Abstract
This project assessed dyspraxia in high-functioning school aged children with autism with a focus on Ideational Praxis. We examined the association of specific underlying motor function including eye movement with ideational dyspraxia (sequences of skilled movements) as well as the possible role of visual-motor integration in dyspraxia. We found that compared to IQ-, sex- and age-matched typically developing children, the children with autism performed significantly worse on: Ideational and Buccofacial praxis; a broad range of motor tests, including measures of simple motor skill, timing and accuracy of saccadic eye movements and motor coordination; and tests of visual-motor integration. Impairments in individual children with autism were heterogeneous in nature, although when we examined the praxis data as a function of a qualitative measure representing motor timing, we found that children with poor motor timing performed worse on all praxis categories and had slower and less accurate eye movements while those with regular timing performed as well as typical children on those same tasks. Our data provide evidence that both motor function and visual-motor integration contribute to dyspraxia. We suggest that dyspraxia in autism involves cerebellar mechanisms of movement control and the integration of these mechanisms with cortical networks implicated in praxis.
Keywords: Autism, Dyspraxia, Motor, Visual-Motor Integration, Eye Movement, Cerebellum
1. INTRODUCTION
Autism is a heterogeneous behavioral disorder characterized by deficits in social interactions and reciprocal or non-verbal communication and by the presence of restricted, repetitive or stereotyped behaviors or interests. The US Center for Disease Control estimates that 1 in 88 children in the USA meet the diagnosis for an Autism Spectrum Disorder (http://www.cdc.gov/Features/CountingAutism/). This represents a 78% increase in rate since the CDC 2007 report and a major public health concern. Increasing rates of autism have made research into the etiology and potential treatment of autism increasingly important.
Currently, motor deficits are not included in the diagnostic criteria of Autism Spectrum Disorder (ASD); however, motor deficits are prominent in the presentation of children and adults with autism. In fact, Dr. Leo Kanner’s initial description of autism noted motor deficits describing the children as “somewhat clumsy in gait and gross motor performance” [1]. Continuing research has only strengthened Dr. Kanner’s observations. Studies have consistently shown motor deficits across the autism spectrum (reviews\ [2-4]) including abnormalities in muscle tone, gross and fine motor skills [5-10], gait [11-15], balance [5, 14, 16], motor planning [17, 18], motor coordination and specific common tasks such as reach-to-grasp [19]. In addition, these deficits often present early in life [20-22] and may be among the most useful predictors of a future autism diagnosis [23-26]. Development of motor skills in toddlers with autism may also predict clinical outcome in middle childhood [27].
Eye movements are an important subset of motor deficits in autism although the literature describing these deficits is not always consistent (review\[28]). Many eye movement studies in autism have addressed the way in which social information affects eye movement and have not examined the characteristics of saccades. For those studies that do test the timing, quality and accuracy of saccades in autism, many have found eye movements to be slow to initiate, inaccurate and highly variable in amplitude [29-35] but some others have found no differences in latency of saccade initiation or saccade accuracy [36, 37].
The correlation between motor deficits and deficits in the praxis system in other pathologies (particularly stroke) has provided a model for the way in which the praxis system may also be implicated in the motor deficits of autism. The term “apraxia” originated in the adult neurology literature to describe an acquired brain lesion which results in an impaired ability to carry out learned skilled movements—in the absence of underlying motor, sensorimotor or cognitive deficits. However, in the pediatric neurology literature, the term “apraxia” is somewhat confusing, and is often modified to “dyspraxia”, describing a less severe developmental form of impairment. In addition, underlying motor deficits are the rule rather than the exception in pediatric cases. Steinman et al. proposed a modification in the definition of developmental dyspraxia to describe impairments in the execution of skilled, purposeful or coordinated motor activity that are out of proportion to any underlying motor deficits [38]. This is the definition used in this manuscript.
Dyspraxia has been widely reported in autism-- particularly ideomotor dyspraxia (impaired performance of skilled motor acts). These deficits take several forms including impaired imitation of skilled gestures, a finding first demonstrated in 1972 and replicated many times since [39-43] (review\[44]. In fact, deficits in imitation have been so widely reported in autism that some authors have suggested that impaired imitation may be a core deficit in autism [45]. Other studies have shown that deficits in gesture in autism are not limited to imitation, but also include impairments in gestures to command as well as gestures with tool use [40, 46-48]. Although less well studied, ideational (sequences of skilled motor acts), limb-kinetic (distal limb movements) and buccofacial (skilled movements involving the face, mouth, tongue, larynx, and pharynx) dyspraxia have also been described in autism [49].
While the skilled movements that represent dyspraxia clearly depend upon basic motor skills that are impaired in autism, a number of studies have demonstrated that these motor deficits alone cannot account for dyspraxia in autism [46, 47, 50]. Mostofsky and colleagues have proposed that in addition to basic motor skill, praxis depends upon “knowledge of representation of movement” and the coding of those representations into motor plans [18, 51, 52]. Other studies have suggested that underlying factors may include problems with sensory feedback [18, 48, 53] or reduced connectivity among networks controlling praxis [51, 54]—a hypothesis that is consistent with more general models of reduced long-distance structural and functional connectivity in autism [55-59]. A review of twenty-one praxis studies done over thirty years with 281 ASD children implicated visuo-motor “coupling” mechanisms as a contributing factor [44]. Problems with sensory-motor integration are quite consistent with a large literature suggesting more general deficits in the long-distance connectivity that supports associative or integrative processes [55-57, 60-63].
Studies developing these models of dyspraxia in autism have examined imitative and non-imitative skilled gestures (ideomotor dyspraxia)--other forms of dyspraxia that are also impaired in autism are less well studied. This project assessed dyspraxia in school aged children with autism with a focus on Ideational Praxis in addition to a set of basic motor tasks to assess specific motor functions underlying the praxis tests. This study was designed to examine the association of specific underlying motor function with ideational dyspraxia and potential additional contributions of oculomotor function and visual-motor integration.
2. METHODS
2.1 Participants (See Table 1)
Table 1.
