Abstract
Aims
Characterize longitudinal patterns of drug use careers and identify determinants of drug use frequency across cohorts of primary heroin, methamphetamine (MA) and cocaine users.
Design
Pooled analysis of prospective cohort studies.
Settings
Illicit drug users recruited from community, criminal justice and drug treatment settings in California, USA.
Participants
We used longitudinal data on from five observational cohort studies featuring primary users of heroin (N=629), cocaine (N=694) and methamphetamine (N=474). The mean duration of follow-up was 20.9 years.
Measurements
Monthly longitudinal data was arranged according to five health states (incarceration, drug treatment, abstinence, non-daily and daily use). We fitted proportional hazards (PH) frailty models to determine independent differences in successive episode durations. We then executed multi-state Markov (MSM) models to estimate probabilities of transitioning between health states, and the determinants of these transitions.
Findings
Across primary drug use types, PH frailty models demonstrated durations of daily use diminished in successive episodes over time. MSM models revealed primary stimulant users had more erratic longitudinal patterns of drug use, transitioning more rapidly between periods of treatment, abstinence, non-daily and daily use. MA users exhibited relatively longer durations of high-frequency use. Criminal engagement had a destabilizing effect on health state durations across drug types. Longer incarceration histories were associated with delayed transitions towards cessation.
Conclusions
PH frailty and MSM modeling techniques provided complementary information on longitudinal patterns of drug abuse. This information can inform clinical practice and policy, and otherwise be used in health economic simulation models, designed to inform resource allocation decisions.
Keywords: drug use careers, longitudinal, health state transitions, proportional hazards frailty, multi-state Markov
1. INTRODUCTION
Drug dependence has been characterized as a chronic relapsing disorder (McLellan, 2002, 2000). Longitudinal studies have characterized drug users as following a recurrent pattern of frequent use, treatment, abstinence and relapse that are of varying duration and acuity (Brecht et al., 2008; Boeri et al., 2011; Dennis et al., 2005; Galai et al., 2003; Genberg et al., 2011; Grella and Lovinger, 2011; Hser et al., 2008; Juon et al., 2011; Scott et al., 2005). Few opioid users are able to abstain from illicit drug use for sustained periods (Bell et al., 2006; Bovasso and Cacciola, 2003; Dobler-Mikola et al., 2005; Galai et al., 2003; Termorshuizen et al., 2005). Instead, switching between periods of treatment and relapse is evident, with relapse typically occurring within five or fewer years of drug use cessation (Termorshuizen et al., 2005).
Trajectories of drug use progression and outcome vary by primary drug of abuse. Prior research has revealed that episodes of high frequency use and incarceration occur more frequently among users of heroin and methamphetamine than cocaine, and patterns of persistent high frequency use occur more frequently for heroin use relative to cocaine or methamphetamine (MA) use (Hser et al., 2008a, 2008b). These prior longitudinal analyses used finite mixture modeling to provide qualitative descriptions of latent trajectories of drug use; focused on periods in which diseases have been symptomatic; or analyzed the time to failure (adverse event, death) from drug treatment initiation, without considering the order and duration of preceding events. Given the inherent limitations of these types of analyses, the dynamic sequences of transitions between health states and their durations, particularly in light of prior events, remain unclear. Few studies have considered the overall dynamic pattern of events as they unfold over an individual's lifetime (Nosyk et al., 2009, 2013; Termorshuizen et al., 2005). A more nuanced understanding of the longitudinal patterns of drug abuse afforded by emerging statistical methods can help inform both policy and clinical practice for chronic drug users.
According to international guidelines and evidence-based standards (Sculpher et al., 2006; Weinstein et al., 2003), health economic evaluations in substance abuse have estimated costs and health outcomes over an extended duration to reflect continued availability and long-term or recurrent use of drug treatment (Nosyk et al., 2012; Zaric et al., 2000; Zarkin et al., 2005). A key input for these models is an empirical basis on the dynamics of transitions from one health state to another. Estimates can be used to develop simulation models that can ultimately provide a sound basis for resource allocation decisions.
Our objectives are to identify, by primary drug type (heroin, methamphetamine (MA), and cocaine), the probability and determinants of transitions between successive durations of drug use, treatment, and incarceration in the process towards drug use cessation. We draw upon a recurrent events framework and apply two complementary forms of analysis, Proportional Hazards (PH) frailty modeling and multi-state Markov (MSM) modeling. MSM modeling can capture inherent competing risks, however this framework cannot easily incorporate patient histories. For instance, accounting for differences in durations of successive episodes of a given health state requires explicit modeling of each successive entry into the health state (i.e., treatmentepisode 1, treatmentepisode 2, ..., treatmentepisode N), with the resulting high level of dimensionality making estimation infeasible. PH frailty modeling, on the other hand, can reveal differences in durations of successive episodes and can handle time-dependency, but it cannot adequately characterize the competing risks of transitioning to multiple different states. Contrasting these methodologies can provide complementary insights into longitudinal drug use careers and, subsequently, yield a better understanding of how these methodological strengths and limitations impact simulation modeling of disease processes.
2. METHODS
2.1 Study population
This analysis employed data on adult drug users combined from non-overlapping samples from five studies that collected longitudinal information using the Natural History Interview (described below and in Table 1). All studies were conducted in California. Following our prior work with this sample (Brecht et al., 2008; Evans et al., 2013; Hser et al., 2008a, 2008b), we selected from each study those subjects who reported a primary drug problem of heroin, cocaine, or methamphetamine use. Projects included: (1) 33-year heroin follow-up study (Hser et al., 2001); cocaine treatment evaluation (Hser et al., 2006), methamphetamine natural history study (Brecht et al., 2004), treatment process study (Hser et al., 2004), and treatment utilization and effectiveness study (Hser et al., 2003). Studies included subjects recruited from drug treatment and non-drug treatment (e.g., emergency rooms, sexually transmitted disease clinics, jails) settings. Written informed consent was obtained. Use of these data for the current analysis was reviewed and approved by the University of California Los Angeles Institutional Review Board.
Table 1.
Description of Prospective Cohort Studies from which the study populations were drawn.
