Abstract
There has been growing interest in the role of viral infections and their association with adverse pregnancy outcomes. However, little is known about the impact viral infections have on the fetal membranes (FM). Toll-like receptors (TLR) are thought to play a role in infection-associated inflammation at the maternal–fetal interface. Therefore, the objective of this study was to characterize the cytokine profile and antiviral response in human FMs exposed to viral dsRNA, which activates TLR3, and viral ssRNA, which activates TLR8; and to determine the mechanisms involved. The viral dsRNA analog, Poly(I:C), induced up-regulated secretion of MIP-1α, MIP-1β, RANTES and TNF-α, and down-regulated interleukin (IL)-2 and VEGF secretion. In contrast, viral ssRNA induced a broader panel of cytokines in the FMs by up-regulating the secretion of IL-1β, IL-2, IL-6, G-CSF, MCP-1, MIP-1α, MIP-1β, RANTES, TNF-α and GRO-α. Using inhibitory peptides against TLR adapter proteins, FM secretion of MIP-1β and RANTES in response to Poly(I:C) was MyD88 dependent; MIP-1α secretion was dependent on MyD88 and TRIF; and TNF-α production was independent of MyD88 and TRIF. Viral ssRNA-induced FM secretion of IL-1β, IL-2, IL-6, G-CSF, MIP-1α, RANTES and GRO-α was dependent on MyD88 and TRIF; MIP-1β was dependent upon TRIF, but not MyD88; and TNF-α and MCP-1 secretion was dependent on neither. Poly(I:C), but not ssRNA, induced an FM antiviral response by up-regulating the expression of IFNβ, myxovirus-resistance A, 2′,5′-oligoadenylate synthetase and apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G. These findings demonstrate that human FMs respond to two viral signatures by generating distinct inflammatory cytokine/chemokine profiles and antiviral responses through different mechanisms.
Keywords: antiviral factors, cytokines, fetal membranes, Toll-like receptors, viral infections
Introduction
Bacterial infection and inflammation of the placental fetal membranes (FM) play a major role in chorioamnionitis, preterm premature rupture of membranes (PPROM) and preterm birth (ACOG Practice Bulletin No. 80, 2007, Goldenberg et al., 2000; Lamont, 2003; Caughey et al., 2008; Goldenberg et al., 2008). The FMs are likely the first tissues colonized by an ascending bacterial infection (Herve et al., 2008). Although most normal term deliveries have evidence of bacteria in the chorioamnion, it is the association with inflammation that correlates with pathology and prematurity (Romero et al., 2007). An intrauterine infection gaining access to the FMs is thought to trigger prematurity by activating innate immune responses (Romero et al., 2007). Indeed, clinical and experimental studies have correlated bacteria/bacterial components and inflammation at the maternal–fetal interface with preterm birth (Elovitz and Mrinalini, 2004; Elovitz et al., 2006; Romero et al., 2006; Pettker et al., 2007; Koga et al., 2009; Pirianov et al., 2009; Burd et al., 2010; Cardenas et al., 2011). However, in spite of this association, no single bacterium has been attributed to preterm birth, and antibiotic interventions have proved unsuccessful (Ganu et al., 2012).
More recently there has been growing interest in the role of viral infections and adverse pregnancy outcomes. The presence of a number of viral infections in the amniotic fluid or gestational tissues have been reported to be linked to increased risk for chorioamnionitis and spontaneous preterm birth, such as adenovirus (Srinivas et al., 2006; Tsekoura et al., 2010); Epstein–Barr virus, cytomegalovirus (CMV), herpes virus (Gibson et al., 2008, 2011), human papillomavirus (Gomez et al., 2008), coxsackie virus, group B type 1 (Strong and Young, 1995), the enterovirus herpangia (Chen et al., 2010) and hepatitis virus (Elefsiniotis et al., 2010; Connell et al., 2011). Infection with adenovirus has also been associated with second trimester pregnancy loss (Srinivas et al., 2006). Similarly, infection with Parvovirus B19 or herpes virus early in pregnancy has been linked to second trimester miscarriage or very preterm birth (Johansson et al., 2008). In addition, women with H1N1 are more likely to have adverse pregnancy outcomes such as spontaneous miscarriages and preterm birth (Creanga et al., 2010; Investigators and Australasian Maternity Outcomes Surveillance, 2010; Siston et al., 2010), and the rate of preterm birth correlates with maternal disease severity (Michaan et al., 2012).
While FMs are permissive to viral infections, including influenza virus (Uchide et al., 2002a, b, 2006, 2009, 2012), CMV (Figueroa et al., 1978; Kumazaki et al., 2002; Matsunaga et al., 2013), and herpes virus (Rokos et al., 1998), and viral infections can trigger an inflammatory cytokine response in the chorioamnion (Uchide et al., 2002b, 2006, 2012), little is known about the mechanisms involved. One way in which infection-associated inflammation at the maternal–fetal interface arises is through activation of the innate immune Toll-like receptors (TLRs) (Abrahams, 2008). We have previously shown that normal human FMs at term constitutively express TLRs 1–10, as well as the two major TLR adapter proteins, MyD88 and TRIF (Hoang et al., 2014). Moreover, in response to bacterial agonists, TLR2, TLR4, TLR5 and TLR9 mediate distinct FM cytokine profiles (Hoang et al., 2014). Thus, we hypothesized that this might also be the case for TLR3, which senses viral dsRNA and TLR8, which detects viral ssRNA (Kumar et al., 2011). Therefore, the objective of this study was to characterize the cytokine profile and antiviral response in human FMs exposed to the viral signatures, dsRNA and ssRNA, and to determine the mechanisms involved.
