Abstract
BACKGROUND
The acute coagulopathy of trauma is present in up to one third of patients by the time of admission, and the recent CRASH-2 and MATTERs trials have focused worldwide attention on hyperfibrinolysis as a component of acute coagulopathy of trauma. Thromboelastography (TEG) is a powerful tool for analyzing fibrinolyis, but a clinically relevant threshold for defining hyperfibrinolysis has yet to be determined. Recent data suggest that the accepted normal upper bound of 7.5% for 30-minute fibrinolysis (LY30) by TEG is inappropriate in severe trauma, as the risk of death rises at much lower levels of clot lysis. We wished to determine the validity of this hypothesis and establish a threshold value to treat fibrinolysis, based on prediction of massive transfusion requirement and risk of mortality.
METHODS
Patients with uncontrolled hemorrhage, meeting the massive transfusion protocol (MTP) criteria at admission (n = 73), represent the most severely injured trauma population at our center (median Injury Severity Score [ISS], 30; interquartile range, 20–38). Citrated kaolin TEG was performed at admission blood samples from this population, stratified by LY30, and evaluated for transfusion requirement and 28-day mortality. The same analysis was conducted on available field blood samples from all non-MTP trauma patients (n = 216) in the same period. These represent the general trauma population.
RESULTS
Within the MTP-activating population, the cohort of patients with LY30 of 3% or greater was shown to be at much higher risk for requiring a massive transfusion (90.9% vs. 30.5%, p = 0.0008) and dying of hemorrhage (45.5% vs. 4.8%, p = 0.0014) than those with LY30 less than 3%. Similar trends were seen in the general trauma population.
CONCLUSION
LY30 of 3% or greater defines clinically relevant hyperfibrinolysis and strongly predicts the requirement for massive transfusion and an increased risk of mortality in trauma patients presenting with uncontrolled hemorrhage. This threshold value for LY30 represents a critical indication for the treatment of fibrinolysis.
Keywords: Thromboelastography, fibrinolysis, mortality, trauma, massive transfusion
The acute coagulopathy of trauma (ACOT) is established in up to one third of patients by the time they reach the hospital.1–5 Two thirds of patients who die of hemorrhage will do so within the first 6 hours after injury, and up to 80% of the blood products transfused will be given with these first 6 hours as well.6,7 Thus, an urgent need exists for the rapid diagnosis and treatment of ACOT. The development and widespread adoption of point-of-care viscoelastic coagulation assays, for example, thromboelastography (TEG) and rotational thromboelastometry (ROTEM), have answered this need and opened new doors both scientifically and clinically for the study of ACOT.8–10 ACOT is multifactorial, and we now have the analytic tools to untangle the multiple factors contributing to an individual patient’s coagulopathy.
Clinical application of these assays has now identified hyperfibrinolysis as a significant contributor to ACOT.2,11,12 Moreover, the recent CRASH-2 and MATTERs trials have shown the spotlight on hyperfibrinolysis and focused worldwide attention on the presumptive treatment of coagulopathic bleeding with antifibrinolytic agents.13–16 However, a scientific basis for defining a threshold for instituting therapy is lacking.17 Clot lysis at 30 minutes after maximum clot strength (LY30) is the standard measure of fibrinolysis by TEG. LY30 offers a rapid measure of fibrinolysis and, as a predictor of this critical component of ACOT, may be a vital early prognostic indicator for the risk of massive hemorrhage. However, it is unclear whether the accepted normal upper bound of LY30 (7.5%, as stated in the TEG instrument reference range documentation, Haemonetics, Niles, IL) is appropriate for guiding therapy in trauma.18,19 Recent data suggest that the risk of death rises at a much lower level of clot lysis than 7.5%, but more detailed investigation is required to establish the utility of TEG LY30 as a test for hyperfibrinolysis and how to use this tool to guide antifibrinolytic therapy.17,19,20
As one of the objectives of a prospective study to characterize ACOT, we sought to determine the clinically relevant threshold for fibrinolysis in the population of severely injured patients who prompt the activation of our massive transfusion protocol (MTP). Specifically, we hypothesized that even small elevations of LY30 would predict massive transfusion requirement and hemorrhage-related death and should therefore serve as a trigger for antifibrinolytic therapy.11,21–26
PATIENTS AND METHODS
Study Population
Consecutive trauma patients (n = 83) admitted to Denver Health Medical Center (DHMC) and warranting activation of our MTP were enrolled from September 2010 to December 2012, under COMIRB protocol number 10-0477. Inclusion criteria were age greater than 18 years, admitted with blunt or penetrating trauma sustained less than 6 hours before admission, and deemed likely to require a massive transfusion by the clinical judgment of the attending trauma surgeon. Exclusion criteria were vulnerable populations (e.g., prisoners), pregnancy, and patients with preexisting coagulation disorders. Of these 83 enrollees, admission blood samples were obtained on 73 patients. The missing samples were at random, owing to the lack of availability of research personnel. Demographically, these patients were similar to our general trauma (GT) population. Mean age was 43.0 ± 16.3 years, and 68% were male, with 84% identifying as white, 16% as African American, and 40% as Hispanic ethnicity. There were 51 blunt (70%) and 22 penetrating injuries, with a median Injury Severity Score (ISS) of 30 (interquartile range [IQR], 20–38), and 46 patients with an admission arterial blood gas data had a median base deficit of −11.5 (IQR, −16 to −9). All patients received a blood transfusion. In addition, a convenience sample of field-collected blood from nonselective, GT patients (n = 216) was available under the same protocol. Owing to our very short average ground transport times (6–8 minutes), the time differential between field and admission samples was considered negligible for the purposes of comparison.