| Project name | Population | % male | Mean age (SD) at baseline | Race/ethnicity | Primar y drug type | Time-points (years of data collection) | Sample size used for this analysis |
|---|---|---|---|---|---|---|---|
| Natural History of Narcotics Addiction 33 Year Follow-Up of Heroin Users (CAP) | Male opioid users treated by the California Civil Addict Program (CAP) | 100 | 25.4 (5.8) | 36.5% White; 55.6% Hispanic; 7.9% African American | Heroin | Baseline (1964) 12 years (1974) 25 years (1986) 35 years (1997) |
472 |
| A 12 Year Follow Up of a Cocaine Dependent Sample (CTE) | Male military veterans treated for cocaine dependence | 100 | 35.0 (6.4) | 24.7% White; 6.9% Hispanic; 67.5% African American; <1% Asian/other | Cocaine | Baseline (1988-1989) 1year (1989-1990) 2years (1990-1991) 12 years (2002-2003) |
319 |
| Methamphetamine Abuse: Natural History, Treatment Effect (Meth) | Methamphetamin e users recruited from from drug treatment and non-drug treatment settings | 57 | 32.6 | 47.1% White; 29.7% Hispanic; 16.6% African American; 6.6% Asian/other | Methamphetamine | Baseline (1995-1997) 3 years (1998-1999) 5.5 years (1999-2002) |
350 |
| Drug Treatment Process (TXPR) | Drug users recruited from drug treatment settings in Los Angeles County | 46 | 35.7 (9.6) | 41.4% White; 18.5% Hispanic; 32.3% African American; 7.9% Asian/other | Heroin, cocaine, methamphetamine | Baseline (1995) 1 year (1996) |
391 |
| Treatment Utilization and Effectiveness Project (TUE) | Drug users, respectively, recruited from drug treatment and non-drug treatment settings in Los Angeles County | 65 | 32.2 (8.9) | 19.4% White; 25.6% Hispanic; 51.8% African American; 3.2% Asian/other | Heroin, cocaine, methamphetamine | Baseline (1993) 1year (1994) 2years (1995) 3years (1996) |
265 |
2.2 Study design
The Natural History Interview (NHI), from which the variables for this analysis were derived, was used in all five studies. The NHI was adapted from instruments designed by Nurco and colleagues (1975) and has been used with various drug-abusing populations. The NHI was designed to collect retrospective longitudinal quantitative data on drug use and related behaviors. The instrument consists of “static” and “dynamic” forms that permit the capture of longitudinal, sequential data on drug use, employment, criminal involvement, treatment, and other behaviors over the life course of research participants (McGlothlin et al., 1977). Using an illustrated time-line, the interviewee notes major life events and then identifies time periods associated with specific behaviors, with periods delineated by changes in behavior. These reported data are translated to longitudinal data of behaviors for each month. The NHI has been shown to have generally high reliability; correlation coefficients of inter-variable relationships, based on 46 variables measured at two interviews 10 years apart, ranged as high as 0.86 and 0.90 (Anglin et al., 1993; Chou et al., 1996; Hser et al., 1992). A comparison of drug use obtained using Addiction Severity Index (ASI) and NHI data showed good correspondence, revealing that the temporal pattern of the trajectories of days of use assessed by the ASI and NHI are comparable for alcohol, heroin, cocaine, methamphetamine, and marijuana use (Murphy et al., 2010).
This study uses self-reported monthly NHI data to construct the durations of these five mutually exclusive health states observed over a drug use career: incarceration; drug treatment; abstinence (0 days of primary drug use within a given month); non-daily use (1-27 days); and daily use (≥28 days). Episodes were defined as consecutive months of incarceration, treatment, and self-reported drug use and abstinence (within the defined strata). Data were structured such that it was possible to detect whether self-reported drug use occurred during episodes of treatment and incarceration, and treatment during incarceration. In such cases, attribution into the incarceration state took primary precedence, followed by treatment. We've modeled incarceration as a health state in this context as incarceration increases the likelihood of severe adverse health consequences (Schnittker and John, 2007) and it requires distinction in the context of health economic models.
Covariates were selected from NHI data, which were then organized into sets of: (i) fixed covariates (gender, age at first use, race/ethnicity, educational attainment, marital status); (ii) incident covariates (employment, crime, incarceration, treatment, drug use, polydrug use), indicating status in the 30 days prior to initiation of a given episode; and (iii) cumulative covariates (crime, incarceration, treatment, polydrug use, time from intake to episode start, age at episode start), indicating status of a covariate over the duration of time from drug use initiation to episode initiation. Continuous variables were categorized or dichotomized according to their empirical distributions.
Informed by the literature on the cumulative effect of drug treatment on drug use patterns (Evans et al., 2013; Hser et al., 2006; Li et al., 2010; Scott et al., 2005), two additional covariates of interest were constructed. First, indicator variables for 1st to ≥5th episode attempts were derived following construction of the repeated-measures dataset. Second, we initially considered an indicator variable for drug treatment (primarily methadone maintenance or detoxification, but also including residential forms of treatment) in the 30 days prior to abstinence episode initiation in preliminary analyses, then expanded our approach to consider the duration of treatment prior to abstinence episode initiation in order to compare the effectiveness of shorter (<6 months) versus longer treatment (≥6months).
2.3 Statistical analysis
Our analysis proceeds in two steps. First, Cox proportional hazards (PH) frailty models were fitted to identify determinants of durations of each of the defined states across multiple episodes (Cook and Lawless, 2007; Sargent, 1998; Vaida and Xu, 2000). Like standard PH applications, the outcome is the bivariate pair (duration, censorship), and like other mixed effects modeling applications with longitudinal data, the PH frailty model captures the correlation in episode lengths within an individual; conditional on the frailty terms, the episode lengths are independent (Vaida and Xu, 2000). The unobserved random effect, or frailty, is assumed to follow a gamma distribution. Conceptually, these terms represent covariates capturing time-invariant unmeasured confounding (Raudenbush and Bryk, 2002). The proportional hazards assumption was tested for each covariate using the weighted residuals score test, (Grambsch and Therneau, 1994) and by inspecting Schoenfeld residual plots (Grambsch and Therneau, 1994). Hazard ratios > 1 indicated faster time to discontinuation, or shorter episodes, compared to the referent group.
Second, a parametric continuous-time, MSM model (Jackson, 2011; R Development Core Team, 2012) was implemented to estimate the impact of the selected covariates on transitions between states, and estimate transition probabilities between states over time. In this model, a covariate is assumed to affect the baseline intensity by a proportional (constant over time) factor, so that a model with ten transitions requires ten different regression coefficients to be estimated for each covariate. The effects of the different covariates (fixed and time-varying) were assumed to be multiplicative and constant over time, both assumptions being consistent with the conventional proportional hazards model. All baseline intensities and regression coefficients were simultaneously estimated via maximum likelihood estimation.
We chose a common set of covariates across modeling strategies and primary drug use types, with the exception of the episode count variables, as well as prior health state indicators (such as treatment and incarceration), which could not be incorporated in the MSM framework. Covariates were ultimately included if they had a statistically significant effect on the outcome in any primary drug use category (initially at an alpha level of 0.10). Covariates were selected iteratively, first fitting models by variable class ((i)-(iii)), then combining reduced sets of covariates from each class into a final regression model incorporating covariates from each class. For each covariate, the model produced 20 ((5 health states) × (4 possible transitions) = 20) adjusted hazard ratios, for a total of 180 adjusted hazard ratios ((9 covariates) × (20 estimated effects)) for each drug use type. For all hypotheses tested, a significance level of α=0.05 was used. Analyses were conducted using SAS (Statistical Analysis Software, Cary NC, USA) version 9.2 and R (Lucent technologies, Murray Hill NJ, USA) version 2.5.1.
3. RESULTS
From the total sample of 1797 individuals, 629 (35.0%) primarily used heroin, 694 (38.6%) cocaine, and 474 (26.4%) MA. The mean duration of follow-up was 29.3 (SD: 10.9) years for heroin users, 14.8 (7.4) years for MA users, and 17.7 (7.6) years for cocaine users.
Shown in Table 2, heroin users were primarily Hispanic unmarried males with low educational attainment; onset of arrest and drug use occurred, on average, at age 15.5 and 18.9, respectively; about half (55%) had used drugs other than heroin, many had been criminally active (82.4%), and most had been employed, but individuals spent relatively little of the follow-up time-period engaged in each of these activities (polydrug use:18.1%, crime: 23.2%, employment: 29.3% of follow-up time-period, respectively).
Table 2.