Materials and Methods
Fetal membrane collection, preparation and stimulation
FM (n = 16) were collected from uncomplicated normal term pregnancies (39–41 weeks) delivered by elective repeat Cesarean section, without signs of labor, infection or PPROM. No patients received prostaglandins or any other induction agent prior to Cesarean section. Sample collection was approved by Yale University's Human Investigation Committee. After washing the FMs with sterile PBS supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) (Gibco, Grand Island, NY, USA), adherent blood clots were removed and sections where both the chorion and amnion were intact were cut using a 6-mm biopsy punch. The FM explants were then placed in 0.4-μm cell culture inserts (BD Falcon, Franklin Lakes, NJ, USA), with 500-μl Dulbecco's Modified Eagle Medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and these were placed in a 24-well plate containing 500 μl of the DMEM media for 24 h, as previously described (Luo et al., 2010; Hoang et al., 2014). The next day the media was removed and replaced with serum-free OptiMeM media (Gibco). FM explants were then treated with: no treatment (NT); the TLR3 agonist and synthetic analog of viral dsRNA, Poly(I:C), at 20 μg/ml (Invivogen, San Diego, CA, USA); or the TLR8 agonist, viral ssRNA at 5 μg/ml (Invivogen). To determine the involvement of caspase-1, FM explants were pretreated for 1 h with the specific caspase-1 inhibitor, Z-WEHD-FMK at 1 μM (R&D Systems, Minneapolis, MN, USA). To determine the involvement of MyD88 or TRIF, FM explants were pretreated for 1 h with either a MyD88 inhibitor peptide (Pepin-MYD; 10 μM); a TRIF inhibitor peptide (Pepin-TRIF; 10 μM) or a control peptide (Pepin-Control; 10 μM) (Invivogen). The optimal Poly(I:C) and viral ssRNA concentrations were determined in preliminary experiments (data not shown) and the time point determined from previous studies (Hoang et al., 2014). After 24 h of treatment, cell-free culture supernatants were collected and the explants snap frozen. Supernatants and tissues were then stored at −80°C until further analysis was performed.
Cytokine analysis
FM supernatants were analyzed for the following cytokines/chemokines using multiplex analysis (BioRad): IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, G-CSF (CSF3), GM-CSF (CSF2), IFNγ, MCP-1 (CCL2), MIP-1α, MIP-1, β RANTES (CCL5), TNF-α, VEGF and GRO-α as previously described (Luo et al., 2010; Hoang et al., 2014). FM supernatants were also analyzed for IL-1β by ELISA (R&D Systems).
Quantitative real-time RT–PCR
FM explant biopsies were homogenized and total RNA extracted as previously described (Abrahams et al., 2013; Krikun et al., 2013; Hoang et al., 2014). Quantitative real-time PCR was performed using the KAPA SYBR Fast qPCR kit (Kapa Biosystems, Woburn, MA, USA), and PCR amplification performed on the BioRad CFX Connect Real-time System (BioRad, Hercules, CA, USA). Detection of human IFNβ, 2′,5′-oligoadenylate synthetase (OAS), myxovirus-resistance A (MxA) and apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) and secretory leukocyte protease inhibitor (SLPI) was performed using primer sequences as previously described (Krikun et al., 2013). Data were normalized to the housekeeping gene, GAPDH, analyzed using the Δ-ΔCT method and presented as fold change (FC) in the expression of gene of interest relative to the NT control, as previously described (Abrahams et al., 2013; Krikun et al., 2013).
Statistical analysis
Experiments were performed at least three times and data presented as mean ± SEM. Prism from Graphpad Software, Inc. (La Jolla, CA, USA) was used to calculate significance (P < 0.05). Statistical analysis was performed using either the paired t-test, or for multiple comparisons, one-way ANOVA.