Viscolelastic Clotting Assays
Blood was collected from MTP patients in 2.7-mL buffered sodium citrate (3.2%) sample tubes (Vacutainer, Becton-Dickinson, Franklin Lakes, NJ). Samples were run within one half to 1 hour of collection. Citrated kaolin thromboelastography (CK-TEG) assays were recalcified and run according to the manufacturer’s instructions on a TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics).
Data Analysis
CK-TEG LY30 data were initially stratified according to the known performance characteristics of the assay. Detectable fibrinolysis was defined as any LY30 greater than 0%. Our previous healthy controls have demonstrated that an LY30 of up to 3% may exist in normal individuals. Moreover, this effect may be an artifact caused by platelet contraction because it is not observed in Functional Fibrinogen TEG, which contains a platelet inhibitor. Therefore, LY30 of 3% or greater was set as the lower boundary for the next cohort. The TEG instrument manufacturer has established an LY30 of 7.5% as the upper bound of the reference range (0.0–7.4%) within the instrument software, which is based on healthy controls tested during their initial validation process (TEG instrument documentation, Haemonetics).18 Therefore, LY30 of 7.5% or greater was set as the lower boundary for the uppermost cohort. Patients were followed up for outcome until discharge or in-hospital death. Study end points for clinically significant fibrinolysis were (a) the need for massive transfusion within 6 hours and (b) 28-day mortality. Statistical analysis was conducted using SAS statistical analysis software (SAS Institute Incorporated, Cary, NC). Comparisons of binary outcomes used Fisher’s exact test. Statistical performance measures were calculated for LY30 in 1% increments.
RESULTS
Incidence of Fibrinolysis
Of the 73 available MTP-activating patients, 19 (26%) had detectable fibrinolysis (LY30 > 0%); 11 of the 73 patients (15%) had an LY30 of 3% or greater, and 5 (6.8%) had an LY30 of 7.5% or greater. Further analysis was conducted to seek predisposing factors for hyperfibrinolysis. Demographically, there were no significant differences in sex, age, race, ethnicity, body mass index, or mechanism of injury between these four stratified cohorts of LY30. Interestingly, while ISS did not correlate with LY30; the magnitude of hypotension seemed to have a relationship to hyperfibrinolysis. Specifically, in normotensive patients (n = 35), 9% had low-level fibrinolysis (LY30 >0% to <3%), 9% had moderate fibrinolysis (LY30 ≥3% to <7.5%), and none had severe fibrinolysis (LY30 ≥ 7.5%), and in moderately hypotensive patients (n = 17), with systolic blood pressure (SBP) of 90mmHg or lower and greater than 70mmHg, 24% had low-level fibrinolysis, 6% had moderate fibrinolysis, and none had severe fibrinolysis. In contrast, of 21 patients with profound hypotension (SBP ≤ 70 mm Hg), 5% had low-level fibrinolysis, 10% had moderate fibrinolysis, and 24% had severe fibrinolysis. In summary, (a) moderate-to-severe fibrinolysis (LY30 ≥ 3%) is more common in profoundly hypotensive patients than in those with moderate hypotension or normal SBP (p = 0.01), and (b) all patients who had severe fibrinolysis were profoundly hypotensive, and 80% of these had an undetectable SBP on arrival.
Hyperfibrinolysis as a Predictor of Massive Transfusion Requirement
Our next step was to determine a clinically relevant definition of hyperfibrinolysis related to requirement for a massive blood transfusion. Overall, 32 (44%) of 73 MTP-activating patients required a massive transfusion of 10 or more units of packed red blood cells (PRBCs) in 6 hours. MTP-activating patients were stratified according to CK-TEG LY30 into four cohorts, as described in the Data Analysis in the Patients and Methods section earlier: LY30 of 0% (undetectable fibrinolysis), LY30 of greater than 0% to less than 3%, LY30 of 3% or greater to less than 7.5%, and LY30 of 7.5% or greater. These cohorts were compared with respect to the massive transfusion requirement (Fig. 1). In our study population, all patients who died before 6 hours after injury still received a massive transfusion; therefore, there was no survivor bias in this analysis.
