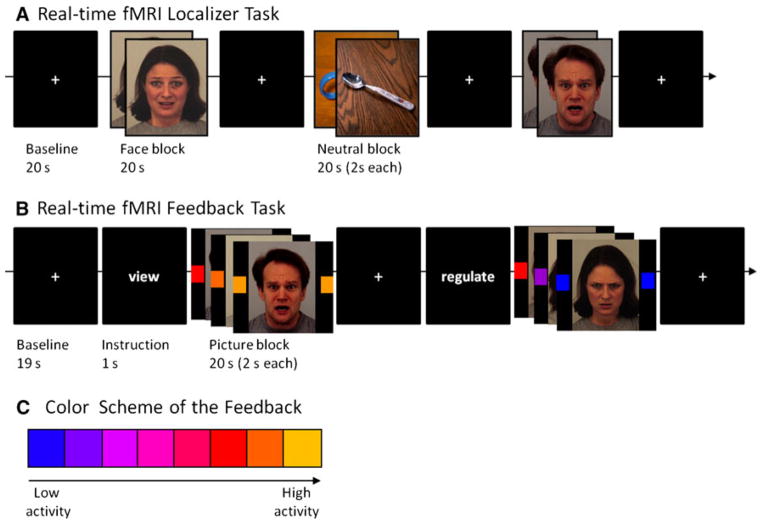
Functional Localizer (Fig. 1a)
Fig. 1.
Tasks and coding of amygdala activity Tasks for localization (a) and feedback (b) of amygdalar activity. The colors indicate the activity of the respective amygdala ROI (c) (Color figure online)
The amygdala was first localized functionally in each participant in each session. Participants were presented negative emotional faces from the Karolinska Directed Emotional Face Set (Lundqvist et al. 1998) and, for contrast, neutral and low arousing pictures from the International Affective Pictures System (IAPS, Lang et al. 2005) for individually localizing the amygdala. Non-facial neutral pictures from the IAPS were chosen to increase the contrast to the negative emotional pictures with respect to amygdala activation (Sabatinelli et al. 2011). Pictures were presented in a blocked design with 10 pictures in each block, each shown for 2 s. After each block a baseline period (fixation cross) of 30 s allowed the blood oxygen level dependent (BOLD) signal to level off before the next condition (total duration of the localizer: about 6 min). In each block, pictures of the same gender and the same emotional valence were presented. To achieve intensive activation of the amygdala in the localizer, only fearful, sad and angry expressions were shown. Subjects were instructed to passively observe the pictures. In total, nine trials of pictures and baseline were shown in a pseudo-randomized counterbalanced order, three depicting neutral pictures, and six with emotional faces.
Feedback Task (Fig. 1b)
The feedback task (Fig. 1b) was constructed similar to the localizer task in a blocked design, but without neutral IAPS stimuli. Each single feedback period consisted of emotional faces of the same gender and the same emotional valence (angry, fearful). Within one run, 16 periods of 20 s duration, each containing 10 pictures of 2 s duration, total duration of a run about 12 min, were shown. Prior each period, a short written instruction (“view”, “regulate”, duration 1 s) was given on the screen. After each single feedback period a baseline period (fixation cross) was implemented for 29 s (baseline + instruction = 30 s). Each run consisted of six periods of the “view” conditions and ten periods of the “regulate” conditions. This increased weighting of the “regulate” condition was chosen to reduce habituation and to improve training effects. Due to the length of the total measurements and the task, we asked the participants after each run about their subjective tiredness and drowsiness. Depending on their response, they performed two or (optimally) three feedback runs in each session (mean number of feedback runs per session: 2.42). Pictures were randomized and in each session 50 % of the pictures were “new”, prior unseen pictures to prevent habituation and effects of familiarity. Feedback of amygdala activity was recorded from the region identified in the localizer task and was given to the participant during both “regulate” and “view” conditions in form of changing colour of blocks on both sides of the pictures. They were positioned bilaterally at the height of the eyes of the depicted faces to avoid distraction to either side or otherwise away from the eyes (the most significant aspects of faces). Although previous rtfMRI studies use motion-based feedback (Sulzer et al. 2013a), the distraction from the stimulation provided by the motion was not appropriate for this study.
Task Instruction
Prior the first session, all participants were given written instructions and were informed on the 4–6 s delay of the feedback reaction due to the delay of the hemodynamic response function. Participants were instructed to apply cognitive control by reality checking such as “these are pictures, these are actors, this is an experiment” (Herwig et al. 2007). After each session, subjects were interviewed on the used strategies, their experiences and subjective performance during feedback and regulation.
