Abstract
Ceramide is a sphingolipid that activates stress kinases such as p38 and c-JUN N-Terminal Kinase (JNK). Though Chronic Myelogenous Leukemia (CML) derived K562 cells resist killing by short chain C2-ceramide, we report here that longer chain C6-ceramide promotes apoptosis in these cells. C6-ceramide induces cleavage of Caspase-8 and Caspase-9, but only Caspase-8 is required for apoptosis. The sphingolipid killed CML derived KBM5 cells and, to a lesser extent, imatinib-resistant KBM5-STI cells suggesting that BCR-ABL can not completely block C6-ceramide-induced apoptosis but the kinase may regulate the process. BCR-ABL is known to suppress Protein Phosphatase 2A (PP2A) in CML cells. While C6-ceramide can activate PP2A in acute leukemia cells, the sphingolipid did not activate the phosphatase in K562 cells. C6-ceramide did not activate p38 kinase but did promote JNK activation and phosphorylation of JUN. Inhibition of JNK by pharmacological agent protected K562 cells from C6-ceramide suggesting that JNK plays an essential role in C6-ceramide mediated apoptosis. Furthermore, the sphingolipid promoted MCL-1 phosphorylation by a mechanism that, at least in part, involves JNK. The findings presented here suggest that Caspase-8, JNK, and perhaps MCL-1 may play important roles in regulating cell death and may represent new targets for therapeutic strategies for CML.
Keywords: Ceramide, Apoptosis, Casapse-8, JNK, Stress signal pathway, Chronic Myelogenous Leukemia
Introduction
Chronic myelogenous leukemia (CML) is a hematologic malignancy associated with the t(9,22) translocation (i.e the Philadelphia chromosome) that results in the creation of the BCRABL tyrosine kinase1-7. One mechanism to account for malignant transformation in CML involves the constitutive activation of mitogenic signaling by the BCR-ABL kinase8-11. The BCR-ABL oncogene is unique among oncogenes as it is sufficient alone to transform cells. Considering that the BCR-ABL kinase is an aberrantly formed chimera protein that is not found in normal cells, BCR-ABL proved to be a perfect candidate to directly target in the treatment of CML4, 10, 12, 13. The clinically use of imatinib myselate (aka STI571, Gleevec), a potent BCRABL inhibitor, has met with much success13, 14. However, the emergence of imatinib-resistant blasts in some patients and the failure of the drug to achieve long-term responses in patients with advanced stage disease suggest that other targets besides BCR-ABL will be necessary to effectively treat CML4, 13, 15. One study has shown that primitive CML cells are especially resistant to imatinib15.
Ceramide, a pro-apoptotic lipid, regulates diverse signaling pathways. A wide variety of cell types undergo programmed cell death or become growth arrested when treated with ceramide16-19. Ceramide-mediated programmed cell death can involve the intrinsic and/or extrinsic apoptotic pathways20, 21. Caspase activation during apoptosis can involve receptor-mediated death pathways where the TNF family of death receptors activate initiator Caspase-8 (i.e. extrinsic pathway) or can involve the mitochondrial-mediated apoptosis pathway where Cytochrome C is released from the mitochondria and activates initiator Caspase-920-22. Both pathways culminate in the activation of the major downstream effector protease, Caspase-3.
The diversity of biological responses of cells to ceramide reflects the complexity of the role of this sphingolipid as a second signal molecule. Recent studies suggest that stress-activated protein kinases such as JNK, ERK or p38 play important roles in triggering apoptosis in response to various cellular stressors including ceramide. The members of BCL-2 family of proteins play pivotal roles in cellular decision to undergo apoptosis23, 24. BCL-2 has been reported to be phosphorylated by JNK in response to different stimuli25, 26. Although the significance of phosphorylation of BCL-2 is controversial, it was suggested that multi-site phosphorylation by JNK within the unstructured loop region of BCL-2 decreases its anti-apoptotic activity25, 27. Anti-apoptotic BCL-2 family proteins thus may be potential mediators of JNK-induced apoptosis. However, little is known about the relation between JNK and the other anti-apoptotic members of the BCL-2 family in the context of ceramide stress-induced apoptosis signaling. MCL-1 is an anti-apoptotic BCL-2 family member that plays an important role in the development of various carcinomas25, 28, 29. Like BCL2, phosphorylation has been implicated in both positively and negatively regulating MCL-1 anti-apoptotic function30-33. While ceramide is known to promote dephosphorylation of BCL234, at present it is not how the sphingolipid affects MCL-1 phosphorylation status and/or function.