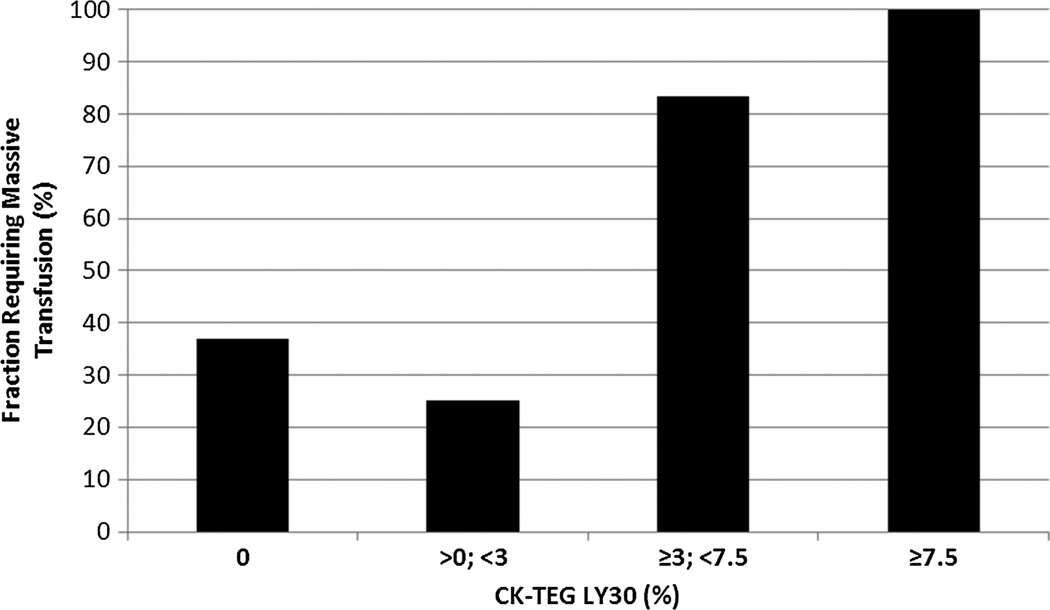
Figure 1.
Massive transfusion risk versus LY30. The risk of requiring a massive transfusion (≥10 U of PRBCs by the 6-hour postinjury time point) rises sharply above the LY30 threshold value of 3% (p = 0.0008). There is no statistically significant difference in the massive transfusion risk between the subcohorts (0% compared with >0% to <3%, p = 0.7, and ≥3% to <7.5% compared with ≥7.5%, p = 1.0).
An increased risk of requiring a massive transfusion was noted in the combined cohort of all patients with LY30 of 3% or greater (i.e., LY30 ≥3% to <7.5%, and LY30 ≥ 7.5%) compared with those with LY30 of less than 3% (90.9% vs. 30.5%, p = 0.0008). No statistically significant difference existed between the subcohorts (LY30 ≥3 to <7.5% compared with LY30 ≥ 7.5%) as seen in Figure 1. A more granular statistical analysis of massive transfusion risk at multiple thresholds of LY30 confirmed that the statistical figures of merit for LY30 as a predictive test for massive transfusion requirement were optimized at this 3% threshold. The positive predictive value (PPV) of LY30 for massive transfusion at this threshold was 91%, and the specificity was 98%, with an acceptable negative predictive value (NPV) of 65% with a sensitivity of 31%. Raising the LY30 threshold created little benefit in PPV at the cost of an unacceptable loss of sensitivity, dropping to 16% (Table 1).
TABLE 1.
Predictive Power of LY30 for Massive Transfusion at Various Threshold Values of LY30 to Define a Positive Test Result
| LY30 Threshold Value | Specificity | PPV | Sensitivity | NPV |
|---|---|---|---|---|
| ≥7.5% | 1.00 | 1.00 | 0.16 | 0.60 |
| ≥3% | 0.98 | 0.91 | 0.31 | 0.65 |
| ≥0.1% | 0.83 | 0.63 | 0.38 | 0.63 |
The LY30 test is best suited to prediction of massive transfusion in the MTP-activating population using a threshold value of 3%. Using a higher LY30 threshold criterion lowers sensitivity with a marginal gain in PPV.
When stratifying the population further by age and injury severity (known independent predictors of poor outcomes in trauma), LY30 becomes an even more powerful predictor of massive transfusion requirement. For patients with an ISS of greater than 25, the PPV of LY30 of 3% or greater rose to 100%, but the sensitivity remained low at 22%. Examining those patients older than 45 years, the same finding holds true: LY30 of 3% or greater remains the optimal threshold, with a PPV of 100% but with sensitivity remaining low at 22%.