Study subject characteristics
| Primary Heroin Users | Primary MA Users | Primary Cocaine Users | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| N | 629 | 474 | 694 | |||
| Female | 67 | 10.7 | 217 | 45.8 | 204 | 29.4 |
| Age at first use [Mean (SD)] | 18.9 | 4.7 | 19.6 | 5.5 | 23.0 | 6.8 |
| Age at first arrest [Mean (SD)] | 15.5 | 4.3 | 19.1 | 6.8 | 20.2 | 7.1 |
| Race: Black | 58 | 9.2 | 62 | 13.1 | 457 | 66.0 |
| Hispanic | 336 | 53.4 | 126 | 26.6 | 75 | 10.8 |
| White | 223 | 35.5 | 255 | 53.8 | 138 | 19.9 |
| Other | 12 | 1.9 | 31 | 6.5 | 22 | 3.2 |
| Education: College | 116 | 19.7 | 167 | 35.2 | 332 | 47.9 |
| High School/GED | 195 | 33.1 | 151 | 31.9 | 204 | 29.4 |
| Less than High School | 279 | 47.3 | 156 | 32.9 | 157 | 22.7 |
| Marital Status: Married | 154 | 25.8 | 26 | 5.5 | 153 | 22.1 |
| Divorced/Separated | 335 | 56.2 | 431 | 90.9 | 350 | 50.5 |
| Single | 107 | 18.0 | 17 | 3.6 | 190 | 27.4 |
| Polydrug Use: Ever | 349 | 55.5 | 365 | 77.0 | 265 | 38.2 |
| Months of follow-up of polydrug use [Mean (SD)] | 18.1 | 20.0 | 21.5 | 21.4 | 18.2 | 21.0 |
| Crime: Ever | 518 | 82.4 | 332 | 70.2 | 349 | 50.3 |
| Months of follow-up criminally active [Mean (SD)] | 23.2 | 17.5 | 34.8 | 25.4 | 23.7 | 22.5 |
| Employed: Ever | 598 | 95.1 | 433 | 91.4 | 622 | 89.6 |
| Months of follow-up employed [Mean (SD)] | 51.2 | 26.9 | 44.0 | 28.7 | 60.4 | 32.6 |
| Duration of Follow-up (years) [Mean (SD)] | 29.3 | 10.9 | 14.8 | 7.4 | 17.7 | 7.6 |
| Study: CAP | 472 | 75.0 | 0 | 0.0 | 0 | 0.0 |
| CTE | 0 | 0.0 | 0 | 0.0 | 319 | 46.0 |
| METH | 0 | 0.0 | 350 | 73.8 | 0 | 0.0 |
| TUE | 42 | 6.7 | 30 | 6.3 | 193 | 27.8 |
| TXPR | 115 | 18.3 | 94 | 19.8 | 182 | 26.2 |
CAP: Civil Addict Program; CTE: cocaine treatment evaluation; METH: methamphetamine natural history study; TUE: treatment utilization and effectiveness study; TXPR: treatment process study.
In contrast, MA users were primarily white and unmarried, with a high school or college education; onset of arrest and drug use occurred at about the same age (19.1, 19.6); many had used drugs other than MA (77.0%) and been criminally active (70.2%), and most had been employed at some point during follow-up. Most cocaine users were African American unmarried males with a high school or college education; onset of first arrest (among those arrested) and drug use occurred at age 20.2 and 23.0, respectively; some had used drugs other than cocaine (38.2%) and half had been criminally active (50.3%).
3.1 Median episode durations
Summary statistics on duration of health state episodes by drug type (Table 3) showed that abstinence episodes were least common (1424 of 13,862 episodes) but longest in duration (median 12 vs. 7 months) among heroin users compared to MA and cocaine users, who also had the longest duration of incarceration episodes (median 6 vs. 4 months). In comparison, MA and cocaine users (hereafter called stimulant users when patterns are similar) had longer episodes of daily use compared to heroin users (median 9 vs. 7 months) and shorter episodes of treatment (median 3 vs. 10 months).
Table 3.
Summary statistics on health state episode durations
| Episode Duration (months) | Censored | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | Median | Q1 | Q3 | N | % | |
| Primary Heroin Users [N=629] | |||||||
| Incarceration | 3422 | 11.6 | 6 | 3 | 13 | 95 | 3.0 |
| Treatment | 2546 | 16.5 | 10 | 3 | 18 | 169 | 7.0 |
| Abstinence | 1424 | 37.1 | 12 | 4 | 35 | 243 | 17.0 |
| Non-Daily Use | 2268 | 14.7 | 8 | 3 | 18 | 65 | 3.0 |
| Daily Use | 4202 | 12.7 | 7 | 3 | 15 | 57 | 1.0 |
| All Episodes | 13862 | 15.9 | 8 | 3 | 17 | 629 | 4.5 |
| Primary Methamphetamine Users [N=474] | |||||||
| Incarceration | 1101 | 7.6 | 4 | 2 | 9 | 65 | 6.0 |
| Treatment | 880 | 5.1 | 3 | 2 | 6 | 37 | 4.0 |
| Abstinence | 1613 | 18.0 | 7 | 3 | 20 | 283 | 18.0 |
| Non-Daily Use | 1260 | 15.1 | 6 | 1.5 | 18.5 | 72 | 6.0 |
| Daily Use | 1346 | 17.3 | 9 | 4 | 21 | 17 | 1.0 |
| All Episodes | 6200 | 13.6 | 6 | 2 | 14 | 474 | 7.6 |
| Primary Cocaine Users [N=694] | |||||||
| Incarceration | 1073 | 7.8 | 4 | 2 | 10 | 64 | 6.0 |
| Treatment | 1297 | 5.8 | 3 | 1 | 7 | 80 | 6.0 |
| Abstinence | 2759 | 21.3 | 7 | 3 | 21 | 432 | 16.0 |
| Non-Daily Use | 2724 | 15.7 | 6 | 1 | 18 | 88 | 3.0 |
| Daily Use | 1677 | 18.1 | 9 | 4 | 21 | 30 | 2.0 |
| All Episodes | 9530 | 15.5 | 6 | 2 | 16 | 694 | 7.3 |
Q1 indicates the lower (first) quartile and Q3 the upper (third) quartile.
3.2 Changes in duration across successive episodes
Selected results of the PH frailty analysis were presented in Table 4. Hazard ratios > 1 indicated faster time to discontinuation, or shorter episodes, compared to the referent group and hazard ratios < 1 indicated slower time to discontinuation, or longer episodes, compared to the referent group. Consistent with standard legislative and judicial processes, episodes of incarceration tended to increase in duration in subsequent visits for each drug type cohort, with the exception of ≥5th incarceration episodes for heroin users – a result due to small sample sizes for these strata. The increases in incarceration durations were substantially longer for stimulant users in comparison to heroin users. Further, episodes of daily use tended to decrease in duration across successive episodes for each primary drug use cohort. This was most pronounced among MA users, with the 2nd daily use episode (HR: 1.49 (1.25,1.79)), and the ≥6th episode (2.41 (1.84, 3.16)). No clear pattern emerged across primary drug use cohorts in durations of episodes of treatment, abstinence, and non-daily use and, in many cases, duration did not change in successive episodes.
Table 4.