Image Acquisition
Imaging was performed with a 3.0 T Philips Achieva Scanner (Philips Medical Systems, Best, The Netherlands, equipped with an 8-channel receive head-coil array). Echo-planar imaging was performed for functional MR imaging [repetition-time (TR)/echo-time (TE) 2,000/25 ms, 30 sequential axial slices, whole brain, slice thickness: 3.0 mm, gap 1.1 mm, field of view (FOV): 240 × 240 mm, matrix 80 × 80 voxel, resulting voxel size: 3 × 3 × 3 mm, axial orientation, SENSE-factor: 2.0]. The localizer run consisted of 170 volumes, the feedback runs of 330 volumes each. High-resolution 3-D T1 weighted anatomical volumes were acquired (TR/TE 6.73/3.1 ms; voxel size 1 × 1 × 1 mm, 145 slices, axial orientation) for coregistration with the functional data. Stimuli were presented via digital goggles (Resonance Technologies, Northridge, CA, USA).
FMRI Analysis and Statistics
Online Real-time Analysis and Statistics
Functional data were analyzed online during fMRI with Turbo Brain voyager (TBV) Version 3.0.0 (Brain Innovation, Maastricht, NL, USA). The processing has been described previously (Goebel 2001; Caria et al. 2010). Real-time data analysis comprised incremental 3D motion detection and correction and drift removal and resulted in incrementally computed statistical maps based on the general linear model (GLM) and event-related averages. These analyses were performed in native space.
After the localizer scan, a region of interest (ROI) was placed in the anatomical region of the right amygdala extending over 3 slices (=9 mm) using a t-value threshold of 2.0. The size and centers of these localizer ROIs are given in Table 1. The individual maximal activation for the calculation of the colour range for the feedback was determined from the event related averaging of the individual amygdala ROI. This event-related average is calculated by TBV in parallel to the typical averaging performed in the analysis of event-related potentials according to the formula (value baseline)/baseline. As the sessions were each separated by about a week, we determined the specific ROIs individually for each session. The BOLD signal of these ROIs was extracted during the feedback sessions by TBV and then transferred to Visual Studio, where the information was converted into the change of the color blocks as described above.
Table 1.
Localizer regions of interest (ROIs). Given are the Talairach coordinates of the center
| Subject no. | Session | Talairach X/Y/Z | Vol (mm3) | % Signal change localizer |
|---|---|---|---|---|
| 01 | 1 | 19/−1/−12 | 478 | 1.0 |
| 2 | 18/−6/−9 | 1,996 | 0.8 | |
| 3 | 23/−1/−14 | 2,083 | 0.8 | |
| 4 | 21/−1/−11 | 477 | 1.0 | |
| 02 | 1 | 23/−6/5 | 1,798 | 1.2 |
| 2 | 19/−2/5 | 2,151 | 2.0 | |
| 3 | 21/−2/4 | 918 | 1.5 | |
| 4 | 23/−8/5 | 2,269 | 1.7 | |
| 03 | 1 | 26/1/−11 | 1,064 | 1.3 |
| 2 | 22/−5/−10 | 2,772 | 1.2 | |
| 3 | 27/−4/−12 | 963 | 1.0 | |
| 4 | 21/1/−8 | 659 | 1.4 | |
| 04 | 1 | 19/1/−8 | 797 | 1.0 |
| 2 | 21/2/−7 | 124 | 0.7 | |
| 3 | −19/−10/−8 | 224 | 1.0 | |
| 4 | −22/−5/−9 | 242 | 0.5 | |
| 05 | 1 | 20/−5/−10 | 442 | 1.8 |
| 2 | 20/−0/−11 | 623 | 1.8 | |
| 3 | 24/−1/−11 | 275 | 1.8 | |
| 4 | 21/0/−15 | 862 | 1.0 | |
| 06 | 1 | 25/6/−12 | 363 | 0.8 |
| 2 | 27/4/−13 | 891 | 0.8 | |
| 3 | 22/2/−11 | 354 | 0.7 | |
| 4 | 24/−2/−13 | 1,223 | 0.6 |
To provide a sensitive and also reliable and informative feedback of the brain activity and the effects of regulation, we fitted the range of the feedback colours to the individual maximal activation. The inter-individual variability of stimulus-related BOLD responses can vary by a factor of more than two (e.g. Liu et al. 2011, Handwerker et al. 2004, Raemaekers et al. 2012). Using a fixed assignment of % signal change to a colour would in participants with a high amplitude of BOLD signal change have resulted in quickly reaching the ceiling of the colour spectrum but not getting a fine-grained feedback on their performance, whereas in participants with a low amplitude their activation and regulation would have been represented by only slight colour changes in the blue-violet colour range. Therefore, we computed the individual reactivity of the amygdala from the localizer using the average percent signal change from baseline in the chosen amygdala ROI. This was entered in the computation of the range of colours of the feedback blocks as maximum value (=bright orange), determined on a subject-wise basis during the localizer. The feedback was first normalized based on the percent signal increase from the previous baseline condition (last five volumes), then three-point averaged (averaging the current value with the previous two) to reduce noise and strong fluctuations of the feedback (in parallel to Sulzer et al. 2013b). This feedback signal was computed and presented by custom-made software running on Visual-Studio® (Microsoft, Redmond, WA, USA).