Hyperfibrinolysis as a Predictor of Mortality
Both all-cause 28-day mortality and mortality caused by hemorrhage were analyzed in relation to the degree of fibrinolysis, using the same cohorts defined for massive transfusion risk previously mentioned (Fig. 2). The overall risk of mortality in the MTP-activating population was 24.7% (18 of 73 patients), and all but three of these patients received a massive transfusion of at least 10 U of PRBCs. Of the 18 fatalities, 8 deaths were clearly attributable to hemorrhagic shock without confounding factors such as traumatic brain injury. Of these hemorrhagic deaths, five (62%) died within 6 hours of injury, and the remainder died within 14 hours. All of these patients received a massive transfusion of between 12 U and 54 U of PRBCs before they died.
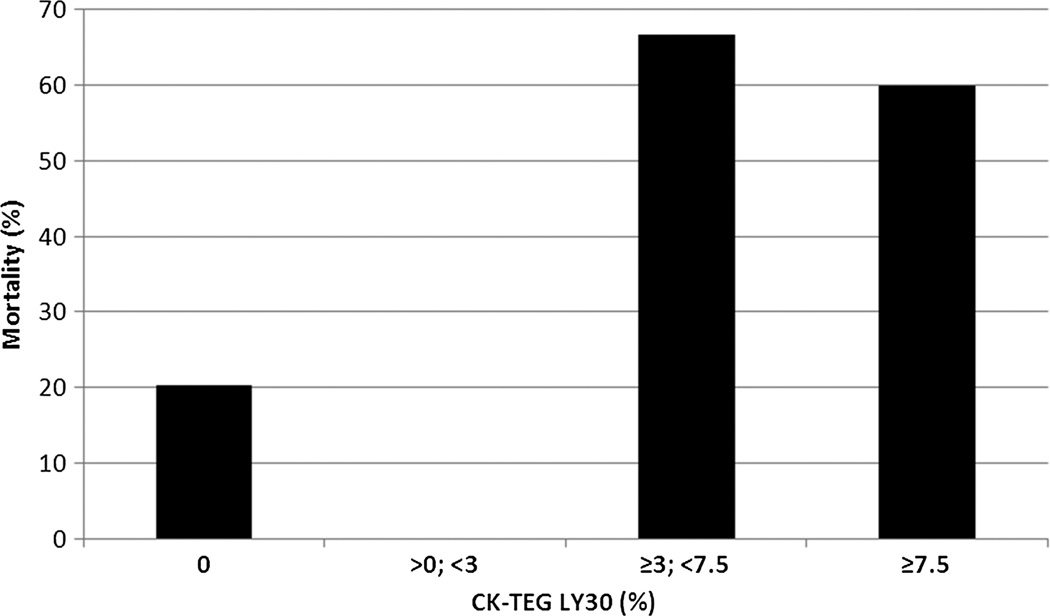
Figure 2.
All-cause 28-day mortality versus LY30. The risk of death rises sharply above the LY30 threshold value of 3% (p = 0.0034). There is no statistically significant difference in the mortality risk between the subcohorts (0% compared with >0% to <3%, p = 1.0, and ≥3% to <7.5% compared with ≥7.5%, p = 0.33).
The cohort of patients with an LY30 of 3% or greater were at a much higher risk of all-cause death (63.6% vs. 17.7%, p = 0.0034) than those with an LY30 of less than 3%. The risk of death as an immediate consequence of uncontrollable hemorrhage (Fig. 3) followed a similar pattern of marked elevation of risk in the cohort of LY30 of 3% or greater compared with the cohort of LY30 of less than 3% (45.5% vs. 4.8%, p = 0.0014). Again, no statistically significant difference in mortality existed between the subcohorts (LY30 ≥3 to <7.5% compared with LY30 ≥ 7.5%).
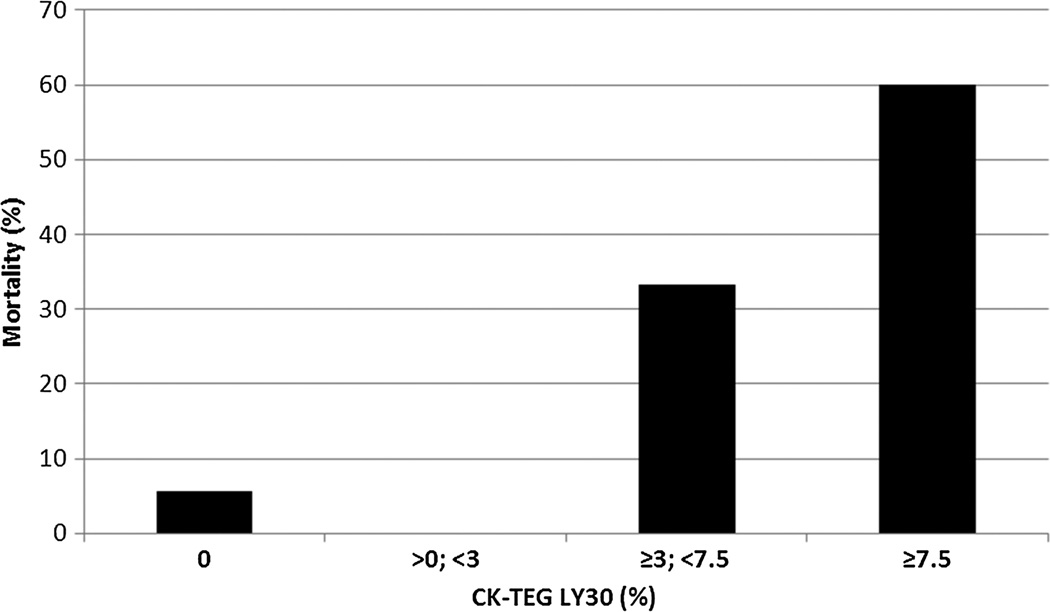
Figure 3.