Results of Multivariate Cox Proportional Hazards Frailty Models
| Primary Heroin Users | Primary MA Users | Primary Cocaine Users | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AHR | 95% CI | AHR | 95% CI | AHR | 95% CI | ||||
| Outcome: Incarceration Episode Duration | |||||||||
| Episode 1 | 1 | 1 | 1 | ||||||
| Episode 2 | 0.87 | 0.76 | 0.99 | 0.73 | 0.59 | 0.91 | 0.81 | 0.67 | 0.99 |
| Episode 3 | 0.80 | 0.69 | 0.93 | 0.55 | 0.43 | 0.71 | 0.62 | 0.49 | 0.79 |
| Episode 4 | 0.82 | 0.69 | 0.96 | 0.49 | 0.37 | 0.64 | 0.47 | 0.36 | 0.62 |
| Episode 5 | 0.87 | 0.73 | 1.04 | 0.40 | 0.29 | 0.55 | 0.52 | 0.37 | 0.72 |
| Episode ≥6 | 0.87 | 0.74 | 1.03 | 0.32 | 0.24 | 0.43 | 0.41 | 0.30 | 0.56 |
| Outcome: Treatment Episode Duration | |||||||||
| Episode 1 | 1 | 1 | 1 | ||||||
| Episode 2 | 1.14 | 1.00 | 1.30 | 0.86 | 0.72 | 1.02 | 0.73 | 0.62 | 0.85 |
| Episode 3 | 1.32 | 1.14 | 1.55 | 0.76 | 0.59 | 0.97 | 0.61 | 0.50 | 0.76 |
| Episode 4 | 1.14 | 0.96 | 1.36 | 0.75 | 0.53 | 1.06 | 0.56 | 0.43 | 0.74 |
| Episode 5 | 0.95 | 0.78 | 1.14 | 0.87 | 0.55 | 1.38 | 0.80 | 0.57 | 1.12 |
| Episode ≥6 | 1.20 | 1.01 | 1.42 | 0.82 | 0.48 | 1.40 | 0.63 | 0.45 | 0.89 |
| Outcome: Abstinence Episode Duration | |||||||||
| Episode 1 | 1 | 1 | 1 | ||||||
| Episode 2 | 1.09 | 0.91 | 1.29 | 1.24 | 1.05 | 1.47 | 1.26 | 1.11 | 1.44 |
| Episode 3 | 1.08 | 0.88 | 1.34 | 1.20 | 0.98 | 1.46 | 1.23 | 1.07 | 1.43 |
| Episode 4 | 0.89 | 0.69 | 1.14 | 1.21 | 0.97 | 1.52 | 1.28 | 1.08 | 1.51 |
| Episode 5 | 0.78 | 0.57 | 1.07 | 1.74 | 1.33 | 2.27 | 1.51 | 1.25 | 1.82 |
| Episode ≥6 | 1.08 | 0.80 | 1.47 | 1.33 | 1.05 | 1.69 | 1.27 | 1.08 | 1.50 |
| Outcome: Non-Daily Use Episode Duration | |||||||||
| Episode 1 | 1 | 1 | 1 | ||||||
| Episode 2 | 0.90 | 0.77 | 1.05 | 1.01 | 0.83 | 1.22 | 0.94 | 0.83 | 1.07 |
| Episode 3 | 0.96 | 0.80 | 1.15 | 1.06 | 0.84 | 1.34 | 0.99 | 0.85 | 1.15 |
| Episode 4 | 0.98 | 0.80 | 1.21 | 1.20 | 0.91 | 1.57 | 1.34 | 1.12 | 1.60 |
| Episode 5 | 1.03 | 0.82 | 1.29 | 1.03 | 0.74 | 1.43 | 1.32 | 1.08 | 1.61 |
| Episode ≥6 | 1.01 | 0.82 | 1.25 | 1.44 | 1.02 | 2.04 | 1.37 | 1.15 | 1.64 |
| Outcome: Daily Use Episode Duration | |||||||||
| Episode 1 | 1 | 1 | 1 | ||||||
| Episode 2 | 1.12 | 0.97 | 1.28 | 1.49 | 1.25 | 1.79 | 1.05 | 0.90 | 1.23 |
| Episode 3 | 1.35 | 1.16 | 1.58 | 1.53 | 1.24 | 1.90 | 1.25 | 1.03 | 1.51 |
| Episode 4 | 1.30 | 1.09 | 1.54 | 2.16 | 1.68 | 2.77 | 1.28 | 1.02 | 1.61 |
| Episode 5 | 1.51 | 1.25 | 1.81 | 2.12 | 1.60 | 2.81 | 1.33 | 1.02 | 1.75 |
| Episode ≥6 | 1.55 | 1.30 | 1.85 | 2.41 | 1.84 | 3.16 | 1.31 | 1.01 | 1.70 |
AHR (95% CI): Adjusted Hazard Ratio (95% Confidence Interval); P: p-value. Individual regression multiple regression models executed for each health state and primary drug of abuse. All models controlling for: female gender, white race, age at initiation, crime and treatment utilization in the 30 days preceding the selected episode, measures of cumulative durations of incarceration (> or ≤25% of follow-up), polydrug use (> or ≤40% of follow-up) and crime ((> or ≤35% of follow-up), as well as an indicator of time since drug use initiation (> or ≤5 years).
3.3 Factors associated with transitions towards cessation
Selected results of the multivariate MSM analysis indicating progression towards cessation were presented in Table 5. Cumulative and incident criminal activity provided mixed results. Incident crime delayed transition from non-daily use to treatment in the primary heroin cohort by 43% (HR: 0.57 (95% CI: (0.34, 0.93)) and from non-daily use to abstinence in the primary MA cohort by 33% (HR: 0.67 (0.47, 0.95)); no statistically significant effects were observed in the primary cocaine cohort. In contrast, more cumulative criminal involvement tended to hasten transitions to treatment and abstinence, particularly among stimulant users (MA, non-daily-treatment, 86% faster time to discontinuation: 1.86 (1.28,2.69); cocaine, daily-treatment, 62% faster time to discontinuation: 1.62 (1.26,2.08)).
Table 5.