Offline Analysis and Statistics
After scanning, the acquired images were processed offline using BrainVoyagerQX 2.4 (Brain Innovation, Maastricht, NL, USA, Goebel et al. 2006). Standard preprocessing with BrainVoyagerQX included motion correction, slice scan-time correction, high-frequency temporal filtering and removal of linear trends (as described in Herwig et al. 2007). All individual functional datasets were checked for excessive head movements (datasets would have been excluded if sudden movements exceeded 3 mm in any direction, however no datasets exceeded this limit). Functional data were co-registered with the individual T1-weighted 3D structural data, resulting in a functional dataset. Structural and functional data were transformed into Talairach space and spatially smoothed with a 4 mm full-width half-maximum Gaussian kernel for subsequent within- and between-subject analysis. The relatively small kernel was chosen with respect to the small size of the ROI. Standard GLM analysis was performed using three regressors of interest (rest, regulate and view) convolved with the hemodynamic response function, and six head movement regressors representing translation and orientation as regressors of no interest.
Learned regulation was determined as a significant linear decrease in ROI activity over sessions. The primary outcomes, amygdala parameter estimates (beta values), were extracted from the defined functional area within the right amygdala, adapted to each session. The anatomical area of the amygdala was defined based on structural images, confirmed using the Talairach client (Lancaster et al. 2000) and the Talairach atlas (Talairach and Tournoux 1988), and confined to a 20 × 20 × 20 mm volume. Beta values were then extracted from functional ROIs, obtained from the contrast “view >regulate” in each session for each participant, with a statistical threshold of p <0.005 (uncorrected, Talairach-coordinates and size: see Table 2). Comparing the “regulate” to the “view” condition instead of a comparison to “rest” ensured control for the stimulation and its associated effects, as well as habituation to the environment, habituation to the stimuli and effects of exhaustion and drowsiness. The beta values were then used in a single factor (session, four levels) repeated measures ANOVA, controlling for the varying size of the amygdala ROI, including confirmation of normality (Kolmogorov–Smirnov) and sphericity (Mauchly) using SPSS 21 (IBM, Armonk, NY, USA). Post-hoc two-tailed paired t-tests and effect sizes (Cohen’s d) were calculated in those comparisons where the main effect of session was significant.
Table 2.
Reduced activity in the contrast “regulate >view” (p <0.005) in the amygdala in each subject and each session (paired t test)
| Subject no. | Session | Talairach X/Y/Z | Vol (mm3) | r >v beta weights mean (SE) | r >v t/p |
|---|---|---|---|---|---|
| 01 | 1 | 27/−4/−6 | 849 | −0.528 (0.089) | −5.93/ <0.000 |
| 2 | 24/−8/−9 | 2,097 | −0.657 (0.091) | −7.21/ <0.000 | |
| 3 | 21/−4/−15 | 3,716 | −0.847 (0.096) | −8.83/ <0.000 | |
| 4 | 27/1/−18 | 1,777 | −0.905 (0.117) | −7.71/ <0.000 | |
| 02 | 1 | 16/−9/−18 | 181 | −0.461 (0.093) | −4.98/ <0.000 |
| 2 | 19/−1/−18 | 2,637 | −0.637 (0.094) | −6.75/ <0.000 | |
| 3 | 22/4/−22 | 1,634 | −0.681 (0.093) | −7.32/ <0.000 | |
| 4 | 23/9/−5 | 264 | −0.738 (0.115) | −6.39/ <0.000 | |
| 03 | 1 | 19/−3/−11 | 1,396 | −0.795 (0.094) | −8.43/ <0.000 |
| 2 | 16/−5/−14 | 4,138 | −0.95 (0.094) | −10.10/ <0.000 | |
| 3 | 17/−4/−10 | 1,540 | −0.616 (0.096) | −6.38/ <0.000 | |
| 4 | 18/−2/−11 | 1,319 | −0.879 (0.117) | −7.54/ <0.000 | |
| 04 | 1 | 30/−7/−25 | 72 | −0.32 (0.103) | −3.11/0.002 |
| 2 | 17/−4/−22 | 298 | −0.359 (0.089) | −4.04/ <0.000 | |