Participants’ demographic characteristics, Mean (Standard Deviation): IQ [58], ADOS diagnostic scores [87], and social responsiveness [57]
| TD | ASD | T-Test | |
|---|---|---|---|
| N | 20 | 20 | |
| Sex | 14 M, 6 F | 17 M, 3 F | |
| Age | 11.53 (2.5) | 12.06 (2.2) | NS |
| WASI-II Verbal IQ | 115.8 (14) | 97.4 (18) | p < 0.001 |
| WASI-II Performance IQ | 107.7 (13) | 98.5 (20) | NS |
| ADOS Social (Cutoff= 4) | 8.4 (2) | ||
| ADOS Communication (Cutoff= 2) | 4.0 (2) | ||
| ADOS SB &RI | 3.4 (1) | ||
| SRS Total Score | 47.4 (8) | 83.1 (11) | p < 0.0001 |
Twenty children (17 male) with autism (ASD), age 8-15 (mean age= 12.1 ± 2) were recruited through an existing sample of children enrolled in UCSD’s Project in Cognitive and Neural Development (PCND) and children participating in on-going studies at the UCSD Research on Autism and Development Laboratory. Children met diagnostic criteria for Autistic Disorder based on the Autism Diagnostic Interview-Revised (ADI-R) and/or the Autism Diagnostic Observation Schedule-Generic (ADOS-G). Individuals had no history of Fragile X syndrome, tuberous sclerosis, fetal CMV, encephalitis or other major medical conditions. Video from the praxis battery was corrupted for one female participant so that for some subtests there are only 19 ASD participants.
Twenty age- and performance-IQ matched typically developing (TD) children (14 male) children, age 7.7-15 (mean age= 11.5 ± 2) were recruited as control subjects through an existing sample of children enrolled in UCSD’s Project in Cognitive and Neural Development (PCND) and children participating in on-going studies at the UCSD Research on Autism and Development Laboratory. Typical control children had no history of learning disability or psychiatric disorders.
All participants had normal or corrected to normal hearing and vision. One participant in each group (ASD, TD) was left-handed others were right hand dominant. All participants received IQ testing and their parents completed the Social Responsiveness Scale (SRS) [64, 65].
2.2 Tests and Procedures
2.2.1 Praxis Testing
Dyspraxia was assessed using a 30-item test compiled from items used in previous tests [47, 48]. This battery was designed to assess ideomotor, ideational and buccofacial dyspraxia as well as basic (simple) motor function (Table 2). Functioning was assessed under multiple conditions (i.e. response to verbal command, imitation, actual tool use etc.) and included both transitive (imitation of actions involving objects) and intransitive skills. The study focus was on performance of skilled learned movements and so non-symbolic gestures were not assessed. Both the number and type of errors were scored. Finally, a general assessment of inherent distal limb deftness was made by evaluating repetitive and sequential finger thumb apposition.
Table 2.
Praxis and Basic Motor Tests.
| Basic Motor | Ideomotor | Ideational | Buccofacial |
|---|---|---|---|
| Pick up Skittles | Blow out Candle | Luria | Stick out Tongue |
| Stack blocks | Wave Goodbye | 3-block bridge | Wiggle Tongue |
| Walk | Close Eyes | 6-block pyramid | Puff out Cheeks |
| Run | Brush Hair | Tandem Gait | Curl Tongue |
| FTAR | Brush Teeth | FTAS | Press Tongue to Cheeks |
| Use Hammer | Pa (say repeatedly at sample pace) | ||
| Use Spoon | Ta (say repeatedly at sample pace) | ||
| Use Hairbrush | Kae (say repeatedly at sample pace) | ||
| Use Toothbrush | Click Tongue | ||
| Use Screwdriver | Use Straw |
Each participant was videotaped performing the praxis battery. Two trained, independent raters who were blind to participant diagnosis reviewed each participant video and scored individual test items using scoring metrics described below. After both videos were scored, individual item scores were compared. Any individual discrepancy score of 2 or greater was reviewed a third time. Obvious scoring mistakes were corrected, but judgment decisions on the part of the scorers were allowed to stand and were averaged to calculate a final dyspraxia score.
Ideomotor dyspraxia was assessed with tasks that followed the general pattern “Show me how to … (e.g. brush your hair).” Participants were asked to pantomime five common transitive and intransitive movements to oral command. If the participant failed to properly pantomime the task, they were instructed to imitate the examiner performing the task. In addition, subjects were asked to demonstrate correct usage of five common tools. A numerical score was assigned to each individual task (2= correct, 1= distorted/incorrect, 0= not completed).
Ideational dyspraxia tasks required the participant to perform a sequence of actions in a prescribed order. Five individual tasks assessed ideational dyspraxia including: finger thumb apposition-sequential (FTAS); the Luria fist test (repeated sequence of 3 movements, fist, open hand, side hand); 3-block bridge building, 6-block pyramid building; and tandem gait. While Tandem Gait is clearly a test of balance, our rationale for including it in the Ideational Praxis battery is that is does require a sequence of movements. We observed that many children had some difficulty with the sequence (e.g., placing foot behind rather than in front). Except for FTAS and Tandem Gait, all tasks were scored subjectively and rated with scores ranging from 0-3 (3 = Subject correctly performs the task with 0 repeated demonstration; 2 = Subject correctly performs the task with 1 repeat demonstration; 1 = Subject correctly performs the task with 2 repeat demonstrations; 0 = Subject unable to correctly perform the task).
FTAS was scored as the average number of correct sequences completed in two 10-second trials for each hand. In addition to quantitative scoring, FTAS was assessed qualitatively with a standard descriptor (regular/rhythmic, irregular/dysrhythmic or slow/halting). FTAS error types were tabulated and classified as specific sequencing errors (e.g., start on wrong finger, omit a step, duplicate a step, ‘slur’ a transition). Tandem gait was qualitatively assessed with a standard descriptor (stable gait/balance, clumsy gait or poor balance) and rated with numerical scores assigned to the participants starting position (1=correct, 0=incorrect) and dynamic positioning (2=correct, 1=incorrect/distorted, 0=no attempt). These scores were summed for analysis in the battery.
Buccofacial dyspraxia tests required the subject to perform with ten common tasks involving the tongue, lips and muscles of facial expression. Each individual task was assessed a numerical score (2=correct, 1= distorted, 0= not completed). Errors were classified according to common error types (e.g., perseverative or verbal description instead of movement).
Basic (Simple) motor function was assessed with a series of five tasks: Pick up Skittles (Use a pincer grasp to relocate a small object (i.e. Skittles, Goldfish etc) from the table to a nearby cup), Stack Blocks (Stack 6 1×1 cm blocks on top of each other to form a tower), Walk (Walk 15′), Run (Run 15′) and Finger Thumb Apposition Repetitions (FTAR, touch the thumb (finger 1) to the index finger (finger 2 ) as many times as possible in ten seconds). Pick up Skittles, Stack Blocks, Walk and Run were rated (2=correct, 1= distorted, 0= not completed). FTAR was scored as a total number of repetitions completed in two 10-second trials with each hand and the results averaged. Qualitatively, FTAR was assessed with a standard descriptor (regular/rhythmic, irregular/dysrhythmic or slow/halting).