Selected results of Multi-state markov analysis: Transitions towards drug use cessation
| Primary Heroin | Primary MA | Primary Cocaine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||||
| Crime, past 30 days | |||||||||||
| Treatment | - | Abstinence | 0.83 | 0.66 | 1.04 | 1.17 | 0.93 | 1.47 | 1.19 | 0.96 | 1.47 |
| Non-daily | - | Treatment | 0.57 | 0.34 | 0.93 | 0.87 | 0.53 | 1.44 | 0.62 | 0.35 | 1.10 |
| Non-daily | - | Abstinence | 0.59 | 0.28 | 1.23 | 0.67 | 0.47 | 0.95 | 0.80 | 0.57 | 1.12 |
| Daily | - | Treatment | 1.15 | 0.96 | 1.36 | 0.84 | 0.61 | 1.15 | 0.93 | 0.66 | 1.32 |
| Daily | - | Abstinence | 1.03 | 0.64 | 1.66 | 0.81 | 0.55 | 1.18 | 1.13 | 0.73 | 1.73 |
| Daily | - | Non-daily | 1.74 | 1.02 | 2.95 | 1.54 | 0.84 | 2.81 | 1.43 | 0.81 | 2.51 |
| Cumulative Crime (>35% offollow-up) | |||||||||||
| Treatment | - | Abstinence | 0.92 | 0.74 | 1.14 | 0.83 | 0.67 | 1.03 | 0.78 | 0.62 | 0.98 |
| Non-daily | - | Treatment | 1.33 | 1.08 | 1.63 | 1.86 | 1.28 | 2.69 | 1.54 | 1.12 | 2.11 |
| Non-daily | - | Abstinence | 1.02 | 0.72 | 1.45 | 1.54 | 1.20 | 1.96 | 0.82 | 0.63 | 1.06 |
| Daily | - | Treatment | 1.03 | 0.92 | 1.16 | 1.37 | 1.06 | 1.77 | 1.62 | 1.26 | 2.08 |
| Daily | - | Abstinence | 0.81 | 0.59 | 1.11 | 1.15 | 0.85 | 1.55 | 0.88 | 0.61 | 1.27 |
| Daily | - | Non-daily | 0.81 | 0.54 | 1.22 | 1.02 | 0.59 | 1.79 | 0.69 | 0.40 | 1.19 |
| Cumulative Incarceration (>25% offollow-up) | |||||||||||
| Treatment | - | Abstinence | 1.01 | 0.82 | 1.25 | 1.14 | 0.86 | 1.51 | 0.72 | 0.47 | 1.10 |
| Non-daily | - | Treatment | 1.03 | 0.83 | 1.26 | 1.72 | 1.01 | 2.93 | 0.49 | 0.25 | 0.97 |
| Non-daily | - | Abstinence | 0.58 | 0.39 | 0.86 | 1.12 | 0.74 | 1.69 | 0.36 | 0.22 | 0.61 |
| Daily | - | Treatment | 0.90 | 0.80 | 1.01 | 1.12 | 0.77 | 1.63 | 0.60 | 0.38 | 0.96 |
| Daily | - | Abstinence | 0.81 | 0.60 | 1.11 | 1.06 | 0.65 | 1.71 | 0.66 | 0.37 | 1.16 |
| Daily | - | Non-daily | 0.64 | 0.41 | 0.99 | 0.12 | 0.02 | 1.04 | 0.61 | 0.27 | 1.41 |
| Cumulative Polydrug Use (>40% offollow-up) | |||||||||||
| Treatment | - | Abstinence | 1.81 | 1.29 | 2.55 | 1.35 | 1.10 | 1.65 | 0.77 | 0.58 | 1.02 |
| Non-daily | - | Treatment | 0.83 | 0.50 | 1.37 | 0.74 | 0.49 | 1.12 | 0.67 | 0.47 | 0.95 |
| Non-daily | - | Abstinence | 1.57 | 0.88 | 2.81 | 0.87 | 0.67 | 1.13 | 0.80 | 0.64 | 0.99 |
| Daily | - | Treatment | 1.24 | 1.05 | 1.48 | 0.95 | 0.72 | 1.24 | 0.75 | 0.54 | 1.05 |
| Daily | - | Abstinence | 0.90 | 0.55 | 1.45 | 0.73 | 0.53 | 1.02 | 0.60 | 0.39 | 0.92 |
| Daily | - | Non-daily | 1.00 | 0.57 | 1.76 | 0.68 | 0.39 | 1.20 | 0.89 | 0.54 | 1.47 |
| Time since drug use initiation (>5 years vs. <5 years) | |||||||||||
| Treatment | Abstinence | 0.82 | 0.61 | 1.09 | 0.91 | 0.73 | 1.14 | 0.64 | 0.52 | 0.79 | |
| Non-daily | - | Treatment | 3.18 | 2.36 | 429 | 1.31 | 0.89 | 1.93 | 2.08 | 1.66 | 2.61 |
| Non-daily | - | Abstinence | 0.88 | 0.55 | 1.41 | 1.10 | 0.87 | 1.39 | 1.34 | 1.18 | 1.53 |
| Daily | - | Treatment | 1.87 | 1.61 | 2.17 | 1.38 | 1.07 | 1.78 | 1.80 | 1.45 | 2.24 |
| Daily | - | Abstinence | 0.77 | 0.53 | 1.12 | 1.29 | 0.99 | 1.69 | 1.31 | 1.02 | 1.67 |
| Daily | - | Non-daily | 0.61 | 0.38 | 0.96 | 1.83 | 1.13 | 2.96 | 1.40 | 1.00 | 1.96 |
Controlling also for age at drug use initiation, gender and race (white vs. non-white), crime in the past 30 ays, cumulative durations of incarceration (> or <25% of follow-up), polydrug use (> or <40% of follow-up) and rime (> or <35% of follow-up), as well as an indicator of time since drug use initiation (> or <5 years).
More cumulative time incarcerated delayed transitions to drug use cessation in the primary heroin [from non-daily use to abstinence: delayed by 42% (0.58 (0.39,0.86)) and from daily to non-daily use: delayed by 36% (0.64 (0.41, 0.99))] and cocaine [non-daily and daily use to treatment and abstinence] cohorts.
More cumulative polydrug use hastened transitions from treatment to abstinence in the primary heroin (81% faster time from treatment to abstinence; 1.81 (1.29,2.55)) and MA cohorts (35% faster time from treatment to abstinence; 1.35 (1.10,1.65)), and transition from daily use to treatment among heroin users (24% faster time from daily use to treatment; 1.24 (1.05,1.48)); however, this factor delayed transitions to treatment and abstinence among cocaine users (non-daily-abstinence: 0.80 (0.64, 0.99); daily–abstinence: 0.60 (0.3.9, 0.92), representing 20% and 40% delays, respectively).
Finally, transitions towards cessation were accelerated among more experienced users (>5 vs. <5 years since primary drug use initiation), particularly among cocaine users, for whom five of six transitions among experienced (vs. less experienced) users towards cessation were accelerated (AHR>1).