Results
Viral signatures induce distinct FM cytokine profiles
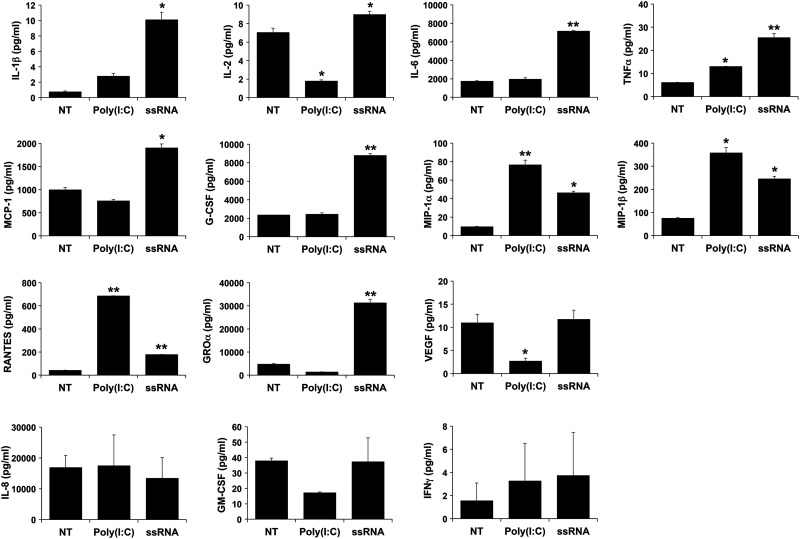
In a previous study we established that human FMs from normal term pregnancies constitutively express the viral sensors, TLR3 and TLR8, as well as the TLR adapter proteins MyD88 and TRIF (Hoang et al., 2014). Therefore, in this study, FMs were treated with the TLR3 agonist, Poly(I:C), and the TLR8 agonist, viral ssRNA (Kumar et al., 2011). After treatment with Poly(I:C), FMs secreted significantly higher levels of TNF-α (2.1-fold), MIP-1α (8-fold), MIP-1β (4.8-fold), and RANTES (16.4-fold) compared with the NT control. In parallel, FM secretion of IL-2, and VEGF was significantly dampened by 4-fold and 4.1-fold, respectively, after exposure to Poly(I:C) (Fig. 1). FM secretion of IL-1β, IL-6, G-CSF, MCP-1, GRO-α, IL-8, GM-CSF and IFNγ were not significantly changed after Poly(I:C) treatment (Fig. 1). After treatment with viral ssRNA, FMs secreted significantly higher levels of IL-1β (13.8-fold), IL-2 (1.3-fold), IL-6 (4.1-fold), TNF-α (4.2-fold), MCP-1 (1.9-fold), G-CSF (3.8-fold), MIP-1α (4.8-fold), MIP-1β (3.3-fold), RANTES (4.3-fold) and GRO-α (6.6-fold) (Fig. 1). FM secretion of IL-8, GM-CSF, IFNγ and VEGF were not significantly changed after ssRNA treatment (Fig. 1). Levels of IL-4, IL-10, IL-12 and IL-17 were below the assay's detection limit.
Figure 1.
Cytokine profile of FM after exposure to Poly(I:C) and viral ssRNA. FM explants were treated with NT, Poly(I:C) or viral ssRNA. Bar charts show levels of IL-1β, IL-2, IL-6, TNFα, MCP-1, G-CSF, MIP-1α, MIP-1β, RANTES, GROα, VEGF, IL-8, GM-CSF and IFNγ (*P < 0.05, **P < 0.001; n = 6).
Role of MyD88 and TRIF in viral dsRNA- and ssRNA-induced FM cytokine secretion
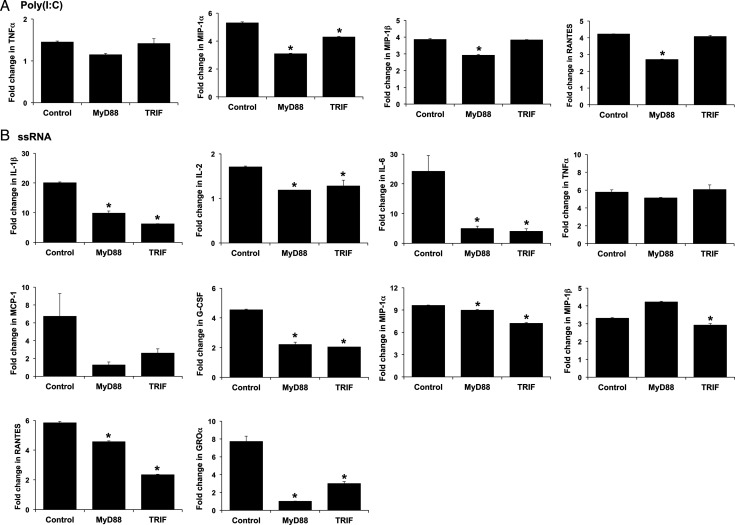
Having established that human FMs respond to the viral TLR agonists, Poly(I:C) and ssRNA by secreting cytokines and chemokines, we next sought to determine the role of the adapter proteins MyD88 and TRIF. Typically, TLR3 utilizes the adapter protein TRIF (Yamamoto et al., 2002; Oshiumi et al., 2003), while TLR8 signals through MyD88 (Han et al., 2012; Heil et al., 2004). As shown in Fig. 2A, the Poly(I:C)-induced up-regulation of FM MIP-1α, MIP-1β and RANTES in the presence of the control peptide was significantly reduced by the presence of the MyD88 inhibitor. The TRIF inhibitor also significantly reduced Poly(I:C)-induced secretion of MIP-1α, but not MIP-1β or RANTES (Fig. 2A). Neither the MyD88 nor the TRIF inhibitor had any significant effect on the FMs Poly(I:C)-induced TNF-α response (Fig. 2A). As shown in Fig. 2B, the ssRNA-induced up-regulation of IL-1β, IL-2, IL-6, G-CSF, MIP-1α, RANTES and GRO-α secretion by the FMs was significantly reduced by both the MyD88 inhibitor and the TRIF inhibitor, when compared with the control peptide. Viral ssRNA-induced up-regulation of MIP-1β secretion by the FMs was significantly inhibited by the presence of the TRIF inhibitor, but not the MyD88 inhibitor (Fig. 2B). ssRNA-induced TNF-α and MCP-1 secretion was not significantly altered by ether inhibitor (Fig. 2B).