A recent study has found that Glucosylceramide synthase (GCS), an enzyme that glucosylates the sphingolipid and abrogates its toxicity, can promote chemoresistance in K562 cells and in CML cells35. In the present study, we investigated the mechanism of C6-ceramide mediated apoptosis in CML-derived K562 cells. We have found that the sphingolipid promoted cell death by a mechanism involving the extrinsic apoptotic pathways since suppression of Caspase-8 protected K562 cells. We have also found that C6-ceramide was more effective at killing CML derived KBM5 cells compared to an imatinib variant of the cell line (i.e. KBM5-STI). While BCR-ABL does not appear to fully protect cells from ceramide, the kinase may serve a protective function. Perrotti’s group has demonstrated that BCR-ABL suppresses PP2A in Philadelphia chromosome positive leukemias such as CML36-38. BCR-ABL supports activation of the SET protein which is a potent inhibitor of PP2A36. While ceramide can potently activate PP2A in ALL and AML cell lines16, 34, 39, C6-ceramide did not activate PP2A in the K562 cells suggesting that the sphingolipid cannot overcome the activation of SET by BCR-ABL in these cells. C6-ceramide did promote activation of the JNK kinase with concomitant phosphorylation of the JNK substrate c-JUN. Inhibition of JNK using SP600125 protected K562 cells from the sphingolipid’s toxic effects. Ceramide was found to promote phosphorylation of the anti-apoptotic BCL2 family member, MCL-1 by a mechanism involving JNK. The results indicate that C6-ceramide induced apoptosis in K562 cells involves Caspase-8 and JNK.
Materials and Methods
Materials
All reagents used were purchased from commercial sources unless otherwise stated. Cell Lines – K562 cells were obtained from the American Type Culture Collection (Rockvile, MD) and maintained in RPMI medium 1640 supplemented with 5% fetal bovine serum and 5% calf serum at 37° C in 5% CO2. KBM5 and KBM5-STI cells were kindly provided by Dr. Miloslav Beran (MD Anderson Cancer Center, Houston, TX). KBM5 is a cell line derived from a CML patient and contains multiple copies of the Philadelphia chromosome and lacks the normal ABL gene. KBM5 cells resistant to imatinib (KBM5-STI) were derived by Ricci et al. by chronic exposure of KBM5 cells to imatinib and have been previously described40. Cells were grown in RPMI 1640 with 10% fetal bovine serum, 1% glutamine, and 100 units/ml penicillin at 37° C in 5% CO2. KBM5-STI cells were maintained in the presence of 2 μM imatinib and were maintained at this concentration.
Analysis of Cell Viability and Apoptosis
Cells were treated with various doses of C6-ceramide (BioMol, Plymouth Meeting, PA) for up to 72 hours. Where appropriate, cells were pretreated for 1 hour prior to C6-ceramide addition with 40 μM Caspase inhibitor or 20 μM SP600125 (Calbiochem, La Jolla, CA). The Caspase inhibitors used were all from Calbiochem and included Caspase-3 Inhibitor II (Z-DEVD-FMK), Caspase-8 Inhibitor II (Z-IETD-FMK), and Caspase-9 Inhibitor I (Z-LEHD-FMK). Cell viability was measured by trypan blue dye exclusion assay. Apoptosis was analyzed by cell morphology using light microscopy and flow cytometry analysis to measure DNA fragmentation and Annexin V staining. To observe cell morphology changes, cells were transferred to slides using a cytocentrifuge, fixed, and stained robotically with 20% May-Grunwald-Wright-Giemsa composite stain using an Aerospray Slide Stainer as per the manufacturer’s recommendations (Wescor, Logan, UT). Cells were observed by light microscopy using 40X magnification. To analyze apoptosis by measuring DNA fragmentation using flow cytometry, cells were fixed with 100% methanol at -20° C for at least 24 hours, washed with PBS, incubated in RNase A (100μg/ml) for 2 hour, and then labeled with propidium iodide (0.1 mg/ml). DNA fragmentation was analyzed by measuring cell populations containing sub-G0 DNA content using a Beckman Coulter Cytomics 500(Beckman Coulter, Miami, FL). To confirm apoptosis cells were stained with Annexin V / TMRM (tetramethyl rhodamine methyl ester) and the percentages of apoptotic cells were assessed by flow cytometry. Cells were washed in PBS, resuspended in binding buffer containing Annexin V (Roche Diagnostics, Indianapolis, IN) or TMRM. Apoptotic cells were identified as positive for Annexin V or by loss of TMRM staining using a Becton Dickinson LSR II FACScan (Becton Dickinson, San Jose, CA).
Protein Phosphatase Assay
Protein phosphatase activity of nuclear and mitochondrial fractions was determined as previously described39. Generation of free PO4 from the phosphopeptide RRA(pT)VA was measured using the molybdate:malachite green:phosphate complex assay as described by the manufacturer (Promega, Madison, WI). Nuclei and mitochondrial membranes were prepared as previously described39. The phosphatase assay was performed in a PP2A-specific reaction buffer ( final 50 mM imidazole (pH 7.2)/ 0.2 mM EGTA/ 0.02 % 2-mercaptoethanol/ 0.1 mg/ml BSA) using 100 μM phosphopeptide substrate and 2 μg of protein isolated from the nuclei or mitochondria. After incubation at 30° C for 30 minutes, molybdate dye was added and free phosphate was measured by absorbance at 590 nM. A standard curve with free phosphate was used to determine the amount of free phosphate generated. Phosphatase activity was defined as pmole free PO4 generated/μg protein/ min.