This set of fine and gross motor tasks served as baseline tasks for the praxis battery and particularly for ideational praxis, representing the simple movements underlying the complex ideational praxis tasks as follows: Pick up Skittles and FTAR used the simple pincer grasp/finger thumb apposition required for FTAS; Stack Blocks used the same movements used in the more complex 3-block bridge building and 6-block pyramid building; walk and run served as basic gross motor and balance controls for the more complex tandem gait task.
2.2.2 Beery VMI
The VMI, VMI Supplemental Developmental Test of Visual Perception, and VMI Supplemental Developmental Test of Motor Coordination tests were administered to each participant as described in the VMI Administration, Scoring, and Teaching Manual [66]. Each test was scored by two individual raters in accordance with the VMI Administration, Scoring and Teaching Manual. The VMI is a normed test used to assess visual-motor integration ability, in this case, the coordination of visual perception and finger-hand movements. Performance on the VMI requires participants to copy geometric forms of increasing difficulty with paper and pencil. During the Visual Perception test, participants are given a sample shape at the top of the list of shapes and are asked to identify its exact match from a list of similar looking forms in a specified period of time. The Motor Coordination test is also timed and the task is to trace within the boundaries of a given shape without going outside the lined paths. Starting and ending dots are also provided to indicate where the trace should start and end for each line segment that comprises each geometric form in order to lessen the perceptual requirements of the task. The Developmental Test of Visual Perception and the Developmental Test of Motor Coordination utilize subsets of the same geometric forms presented during the VMI, but are designed to minimize either motor or visual-perceptual demands in order to determine whether a given participant’s performance on the VMI test is affected by a difficulty with either visual perception or motor coordination, or whether the participant has difficulty with integrating the two domains effectively.
2.2.3 Gap/Null/Overlap Paradigm
This paradigm is commonly used to assess eye movement and attention and has been employed in a number of autism studies [29, 37, 67-69]. In this study, subjects were seated approximately 500 mm from a standard 16” computer monitor. Visual stimuli consisted of a central fixation target surrounded by possible saccade target locations on two concentric circles located at of 4.9° and 9.8° from the central fixation target. Eight possible target locations were located on each concentric circle for a total of 16 total peripheral target locations. The central fixation target either 1) extinguished 200 ms before the peripheral target appeared (gap condition), 2) extinguished simultaneously with the onset of the peripheral target (null condition), or 3) extinguished 200 ms after the peripheral target appeared (overlap condition). Each trial began with the participant focused on a central fixation target. And subjects were instructed to move their eyes to the peripheral target “as quickly as possible.” Two trials of each condition were presented at each of the 16 peripheral target locations for a total of 96 trials. Eye movement dynamics (saccade latency, accuracy etc.) were monitored using the EyeLink 1000 remote eye-tracking system. Only the Null condition results are reported here to assess oculomotor function. The Gap and Overlap conditions will be reported separately in an examination of eye movement and spatial attention.
3. RESULTS
Statistical analyses used BMDP Statistical software [70]. In analyses described below, if sample variances of the autism and typical groups were unequal (based on the Levene F-test), a separate variance t-test (Welch) which does not assume variance equality is reported.
3.1 Praxis (See Figure 1-2)
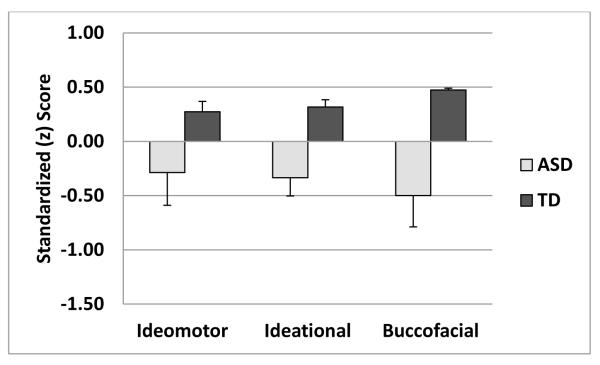
Figure 1.
shows standardized scores for three praxis categories. ASD children have significantly worse performance than typical children on Ideational and Buccofacial Praxis, but not Ideomotor.
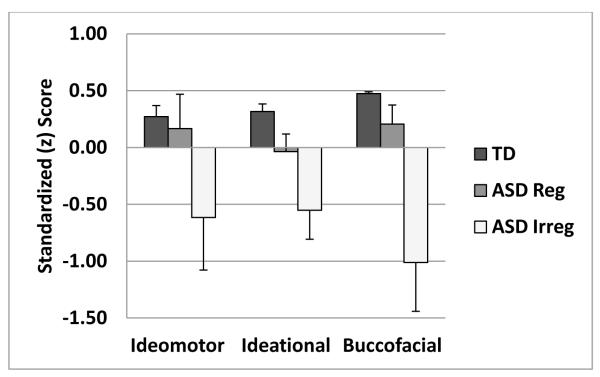
Figure 2.
shows praxis data as a function of rhythmicity/regularity of pacing in Finger-Thumb Apposition Sequencing. ASD children whose movements were judged to be irregular were significantly worse than typical children on all three praxis categories. ASD children whose movements were judged to be rhythmic/regular did not differ significantly from typical children on any of the praxis categories.
Subtests that were combined to index each of the three categories of praxis we tested (Ideomotor, Ideational, Buccofacial and Simple Motor control tasks). Individual tests scores have different scales and so were transformed to z-scores based on the mean and standard deviation of the entire sample. These standardized scores for components of each praxis type were averaged to produce a single (z-score) index for each subject for each category. Analyses examining the effect of age as a covariate showed no significant relationship between age and praxis in this sample, so age was not used as a covariate in the following analyses.
A multivariate analysis (Hotelling T2) using all three praxis categories showed that compared to typical children, those with autism showed significantly greater levels of dyspraxia (F(3,36)=4.72, p < 0.007). Follow-up tests for each category found worse function for children with autism for Ideational Dyspraxia (ASD mean z-score=−0.34 ± 0.7, TD mean z-score =0.32 ± 0.3; t(37)=−3.65, p < 0.001 and Buccofacial Dyspraxia (ASD mean z-score=−0.50 ± 1.3, TD mean z-score =0.47 ± 0.1; t(37)=3.36, p < 0.004, corrected for unequal group variances), with a trend for group differences in Ideomotor Dyspraxia (ASD mean z-score=−0.28 ± 1.3, TD mean z-score =−0.27 ± 0.4; t(37)=−1.75, p < 0.09).