3.4 Estimated transition probability matrices
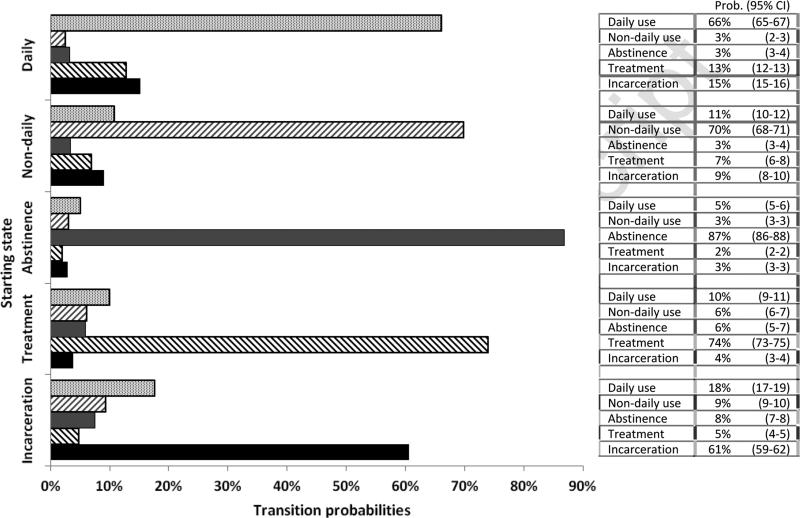
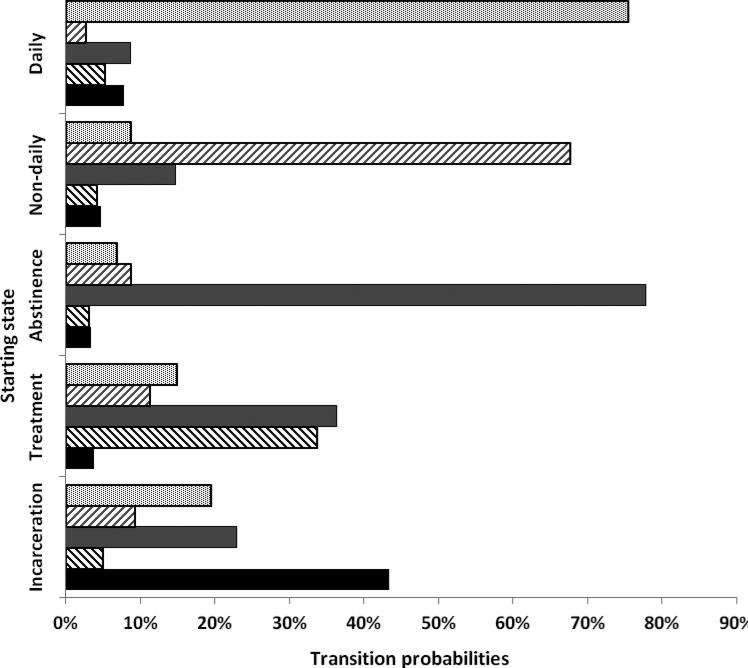
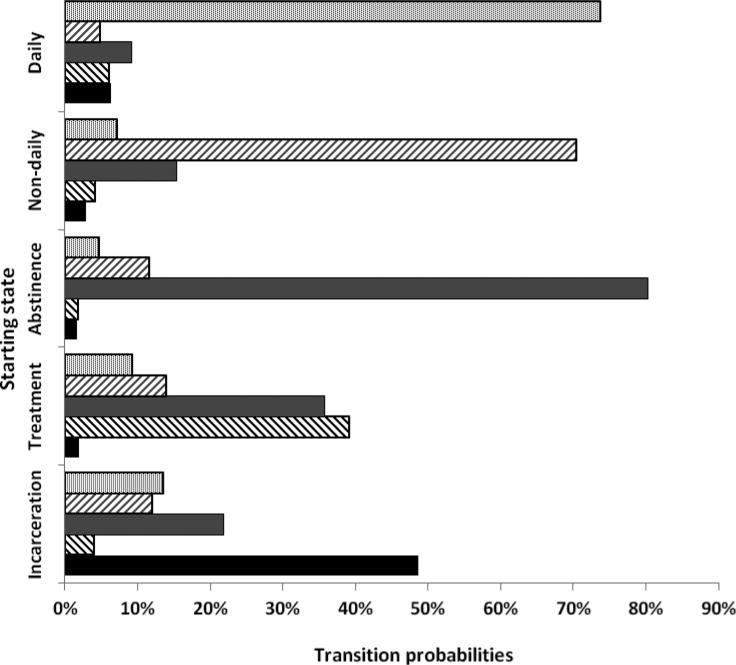
Finally, 12-month transition probability matrices for each of the primary drug use cohorts, drawn from the MSM analysis, are presented in Figure 1. Regardless of drug type, about one quarter of individuals transitioned from incarceration to either daily- or non-daily use (heroin 9.39+17.64=27.03%, MA 28.71%, cocaine 25.55%).
Figure 1.
Estimated health state transition probabilities, by primary drug of abuse
Heroin users had the highest probability of remaining abstinent at 12 months (86.91% vs. 77.83%, 80.25%) and the lowest probability of relapse into non-daily or daily use (8.15% vs. 15.63%, 16.21%). Further, the 12-month probability of remaining in treatment was nearly twice as high for heroin users compared to stimulant users (74.03% vs. 33.65%, 39.16%) and heroin users' rates of relapse to daily or non-daily use were lower ((6.13+10.07=16.20) vs. 26.19%, 23.19%). However, heroin users were half as likely as stimulant users to transition from incarceration to treatment or abstinence (4.83+7.54=12.37% vs. 27.91%, 25.82%).
4. DISCUSSION
To summarize, longitudinal patterns of transitions between health states were distinctly different between primary heroin and stimulant users. However, regardless of drug type, durations of daily use tended to diminish in successive episodes over time. Individuals who used primarily stimulants tended to have more erratic longitudinal patterns of drug use, transitioning more rapidly between periods of treatment, abstinence, non-daily use, and daily use.
Sustained cessation is typically achieved following a process characterized by periods of frequent use, treatment, abstinence and relapse that are of varying duration and acuity. Prior PH frailty analyses demonstrated successively longer durations of methadone maintenance treatment (Nosyk et al., 2013) and abstinence (Nosyk et al., 2009), and shorter durations of relapse following treatment (Nosyk et al., 2012) among opioid users. The current study expands not only the scope of analysis to consider a broader range of health states, but also provides complementary information from the Multi-state Markov analyses. Using the same dataset, the PH models have identified changes in successive durations of incarceration (increasing) and frequent drug use (decreasing), while the MSM analyses highlighted frequent transitions from incarceration to daily and non-daily use. Secondary analyses of longitudinal data using different statistical techniques can thus reveal a number of important inferences regarding individual drug use careers.
Our findings are consistent with the notion that heroin dependence is a chronic condition that is best treated by long-term care strategies (Bart, 2012; Hser et al., 2007). A key component of effective heroin dependence treatment (Vocci et al, 2005), methadone maintenance reduces risk of mortality (Degenhardt et al., 2011), as well as a range of other health benefits (Amato et al., 2005). In contrast, effective pharmacotherapy options to enhance treatment for stimulant dependence are still in development (Brackins et al., 2011; Ross and Peselow, 2009; Vocci et al., 2005). Psychosocial interventions for stimulant dependence are moderately effective (Vocci and Montoya, 2009) but challenged by poor rates of treatment induction and retention (Shearer, 2007), as observed in our study.
These findings also indicated that across drug types a significant proportion of individuals use drugs after release from incarceration and, compared to stimulant users, heroin users are least likely to achieve abstinence 12 months after incarceration. Use of medication-assisted treatments for opioid dependence during the period when incarcerated individuals reenter the community is uncommon (Friedmann et al., 2012). Arrest and incarceration are underutilized opportunities for early intervention or treatment diversion strategies (Kubiak et al., 2006). New initiatives for infectious disease control provide optimism for jail- and prison-based drug treatment interventions (Rich et al., 2011).
Our analyses provide estimates of transitions between health states that can be used directly in simulation models to support health economic evaluation. In particular, while several models have been developed to evaluate forms of treatment for opioid dependence (Nosyk et al., 2012; Zaric et al., 2000; Zarkin et al., 2005) , to date, no such models have been developed for stimulant dependence. As new pharmacological treatment modalities for stimulant dependence are tested their long-term outcomes will need to be considered to provide an adequate basis for health resource allocation decisions. The contrasting methods may be used in sensitivity analyses to test the influence of various limitations in modeling the disease processes modeled herein.
It is critical to note that the long duration between follow-up interviews (from 1-10 years) precluded our ability to model mortality explicitly, as the intervals between the last follow-up date and death were not observed. The results are therefore representative of individuals surviving beyond one or more follow-up intervals, and may misrepresent longitudinal patterns leading to mortality. A previous article modeling mortality as a function of baseline covariates revealed that 16.1% of heroin, 6.5% of cocaine and 1.5% of primary MA users suffered mortality within 30 years of drug use initiation (Liang et al., 2010). In contrast, recent meta-analyses estimated a standardized mortality ratio of 14.7 in opioid users (Degenhardt et al., 2011) and 4-8 in cocaine and amphetamine users (Balster et al., 2009; Degenhardt et al., 2011).
In applied simulation modeling studies, the standard solution to this problem is to multiply existing transition probabilities by standardized mortality ratios or relative risks of mortality for each of the given health states (Briggs et al., 2006; Nosyk et al., 2012). Nonetheless, external validation and potential subsequent refinements in these methods are necessary next steps for informed decision-making.