Hemorrhagic mortality versus LY30. Similar to all-cause mortality, the risk of death clearly attributable to bleeding is markedly higher above the LY30 threshold value of 3% (p = 0.0014). There is no statistically significant difference in the mortality risk between the subcohorts (0% compared with >0% to <3%, p = 0.57, and ≥3% to <7.5% compared with ≥7.5%, p = 1.0).
The same granular analysis of statistical performance of LY30 as a predictive test for mortality (Table 2) was performed as for massive transfusion prediction. In an attempt to optimize the figures of merit (sensitivity, specificity, PPV, and NPV), these parameters were calculated while varying the threshold value of LY30. In the case of mortality, the PPV of LY30 was maximized at 71% at a threshold value of 5% or greater. This reflects a very high specificity of 96%; however, the sensitivity was poor at this threshold at 28%. The sensitivity rises substantially to 39% with a sacrifice in PPV (dropping to 64%) when the LY30 threshold is set at 3% or greater. Importantly, the sensitivity and NPV for hemorrhagic death (i.e., a potentially preventable death with early correction of coagulopathy) are maximized for LY30 of 3% or greater at 63% and 95%, respectively. It is conceivable that this risk is even greater at higher values of LY30, but the exceedingly small number of patients with higher LY30s makes statistical distinction of these subgroups impossible with our current sample size.
TABLE 2.
Predictive Power of LY30 for (A) All-Cause and (B) Hemorrhagic Mortality at Various Threshold Values of LY30 to Define a Positive Test
| A. All-Cause 28-d Mortality | ||||
|---|---|---|---|---|
| LY30 Threshold | Specificity | PPV | Sensitivity | NPV |
| ≥7.5% | 0.96 | 0.60 | 0.17 | 0.78 |
| ≥3% | 0.93 | 0.64 | 0.39 | 0.82 |
| ≥0.1% | 0.78 | 0.37 | 0.39 | 0.80 |
| B. Hemorrhagic Mortality | ||||
| LY30 Threshold | Specificity | PPV | Sensitivity | NPV |
| ≥7.5% | 0.97 | 0.60 | 0.38 | 0.93 |
| ≥3% | 0.91 | 0.45 | 0.63 | 0.95 |
| ≥0.1% | 0.78 | 0.26 | 0.63 | 0.94 |
The LY30 test is best suited to prediction of both (A) all-cause and (B) hemorrhagic death in the MTP-activating population using a threshold value of 3%, with very high specificity. Using a higher LY30 threshold criterion lowers sensitivity unacceptably with only a modest gain in PPV.
When the patient population is stratified by age and injury severity, again, the performance of the LY30 test is improved, and the optimal threshold for predicting all-cause mortality is 3% or greater. In patients with an ISS greater than 25, the PPV is maximized at LY30 of 3% or greater at 83%, with specificity of 96% and improved sensitivity at 42%. In older patients, older than 45 years, the PPV is maximized at LY30 of 3% or greater at 100%, with specificity of 100% and sensitivity at 38%.
Fibrinolysis as a Predictive Tool in the GT Population
Having established an LY30 of 3% or greater as a clinically useful definition of hyperfibrinolysis in our severely injured MTP-activating population, we then sought to determine the generalizability of this threshold value to a non-selective population. This GT population consisted of 216 patients taken as a convenience sample of all trauma activations and alerts at DHMC with available field-drawn blood, collected during the same period as our MTP-activating population. This population was, in the aggregate, much less severely injured and much more heterogeneous with respect to injury severity than our MTP-activating population (median ISS, 9; IQR, 1–17; mean, 11.4 ± 12.4). Demographically, the GT population was 75% male with a median age of 40 ± 17 years. With regard to fibrinolysis, 94 (43.5%) of 216 GT patients had a detectable LY30, while 24 (11.1%) had LY30 of 3% or greater, and 7 (3.2%) had an LY30 of 7.5% or greater.