Children with autism also showed worse performance than typical children on tasks assessing basic motor function (ASD mean z-score=−0.16 ± 0.6, TD mean z-score =0.17 ± 0.3; t(35)=−2.16, p < 0.04).
Qualitative scoring for praxis subtests also highlighted differences between children with autism and typically developing children. In the Finger-Thumb Apposition Repetitions (FTAR) subtest of the Basic Motor tasks, 53% of ASD children were judged by raters (blinded to the child’s diagnostic category) to have movements that were irregular, dysrhythmic or slow and halting. All TD children’s movements were judged to be rhythmic and regular. ASD children whose movements were judged to be dysrhythmic, irregular or slow/halting produced fewer repetitions than did other ASD or TD children whose movements were judged to be rhythmic and regular (58± 19 vs. 67± 4, 72± 14, respectively).
There were also qualitative differences between ASD and TD children in the Finger-Thumb Apposition-Sequential (FTAS) subtest of the Ideational Dyspraxia Index. In the FTAS, 58% of ASD children and 10% of TD children were judged by raters (blinded to the child’s diagnostic category) to have movements that were irregular, dysrhythmic or slow and halting. ASD and TD children whose movements were judged to be dysrhythmic, irregular or slow/halting produced fewer correct sequences (Dysrhythmic: ASD, 7.6 ± 4, TD, 5.9 ± 1; Rhythmic: ASD 10.5 ± 2, TD 13.2 ± 4) and made more sequencing errors (dysrhythmic: ASD, 4.3 ± 3, TD, 8.1 ± 3; Rhythmic: ASD 1.4 ± 1, TD 1.7 ± 2) than did children whose movements were judged to be rhythmic and regular. ASD children whose movements were judged to be dysrhythmic performed worse on all three praxis tests than did TD children (p < 0.05 for all) while ASD children whose movements were judged to be rhythmic performed similarly to TD children (p > 0.1 for all), see Figure 2.
Finally, raters also noted whether or not participants looked at their hands during the FTAR and the FTAS. During FTAR, 58% of ASD and 35% of TD participants looked at their hands. During FTAS the majority of children in both groups looked at their hands (84% ASD, 75% TD).
3.2 Eye Movement (See Figure 3)
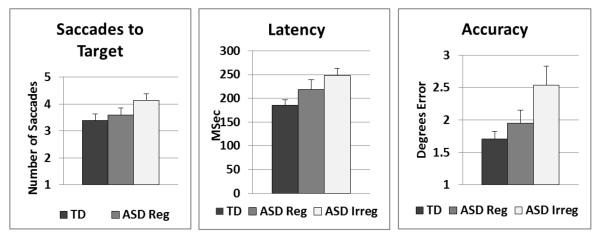
Figure 3.
shows eye movement data as a function of rhythmicity/regularity of pacing in Finger-Thumb Apposition Sequencing. Compared to typical children, ASD children whose movements were judged to be irregular were significantly slower to initiate saccades (Latency), had larger error in saccades to the target (Accuracy) and required more saccades to reach the target (Saccades to Target). ASD children whose movements were judged to be rhythmic/regular did not differ significantly from typical children on any of the eye movement measures.
Eye movement variables analyzed were Saccade Latency (time to initiate the first saccade to the target), Saccade Accuracy (distance in degrees from the target for the first saccade to the target) and the Number of Saccades required to reach the target (within 1 degree). For each of these measures, variability was greater in the ASD than in the TD groups, although only variability for latency reached significance at alpha of 0.05. All tests are corrected for unequal group variances. For Latency, standard deviation = 199.7 msec ASD and 72.5 msec TD (t(37) = 2.66, p < 0.015). For Accuracy, standard deviation = 2.3 degrees ASD and 1.4 degrees TD (t(37) = 1.70, p < 0.10). For Number of saccades, standard deviation = 1.8 ASD and 1.3 TD (t(37) = 1.80, p < 0.08).
Multivariate analysis (Hotelling T2) comparing these three measures in children with autism and typical children showed overall worse eye movement performance in those with autism (F(3,36)=4.68, p < 0.008). Follow-up tests showed that compared to typical children, those with autism were slower to initiate saccades (ASD mean= 238.2 ± 70 msec, TD mean= 179.9 = ± 21 msec; t(37)=3.58, p < 0.002, corrected for unequal group variances), less accurate (ASD mean degrees error= 2.29 ± 1.7, TD mean degrees error= 1.71 = ± 0.3; t(37)=2.20, p < 0.04, corrected for unequal group variances) and required more saccades to reach the target (ASD mean = 4.1 ± 1, TD mean = 3.4 = ± 1; t(38)=2.23, p < 0.035). As for praxis measures above, when the ASD group was separated as a function of regular/rhythmic or irregular/dysrhythmic movement on the FTAS, ASD children whose movements were judged to be dysrhythmic performed worse on all three eye movement functions than did TD children (p < 0.05 for all) while ASD children whose movements were judged to be rhythmic performed similarly to TD children (p > 0.1 for all), see Figure 3.
3.3 Visual-Motor Integration
Standard scores (SS) from the Beery VMI [66] showed worse performance for children with autism compared to typical children on the test of Visual-Motor Integration (ASD mean SS=84.7 ± 18, TD mean SS=104.7 ± 15; t(37)=3.42, p < 0.0017) and the supplemental test of Motor Coordination (ASD mean SS=74.0 ± 17, TD mean SS=84.2 ± 10; t(37)=2.30, p < 0.03, corrected for unequal group variances). However, there were no significant differences between groups on the supplemental test of Visual Perception (ASD mean SS=93.2 ± 15, TD mean SS=100.7 ± 10; t(37)=1.78, p > 0.08).
3.4 Correlation Analyses (See Figure 4-5)
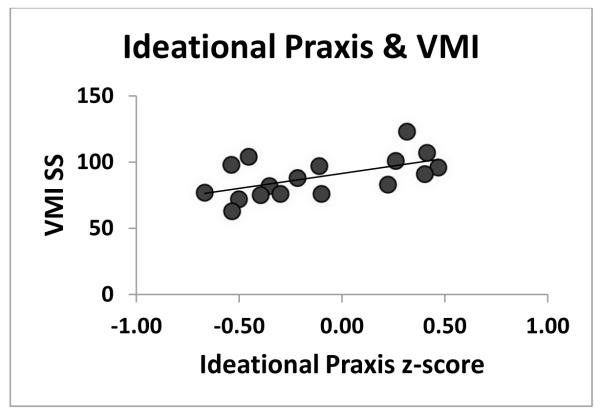
Figure 4.
shows better performance on the Visual-Motor Integration test Standard Score [66] associated with better performance on the Ideational Praxis battery. Two extreme scores (very low Praxis and very low VMI performance) that influenced the size of the correlation coefficient were omitted. The resulting correlation was significant (r(15)=0.57, p < 0.009) as was the non-parametric correlation using all data reported in the results (rs (17)=0.63, p < 0.002).