Figure 2.
FM cytokine responses to Poly(I:C) and viral ssRNA are regulated by MyD88 and TRIF. FM explants were treated with NT, or either (A) Poly(I:C) or (B) viral ssRNA all in the presence of either a control peptide (Control), a MyD88 inhibitor peptide (MyD88) or a TRIF inhibitor peptide (TRIF) (10 μM). Bar charts show cytokine secretion as FC relative to the NT controls (*P < 0.05 relative to the control; n = 5).
FM IL-1β secretion in response to viral ssRNA is caspase-1 dependent
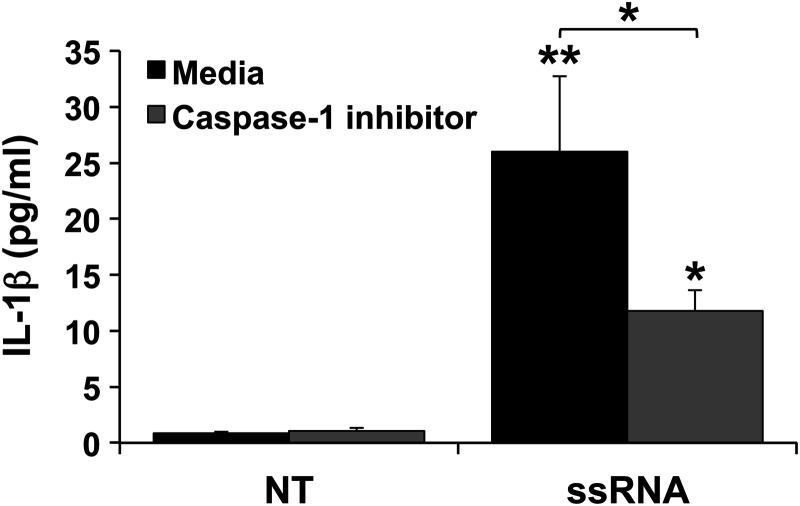
Since viral ssRNA, but not Poly(I:C), induced the FMs to produce IL-1β, we further examined the mechanism involved. We previously demonstrated that human FMs express the inflammasome components Nalp1, Nalp3, ASC (apoptosis-associated speck-like protein containing a CARD) and caspase-1 (Hoang et al., 2014), which mediate the processing of intracellular pro-IL-1β into its active, secreted form (Agostini et al., 2004; Martinon and Tschopp, 2004). In the presence of a caspase-1 inhibitor, IL-1β secretion induced by viral ssRNA was significantly reduced by 54.7% (Fig. 3).
Figure 3.
Viral ssRNA induces FM IL-1β secretion in a caspase-1 dependent manner. FM explants (n = 10) were treated with NT or ssRNA in the presence of media or a caspase-1 inhibitor after which supernatants were measured for IL-1β (*P < 0.05, **P < 0.001 relative to NT unless otherwise indicated).
Poly(I:C), but not viral ssRNA, induces an FM antiviral response
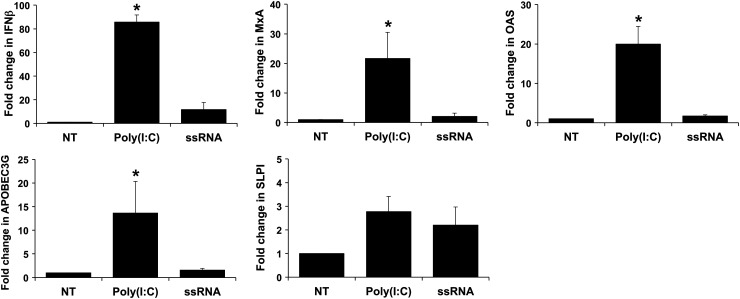
TLR3 and TLR8 activation can induce a type I interferon (IFN) response (Arpaia and Barton, 2011), and the subsequent production of antiviral factors, such as IFN-inducible OAS, MxA and APOBEC3G (Samuel, 2001; Turelli et al., 2004; Abrahams et al., 2006; Bonvin et al., 2006; Krikun et al., 2013) and SLPI (Schaefer et al., 2005; Abrahams et al., 2006; Krikun et al., 2013). As shown in Fig. 4, Poly(I:C), but not ssRNA, significantly induced FMs to express elevated levels of IFNβ, MxA, OAS and APOBEC3G mRNA. In contrast, neither Poly(I:C) nor ssRNA induced a significant increase in SLPI mRNA levels (Fig. 4).
Figure 4.
Poly(I:C) and viral ssRNA trigger distinct FM antiviral responses. FMs explants were incubated with NT, Poly(I:C) or viral ssRNA and then analyzed for IFNB, MxA, OAS, APOBEC3G and SLPI mRNA levels by qRT–PCR. Bar charts show mRNA expression as FC relative to the NT control (*P < 0.05; n = 3–6).