We evaluated the GT patients for outcomes retrospectively, using the same cohort strategy as for the MTP-activating population analysis, starting with transfusion requirement. The overall mortality was 6.5%, and the overall risk of requiring a massive transfusion was 3.7%. Thus, the pretest probability of death or massive transfusion was much lower than for the MTP-activating population.
As seen in Figure 4A, the risk of requiring a massive transfusion again rises markedly at or above an LY30 of 3% and even more so at the 7.5% threshold. When dividing the population into two cohorts at the threshold of LY30 of 3% or greater, the risk of massive transfusion is 16.7% for the upper versus 2.1% for the lower (p = 0.006). This low PPV is a result of the low pretest probability in this population, as the specificity remains quite good at 90% with an acceptable sensitivity of 50% and an NPV of 98%.
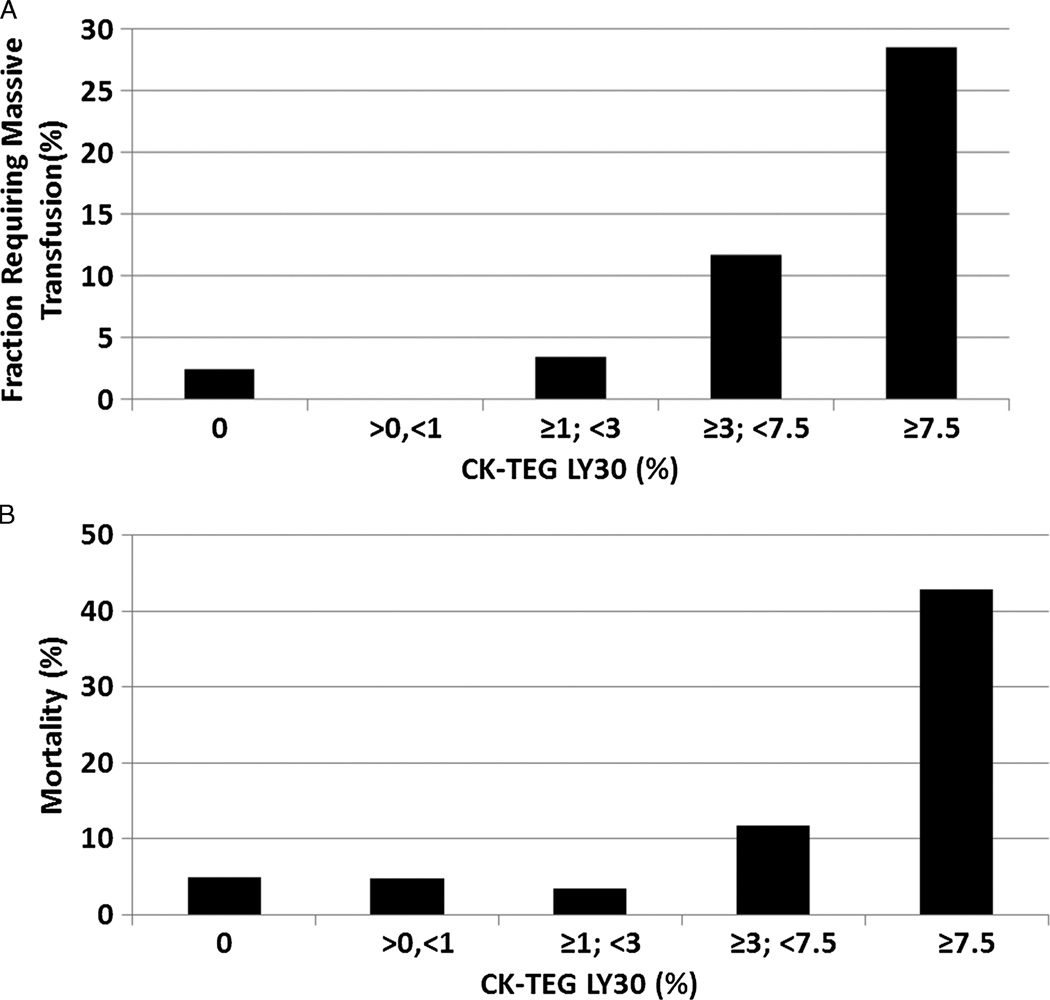
Figure 4.
Outcomes versus LY30 in the GT population. A, Plot of massive transfusion risk versus LY30 shows trend of increasing risk with increasing LY30. B, Plot of all-cause 28-day mortality versus LY30 demonstrates trend of increasing mortality with increasing LY30. Both of these outcomes display the same pattern of a sharp increase in risk at the threshold of LY30 of 3% or greater as in the MTP-activating population; however, the effect is much more marked at the 7.5% threshold, reflecting the lower pretest probability of massive transfusion or death in the highly heterogeneous GT population.