| 3 | 22/2/−17 | 362 | −0.428 (0.111) | −3.86/ <0.000 | |
| 4 | 29/4/−19 | 501 | −0.668 (0.157) | −4.26/ <0.000 | |
| 05 | 1 | 24/0/−16 | 73 | −0.327 (0.111) | −2.95/0.003 |
| 2 | 25/3/−12 | 28 | −0.522 (0.113) | −4.61/ <0.000 | |
| 3 | 15/−2/−19 | 313 | −0.651 (0.111) | −5.87/ <0.000 | |
| 4 | 24/7/−26 | 120 | −0.515 (0.171) | −3.02/0.002 | |
| 06 | 1 | 21/−2/−13 | 378 | −0.805 (0.113) | −7.14/ <0.000 |
| 2 | 19/−5/−22 | 2,178 | −0.905 (0.113) | −8.04/ <0.000 | |
| 3 | 18/−1/−20 | 1,181 | −0.848 (0.110) | −7.73/ <0.000 | |
| 4 | 13/0/−19 | 1,899 | −0.945 (0.108) | −8.76/ <0.000 |
Secondary post hoc repeated measures analysis was conducted on a DMPFC ROI to examine whether learned down-regulation also involved central emotion regulation network represented by the DMPFC (Buhle et al. 2013; Kalisch 2009; Diekhof et al. 2011). To test for related effects in the left amygdala, we also analyzed activation in an anatomically defined cubic ROI (edge length 9 mm, volume 729 mm3) in the left amygdala centered at x/y/z = −19/−8/−15 using repeated measures analyses and bivariate correlations with the respective beta values of the right amygdala ROIs. Furthermore, to test for non-specific effects of training and repeated exposure to the task in rather unrelated brain regions, we also computed post hoc repeated measures analyses on ROIs positioned in the primary visual (V1) and somatosensory (S1) cortex. The DMPFC was individually defined due to the contrast “view >regulate” according to literature (Buhle et al. 2013; Kalisch 2009; Diekhof et al. 2011). The sum of the individual ROIs covered the medial and superior frontal gyrus (Brodmann area 6, placed around the mean (SD) center coordinates x/y/z = −2 (7.1)/ −5 (8.8)/57 (8.6), maximal extension: x = 13 to −18/y = 12 to −23/ z = 38–70); mean size 2,795 mm3 [60–7,989 mm3 (Supplemental Fig. 1)], bordering caudally to the anterior and middle cingulate cortex (BA 31, 32), frontally to the upper part of the superior frontal gyrus (BA 6) and occipitally to the precentral gyrus (BA 4). Both V1 and S1 were defined using spherical ROIs, the former centered at x/y/z = ±11/−90/−3, (16 mm diameter) and the latter at x/y/z = ± 33/−24/62 (10 mm diameter).
Modulatory effects of neurofeedback on amygdala activity were investigated using a psychophysiological interaction analysis (PPI, Friston et al. 1997), with the expectation that neurofeedback modulates connectivity between the amygdala and DMPFC (Kanske et al. 2011). As typical in a PPI analysis, time courses for each amygdala ROI were extracted, followed by its dot product with the two task regressors (i.e. view and regulate). The interaction regressors of interest were included in a design matrix along with task, seed region time course, and head movement regressors of no interest. PPI analysis of each subject was restricted to the DMPFC ROI obtained earlier. The resulting beta values were extracted from the DMPFC and evaluated for significant changes using a repeated-measures ANOVA.
Results
The subjects used mostly cognitive (i.e. reality check) and attentional strategies (thinking about something else, thought distraction).
There was no significant effect of session on the size of the amygdala ROI resulting from the localizer session [F(3,15) = 0.119, p = 0.747, partial η2 = 0.029] as well as no significant linear effect of session on the activity of the amygdala during the localizing session [F(3,15) = 1.220, p = 0.320, partial η2 = 0.196]. The respective regions were then used as source of the feedback signal. The analysis of probabilistic overlap between these feedback ROIs revealed a maximal probabilistic overlap of 50 %, and at a threshold of >35 % overlap we found one cluster centered at x/y/z = 20/−2/−12 with a volume of 145 mm3.