Discussion
Infection-associated pregnancy complications such as chorioamnionitis, PPROM and preterm birth have been strongly associated with bacterial infection and inflammation of the FMs (ACOG Practice Bulletin No. 80, 2007, Goldenberg et al., 2000; Lamont, 2003; Caughey et al., 2008; Goldenberg et al., 2008). Although, much less is known about the role of viral infections, there is growing evidence to suggest an association with pregnancy mortality and morbidity and gestational tissues including the FMs may be a target (Figueroa et al., 1978; Rokos et al., 1998; Kumazaki et al., 2002; Uchide et al., 2002a, b, 2006, 2009, 2012; Matsunaga et al., 2013). The functional role of innate immune pattern recognition receptors, such as the TLRs and Nod proteins, in response to bacterial components in the FMs has been described (Kim et al., 2004; Adams et al., 2007; Leroy et al., 2007; Abrahams et al., 2013; Lappas, 2013, 2014; Hoang et al., 2014), however, little is known about this tissue's response to viral components or the mechanisms involved. In this current study we have demonstrated that FMs exposed to the TLR3 agonist, viral dsRNA, and the TLR8 agonist, viral ssRNA, generate distinct cytokine/chemokine and antiviral profiles. Furthermore, we have demonstrated a differential role for the TLR adapter proteins, MyD88 and TRIF, in mediating FM cytokine/chemokine production in response to these two viral signatures. We have also demonstrated a role for the inflammasome component, caspase-1, in mediating FM IL-1β production following exposure to viral ssRNA.
When we compared the cytokine profile generated by human FMs after exposure to the viral TLR3 and TLR8 agonists, Poly(I:C) and viral ssRNA, respectively, these two viral signatures induced quite different cytokine/chemokine responses. Poly(I:C) up-regulated the secretion of the pro-inflammatory cytokine, TNF-α, and the inflammatory chemokines MIP-1α, MIP-1β and RANTES, while down-regulating the constitutive of IL-2 and VEGF. In contrast viral ssRNA triggered a much broader response by increasing the tissue's section of the pro-inflammatory cytokines, IL-1β, TNF-α, IL-6 and IL-2; the inflammatory chemokines, MCP-1, MIP-1α, MIP-1β, RANTES and GRO-α and the growth factor, G-CSF. So while both viral components triggered distinct profiles, both responses were a combination of pro-inflammatory cytokines and chemokines.
The production of the chemokines, MCP-1, MIP-1α, MIP-1β, RANTES and GRO-α by FMs exposed to viral RNA, suggests that in the context of a viral infection, the chorioamnion may play a role in immune cell recruitment. Although histologic chorioamnionitis is characterized by a neutrophil infiltrate, and the classic chemokine for this, IL-8, is often elevated in these cases (Menon et al., 2010), in our studies FMs treated with Poly(I:C) or ssRNA did not produce elevated levels of IL-8. Interestingly, basal IL-8 levels produced by the FMs were much higher than the other chemokines detected. Neutrophil chemotaxis can, however, be regulated by a number of other chemokines. In mice, for example, it has been shown that neutrophils express two major chemokine receptors, CCR1 and CXCR2 (Chou et al., 2010; McDonald and Kubes, 2010). CCR1 can be ligated by MIP-1α, MIP-1β and RANTES, while CXCR2 can be activated by GRO-α (Chou et al., 2010; McDonald and Kubes, 2010). Thus, it seems that after TLR3 and TLR8 stimulation, human FMs are producing mostly CCR1 ligands and may recruit neutrophils preferentially through this receptor.
The production of the pro-inflammatory cytokine, TNF-α by the FMs in response to TLR3 and TLR8 activation, and IL-1β and IL-6 in response to viral ssRNA also suggests a role for the FMs in contributing to preterm birth. Levels of IL-1β, IL-6 and TNF-α are elevated in the amniotic fluids of patients with preterm birth and intra-amniotic infection (Romero et al., 1989, 1990, 1992a, b) and IL-1β plays a role in promoting preterm labor (Sadowsky et al., 2006; Christiaens et al., 2008; Kemp et al., 2010). These findings are also in keeping with previous reports that infection of human chorion cells with influenza virus, which is an ssRNA virus, induces the production of the pro-inflammatory cytokines, IL-1β, IL-6 and TNFα (Uchide et al., 2002b, 2006).
In order to achieve IL-1β secretion, intracellular pro-IL-1β must be processed into its active, secreted form (Agostini et al., 2004; Martinon and Tschopp, 2004), and this is often mediated by caspase-1 (Netea et al., 2010) that associates with the other inflammasome components, Nalp1 or Nalp3 and ASC (Agostini et al., 2004; Martinon and Tschopp, 2007). We have previously reported that normal human FMs at term express all these components and in response to the bacterial TLR agonists, lipopolysaccharide (LPS), peptidoglycan and flagellin induce IL-1β processing and secretion via caspase-1 (Hoang et al., 2014). A recent study by Lappas, also showed LPS-induced IL-1β by FMs to be mediated by caspase-1 (Lappas, 2014). In this current study we demonstrated that human FMs exposed to viral ssRNA secrete IL-1β in a caspase-1 dependent manner, suggesting that TLR8 activation may induce inflammasome activity. Indeed, the TLR8 agonist, imidazoquinoline, can activate monocyte IL-1β production via caspase-1 (Philbin et al., 2012), and viral ssRNA-mediated IL-1β release by monocytes is dependent on the Nalp3 inflammasome (Allen et al., 2011).