Metabolic Labeling, Immunoprecipitation, and Immunoblotting Analysis
K562 cells were pretreated with 20 μM SP600125 for 1 hr followed by treatment with 25 μM C6-ceramide for 3 hours during metabolic labeling with 32P-orthophosphate and BCL-XL and MCL-1 were analyzed by immunoprecipitation by a method similar to that described for studies with BCL234. Anti-sera from Cell Signaling (Beverly, MA) was used to immunoprecipitate BCL-XL and antisera from Chemicon (Temacula, CA) was used to immunoprecipitate MCL-1. Immunoprecipitated protein was electrophoresed in a 12% acrylamide/0.1% SDS gel, transferred to nitrocellulose, and exposed to Kodak X-Omat film at -80°C. To confirm the identity of phosphorylated bands as either BCL-XL or MCL-1, the same blot was used for Western blotting with antibodies that were different than the one used for immunoprecipitation. Anti-BCL-XL anti-sera (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-MCL-1 anti-sera (Santa Cruz Biotechnology) was used in Western analysis.
Western Blot Analysis
K562 cells were sonicated in 200 μl lysis buffer (62.5 mM Tris (pH 8.0), 2% SDS, 10% glycerol, 100 μM AEBSF, 80nM Aprotinin, 5μM Bestatin, 1.5 μM E-64, 2 μM leupeptin, 1 μM Pepstatin, 500 uM sodium orthovanadate, 500 μM glycerol phosphate, 500 μM sodium pyrophosphate and 50 μM DTT) and protein (5 × 105 cell equivalents) was subjected to electrophoresis using 10-14% acrylamide/ 0.1% SDS gels. Proteins were transferred to a nitrocellulose membrane and Western blotting analysis was performed with antibodies against PARP (Santa Cruz Biotechnology), Caspase-8 (Santa Cruz Biotechnology), Caspase-9 (Santa Cruz Biotechnology), phospho-JNK (Cell Signaling), JNK (Cell Signaling), phospho-p38 (Cell Signaling), p38 (Cell Signaling), phospho-ERK (Cell Signaling), ERK (Cell Signaling), GAPDH (Chemicon) and Actin (Sigma, St. Louis, MO). Western blots were developed using an ECL kit (Amersham, Piscataway, NJ).
Statistics
Statistical analysis was performed using standard t test analysis with Sigma Stat computer software (SSPS, Chicago, IL).
Results
Ceramide Promotes Apoptosis in K562 Cells by a Caspase-dependent Mechanism
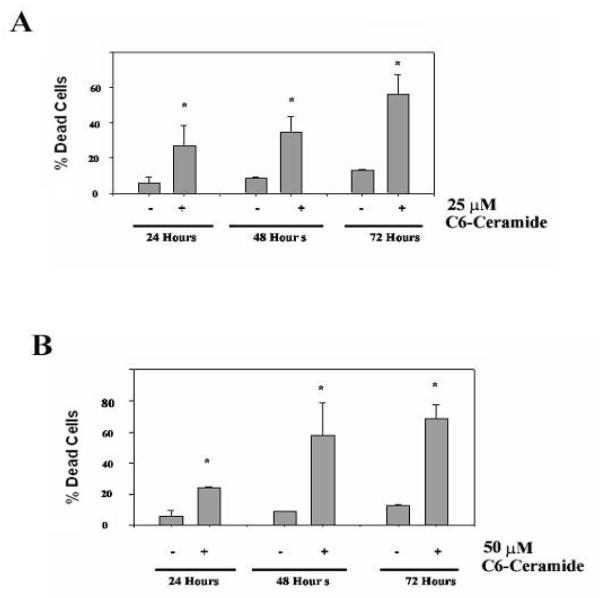
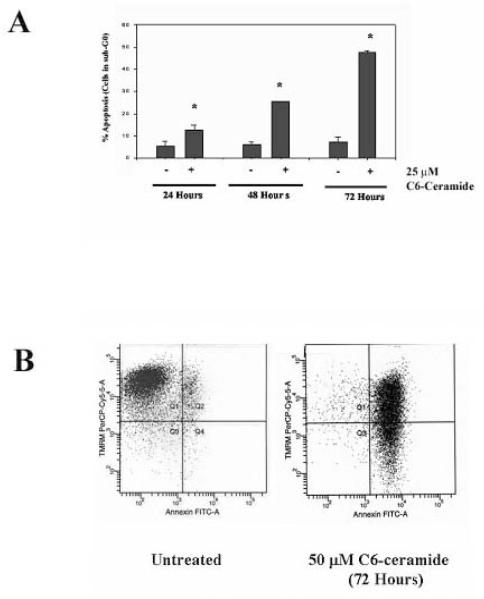
Previous studies on the effect of ceramide on K562 cells have indicated that the cells are resistant to the sphingolipid41. Furthermore, irradiation of K562 cells fails to induce production of ceramide species associated with apoptosis which may explain the resistance of these cells to irradiation-induced apoptosis42. However, the study of ceramide-induced apoptosis in K562 cells was performed using C2-ceramide rather than C6-ceramide41. Recent studies have indicated that short chain ceramides are converted to more physiologically relevant long chain ceramides by a mechanism that involves recycling the sphingosine backbone of short chain ceramides for use in generating long chain molecules43. While C2-ceramide is more soluble in aqueous solution than C6-ceramide, the longer fatty acid chain promotes greater cell permeability. Thus, C6-ceramide could prove more effective than C2-ceramide to treat cells. To support this notion, C6-ceramide has been shown to be at least 6X more effective at suppressing cell growth in mouse leukemia cells compared to C2-ceramide44. Studies were performed to determine if C6-ceramide could promote apoptosis in K562 cells. In a time course experiment using trypan blue dye exclusion assay, both 25 μM (Figure 1A) and 50 μM C6-ceramide (Figure 1B) were effective at promoting cell death in K562 cells. The mechanism of cell killing was determined to be apoptosis since K562 cell treated with C6-ceramide display DNA fragmentation. As shown in Figure 2A, flow cytometry analysis after cell staining with propidium iodide indicates that chromosomal DNA is intact in untreated cells (i.e. sub-G1 < 5%) whereas significant DNA fragmentation occurs in ceramide-treated cells (i.e. > 25% sub-G1 after 48 hours with 25 μM C6-ceramide). In addition, an apoptotic mechanism is indicated since C6-ceramide promoted inversion of the plasma membrane as shown by Annexin V staining of the K562 cells. Flow cytometry analysis of untreated K562 cells and cells treated with 50 μM C6-Ceramide for 72 hours indicate that nearly all the cells are Annexin V positive after staining (Figure 2B). As shown in Figure 2B, 72 hour treatment of K562 cells with 50 μM C6-Ceramide also promoted loss of mitochondrial membrane permeability as indicated by loss of TMRM (tetramethyl rhodamine methyl ester) staining. These data clearly indicate that C6-Ceramide promotes apoptosis in K562 cells.