In Figure 4B, the same analysis for all-cause mortality shows a trend almost identical to that observed for massive transfusion requirement, with mortality rising at the 3% threshold of LY30, then increasing dramatically again at 7.5%. The cohort of LY30 of 3% or greater had a mortality of 20.8% versus 4.7% for the cohort of LY30 of less than 3% (p = 0.011). The sensitivity for mortality was poor at 36% (again reflecting the heterogeneity of this population and their etiologies of death), but specificity and NPV were sound at 91% and 95%, respectively.
DISCUSSION
In this prospective study of severely injured trauma patients, we found that an admission CK-TEG LY30 value of 3% or greater was a powerful predictor of requiring a massive transfusion within 6 hours of injury (PPV, 91%). This reflects a high specificity of 98% for predicting massive transfusion requirement as well as a moderately high pretest probability of requiring a massive transfusion in this population (44%). These patients were all deemed likely enough to require a massive transfusion to activate DHMC’s MTP, but a positive test finding for LY30 of 3% or greater is a vastly superior predictor to clinical judgment alone. The sensitivity of the test is poor, however. This is not surprising because ACOT is a multifactorial entity and not all occurrences of ACOT necessarily manifest hyperfibrinolysis; moreover, not all massive traumatic bleeding is necessarily coagulopathic.27 Thus, LY30 of 3% or greater rules in the need to prepare for massive transfusion; it does not rule it out.
It is important to observe that the apparently weak PPV of LY30 for all-cause and hemorrhagic death belies a very high specificity for mortality (93% for all-cause and 91% for hemorrhagic). False-positive rates for death using an LY30 of 3% or greater are rare, and only the overall relative low mortality rate in our trauma population keeps the observed PPV low. This is further reflected by the roughly fourfold increase in relative risk of all-cause death and nearly 10-fold increase in the relative risk of hemorrhagic death with a positive test result at the threshold of LY30 of 3% or greater. It is these hemorrhagic deaths that should concern us most because they are the mostly likely preventable.
This finding of an elevated risk of mortality with LY30 of 3% or greater is consonant with the findings at other institutions.19,20 Given the grave risk of hemorrhagic death in this cohort and the relatively benign adverse effect profile of tranexamic acid or ε-aminocaproic acid, in severely injured patients with an LY30 of 3% or greater, the risk-benefit analysis favors not only the activation of an MTP but also the administration of antifibrinolytic agents.
As noted in Patients and Methods section, one of the limitations of this technology is its inability to differentiate, at low levels of LY30, between genuine fibrinolysis and platelet-mediated effects. Interestingly, upon further analysis of the single false-positive prediction for massive transfusion, analysis of the raw TEG tracing revealed a pattern we believe to be indicative of platelet-mediated clot retraction (which also increases LY30) rather than fibrinolysis. This was confirmed by examining this patient’s Functional Fibrinogen TEG tracing (which is platelet independent) and observing an LY30 of 0%. This finding highlights the gaps in our knowledge about the process of clot lysis and the need for further mechanistic research in this arena. While this current study provides further evidence that profound hypotension is linked to hyperfibrinolysis, neither the nature of the linkage nor the direction of the causality is clear.3,12,19,27 Complicating these investigations are not only platelet effects but also the possibility of proteases other than plasmin being involved in pathologic fibrinolysis. It must be also admitted that the clinical utility of the LY30 is somewhat hampered by the relatively slow time to result, intrinsic to the TEG methodology. We are currently engaged in developing more rapid and specific assays for fibrinolysis that will obviate these problems and hopefully point toward an underlying mechanism (manuscript in preparation).28
With regard to the general application of the criterion LY30 of 3% or greater to the “all comers” trauma population, it is important to note the role that clinical judgment plays in using this test. The MTP-activating population to which we applied this measurement is defined by the patient’s presenting condition and a rapid estimation of their severity of injury and likelihood of bleeding. While the GT population shows the same marked rise in relative risk of massive transfusion and with LY30 of 3% or greater, the PPV is quite low, owing to this broader population’s generally low pretest probability of massive hemorrhage and mortality. Work remains to be done with this population to define corroborating findings predictive of poor outcomes; however, there is clear evidence in our MTP-activating population that the predictive value of LY30 for hemorrhage and death increases with age and ISS. Pending collection of sufficient numbers of patients to conduct a multivariate regression analysis, these known predictors of poor outcome as well as evident hemorrhage or surgical oozing should increase the clinician’s index of suspicion (and thus the pretest probability) of an impending massive transfusion requirement when applying the LY30 parameter as a predictive test.
Acknowledgments
DISCLOSURE
This study was supported by the National Institutes of Health (P50 GM049222, T32 GM008315).
We receive research support from the Haemonetics Corporation, Niles, Illinois.