To further explore the mechanisms by which viral dsRNA and ssRNA induce FM cytokine/chemokine production, we determined the functional role of the TLR adapter proteins, MyD88 and TRIF in this response. TLR3 is known to signal through TRIF (Yamamoto et al., 2002; Oshiumi et al., 2003), while TLR8 signals through MyD88 (Heil et al., 2004; Han et al., 2012). In this study we found that Poly(I:C) induced MIP-1α, MIP-1β and RANTES production via MyD88, MIP-1α production was also mediated by TRIF, but TNFα production was dependent upon neither adapter protein. This suggests that in FMs, Poly(I:C), in addition to activating the TLR3/TRIF pathway, may also be activating another receptor utilizing MyD88. Alternatively, in human FMs, TLR3 may be able to signal through both adapter proteins, similarly to TLR4 (Yamamoto et al., 2003). Indeed an early study reported that TLR3 could indeed utilize MyD88 (Alexopoulou et al., 2001). The lack of dependency for either MyD88 or TRIF for Poly(I:C)-induced TNFα could again suggest activation of an alternative, TLR3-independent pathway (Hoebe et al., 2003), such as the cytosolic RIG-like receptors (RLR) and non-RLR helicases (Vabret and Blander, 2013). We found a similar differential usage of MyD88 and TRIF in FMs exposed to viral ssRNA. Both adapter proteins played a role in the secretion of the majority of cytokines and chemokines induced by ssRNA, except for MIP-1β, which was only dependent upon TRIF. This suggests that in the FMs, TLR8 can also utilize both MyD88 and TRIF, or that an additional TRIF-dependent pathway is activated. Indeed, Marshall-Clarke et al. (2007), reported that TLR3 can sense both dsRNA and ssRNA. However, similarly to the Poly(I:C) response, TNFα was dependent upon neither adapter protein, as was MCP-1 production, again suggesting activation of a TLR-independent pathway (Vabret and Blander, 2013).
Our last observation in this study was that Poly(I:C) triggered the expression of the type interferon, IFNβ, and the IFN-inducible antiviral factors, OAS, MxA and APOBEC3G (Samuel, 2001; Turelli et al., 2004; Abrahams et al., 2006; Bonvin et al., 2006). However, the antimicrobial peptide SLPI, which is not regulated by type I IFNs, but has been shown to be regulated by TLR3 in other tissues (Schaefer et al., 2005; Abrahams et al., 2006), was not induced in Poly(I:C)-treated FMs. Furthermore, and in contrast to the Poly(I:C)-induced response, treatment of FMs with viral ssRNA did not induce the expression of IFNβ or the antiviral factors. That FMs can generate this antiviral response to viral dsRNA is in keeping with observations that infection of human chorion cells with influenza induces IFNβ (Uchide et al., 2002b), and treatment of FM explants with Poly(I:C) elevates the production of IL-29 (IFN-lambda3) (Nace et al., 2010), a virally induced type III IFN (Li et al., 2013).
In summary, we have demonstrated that viral dsRNA and ssRNA induce distinct pro-inflammatory cytokine and chemokine responses in an MyD88/TRIF-dependent and independent manner. Thus, FM inflammatory responses to viral dsRNA and ssRNA may be both TLR dependent and TLR independent. The FM response to viral dsRNA [Poly(I:C)] appears more protective since a strong type I IFN and antiviral response is also generated, while the ssRNA-induced response is predominantly pro-inflammatory, and FM sensing of viral ssRNA may activate the inflammasome giving rise to IL-1β secretion. Together these findings provide further evidence of how different pattern recognition receptors can generate distinct responses in the FMs through distinct mechanisms, and suggest a role for viruses in promoting inflammation of the chorioamnion.
Acknowledgements
The authors would like to thank L. Coraluzzi and Z. Tang for their help with tissue collection.
Authors’ roles
S.L.B and V.M.A. participated in the study design, data analysis and manuscript drafting. S.L.B, J.A.P. and M.H. performed the experiments. S.G. and C.S.H. facilitated tissue collection. S.G., E.R.N. and C.S.H. contributed to the final manuscript and critical discussion.
Funding
This study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH [PO1HD054713 to V.M.A., R01HD049446 to V.M.A.].
Conflict of interest
None declared.