Figure 1. C6-ceramide promotes cell death in K562 cells.
K562 cells were untreated or treated with 25 μM C6-ceramide (A) or 50 μM C6-ceramide (B) for 24, 48 and 72 hours. Cell death was assessed by trypan blue exclusion assay. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that *p < 0.05 for all ceramide treated cells compared to relevant untreated samples.
Figure 2. C6-ceramide promotes apoptosis in K562 cells.
A) K562 cells were untreated or treated with 25 μM C6-ceramide for 24, 48 and 72 hours. Cell apoptosis (sub-G1 phase) was assessed by flow cytometry after propidium iodide staining. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that *p < 0.05 for all ceramide treated cells compared to relevant untreated samples. B) K562 cells were untreated or treated with 50 μM C6-ceramide for 72 hours, harvested and double-stained with Annexin V and TMRM (tetramethyl rhodamine methyl ester) and flow cytometry was performed.
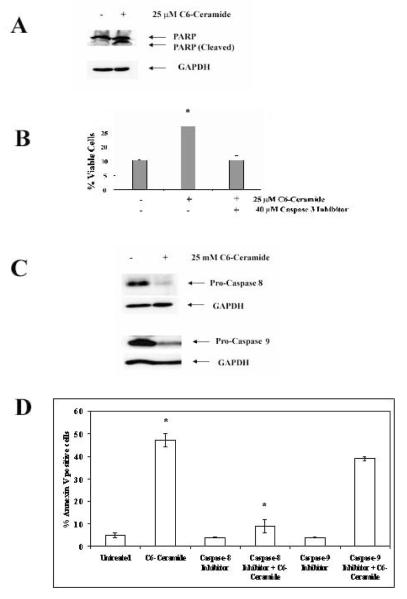
Another feature of apoptosis is the activation of the Caspase family of proteases20-22. As shown in Figure 3A, Western blot analysis of protein isolated from untreated K562 cells or cells treated with 25 μM C6-ceramide demonstrates Caspase-3 activation as observed by cleavage of PARP (poly-ADP-ribose polymerase; a caspase-3 substrate). Next we investigated the effect of caspase-3 inhibitor on the C6-ceramide-induced apoptosis. Supporting a role for Caspase-3 in ceramide-induced apoptosis in K562 cells, Caspase-3 inhibitor protected K562 cells from 25 μM C6-Ceramide when the cells were pretreated with the Caspase inhibitor compared to C6-ceramide treatment alone (Figure 3C). It is not known if C6-Ceramide induced apoptosis in K562 cells involves the intrinsic (i.e. mitochondria mediated) apoptotic pathway or the extrinsic (i.e. death receptor mediated) apoptotic pathway. Ceramide has been implicated in the activation of both pathways45-47. Mitochondrial release of Cytochrome C and other molecules activates Caspase-9 and is an integral part of intrinsic apoptotic pathways20-22. On the other hand, extrinsic apoptotic pathways involve activation of Caspase-8 by death receptors such as FADD and TRADD. The detection of PARP cleavage and suppression of apoptosis by the Caspase-3 inhibitor during ceramide treatment in K562 cells demonstrates that Caspases are activated; however, it is not known which apoptotic pathway is involved. As shown in Figure 3C, after 25 μM C6-Ceramide treatment for 24 hours both Caspase-8 and Caspase-9 were activated as demonstrated by the cleavage of the procaspase forms of these enzymes. Next we investigated the effect of Caspase-8 and Caspase-9 inhibitors on the death determined by ceramide. Apoptosis assay measuring Annexin V positive cells revealed that pretreatment of K562 cells with 40 μM Caspase-8 inhibitor but not 40 μM Caspase-9 inhibitor protected cells from treatment with 25 μM C6-Ceramide for 48 hours (Figure 3D). These data taken together suggest that C6-ceramide induced cells death in K562 cells involves a mechanism of apoptosis that is dependent on Caspase-3 and Caspase-8 (i.e. the extrinsic pathway).