Footnotes
This study was presented at the 43rd annual meeting of the Western Trauma Association, March 3Y8, 2013, in Snowmass, Colorado.
AUTHORSHIP
E.E.M., A.B., C.C.S., A.G., and M.P.C. designed this study. A.G, J.N.H., and T.L.C. designed and maintained the study database. M.P.C., E.E.M., C.R.R., and A.S. analyzed and interpreted the data. M.P.C., J.R.S., and E.E.M. prepared the manuscript and figures.
REFERENCES
- 1.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–231. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am. 2012;92(4):877–891. doi: 10.1016/j.suc.2012.06.001. viii. [DOI] [PubMed] [Google Scholar]
- 5.Ganter MT, Pittet JF. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol. 2010;24(1):15–25. doi: 10.1016/j.bpa.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, et al. Freshfrozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 7.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–271. [DOI] [PubMed] [Google Scholar]
- 8.Gorlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schochl H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother. 2012;39(2):104–113. doi: 10.1159/000337186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251(4):604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 10.Schochl H, Maegele M, Solomon C, Gorlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252(3):434–442. doi: 10.1097/SLA.0b013e3181f09191. discussion 443–444. [DOI] [PubMed] [Google Scholar]
- 12.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anti-coagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. discussion 1217. [DOI] [PubMed] [Google Scholar]
- 13.CRASH-2 collaborators. Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–1101. doi: 10.1016/S0140-6736(11)60278-X. 1011.e1–1011.e2. [DOI] [PubMed] [Google Scholar]
- 14.collaborators C-t. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 15.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MAT-TERs) Study. Arch Surg. 2012;147(2):113–119. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 16.Roberts I, Perel P, Prieto-Merino D, Shakur H, Coats T, Hunt BJ, et al. Effect of tranexamic acid on mortality in patients with traumatic bleeding: prespecified analysis of data from randomised controlled trial. BMJ. 2012;345:e5839. doi: 10.1136/bmj.e5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haemonetics. TEG 5000 System User Manual. P/N 06-510-US, Manual revision: AC. Niles, IL: Haemonetics Corporation, Haemoscope Division; 2010. [Google Scholar]
- 19.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schochl H, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365–370. doi: 10.1097/TA.0b013e31825c1234. discussion 370. [DOI] [PubMed] [Google Scholar]
- 20.Tapia NMCA, Norman MA, Welsh FJ, Scott BG, Wall MJ, Mattox KL, Suliburk JW, editors. Hyperfibrinolysis on Thromboelastogram (TEG) Predicts Mortality in Massively Transfused Trauma Patients. Chicago, IL: American College of Surgeons; 2012. p. S52. [Google Scholar]
- 21.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, et al. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73(2):358–364. doi: 10.1097/TA.0b013e31825889ba. discussion 364. [DOI] [PubMed] [Google Scholar]
- 22.Mitra B, Cameron PA, Gruen RL, Mori A, Fitzgerald M, Street A. The definition of massive transfusion in trauma: a critical variable in examining evidence for resuscitation. Eur J Emerg Med. 2011;18(3):137–142. doi: 10.1097/MEJ.0b013e328342310e. [DOI] [PubMed] [Google Scholar]
- 23.Mitra B, Rainer TH, Cameron PA. Predicting massive blood transfusion using clinical scores post-trauma. Vox Sang. 2012;102(4):324–330. doi: 10.1111/j.1423-0410.2011.01564.x. [DOI] [PubMed] [Google Scholar]
- 24.Neal MD, Hoffman MK, Cuschieri J, Minei JP, Maier RV, Harbrecht BG, et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: when a little goes a long way. J Trauma Acute Care Surg. 2012;72(4):892–898. doi: 10.1097/TA.0b013e31823d84a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal MD, Marsh A, Marino R, Kautza B, Raval JS, Forsythe RM, et al. Massive transfusion: an evidence-based review of recent developments. Arch Surg. 2012;147(6):563–571. doi: 10.1001/archsurg.2011.2212. [DOI] [PubMed] [Google Scholar]
- 26.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 27.Morton AP, Moore EE, Wohlauer MV, Lo K, Silliman CC, Burlew CC, et al. Revisiting early postinjury mortality: are they bleeding because they are dying or dying because they are bleeding? J Surg Res. 2013;179(1):5–9. doi: 10.1016/j.jss.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman MPME, Harr JN, Wohlauer M, Ramos C, Haney E, Norem K, Omert L, Silliman CC, Banerjee A. Differential Tranexamic Acid-Inhibited Functional Fibrinogen Thromboelastography (TEG) Is a Better Guide for Anti-Fibrinolytic Therapy Than Traditional Kaolin TEG. European Congress of Trauma and Emergency Surgeons. Lyon, France: European Society of Trauma and Emergency Surgeons; 2013. [Google Scholar]