References
- Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Bole-Aldo P, Romero R, Wira CR, Mor G. Expression and secretion of anti-viral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I:C) Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG Practice Bulletin No. 80: Premature Rupture of Membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- Adams KM, Lucas J, Kapur RP, Stevens AM. LPS induces translocation of TLR4 in amniotic epithelium. Placenta. 2007;28:477–481. doi: 10.1016/j.placenta.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Allen MC, Cristofalo EA, Kim C. Outcomes of preterm infants: morbidity replaces mortality. Clin Perinatol. 2011;38:441–454. doi: 10.1016/j.clp.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Barton GM. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011;1:447–454. doi: 10.1016/j.coviro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian JP, et al. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology. 2006;43:1364–1374. doi: 10.1002/hep.21187. [DOI] [PubMed] [Google Scholar]
- Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, Yudkoff M, Elovitz MA. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas I, Mulla MJ, Myrtolli K, Sfakianaki AK, Norwitz ER, Tadesse S, Guller S, Abrahams VM. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol. 2011;187:980–986. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- Caughey AB, Robinson JN, Norwitz ER. Contemporary diagnosis and management of preterm premature rupture of membranes. Rev Obstet Gynecol. 2008;1:11–22. [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Lin HC, Lin HC. Increased risk of adverse pregnancy outcomes among women affected by herpangina. Am J Obstet Gynecol. 2010;203:49 e1–7. doi: 10.1016/j.ajog.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31:1163–1170. doi: 10.1111/j.1478-3231.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, Chu SY, Sackoff JE, Jamieson DJ, Fine AD, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- Elefsiniotis I, Tsoumakas K, Vezali E, Glynou I, Drakoulis N, Saroglou G. Spontaneous preterm birth in women with chronic hepatitis B virus infection. Int J Gynaecol Obstet. 2010;110:241–244. doi: 10.1016/j.ijgo.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatric Res. 2006;59:50–55. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- Figueroa ME, Geder L, Rapp F. Infection of human amnion cells with cytomegalovirus. J Med Virol. 1978;2:369–375. doi: 10.1002/jmv.1890020410. [DOI] [PubMed] [Google Scholar]
- Ganu RS, Ma J, Aagaard KM. The role of microbial communities in parturition: is there evidence of association with preterm birth and perinatal morbidity and mortality? Am J Perinatol. 2013;30:613–624. doi: 10.1055/s-0032-1329693. [DOI] [PubMed] [Google Scholar]
- Gibson CS, Goldwater PN, MacLennan AH, Haan EA, Priest K, Dekker GA. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population. BJOG. 2008;115:492–500. doi: 10.1111/j.1471-0528.2007.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CS, Maclennan AH, Haan EA, Priest K, Dekker GA. Fetal MBL2 haplotypes combined with viral exposure are associated with adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:847–854. doi: 10.3109/14767058.2010.531324. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23:709–715. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- Han X, Li X, Yue SC, Anandaiah A, Hashem F, Reinach PS, Koziel H, Tachado SD. Epigenetic regulation of tumor necrosis factor alpha (TNFalpha) release in human macrophages by HIV-1 single-stranded RNA (ssRNA) is dependent on TLR8 signaling. J Biol Chem. 2012;287:13778–13786. doi: 10.1074/jbc.M112.342683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Herve R, Schmitz T, Evain-Brion D, Cabrol D, Leroy MJ, Mehats C. The PDE4 inhibitor rolipram prevents NF-kappaB binding activity and proinflammatory cytokine release in human chorionic cells. J Immunol. 2008;181:2196–2202. doi: 10.4049/jimmunol.181.3.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, Abrahams VM. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod. 2014;27:90. doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- Investigators AI, Australasian Maternity Outcomes Surveillance S. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Buchmayer S, Harlid S, Iliadou A, Sjoholm M, Grillner L, Norman M, Sparen P, Dillner J, Cnattingius S. Infection with parvovirus B19 and herpes viruses in early pregnancy and risk of second trimester miscarriage or very preterm birth. Reprod Toxicol. 2008;26:298–302. doi: 10.1016/j.reprotox.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17:619–628. doi: 10.1177/1933719110373148. [DOI] [PubMed] [Google Scholar]
- Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–1355. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Potter JA, Abrahams VM. Human endometrial endothelial cells generate distinct inflammatory and antiviral responses to the TLR3 agonist, Poly(I:C) and the TLR8 agonist, viral ssRNA. Am J Reprod Immunol. 2013;70:190–198. doi: 10.1111/aji.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Kumazaki K, Ozono K, Yahara T, Wada Y, Suehara N, Takeuchi M, Nakayama M. Detection of cytomegalovirus DNA in human placenta. J Med Virol. 2002;68:363–369. doi: 10.1002/jmv.10212. [DOI] [PubMed] [Google Scholar]
- Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64:644–647. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- Lappas M. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod. 2013;89:14. doi: 10.1095/biolreprod.113.110056. [DOI] [PubMed] [Google Scholar]