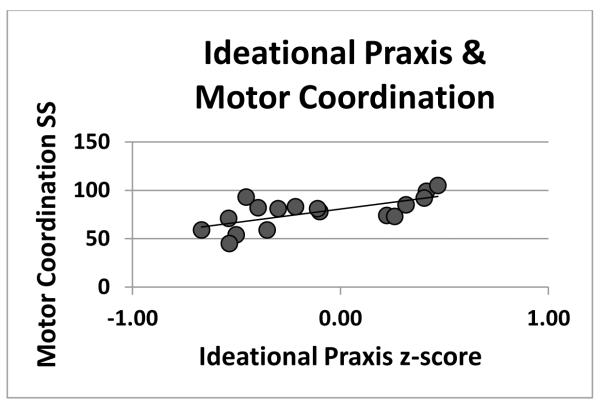
Figure 5.
shows better performance on the VMI Motor Coordination test Standard Score [66] associated with better performance on the Ideational Praxis battery. Two extreme scores (very low Praxis and very low Motor Coordination performance—the same participants as in Figure 4) that influenced the size of the correlation coefficient were omitted. The resulting correlation was significant (r(15)=0.67, p < 0.002) as was the non-parametric correlation using all data reported in the results (rs (17)=0.75, p < 0.0001).
As there are significant group differences in the dependent measures, correlations were computed within group. Because these are small samples with some extreme data points, correlations were analyzed using non-parametric Spearman Ranked (rs) statistics (in which the influence of extreme data points is minimized).
There was no association in either the ASD or the TD groups between Ideational Dyspraxia and the simple motor control index. However, in the children with autism, greater Ideational Dyspraxia was significantly associated with worse performance on standardized tests of both Visual-Motor Integration (rs (17)=0.63, p < 0.002) and Motor Coordination (rs (17)=0.75, p < 0.0001). These measures were not correlated in TD children.
In the children with autism, greater Ideational Dyspraxia was associated with increased autistic mannerisms (SRS subscale) (rs (17)=−0.40, p < 0.05) and with increased repetitive behaviors and restricted interests (ADOS-G subscale) (rs(17)=−0.47, p < 0.02).
4. DISCUSSION
Echoing previous studies of motor abilities and praxis in autism, we found significant differences between ASD and typically developing children across a broad range of motor tests, including measures of simple motor skill, praxis, saccadic eye movements, motor coordination and visual-motor integration.
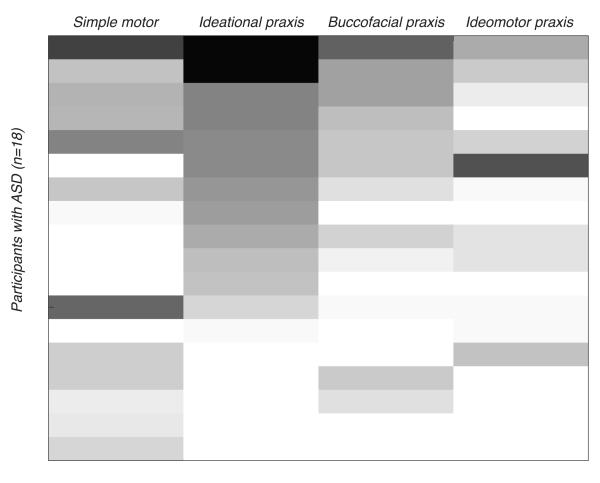
Departing from past reports, we found group differences in ideational and buccofacial dyspraxia, but not ideomotor dyspraxia. However, while we found no group differences in the type of skilled movements that involve imitation, using tools or pantomiming the use of tools that make up the ideomotor praxis scale, performance on these tasks was highly variable in the ASD group. As Figure 6 shows, both the type of praxis and the number of affected subtests in each category varied widely in our sample, with a few children showing little to no impaired skills and others showing deficits in the majority of skills tested.
Figure 6.
shows variability within and across ASD participants for simple motor and praxis indices. Shading represents the number of subtests affected (typical/atypical) on each praxis category and simple motor index. Scores on each dyspraxia battery subtest were summed by first normalizing the scores for each test and then averaging the tests for each subscale. For the numerical tasks like FTAR and FTAS, participants scored a “1” for normal if their score was with one standard deviation of the mean of the typical participants and “0” or atypical if it was outside that range. Lightest shade represents least impaired performance (lowest number of atypical subtests)—darkest represents most impaired (highest number of atypical subtests).
Previous studies, e.g. , [47, 50, 51] have found an association with basic motor skill level and dyspraxia that suggests underlying motor function can explain dyspraxia at least in part. While we found no such association with our simple motor tasks and dyspraxia, we did find evidence of association with dyspraxia and motor coordination. The lack of association with dyspraxia and our set of basic motor tasks could be due to the generally high level of function in our ASD children and differences between our assessments of motor function which involved only a few tasks compared to other commonly used batteries (e.g., PANESS, BOT, M-ABC) [71-73]. We did find better performance on a standardized test of fine motor coordination was strongly associated with better performance on praxis tasks. Also, as an additional measure of underlying motor skill, we examined the praxis data as a function of a qualitative measure representing motor timing (a rating of regular pacing or rhythmicity in the Finger-Thumb Apposition Sequencing task) and found that children with poor timing performed worse on all praxis categories while those with regular timing performed as well as typical children on those same praxis categories. The children with dysrhythmic movements were also more impaired on all of the eye movement measurements (number of saccades to reach the target, accuracy and timing). Given that both eye movement and the complex movements that make up the praxis tests involve cerebellar circuitry [74-78], associations of motor coordination and timing with dyspraxia in autism suggest underlying cerebellar dysfunction.