Figure 3. Ceramide-induced cell death in K562 cells is Caspase dependent and requires Caspase-8.
A) K562 cells were untreated or treated with 25 μM C6-ceramide for 24h and PARP cleavage was assessed by Western blot analysis using anti-PARP antibody. As a loading control the same blot was probed with antibody against GAPDH. B) K562 cells were untreated or treated with 25 μM C6-ceramide for 24 hours. Where appropriate, cells were pre-treated with Caspase-3 inhibitor (Z-DEVD-FMK) for one hour before adding ceramide. Cell apoptosis was assessed by flow cytometry after propidium iodide staining. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that there was a statistical difference in untreated cells and cells treated with C6-ceramide (*p = 0.009). There was no statistical difference between untreated cells and ceramide treated cells that were pretreated with Caspase-3 inhibitor (p > 0.05). C) K562 cells were untreated or treated with 25 μM C6-ceramide for 24 hours. Pro-Caspase-8 and Pro-Caspase-9 cleavage were assessed by Western blot analysis. As a loading control the same blot was probed with antibody against GAPDH. D) K562 cells were untreated or treated with 25 μM C6-ceramide for 48 hours. Where appropriate, cells were pre-treated with Caspase-8 Inhibitor II (Z-IETD-FMK) or Caspase-9 Inhibitor I (Z-LEHD-FMK) for one hour before adding ceramide. Cell apoptosis was assessed by flow cytometry after Annexin V staining. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that there was a statistical difference in cells treated with C6-ceramide and cells treated with Caspase-8 inhibitor (*p = 0.013). There was no statistical difference between cells treated with C6-ceramide and cells treated with Caspase-9 inhibitor (p > 0.05).
Ceramide kills myeloid leukemia cells independent of BCR-ABL status
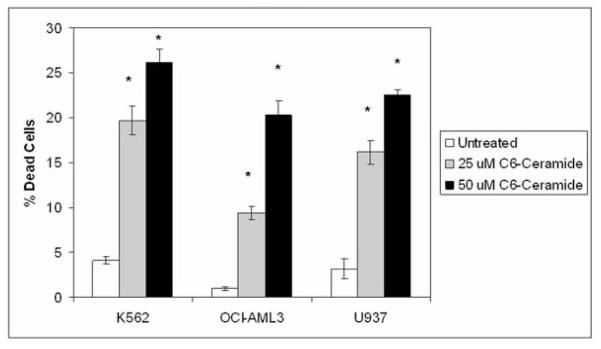
The presence of active BCR-ABL kinase in CML derived cells potentially can suppress death pathways in response to apoptotic agents due to the effect of the kinase on pro-survival signaling8-11. To determine if BCR-ABL affects C6-ceramide induced cell death in malignant myeloid cell lines, C6-ceramide was used to treat the BCR-ABL positive K562 cells and two BCR-ABL negative cell lines (i.e. OCI-AML3 and U937). It has previously been documented that short chain ceramide (i.e. C2-ceramide) can effectively kill AML derived HL60 cells48, so it would be not be unusual if AML derived cell lines such as OCI-AML3 and U937 would be sensitive to C6-ceramide. K562 cells, OCI-AML3 cells, and U937 cells were treated with 25 μM or 50 μM C6-ceramide for 24 hours and cell death was assessed by trypan blue staining. At either concentration of the sphingolipid, K562 cells were more sensitive to C6-ceramide compared to both of the two AML derived cell lines (Figure 4). This result suggests that C6-ceramide induced cell death involves a mechanism that is independent of BCR-ABL.
Figure 4. C6-ceramide promotes apoptosis in BCR-ABL positive and BCR-ABL negative malignant myeloid cells.
BCR-ABL positive K562 cells and BCR-ABL negative OCI-AML3 and U937 cells were untreated or treated with 25 μM, or 50 μM C6-ceramide for 48 hours. Cell death was assessed by trypan blue exclusion assay. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that *p < 0.05 for all ceramide treated cells compared to relevant untreated samples.