Our study had several additional limitations that require consideration. As noted, data came from self-reported interviews at 10-year intervals from which monthly records of the outcome and measures of exposure were constructed. The temporal ordering of events within the recurrent event process may have been influenced by rounding error and recall bias given the long duration between follow-up interviews, and the level of error likely increased as a function of time from the interview date. As noted, recall bias was minimized by using records-based anchors. We have no reason to believe the resulting bias in either case was differential, resulting in attenuation of hazard ratios towards the null hypothesis. In addition, data were provided by five different studies. Differences between estimated trajectories by primary drug type were likely influenced by the design, selection criteria and duration of follow-up of individual studies. Therefore comparisons across types, and representativeness to other heroin, cocaine and MS-using individuals should be interpreted cautiously. Further, as in any non-experimental study, ours may be subject to residual and/or unmeasured time variant confounding (Grimes and Schulz, 2002). Data on other predictors of durations of abstinence such as motivational status, and social supports were unavailable for the duration of follow-up. Though we cannot ascertain the individual effects of the unobserved factors, we can confidently state that their omission did not bias the coefficients on the existing fixed effects included in the PH frailty analysis.
Nonetheless, the cohort studies on which these analyses are based are among the longest and most comprehensive follow-up studies of heroin and stimulant users available. While we have considered individual-level factors associated with longitudinal drug use patterns, the process towards drug use cessation may also be influenced by external factors such as state legislation on drug-related crime and repeat offenders, and changes in these factors over time.
Further study is required to define the heterogeneous drug use cessation process over time and across settings, particularly in the context of current policy changes that may serve to diminish barriers to substance abuse treatment among a growing segment of the US population (Buck, 2011).
Acknowledgments
Role of Funding Source Funding for this study was provided by US National Institutes of Health, National Institute on Drug Abuse [R01DA031727-01; R01DA032551]; and for the UCLA ISAP Center for Advancing Longitudinal Drug Abuse Research (CALDAR) [P30 DA016383]; the funders had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest No conflict declared.
REFERENCES
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J. Subst. Abuse Treat. 2005;28:321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Anglin MD, Hser YI, Chou C. Reliability and validity of retrospective behavioral self-report by narcotics addicts. Eval. Rev. 1993;17:91–108. [Google Scholar]
- Balster RL, Singleton J, Degenhardt L, Hall W, Zabransky T. Mortality among amphetamine users: a systematic review of cohort studies. Drug Alcohol Depend. 2009;105:1–8. doi: 10.1016/j.drugalcdep.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J. Addict. Dis. 2012;31:207–225. doi: 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Burrell T, Indig D, Gilmour S. Cycling in and out of treatment; participation in methadone treatment in NSW, 1990-2002. Drug Alcohol Depend. 2006;81:55–61. doi: 10.1016/j.drugalcdep.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Boeri M, Whalen T, Tyndall B, Ballard E. Drug use trajectory patterns among older drug users. Subst. Abuse Rehabil. 2011;2:89–102. doi: 10.2147/SAR.S14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovasso G, Cacciola J. The long-term outcomes of drug use by methadone maintenance patients. J. Behav Health Serv. Res. 2003;30:290–303. doi: 10.1007/BF02287318. [DOI] [PubMed] [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J. Pharm. Pract. 2011;24:541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Brecht ML, Huang D, Evans E, Hser Y-I. Polydrug use and implications for longitudinal research: ten-year trajectories for heroin, cocaine, and methamphetamine users. Drug Alcohol Depend. 2008;96:193–201. doi: 10.1016/j.drugalcdep.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, O'Brien A, Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict. Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. In: Glick HA, Doshi JA, Seema SS, Polsky D, editors. Economic Evaluation in Clinical Trials. Oxford University Press; Oxford: 2006. pp. 45–76. [Google Scholar]
- Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Aff. 2011;30:1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, Inter-university consortium for Political and Social Research . National Survey of Substance Abuse Treatment Services (NSSATS): 2010. Substance Abuse and Mental Health Services Administration. Inter-university Consortium for Political and Social Research; Ann Arbor: 2011. [Google Scholar]
- Chou CP, Hser YI, Anglin MD. Pattern reliability of narcotics addicts’ self-reported data: a confirmatory assessment of construct validity and consistency. Subst. Use Misuse. 1996;31:1189–1216. doi: 10.3109/10826089609063972. [DOI] [PubMed] [Google Scholar]
- Cook RJ, Lawless JF. The Statistical Analysis of Recurrent Events. Springer; New York: 2007. [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Singleton J, Calabria B, McLaren J, Kerr T, Mehta S, Kirk G, Hall WD. Mortality among cocaine users: a systematic review of cohort studies. Drug Alcohol Depend. 2011;113:88–95. doi: 10.1016/j.drugalcdep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Scott CK, Funk R, Foss MA. The duration and correlates of addiction and treatment careers. J. Subst. Abuse Treat. 2005;28:S51–S62. doi: 10.1016/j.jsat.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Dobler-Mikola A, Hattenschwiller J, Meili D, Beck T, Boni E, Modestin J. Patterns of heroin, cocaine and alcohol abuse during long-term methadone maintenance treatment. J. Subst. Abuse Treat. 2005;29:259–265. doi: 10.1016/j.jsat.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Evans E, Li L, Grella C, Brecht ML, Hser YI. Developmental timing of first drug treatment and 10-year patterns of drug use. J. Subst Abuse Treat. 2013;44:271–279. doi: 10.1016/j.jsat.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Jr., Stein LAR, Gordon M, Schwartz R, Kinlock T, Knight K, Flynn PM, Welsh WN, Sacks S, Oconnell DJ, Knudsen HK, Shafer MS, Hall E, Frisman LK. Medication-assisted treatment in criminal justice agencies affiliated with the Criminal Justice-Drug Abuse Treatment Studies (CJ-DATS): availability, barriers, and intentions. Subst. Abuse. 2012;33:9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galai N, Safaeian M, Vlahov D, Bolotin A, Celetano DD. Longitudinal patterns of drug injection behaviour in the ALIVE study cohort, 1988-2000: description and determinants. Am. J. Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, Latkin CA, Mehta SH. The effect of neighborhood deprivation and residential relocation on long-term injection cessation among Injection Drug Users (IDUs) in Baltimore, Maryland. Addiction. 2011;106:1966–1974. doi: 10.1111/j.1360-0443.2011.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- Grella CE, Lovinger K. 30-year trajectories of heroin and other drug use among men and women sampled from methadone treatment in California. Drug Alcohol Depend. 2011;118:251–258. doi: 10.1016/j.drugalcdep.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Fountain G. Economic cost of alcohol and drug abuse in the United States, 1992: a report. Addiction. 1999;94:631–635. doi: 10.1080/09652149933450. [DOI] [PubMed] [Google Scholar]
- Hser YI, Anglin MD, Chou C. Reliability of retrospective self-report by narcotics addicts. Psychol. Assess. 1992;4:207–213. [Google Scholar]