Schmahmann and colleagues [79-81] have provided evidence for anatomic and functional pathways from the cerebellum to frontal and parietal cortices via the pons and brainstem that suggest the way in which networks for motor dysfunction and dyspraxia may interact in autism. Bostan, Dum and Strick review cerebro-cerebellar loops that provide additional evidence for pathways that support motor and non-motor processes mediated by frontal and parietal cortices [82]. Mosconi and colleagues [78] have implicated cerebellar vermis dysfunction using a saccade adaptation task and in the same report also note that poor performance on a pegboard task was related to weak adaptation performance. These results suggest that the mechanisms that support the error-based feedback, often thought of as an ‘internal model’, could be deficient in ASD, including mechanisms residing in the cerebellum, signals arising from frontal motor areas, or feedback from the sensory periphery. In addition, reports of long-range connectivity differences in individuals with ASD [55-57, 60-63] would suggest that any modulatory influences the cerebellum might typically have on fronto-parietal circuitry could be unreliable in quality or timing. Given the weight of evidence for cerebellar pathology in autism from postmortem and imaging studies [74, 83-89], hypotheses implicating cerebellar modulation of movement plans are particularly appealing.
Some have suggested that in addition to underlying motor dysfunction, dyspraxia involves an additional component, perhaps an inaccurate internal model of movement [47, 51]. Those studies have, however, primarily assessed imitation and pantomiming (Ideomotor Dyspraxia), and specifically found a high proportion of body part-for-tool errors. We did not see differences in ideomotor dyspraxia, nor body part-for-tool errors. The ideational praxis tests, on which we did observe group differences, involved sequences of actions required to perform somewhat complex tasks. In these tasks a substantial amount of sensorimotor integration (visual for most tasks) is required in addition to effective motor sequencing. While some tasks in our battery could be done without visual feedback (e.g., FTAS), we noted that the majority of children did not depend upon somatosensory feedback alone, but also looked at their hands. In fact, in our data, the strongest associations with dyspraxia were observed in the standardized test for visual-motor integration so that those with better visual-motor integration had better performance on ideational praxis tasks.
Developmental dyspraxia has become increasingly equated with developmental coordination disorder (DCD;[90]). Although there is agreement that developmental dyspraxia is quite different from acquired apraxia in adults, there remains considerable discussion over the details of the definition. Although definitions for DCD or developmental dyspraxia are evolving, attempts at consensus highlight the inability to use voluntary motor skills effectively, especially when demand for precise sensorimotor integration is high [90]. The link between dyspraxia in ASD and DCD is useful because a number of quantitative motor studies have been conducted in children with DCD, and there are just a few in children with ASD. Steinman and colleagues [38] have suggested that developmental dyspraxia be thought of as a neurological sign and not a disorder unto itself. This view seems most appropriate in considering individuals with ASD. In our sample, some individuals with ASD clearly show significant motor and praxis challenges (see Figure 6). Much like other symptoms in autism, however, motor and praxis skills present along a spectrum.
Taken together, the most remarkable finding of our study is a deficit with visual-motor integration, which we believe highlights one of the most important aspects of praxis problems in children with ASD: integration. It also dovetails with recent findings from Haswell et al. [52] and Izawa et al. [53] showing that children with ASD tend to bias proprioceptive over visual feedback when planning a movement. What does it mean to have poor visual-motor integration and how might that effect the results that we observed here?
Poor sensorimotor integration can manifest as difficulty with motor control and praxis in many ways and several examples from the literature are instructive. A recent study in children with DCD showed that the integration of visual and proprioceptive information was atypical [91]. Specifically, children with DCD weighted information from proprioception and visual information differently while attempting to maintain a steady posture. This weighting could be due to differential quality of the two types of sensory information, such as variability in sensory feedback or its timing. Another study reported that children with ASD had difficulty tapping in a steady rhythm and that this finding was correlated with overall motor ability [92]. We also found a similar result in that children with dysrhythmic sequential finger tapping (FTAS) had greater praxis and eye movement problems, underscoring the apparently common problem of timing motor acts in children with ASD. Variability in both timing and accuracy of movement can interfere with the brain’s ability to build appropriate predictions of body position at the end of the movement—what we often refer to as an internal model with an underlying set of cerebellar computations [93, 94]. Bastian and colleagues [95] have recently shown that patients with cerebellar damage appear to rely more heavily on peripheral proprioceptive information, even though it is delayed—a bias that produces the highly variable behavioral results that are typical in patients with acquired cerebellar pathology and in individuals with autism who share this bias [52].
5. Conclusions
In summary, this study has expanded our understanding of the scope of praxis deficits in children with autism by focusing on ideational praxis and aspects of saccadic eye movement control. We report that although as a group children with ASD performed worse than the group of typical controls on all motor and praxis tests, this statement masks a tremendous amount of heterogeneity of deficits both across individuals and across tasks. The presence of dysrhythmic finger-thumb movements which may reflect cerebellar dysfunction in ASD appears to be a feature that identifies impairment in both praxis and eye movement. Although our sample size was somewhat small for examining correlations between measures, strong correlations were observed in the ASD group on measures of visual-motor integration and motor coordination with praxis. These findings suggest closer examinations of sensory-motor integration and similarly integration across multiple movements (coordination) as a useful target for future studies. Taken together with the importance of dysrhythmia, these findings suggest a focus on cerebellar mechanisms of movement control and the integration of these mechanisms with cortical networks implicated in praxis.
Highlights.
Children with autism have difficulty with skilled movements (dyspraxia).
These children also have deficits in basic motor skills including eye movements.
Those with poor motor timing have the greatest motor and praxis deficits.
Deficits in motor skill and visual-motor integration contribute to dyspraxia.