Ceramide promotes apoptosis in KBM5 cells and in imatinib-resistant KBM5-STI cells
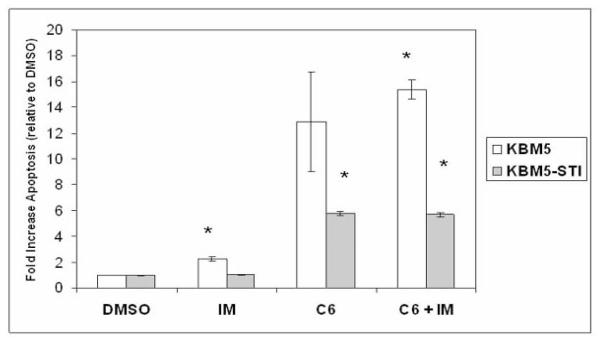
KBM5 cells are a BCR-ABL positive CML cell line. An imatinib-resistant variant of the cell line (KBM5-STI) was developed by Ricci and colleagues40. KBM5-STI have developed drug resistance to imatinib due to mutation of the BCR-ABL kinase (i.e. a threonine-to-isoleucine point mutation at position 315 of ABL). KBM5 and KBM5-STI cells were treated with 50 μM C6-ceramide, 0.5 μM imatinib, or with a combination of both compounds for 48 hours and apoptosis was measured by Annexin V staining using FACSCAN. The induction of apoptosis was measured as an increase in Annexin V positive cells relative to cells treated with DMSO. As shown in Figure 5, the KBM5 cells are sensitive to imatinib (i.e. > 2 fold induction of apoptosis; p < 0.001) while there is no significant Annexin V staining of KBM5-STI cells with the compound. Apoptosis was potently induced in both KBM5 and KBM5-STI cells after treatment with 50 μM C6-ceramide for 48 hours. C6-ceramide promoted a > 12 fold increase in apoptotic cells compared to vehicle in the KBM5 cells and a > 5 fold increase in apoptotic cells compared to vehicle in the KBM5-STI cells (Figure 5). This finding suggests that the KBM5 cells are more sensitive to the sphingolipid compared to the KBM5-STI cells. As shown in Figure 5, the co-treatment of KBM5 cells with C6-ceramide and imatinib resulted in an increase in apoptosis compared to C6-ceramide treatment alone (i.e. on average 15.9 fold versus an average of 12.9 fold increase in apoptosis, respectively) though this difference in apoptotic induction was not statistically significant (p = 0.34). There was no difference in apoptotic induction in KBM5-STI cells treated with C6-ceramide compared to cells treated with the combination of the sphingolipid and imatinib (Figure 5). These results suggest that BCR-ABL may regulate ceramide-induced apoptosis; however more work will be necessary to determine the mechanism involved.
Figure 5. C6-ceramide promotes apoptosis in KBM5 and imatinib-resistant KBM5-STI cells.
KBM5 and KBM5-STI cells were treated with 0.1% DMSO, with 0.5 μM imatinib (IM), with 50 μM C6-ceramide (C6), or with a combination of both compounds (C6 + IM) for 48 hours. Cell apoptosis was assessed by flow cytometry after Annexin V staining. Apoptotic induction was determined by the ratio of apoptotic cells after treatment relative to DMSO treated cells. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that there was a statistical difference in KBM5 cells treated with imatinib compared to cells treated with DMSO (*p < 0.001) and with the combination of imatinib and C6-ceramide compared to cells treated with DMSO (*p < 0.001). Student t test also reveals that there was a statistical difference in KBM5-STI cells treated with C6-ceramide compared to cells treated with DMSO (*p < 0.001) and with the combination of imatinib and C6-ceramide compared to cells treated with DMSO (*p < 0.001).
Ceramide does not activate PP2A in K562 cells
Protein phosphatase 2A (PP2A) is a phosphatase that regulates cell proliferation, survival, and differentiation49, 50. Loss of PP2A function has been associated with cell transformation49. Studies have shown that in BCR-ABL + cell lines and in patient-derived CML CD34+ cells, the phosphoprotein SET expression is enhanced by BCR-ABL and increases during CML disease progression36. This results in progressive loss of PP2A tumor-suppressive activity. Restoring PP2A activity in these cells induces inactivation and downregulation of the BCR-ABL oncogene itself.
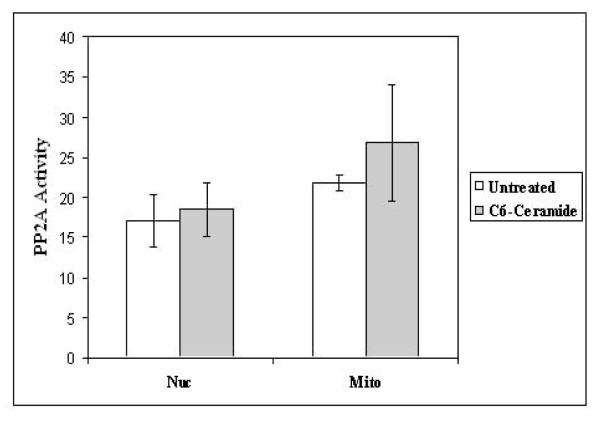
It is well known that phosphatases play an important role in ceramide-mediated processes. Ceramide has been found to be a potent activator of protein phosphatases like PP2A and PP116. Ceramide activation of PP2A results in inactivation of BCL2 and apoptosis34, 39. Ceramide potently kills acute myeloid leukemia and acute lymphoblastic leukemia cell lines such as REH and HL60 by a mechanism that involves activation of a mitochondrial PP2A34, 39. Protein phosphatase assay was performed using protein lysates from mitochondria and nuclei from untreated K562 cells or cells treated with C6-ceramide to determine if ceramide promotes mitochondrial PP2A activity. As shown in Figure 6, ceramide did not significantly promote PP2A activity in either the nucleus or mitochondria. These findings suggest that PP2A is not likely to be involved in ceramide-induced apoptosis. Since BCR-ABL in K562 cells should suppress PP2A activity in these cells36, these results suggest that C6-ceramide cannot overcome suppression of the protein phosphatase by BCR-ABL.
Figure 6. PP2A activity is not significantly increased in K562 cells after ceramide treatment.