The average (SD) Talairach coordinate of all ROIs used in the analysis of the regulation effect of all subjects and sessions was x = 21 (4.5), y = −1 (4.6), z = −16 (5.6), mean size 1,213 mm3. The repeated measures ANOVA on the beta weights of the contrast “view >regulate” in the right amygdala ROI revealed a significant main effect of the factor session [F(3,12) = 4.771, p = 0.021, partial η2 = 0.544]. The repeated measures ANOVA on this contrast in the DMPFC ROI was not significant [F(3,15) = 0.638, p = 0.576, partial η2 = 0.120]. The effect of “session” on amygdalar activity during the viewing condition alone (beta-weights calculated against baseline) was not significant [F(3,15) = 0.466, p = 0.525, partial η2 = 0.085]. The detailed analysis of the main effect of the “session” in the amygdala ROI showed that:
all subjects managed down-regulation of amygdala activity assisted with rtfMRI neurofeedback during stimulation with negative emotional faces (Fig. 2a, b, Table 2) and
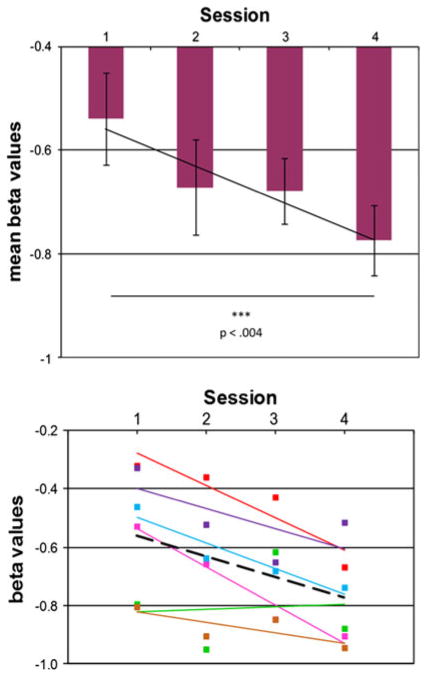
this down-regulation increased and therefore improved significantly from session 1 to 4 [two-tailed paired t test: t(5) = −4.924, p = 0.004, mean difference = −0.236, standard deviation = 0.117, effect size d = 1.34, mean change 30 %, Fig. 2a, Table 1]. On the individual level, this effect was significant in five of the six subjects, only in one subject the trend-line over all sessions did not significantly differ from zero slope (Fig. 2b).
Fig. 2.
Effect of real-time fMRI neurofeedback training over four sessions in the right amygdala increasing ability to down-regulate amygdala activity during stimulation with negative facial expressions. a In the whole group (mean + standard deviation), b individual values and trend-lines (black dotted line trend-line). Given are the beta-weights of the contrast “view >regulate”
In the single individual datasets, the DMPFC was more active during regulating versus passive viewing in 23 of 24 sessions, but without a significant and consistent effect of repeated training.
The post hoc analysis of correlations between right amygdala and the anatomically placed left amygdala ROI showed rather high correlations in the viewing condition of the corresponding sessions (mean r = 0.71, ranging from 0.47 to 0.92), whereas the correlations in the regulate condition were lower and more variable (mean r = 0.36, ranging from 0.14 to 0.81). There was no significant effect of the factor “session” in the repeated measures ANOVA in the left amygdala ROI (Table 3).
Table 3.
Results of the repeated measures GLM in the other ROIs (main effect of the factor “session”) during the regulation and the viewing condition
| ROI | Talairach X/Y/Z | F(3,15) | p | Partial η2 |
|---|---|---|---|---|
| Regulate condition | ||||
| Dorsomedial prefrontal cortexa | −2/−3/55 | 1.379 | 0.287 | 0.216 |
| Visual cortex R (V1) | 11/−90/−3 | 0.357 | 0.785 | 0.067 |
| Visual cortex L (V1) | −11/−90/−3 | 0.437 | 0.730 | 0.080 |
| Sensory cortex R (S1) | 33/−24/62 | 1.064 | 0.394 | 0.176 |
| Sensory cortex L (S1) | −33/−24/62 | 0.118 | 0.948 | 0.023 |
| Anterior insula/VLPFC R | 34/16/4 | 2.063 | 0.148 | 0.292 |
| Anterior insula/VLPFC L | −34/16/4 | 2.716 | 0.082 | 0.352 |
| Amygdala L | −19/−8/−15 | 1.926 | 0.169 | 0.278 |
| View condition | ||||
| Dorsomedial prefrontal cortexa | −2/−3/55 | 1.513 | 0.252 | 0.232 |
| Visual cortex R (V1) | 11/−90/−3 | 2.042 | 0.140 | 0.276 |
| Visual cortex L (V1) | −11/−90/−3 | 2.001 | 0.157 | 0.247 |
| Sensory cortex R (S1) | 33/−24/62 | 0.315 | 0.814 | 0.059 |
| Sensory cortex L (S1) | −33/−24/62 | 0.388 | 0.764 | 0.072 |
| Anterior insula/VLPFC R | 34/16/4 | 1.147 | 0.362 | 0.187 |
| Anterior insula/VLPFC L | −34/16/4 | 2.447 | 0.104 | 0.329 |
| Amygdala L | −19/−8/−15 | 1.161 | 0.357 | 0.189 |
All other ROIs were created based on a priori anatomical coordinates. In all ROIs, normality and sphericity were not violated (Kolmogorov–Smirnov test and Mauchly test)
Individual ROIs
The repeated measures ANOVA in the other ROIs revealed no significant effect in either condition (Table 3). There was no significant linear effect of the factor “session” on size of the amygdala ROI used in the post hoc analysis of the feedback session [F(3,15) = 1.176, p = 0.339, partial η2 = 0.227]. Within the participants, the sizes of the localizer ROIs and of the post hoc ROIs of the feedback sessions were not significantly different [t(23) = 1.128, p = 0.271]. There was no significant effect of the training on PPI between the amygdala and the DMPFC ROIs during the “regulate” condition [F(3,15) = 0.018, p = 0.899, partial η2 = 0.004] as well as during the “view” condition [F(3,15) = 2.51, p = 0.638, partial η2 = 0.048].