Acknowledgements
This study was funded by the NIH 2 T35 HL 7491-31 (MM), NINDS P50-NS22343 (DT) and NINDS R21-NS070296 (JT). Tyler Brocklehurst (Institute for Neural Computation, UCSD) and Carin Rojas (School of Medicine, Northwestern University) who served as trained raters scoring praxis videos.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- [2].Chukoskie L, Townsend J, Westerfield M. Motor skill in Autism Spectrum Disorder: a subcortical view. In: Konopka G, editor. International Reviews in Neurobiology: Neurobiology of Autism. Elsevier; In Press. [DOI] [PubMed] [Google Scholar]
- [3].Downey R, Rapport MJ. Motor activity in children with autism: a review of current literature. Pediatr Phys Ther. 2012;24:2–20. doi: 10.1097/PEP.0b013e31823db95f. [DOI] [PubMed] [Google Scholar]
- [4].Maski KP, Jeste SS, Spence SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr. 2011;23:609–15. doi: 10.1097/MOP.0b013e32834c9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miyahara M, Tsujii M, Hori M, Nakanishi K, Kageyama H, Sugiyama T. Brief report: motor incoordination in children with Asperger syndrome and learning disabilities. J Autism Dev Disord. 1997;27:595–603. doi: 10.1023/a:1025834211548. [DOI] [PubMed] [Google Scholar]
- [6].Ghaziuddin M, Butler E, Tsai L, Ghaziuddin N. Is clumsiness a marker for Asperger syndrome? J Intellect Disabil Res. 1994;38(Pt 5):519–27. doi: 10.1111/j.1365-2788.1994.tb00440.x. [DOI] [PubMed] [Google Scholar]
- [7].Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys Ther. 2011;91:1116–29. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- [8].Lane A, Harpster K, Heathcock J. Motor characteristics of young children referred for possible autism spectrum disorder. Pediatr Phys Ther. 2012;24:21–9. doi: 10.1097/PEP.0b013e31823e071a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2013;17:133–46. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haas RH, Townsend J, Courchesne E, Lincoln AJ, Schreibman L, Yeung-Courchesne R. Neurologic abnormalities in infantile autism. J Child Neurol. 1996;11:84–92. doi: 10.1177/088307389601100204. [DOI] [PubMed] [Google Scholar]
- [11].Vilensky JA, Damasio AR, Maurer RG. Gait disturbances in patients with autistic behavior: a preliminary study. Arch Neurol. 1981;38:646–9. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- [12].Damasio AR, Maurer RG. A neurological model for childhood autism. Arch Neurol. 1978;35:777–86. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- [13].Rinehart NJ, Tonge BJ, Iansek R, McGinley J, Brereton AV, Enticott PG, et al. Gait function in newly diagnosed children with autism: Cerebellar and basal ganglia related motor disorder. Dev Med Child Neurol. 2006;48:819–24. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- [14].Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J Autism Dev Disord. 2006;36:613–21. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- [15].Weiss MJ, Moran MF, Parker ME, Foley JT. Gait analysis of teenagers and young adults diagnosed with autism and severe verbal communication disorders. Front Integr Neurosci. 2013;7:33. doi: 10.3389/fnint.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Whyatt CP, Craig CM. Motor skills in children aged 7-10 years, diagnosed with autism spectrum disorder. J Autism Dev Disord. 2012;42:1799–809. doi: 10.1007/s10803-011-1421-8. [DOI] [PubMed] [Google Scholar]
- [17].Hughes C. Brief report: planning problems in autism at the level of motor control. J Autism Dev Disord. 1996;26:99–107. doi: 10.1007/BF02276237. [DOI] [PubMed] [Google Scholar]
- [18].Dowd AM, McGinley JL, Taffe JR, Rinehart NJ. Do planning and visual integration difficulties underpin motor dysfunction in autism? A kinematic study of young children with autism. J Autism Dev Disord. 2012;42:1539–48. doi: 10.1007/s10803-011-1385-8. [DOI] [PubMed] [Google Scholar]
- [19].Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philos Trans R Soc Lond B Biol Sci. 2003;358:393–403. doi: 10.1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. J Autism Dev Disord. 1999;29:213–24. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- [21].Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47:629–38. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- [22].Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29:565–70. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [23].Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci U S A. 1998;95:13982–7. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bhat AN, Galloway JC, Landa RJ. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav Dev. 2012;35:838–46. doi: 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Hill Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. J Child Psychol Psychiatry. 2008;49:43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Esposito G, Venuti P, Maestro S, Muratori F. An exploration of symmetry in early autism spectrum disorders: analysis of lying. Brain Dev. 2009;31:131–8. doi: 10.1016/j.braindev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- [27].Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, et al. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. J Autism Dev Disord. 2007;37:98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- [28].Rommelse NN, Van der Stigchel S, Sergeant JA. A review on eye movement studies in childhood and adolescent psychiatry. Brain Cogn. 2008;68:391–414. doi: 10.1016/j.bandc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- [29].Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–49. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- [30].Johnson BP, Rinehart NJ, Papadopoulos N, Tonge B, Millist L, White O, et al. A closer look at visually guided saccades in autism and Asperger’s disorder. Front Integr Neurosci. 2012;6:99. doi: 10.3389/fnint.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61:474–81. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- [32].Rosenhall U, Johansson E, Gillberg C. Oculomotor findings in autistic children. J Laryngol Otol. 1988;102:435–9. doi: 10.1017/s0022215100105286. [DOI] [PubMed] [Google Scholar]
- [33].Takarae Y, Minshew NJ, Luna B, Sweeney JA. Oculomotor abnormalities parallel cerebellar histopathology in autism. J Neurol Neurosurg Psychiatry. 2004;75:1359–61. doi: 10.1136/jnnp.2003.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kourkoulou A, Kuhn G, Findlay JM, Leekam SR. Eye movement difficulties in autism spectrum disorder: implications for implicit contextual learning. Autism Res. 2013;6:177–89. doi: 10.1002/aur.1274. [DOI] [PubMed] [Google Scholar]
- [35].Kuhn G, Kourkoulou A, Leekam SR. How magic changes our expectations about autism. Psychol Sci. 2010;21:1487–93. doi: 10.1177/0956797610383435. [DOI] [PubMed] [Google Scholar]
- [36].Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–22. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention, and autism: a saccadic reaction time study using the gap and overlap paradigm. Biol Psychiatry. 2001;50:614–9. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- [38].Steinman KJ, Mostofsky SH, Denckla MB. Toward a narrower, more pragmatic view of developmental dyspraxia. J Child Neurol. 2010;25:71–81. doi: 10.1177/0883073809342591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].DeMeyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN, et al. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. J Autism Child Schizophr. 1972;2:264–87. doi: 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- [40].Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Dev. 1996;67:2060–73. [PubMed] [Google Scholar]
- [41].MacNeil LK, Mostofsky SH. Specificity of dyspraxia in children with autism. Neuropsychology. 2012;26:165–71. doi: 10.1037/a0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44:763–81. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- [43].Smith IM, Bryson SE. Gesture imitation in autism. II. Symbolic gestures and pantomimed object use. Cogn Neuropsychol. 2007;24:679–700. doi: 10.1080/02643290701669703. [DOI] [PubMed] [Google Scholar]