PP2A protein was immunoprecipitated from nuclear and mitochondrial fractions and a protein phosphatase assay was performed using a PP2A specific substrate peptide. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that there was no statistical difference in PP2A activity untreated cells and ceramide treated cells (p > 0.05) for either nuclear or mitochondrial lysates.
Ceramide Activates JNK and ERK but not p38
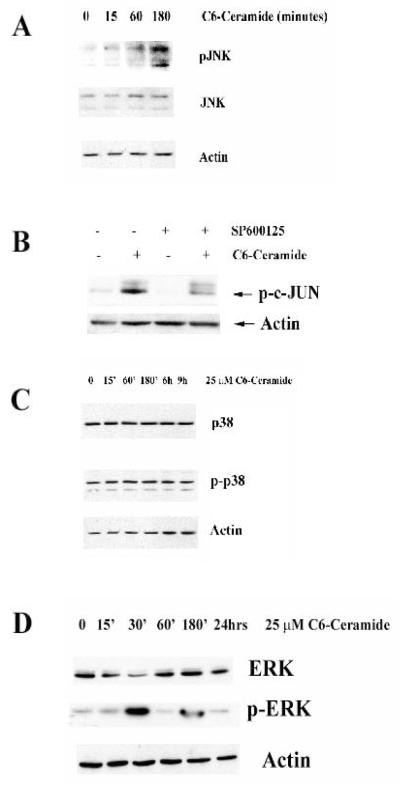
While JNK is known to be regulated by ceramide, the exact role for this enzyme in ceramide-induced cell death in CML-derived K562 cells is unknown. The goal of the present study was to determine what role MAP kinases play in ceramide-induced apoptosis in K562 cells. K562 cells were treated with 25 μM C6-ceramide for varying times and expression and activation (i.e. phosphorylation) of JNK, p38, and ERK were determined by Western blot analysis of protein lysates (Figure 7). C6-ceramide was found to have different effects on each kinase. The sphingolipid promotes activation of the JNK MAP kinase as indicated by phosphorylation after 3 hours treatment (Figure 7A). It is widely reported that JNK phosphorylates the transcription factor c-JUN resulting in its increased transcriptional activity. Consistent with ceramide activation of JNK in K562 cells, results from Western blotting using phosphospecific antibodies demonstrate that 25 μM C6-ceramide induces c-JUN phosphorylation and this phosphorylation can be blocked with the JNK inhibitor SP600125 (Figure 7B). Taken together these results demonstrate that ceramide-induced activation of JNK and its downstream substrate c-JUN in K562 cells may be important in the mechanism in which this compound induces apoptosis.
Figure 7. JNK is activated in K562 cells after C6-ceramide treatment.
A) K562 cells were untreated or treated for various times with 25 μM C6-ceramide. Western blot analysis was performed using antibodies against JNK and phospho-JNK. Actin was used as a loading control. B) K562 cells were treated for 24h with 25 μM C6-ceramide. Where appropriate, cells were pre-treated with 10 μM SP600125. Western blot analysis was performed using phospho-c-Jun anti-sera. Actin was used as a loading control. C) K562 cells were untreated or treated for various times with 25 μM C6-ceramide. Western blot analysis was performed using antibodies against p38 and phospho-p38. Actin was used as a loading control. D) K562 cells were untreated or treated for various times with 25 μM C6-ceramide. Western blot analysis was performed using antibodies against ERK and phospho-ERK. Actin was used as a loading control.
Analysis of p38 and phosphorylated p38 showed that this kinase is not activated during ceramide-induced cell death in K562 cells (Figure 7C). Analysis of ERK demonstrated that the MAP kinase is rapidly phosphorylated and activated after 30 minutes of ceramide treatment, however, phospho-ERK levels come back to the basal level after 1 hour (Figure 7D). It is not clear what role this transient activation of ERK may play in ceramide-induced apoptosis in K562 cells. While p38 does not appear to be a ceramide target in K562 cells, it is clear that the agent potently activates JNK in these cells.
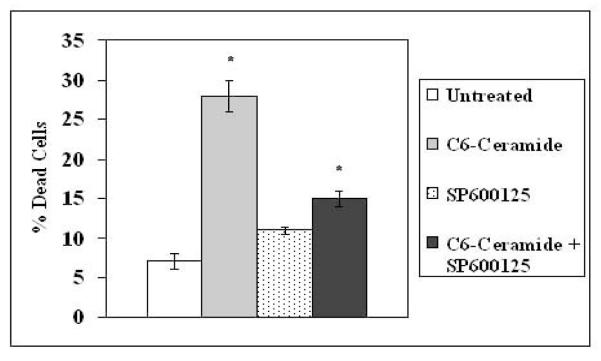
C6-Ceramide induces rapid activation of JNK (Figure 7A) and promotes JNK mediated phosphorylation of c-JUN (Figure 2B). However, it is not known if JNK is required for ceramide-induced apoptosis. The JNK pharmacological inhibitor SP600125 was used to determine if suppression of JNK could protect cells from C6-Ceramide induced apoptosis in K562 cells. Cells were pre-treated for 1 hour with 10 μM SP600125 prior to treatment with 25 μM C6-ceramide for 24 hours. Cell viability was assessed by trypan blue exclusion dye assay. As shown in Figure 8 the JNK inhibitor did indeed protect K562 cells from ceramide-induced death. This result suggests that JNK kinase is necessary for ceramide-induced apoptosis in K562 cells.