Discussion
To our knowledge, this study is the first one to introduce the concept of rtfMRI neurofeedback training for the down-regulation of the amygdala during stimulation with emotionally negative contents. Repeated training successfully enhanced the subjects’ ability to down-regulate their own amygdala activity while being stimulated with negative emotional faces. Down-regulation of the right amygdala in all subjects in the first session is in parallel with previous studies on emotion regulation, showing reduced emotional arousal on the physiological (Ochsner and Gross 2005) and the neural level, particularly the amygdala (Herwig et al. 2007; Kanske et al. 2011; Herwig et al. 2010; Diekhof et al. 2011; Maren and Quirk 2004). However, effects in the first session cannot be specifically attributed to neurofeedback effects, but have been shown before (e.g. Ochsner and Gross 2005; Phan et al. 2005; Herwig et al. 2007) with cognitive emotion regulation strategies such as reality check. Indeed, the improved down-regulation across the sessions, comparing “view” and “regulate” condition, implies a specific training effect of the neurofeedback compared to the purely psychological application of emotion regulation strategies.
Habituation effects, e.g. to stimuli and scanning, would have resulted in a reduced amygdala activation in the “view” condition, which served as an internal control condition (and where we found no effect of the factor session). Habituation could therefore have rather diminished the down-regulation and training effect in the present study design. Additionally, ensuring that 50 % of pictures during each session had not been seen previously, primarily counteracted possible habituation.
The results in the amygdala support the feasibility of rtfMRI neurofeedback training for emotion regulation training. Models of emotion regulation might have suggested an increase of prefrontal cortical activations over the sessions (Diekhof et al. 2011; Ochsner et al. 2012; Maren and Quirk 2004). The DMPFC was active during regulation, but without a consistent modification across the sessions. We also found no changes in connectivity between DMPFC and amygdala over sessions. Therefore, there is insufficient evidence to support the hypothesis that the central emotion network is involved in this training of self-regulation. This could possibly be explained by the subjects’ different and adapting strategies, which could have reduced and interfered with localized effects of learning on brain activity. Studies on brain changes during training and learning revealed early increased and more extended activations (Karni et al. 1995), which later, with consolidation, decreased again (De Weerd et al. 2003). It is possible, that weekly sessions did not capture the zenith of this curve. Thus further research on the aspect of training emotion regulation over time is needed. However, besides this temporal issue it is possible that not so much the DMPFC but perhaps other brain regions such as VMPFC or VLPFC play a stronger role in this regulatory context. Due to the limited power of our current study which focused on feasibility aspects particularly with regard to the amygdala further analyses should be carried out in future studies in larger samples.