- [44].Williams JH, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J Autism Dev Disord. 2004;34:285–99. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- [45].Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25:287–95. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- [46].Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J Int Neuropsychol Soc. 2006;12:314–26. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- [47].Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49:734–9. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- [48].Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr. 2001;22:92–101. doi: 10.1097/00004703-200104000-00002. [DOI] [PubMed] [Google Scholar]
- [49].Dewey D. Error analysis of limb and orofacial praxis in children with developmental motor deficits. Brain Cogn. 1993;23:203–21. doi: 10.1006/brcg.1993.1055. [DOI] [PubMed] [Google Scholar]
- [50].Dewey D, Cantell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2007;13:246–56. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- [51].Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23:563–70. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nat Neurosci. 2009;12:970–2. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Res. 2012;5:124–36. doi: 10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mostofsky SH, Ewen JB. Altered connectivity and action model formation in autism is autism. Neuroscientist. 2011;17:437–48. doi: 10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annual Review of Neuroscience. 2005;28:109–26. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- [56].Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lewis JD, Theilmann RJ, Fonov V, Bellec P, Lincoln A, Evans AC, et al. Callosal fiber length and interhemispheric connectivity in adults with autism: brain overgrowth and underconnectivity. Hum Brain Mapp. 2013;34:1685–95. doi: 10.1002/hbm.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lewis JD, Theilmann RJ, Townsend J, Evans AC. Network efficiency in autism spectrum disorder and its relation to brain overgrowth. Front Hum Neurosci. 2013;7:845. doi: 10.3389/fnhum.2013.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Townsend J, Westerfield M. Autism and Asperger’s Syndrome: A Cognitive Neuroscience Perspective. In: Armstrong C, Morrow L, editors. Handbook of Medical Neuropsychology. Springer Science; New York: 2010. pp. 165–91. [Google Scholar]
- [61].Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- [62].Keehn B, Shih P, Brenner LA, Townsend J, Muller RA. Functional connectivity for an “Island of sparing” in autism spectrum disorder: An fMRI study of visual search. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62:270–3. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Constantino JN. Social Responsiveness Scale (SRS) Western Psychological Services; Los Angeles: 2005. [Google Scholar]
- [65].Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, Harcourt Brace & Company; San Antonio, TX: 1999. [Google Scholar]
- [66].Beery K, Beery NA. The Beery-Buktenica Developmental Test of Visual-Motor Integration with Supplemental Developmental Tests of Visual Perception and Motor Coordination. 5th Edition NCS Pearson, Inc.; Minneapolis, MN: 2006. [Google Scholar]
- [67].Crippa A, Forti S, Perego P, Molteni M. Eye-hand coordination in children with high functioning autism and Asperger’s disorder using a gap-overlap paradigm. J Autism Dev Disord. 2013;43:841–50. doi: 10.1007/s10803-012-1623-8. [DOI] [PubMed] [Google Scholar]
- [68].Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45:1115–22. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- [69].Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, et al. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. 2009;39:1559–66. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dixon W, Brown M, Engelman L, Jennrich R. BMDP Statistical Software Manual (Vols 1-2) University of California Press; Berkeley, CA: 1990. [Google Scholar]
- [71].Bruininks RH. Bruininks-Oseretsky Test of motor proficiency. American Guidance Service; Circle Pines, MN: 1978. [Google Scholar]
- [72].Denckla MB. Revised Neurological Examination for Subtle Signs (1985) Psychopharmacol Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- [73].Henderson SESD. The Movement Assessment Battery for Children. The Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- [74].Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ivry RB. Cerebellar involvement in clumsiness and other developmental disorders. Neural Plast. 2003;10:141–53. doi: 10.1155/NP.2003.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Manto M, Bower JM, Conforto AB, Delgado-Garcia JM, da Guarda SN, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11:457–87. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schlerf JE, Spencer RM, Zelaznik HN, Ivry RB. Timing of rhythmic movements in patients with cerebellar degeneration. Cerebellum. 2007;6:221–31. doi: 10.1080/14734220701370643. [DOI] [PubMed] [Google Scholar]
- [78].Mosconi MW, Luna B, Kay-Stacey M, Nowinski CV, Rubin LH, Scudder C, et al. Saccade adaptation abnormalities implicate dysfunction of cerebellar-dependent learning mechanisms in Autism Spectrum Disorders (ASD) PLoS One. 2013;8:e63709. doi: 10.1371/journal.pone.0063709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schmahmann JD, Rosene DL, Pandya DN. Motor projections to the basis pontis in rhesus monkey. J Comp Neurol. 2004;478:248–68. doi: 10.1002/cne.20286. [DOI] [PubMed] [Google Scholar]
- [80].Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44:1037–66. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–54. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- [84].Bauman ML. Microscopic neuroanatomic abnormalities in autism. Pediatrics. 1991;87:791–6. [PubMed] [Google Scholar]
- [85].Bauman ML, Filipek PA, Kemper TL. Early infantile autism. International Review of Neurobiology. 1997;41:367–86. doi: 10.1016/s0074-7742(08)60360-8. [DOI] [PubMed] [Google Scholar]
- [86].Courchesne E. An MRI study of autism: the cerebellum revisited. Neurology. 1999;52:1106–7. doi: 10.1212/wnl.52.5.1106. [DOI] [PubMed] [Google Scholar]
- [87].Courchesne E, Saitoh O, Townsend JP, Yeung-Courchesne R, Press GA, Lincoln AJ, et al. Cerebellar hypoplasia and hyperplasia in infantile autism. Lancet. 1994;343:63–4. doi: 10.1016/s0140-6736(94)90923-7. [DOI] [PubMed] [Google Scholar]
- [88].Lincoln AJ, Courchesne E, Kilman BA, Elmasian R, Allen M. A study of intellectual abilities in high-functioning people with autism. Journal of Autism and Developmental Disorders. 1988;18:505–24. doi: 10.1007/BF02211870. [DOI] [PubMed] [Google Scholar]
- [89].Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, et al. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25:1–18. doi: 10.1007/BF02178163. [DOI] [PubMed] [Google Scholar]
- [90].Gibbs J, Appleton J, Appleton R. Dyspraxia or developmental coordination disorder? Unravelling the enigma. Arch Dis Child. 2007;92:534–9. doi: 10.1136/adc.2005.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bair WN, Kiemel T, Jeka JJ, Clark JE. Development of multisensory reweighting is impaired for quiet stance control in children with developmental coordination disorder (DCD) PLoS One. 2012;7:e40932. doi: 10.1371/journal.pone.0040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Price KJ, Edgell D, Kerns KA. Timing deficits are implicated in motor dysfunction in Asperger’s Syndrome. Research in Autism Spectrum Disorders. 2012;6:857–60. [Google Scholar]
- [93].Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- [94].Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- [95].Bhanpuri N, Okamura AM, Bastian AJ. Predictive Modeling by the Cerebellum Improves Proprioception. The Journal of Neuroscience. 2013;33:14301–6. doi: 10.1523/JNEUROSCI.0784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]