- Hser YI, Evans E, Huang D, Brecht ML, Li L. Comparing the dynamic course of heroin, cocaine, and methamphetamine use over 10 years. Addict. Behav. 2008a;33:1581–1590. doi: 10.1016/j.addbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch. Gen. Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Hser YI, Huang D, Brecht ML, Li L, Evans E. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J. Addict. Dis. 2008b;27:13–21. doi: 10.1080/10550880802122554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Huang D, Teruya C, Anglin MD. Gender differences in drug abuse treatment outcomes and correlates. Drug Alcohol Depend. 2003;72:255–264. doi: 10.1016/j.drugalcdep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hser YI, Huang D, Teruya C, Anglin MD. Diversity of drug abuse treatment utilization patterns and outcomes. Eval. Program Plann. 2004;27:309–319. [Google Scholar]
- Hser YI, Longshore D, Anglin MD. The life course perspective on drug use: a conceptual framework for understanding drug use trajectories. Eval. Rev. 2007;31:515–547. doi: 10.1177/0193841X07307316. [DOI] [PubMed] [Google Scholar]
- Hser YI, Stark ME, Paredes A, Huang D, Anglin MD, Rawson R. A 12-year follow-up of a treated cocaine-dependent sample. J. Subst. Abuse Treat. 2006;30:219–226. doi: 10.1016/j.jsat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Jackson CH. Multi-State Models for panel data: the MSM package for R. J. Stat. Softw. 2011;38:1–28. [Google Scholar]
- Juon HS, Fothergill KE, Green KM, Doherty EE, Ensminger ME. Antecedents and consequences of marijuana use trajectories over the life course in an African American population. Drug Alcohol Depend. 2011;118:216–223. doi: 10.1016/j.drugalcdep.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak SP, Arfken CL, Swartz JA, Koch AL. Treatment at the front end of the criminal justice continuum: the association between arrest and admission into specialty substance abuse treatment. Subst. Abuse Treat. Prev. Policy. 2006;1:20. doi: 10.1186/1747-597X-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SP, Baldwin S, Lightstone AS, Gelberg L, Diamant AL. Is incarceration a contributor to health disparities? Access to care of formerly incarcerated adults. J. Community Health. 2010;35:268–274. doi: 10.1007/s10900-010-9234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Evans E, Hser YI. A marginal structural modeling approach to assess the cumulative effect of drug treatment on later drug use abstinence. J. Drug Issues. 2010;40:221–240. doi: 10.1177/002204261004000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LJ, Huang D, Brecht ML, Hser YI. Differences in mortality among heroin, cocaine and methamphetamine users: a hierarchical Bayesian approach. J. Drug Issues. 2010;40:121–140. doi: 10.1177/002204261004000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin WH, Anglin MD, Wilson BD. An evaluation of the California Civil Addict Program. National Institute on Drug Abuse; U.S. Government Printing Office; Rockville: Washington DC: 1977. Services Research Monograph Series. DHEW Publication No. (ADM) 78-558. [Google Scholar]
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction. 2002;97:249–252. doi: 10.1046/j.1360-0443.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Murphy D, Hser Y, Huang D, Brecht L, Herbeck DM. Self-report of longitudinal substance use: a comparison of the UCLA Natural History Interview and the Addiction Severity Index. J. Drug Issues. 2010;40:495–516. doi: 10.1177/002204261004000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Anglin MD, Brecht ML, Lima VD, Hser YI. Characterizing durations of heroin abstinence in California Civil Addict Program: results from a 33-year observational cohort study. Am. J. Epidemiol. 2013;177:675–682. doi: 10.1093/aje/kws284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Guh DP, Bansback NJ, Oviedo-Joekes E, Brissette S, Marsh DC, Meikleham E, Schechter MT, Anis AH. Cost-effectiveness of diacetylmorphine versus methadone for chronic opioid dependence refractory to treatment. CMAJ. 2012;184:E317–E328. doi: 10.1503/cmaj.110669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, MacNab YC, Sun H, Fischer B, Marsh DC, Schechter MT, Anis AH. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am. J. Epidemiol. 2009;170:783–792. doi: 10.1093/aje/kwp186. [DOI] [PubMed] [Google Scholar]
- Nurco DN, Bonito AJ, Lerner M, Balter MB. Studying addicts over time: methodology and preliminary findings. Am. J. Drug Alcohol Abuse. 1975;2:183–196. doi: 10.3109/00952997509002733. [DOI] [PubMed] [Google Scholar]
- Office of the National Drug Control Policy . Predicting Heavy Drug Use: Results of Longitudinal Study, Youth Characteristics Describing and Predicting Heavy Drug Use by Adults. Executive Office of the President; Washington, DC: 2004. Publication No. 207303. [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2012. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage; Newbury Park: 2002. [Google Scholar]
- Rich JD, Wohl DA, Beckwith CG, Spaulding AC, Lepp NE, Baillargeon J, Gardner A, Avery A, Altice FL, Springer S. HIV-related research in correctional populations: now is the time. Centers for AIDS Research—Collaboration on HIV in Corrections Working Group. Curr. HIV/AIDS Rep. 2011;8:288–96. doi: 10.1007/s11904-011-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Peselow E. Pharmacotherapy of addictive disorders. Clin. Neuropharmacol. 2009;32:277–289. doi: 10.1097/wnf.0b013e3181a91655. [DOI] [PubMed] [Google Scholar]
- Sargent DJ. A general framework for random effects survival analysis in the cox proportional hazards setting. Biometrics. 1998;54:1486–1497. [PubMed] [Google Scholar]
- Schnittker J, John A. Enduring stigma: the long-term effects of incarceration on health. J. Health Soc. Behav. 2007;48:115–130. doi: 10.1177/002214650704800202. [DOI] [PubMed] [Google Scholar]
- Scott CK, Foss MA, Dennis ML. Pathways in the relapse—treatment--recovery cycle over 3 years. J. Subst. Abuse Treat. 2005;28:S63–S72. doi: 10.1016/j.jsat.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making? Health Econ. 2006;15:677–687. doi: 10.1002/hec.1093. [DOI] [PubMed] [Google Scholar]
- Shearer J. Psychosocial approaches to psychostimulant dependence: a systematic review. J. Subst. Abuse Treat. 2007;32:41–52. doi: 10.1016/j.jsat.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Termorshuizen F, Krol A, Prins M, Geskus R, van den Brink W, van Ameijden EJ. Prediction of relapse to frequent heroin use and the role of methadone prescription: an analysis of the Amsterdam Cohort Study among drug users. Drug Alcohol Depend. 2005;79:231–240. doi: 10.1016/j.drugalcdep.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Termorshuizen F, Krol A, Prins M, van Ameijden EJ. Long-term outcome of chronic drug use: the Amsterdam Cohort Study among drug users. Am. J. Epidemiol. 2005;161:271–279. doi: 10.1093/aje/kwi035. [DOI] [PubMed] [Google Scholar]
- Vaida F, Xu R. Proportional hazards model with random effects. Stat. Med. 2000;19:3309–3324. doi: 10.1002/1097-0258(20001230)19:24<3309::aid-sim825>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am. J. Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr. Opin. Psychiatry. 2009;22:263–268. doi: 10.1097/YCO.0b013e32832a3b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EA, Green J. Incarceration as a key variable in racial disparities of asthma prevalence. BMC Public Health. 2010;10:290. doi: 10.1186/1471-2458-10-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein MC, ISPOR Task Force on Good Research Practices--Modeling Studies. O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--Modeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am. J. Public Health. 2000;90:1100–1111. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkin GA, Dunlap LJ, Hicks KA, Mamo D. Benefits and costs of methadone treatment: results from a lifetime simulation model. Health Econ. 2005;14:1133–1150. doi: 10.1002/hec.999. [DOI] [PubMed] [Google Scholar]