The main limitation of this study is the lack of a control group performing only emotion regulation without contingent feedback. Therefore, we cannot exclude that some of the effects are due to the repeated exertion of cognitive control. Such investigations will be conducted in future studies. Furthermore, due to the proof-of-concept character of this study, we have not tested the transfer of the learned abilities to another situation or task. This will be part of future studies as well. Further limitations are the small number of subjects and the lack of behavioral measures, which was justified due to the main goal of a) proving feasibility and the concept of real-time neurofeedback assisted down-regulation of amygdala activity during stimulation with negative facial expressions and b) proving a training effect of repeated neurofeedback training sessions. Another limitation of this study is the lack of measures of the actual gaze direction and of physiological measures such as breath and heart rate. Prior studies have particularly shown influences of breathing on BOLD responses (Birn et al. 2009) which could have a confounding effect in our paradigm. Furthermore, measuring gaze during the task using eye-tracking techniques could ensure that participants did not influence amygdala activity by changing gaze. However, negative facial expressions, particularly of fear and anger, have been shown to activate the amygdala reliably even if presented subliminally. We have tried to overcome this problem by giving the feedback on both sides of the stimuli and by instructing the participants to focus on the centers of the faces, where furthermore the eyes are positioned as most biologically significant parts of the face (Kret et al. 2013). A marked diversion of gaze would in addition have resulted in reduced activation in the primary visual cortex and also in the left amygdala (which was both not found in the current study). In addition, we cannot completely rule out that subjects possibly changed their centre of focus away from the faces and towards the feedback stimulus. However, such processes might possibly have taken place in a similar way in the view condition. The rather quick change of the faces should furthermore have attracted the attention and visual focus back to the stimuli.
As such, this pilot study is the first combining stimulation and feedback of the amygdala with the instruction to use emotion regulation strategies to reduce amygdala activity. This approach more closely resembles emotion regulation in emotionally activating or even stressful situations than previous studies aiming at up-regulating amygdala activity (Posse et al. 2003; Zotev et al. 2011). Future studies should address aspects of “dosage” (duration and number of sessions) of rtfMRI neurofeedback and optimal integration into established therapies. Furthermore, more extensive research on the question of lateralization of amygdala activation and regulation is necessary, because the available data on lateralization of emotion processing in healthy participants and in patients with affective disorders are not compelling.
Thus, our study introduces the concept of supporting amygdala regulation during stimulation with rtfMRI neurofeedback. Our data support the further development of rtfMRI neurofeedback for improving amygdala regulation as tool for training emotion regulation in affective disorders. It could be used as add-on supporting psychotherapy particularly of affective, anxiety and emotion regulation disorders by improving, focusing, and consolidating individually effective emotion regulation strategies.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s10548-013-0331-9) contains supplementary material, which is available to authorized users.
Contributor Information
Annette Beatrix Brühl, Email: annette.bruehl@puk.zh.ch, Department for Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zürich, Lenggstrasse 31, PO-Box 1931, 8032 Zurich, Switzerland. Department of Psychiatry and Behavioural and Clinical Neuroscience Institute, University of Cambridge, Downing Site, Cambridge CB2 3EB, UK.
Sigrid Scherpiet, Department for Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zürich, Lenggstrasse 31, PO-Box 1931, 8032 Zurich, Switzerland. Department of Neuropsychology, Institute of Psychology, University of Zurich, Binzmühlestrasse, 80, Zurich, Switzerland.
James Sulzer, Department of Health Sciences and Technology, Swiss Federal Institute of Technology, ETH, Leonhardstrasse 27 B9.2, 8092 Zurich, Switzerland. Department of Mechanical Engineering, University of Texas at Austin, 204 E. Dean Keeton St, Austin, TX 78712, USA.
Philipp Stämpfli, MR-Center of the Psychiatric Hospital and the Department of Child and Adolescent Psychiatry, University of Zurich, Lenggstrasse 31, 8032 Zurich, Switzerland.
Erich Seifritz, Department for Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zürich, Lenggstrasse 31, PO-Box 1931, 8032 Zurich, Switzerland.
Uwe Herwig, Department for Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zürich, Lenggstrasse 31, PO-Box 1931, 8032 Zurich, Switzerland. Department of Psychiatry and Psychotherapy, University of Ulm, Ulm, Germany.