- Lappas M. Caspase-1 Activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. Am J Reprod Immunol. 2014;71:189–201. doi: 10.1111/aji.12174. [DOI] [PubMed] [Google Scholar]
- Leroy MJ, Dallot E, Czerkiewicz I, Schmitz T, Breuiller-Fouche M. Inflammation of choriodecidua induces tumor necrosis factor alpha-mediated apoptosis of human myometrial cells. Biol Reprod. 2007;76:769–776. doi: 10.1095/biolreprod.106.058057. [DOI] [PubMed] [Google Scholar]
- Li Q, Kawamura K, Tada Y, Shimada H, Hiroshima K, Tagawa M. Novel type III interferons produce anti-tumor effects through multiple functions. Front Biosci. 2013;18:909–918. doi: 10.2741/4152. [DOI] [PubMed] [Google Scholar]
- Luo G, Abrahams VM, Tadesse S, Funai EF, Hodgson EJ, Gao J, Norwitz ER. Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17:532–539. doi: 10.1177/1933719110363618. [DOI] [PubMed] [Google Scholar]
- Marshall-Clarke S, Downes JE, Haga IR, Bowie AG, Borrow P, Pennock JL, Grencis RK, Rothwell P. Polyinosinic acid is a ligand for toll-like receptor 3. J Biol Chem. 2007;282:24759–24766. doi: 10.1074/jbc.M700188200. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Uchide N, Shono M, Ohyama K, Takeichi M, Toyoda H. Differences in permissive cytomegalovirus infection between primary cultured human fetal membrane chorion and amnion cells. Biol Pharm Bull. 2013;36:1715–1721. doi: 10.1248/bpb.b13-00200. [DOI] [PubMed] [Google Scholar]
- McDonald B, Kubes P. Chemokines: sirens of neutrophil recruitment-but is it just one song? Immunity. 2010;33:148–149. doi: 10.1016/j.immuni.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis—a complex pathophysiologic syndrome. Placenta. 2010;31:113–120. doi: 10.1016/j.placenta.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Michaan N, Amzallag S, Laskov I, Cohen Y, Fried M, Lessing JB, Many A. Maternal and neonatal outcome of pregnant women infected with H1N1 influenza virus (swine flu) J Matern Fetal Neonatal Med. 2012;25:130–132. doi: 10.3109/14767058.2011.562569. [DOI] [PubMed] [Google Scholar]
- Nace J, Fortunato SJ, Maul H, Menon R. The expression pattern of two novel cytokines (IL-24 and IL-29) in human fetal membranes. J Perinat Med. 2010;38:665–670. doi: 10.1515/jpm.2010.093. [DOI] [PubMed] [Google Scholar]
- Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109:739–749. doi: 10.1097/01.AOG.0000255663.47512.23. [DOI] [PubMed] [Google Scholar]
- Philbin VJ, Dowling DJ, Gallington LC, Cortes G, Tan Z, Suter EE, Chi KW, Shuckett A, Stoler-Barak L, Tomai M, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204 e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirianov G, Waddington SN, Lindstrom TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- Rokos K, Wang H, Seeger J, Schafer A, Pauli G. Transport of viruses through fetal membranes: an in vitro model of perinatal transmission. J Med Virol. 1998;54:313–319. doi: 10.1002/(sici)1096-9071(199804)54:4<313::aid-jmv12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992a;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992b;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl. 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, Elovitz MA. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Strong BS, Young SA. Intrauterine coxsackie virus, group B type 1, infection: viral cultivation from amniotic fluid in the third trimester. Am J Perinatol. 1995;12:78–79. doi: 10.1055/s-2007-994407. [DOI] [PubMed] [Google Scholar]
- Tsekoura EA, Konstantinidou A, Papadopoulou S, Athanasiou S, Spanakis N, Kafetzis D, Antsaklis A, Tsakris A. Adenovirus genome in the placenta: association with histological chorioamnionitis and preterm birth. J Med Virol. 2010;82:1379–1383. doi: 10.1002/jmv.21820. [DOI] [PubMed] [Google Scholar]
- Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303:1829. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- Uchide N, Ohyama K, Bessho T, Yuan B, Yamakawa T. Apoptosis in cultured human fetal membrane cells infected with influenza virus. Biol Pharm Bull. 2002a;25:109–114. doi: 10.1248/bpb.25.109. [DOI] [PubMed] [Google Scholar]
- Uchide N, Ohyama K, Yuan B, Sano T, Bessho T, Yamakawa T. Differential mRNA expression of inflammatory cytokines in cultured human fetal membrane cells responding to influenza virus infection. Biol Pharm Bull. 2002b;25:239–243. doi: 10.1248/bpb.25.239. [DOI] [PubMed] [Google Scholar]
- Uchide N, Suzuki A, Ohyama K, Bessho T, Toyoda H. Secretion of bioactive interleukin-6 and tumor necrosis factor-alpha proteins from primary cultured human fetal membrane chorion cells infected with influenza virus. Placenta. 2006;27:678–690. doi: 10.1016/j.placenta.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Uchide N, Ohyama K, Bessho T, Toyoda H. Lactate dehydrogenase leakage as a marker for apoptotic cell degradation induced by influenza virus infection in human fetal membrane cells. Intervirology. 2009;52:164–173. doi: 10.1159/000224644. [DOI] [PubMed] [Google Scholar]
- Uchide N, Ohyama K, Bessho T, Takeichi M, Toyoda H. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomes associated with influenza virus infection. Mediators Inflamm. 2012;2012:270670. doi: 10.1155/2012/270670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N, Blander JM. Sensing microbial RNA in the cytosol. Front Immunol. 2013;4:468. doi: 10.3389/fimmu.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]