References
- Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Baeken C, De Raedt R, Van Schuerbeek P, Vanderhasselt MA, De Mey J, Bossuyt A, Luypaert R. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav Brain Res. 2010;214(2):450–455. doi: 10.1016/j.bbr.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47(3):1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and Habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68(5):425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A, Hyde JS. Real-time functional magnetic resonance imaging. Magn Reson Med. 1995;33(2):230–236. doi: 10.1002/mrm.1910330213. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Reinke K, Ryan L, McIsaac T, Perschler P, Schnyer D, Trouard T, Gmitro A. Cortical mechanisms for acquisition and performance of bimanual motor sequences. Neuroimage. 2003;19(4):1405–1416. doi: 10.1016/s1053-8119(03)00222-2. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. Neuroimage. 2011;54(3):2503–2513. doi: 10.1016/j.neuroimage.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Ellis HD. Recognizing faces. Br J Psychol. 1975;66(4):409–426. doi: 10.1111/j.2044-8295.1975.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24(6):581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29(6):683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, Barale F, Perez J, McGuire P, Politi PL. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci Lett. 2009;452(3):262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Goebel R. Cortex-based real-time fMRI. Neuroimage. 2001;13:S129. [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21(4):1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Hartwell KJ, Canterberry M, Li X, Owens M, LeMatty T, Prisciandaro JJ, Borckardt J, Brady KT, George MS. Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry Res. 2013;213(1):79–81. doi: 10.1016/j.pscychresns.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingh CJ, Ipser J, Tromp S, Syal S, Lochner C, Brooks SJ, Stein DJ. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front Human Neurosci. 2013 doi: 10.3389/fnhum.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, Brühl A, Kottlow M, Schreiter-Gasser U, Abler B, Jäncke L, Rufer M. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007;37(2):652–662. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Jäncke L, Brühl AB. Self-related awareness and emotion regulation. Neuroimage. 2010;50(2):734–741. doi: 10.1016/j.neuroimage.2009.12.089. [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49(1):1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Johnston S, Linden DE, Healy D, Goebel R, Habes I, Boehm SG. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn Affect Behav Neurosci. 2011;11(1):44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21(6):1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y, Rosa MJ, Robineau F, Heinen K, Rieger S, Weiskopf N, Vuilleumier P, Van De Ville D, Scharnowski F. Connectivity-based neurofeedback: dynamic causal modeling for real-time fMRI. Neuroimage. 2013;81:422–430. doi: 10.1016/j.neuroimage.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret M, Stekelenburg J, Roelofs K, De Gelder B. Perception of face and body expressions using electromyography, pupillometry and gaze measures. Front Psychol. 2013 doi: 10.3389/fpsyg.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Center for Research in Psychophysiology. University of Florida; Gainesville: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lee J-H, Kim J, Yoo S-S. Real-time fMRI-based neurofeedback reinforces causality of attention networks. Neurosci Res. 2012;72(4):347–354. doi: 10.1016/j.neures.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, LeMatty T, Brady KT, George MS. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol. 2012;18(4):739–748. doi: 10.1111/j.1369-1600.2012.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7(6):e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X-H, Chen W. Baseline BOLD correlation predicts individuals stimulus-evoked BOLD responses. Neuroimage. 2011;54(3):2278–2286. doi: 10.1016/j.neuroimage.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The karolinska directed emotional faces (KDEF) Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; Stockholm: 1998. [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Morris JS, deBonis M, Dolan RJ. Human amygdala responses to fearful eyes. Neuroimage. 2002;17(1):214–222. doi: 10.1006/nimg.2002.1220. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18:760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Quide Y, Witteveen AB, El-Hage W, Veltman DJ, Olff M. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neurosci Biobehav Rev. 2012;36(1):626–644. doi: 10.1016/j.neubiorev.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, du Plessis S, Ramsey NF, Weusten JMH, Vink M. Test-retest variability underlying fMRI measurements. Neuroimage. 2012;60(1):717–727. doi: 10.1016/j.neuroimage.2011.11.061. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Buyukturkoglu K, Rana M, Birbaumer N, Sitaram R. Real-time fMRI brain computer interfaces: self-regulation of single brain regions to networks. Biol Psychol. 2013 doi: 10.1016/j.biopsycho.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140(2):140–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Bohus M, Esposito F, Treede RD, Di Salle F, Greffrath W, Ludaescher P, Jochims A, Lieb K, Scheffler K, Hennig J, Seifritz E. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63(6):659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sitaram R, Lee S, Ruiz S, Rana M, Veit R, Birbaumer N. Real-time support vector classification and feedback of multiple emotional brain states. Neuroimage. 2011;56(2):753–765. doi: 10.1016/j.neuroimage.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Sulzer J, Haller S, Scharnowski F, Weiskopf N, Birbaumer N, Blefari ML, Bruehl AB, Cohen LG, Decharms RC, Gassert R, Goebel R, Herwig U, Laconte S, Linden D, Luft A, Seifritz E, Sitaram R. Real-time fMRI neurofeedback: progress and challenges. Neuroimage. 2013a doi: 10.1016/j.neuroimage.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer J, Sitaram R, Blefari ML, Kollias S, Birbaumer N, Stephan KE, Luft A, Gassert R. Neurofeedback-mediated self-regulation of the dopaminergic midbrain. Neuroimage. 2013b;75C:176–184. doi: 10.1016/j.neuroimage.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. World Medical Association; Ferney-Voltaire: 2008. [Google Scholar]
- Zotev V, Krueger F, Phillips R, Alvarez RP, Simmons WK, Bellgowan P, Drevets WC, Bodurka J. Self-regulation of amygdala activation using real-time fMRI neurofeedback. PLoS One. 2011;6(9):e24522. doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.