Figure 8. Inhibition of JNK protects K562 cells from C6-ceramide induced apoptosis.
K562 cells were untreated or treated with 25 μM C6-ceramide for 24hours. Where appropriate, cells were pre-treated with 10 μM JNK inhibitor SP600125 for 1 hour prior to ceramide addition. Cell death was assessed by trypan blue exclusion assay. Error bars represent the mean +/− SD from three independent experiments. Student t test reveals that there was a statistical difference in untreated cells and cells treated with C6-ceramide (*p = 0.001) and cells treated with C6-ceramide and cells treated with C6-ceramide and SP6006125 (*p=0.001). There was no statistical difference between untreated cells and cells treated with SP600125 alone (p > 0.05).
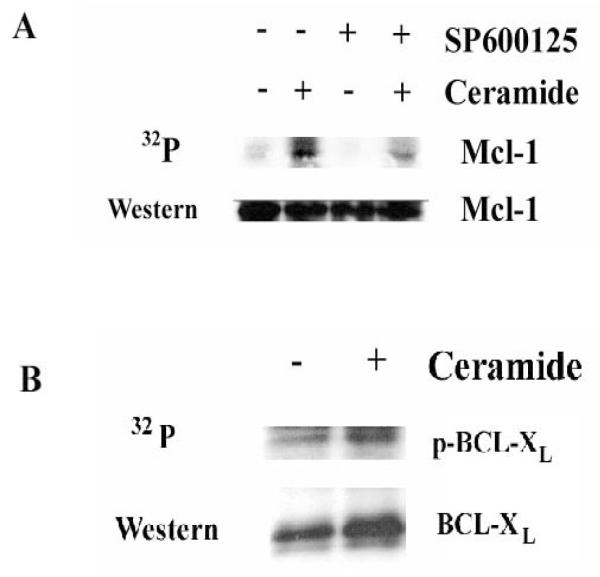
Ceramide promotes phosphorylation of MCL-1
A number of non-c-JUN JNK targets including BCL2, BIM, and MCL-1 have been identified25, 26, 30, 31. While BCL2 is a JNK target, BCL2 was not examined since little if any of the protein is detected in K562 cells (data not shown). Considering that MCL-1 plays a key role in survival of malignant hematopoietic cells28,29 and the molecule is regulated by JNK30,31, it is plausible that ceramide-induced apoptosis in CML cells may involve inactivation the anti-apoptotic molecule by the kinase. A metabolic radio-labeling experiment was performed to determine if MCL-1 was phosphorylated in K562 cells in response to ceramide (Figure 9A). K562 cells were treated with 25 μM C6-ceramide for 3 hours during metabolic labeling with 32P-orthophosphate, and the phosphorylation status of MCL-1 was examined following immunoprecipitation. As shown in Figure 9A, ceramide treatment induces MCL-1 phosphorylation and the Western analysis demonstrates that roughly equivalent levels of MCL-1 protein were immunoprecipitated from control (untreated) and C6-ceramide-treated samples. To determine if ceramide-induced phosphorylation of MCL-1 involves JNK, SP600125 was used in the metabolic radio-labeling experiment to determine if the inhibition of JNK could block ceramide-induced MCL-1 phosphorylation. As shown in Figure 9A, the JNK inhibitor suppresses ceramide-induced phosphorylation of MCL-1 suggesting that JNK kinase phosphorylates the anti-apoptotic molecule in response to ceramide. Considering that the JNK specific inhibitor does protect the cells from ceramide-induced cell death (Figure 8), these findings suggest that MCL-1 may be an important JNK target in the apoptotic process induced by ceramide in K562 cells.
Figure 9. Ceramide promotes phosphorylation of MCL-1 but not BCL-XL by a mechanism involving JNK.
A) K562 cells were untreated or treated with 25 μM C6-ceramide for 3 hours during metabolic labeling with 32P-orthophosphate. Where appropriate, cells were pre-treated with 10 μM SP600125 for 1 hour prior to ceramide addition. The phosphorylation status of MCL-1 was detected by autoradiography and the identity of MCL-1 was confirmed by Western analysis as described in “Materials and Methods”. B) K562 cells were untreated or treated with 25 μM C6-ceramide for 3 hours during metabolic labeling with 32P-orthophosphate. Where appropriate, cells were pre-treated with 10 μM SP600125 for 1 hour prior to ceramide addition. Phosphorylation of BCL-XL was detected by autoradiography and the identity of BCL-XL was confirmed by Western analysis as described in “Materials and Methods”.
Since JNK has been implicated in the inactivation of BCL-XL by multi-site phosphorylation51, the effect of C6-ceramide on BCL-XL phosphorylation status was observed in K562 cells using metabolic radio-labeling (Figure 9B). K562 cells were treated with 25 μM C6-ceramide for 3 hours during metabolic labeling with 32P-orthophosphate, and the phosphorylation status of BCL-XL was examined following immunoprecipitation. Cells treated with C6-ceramide did appear to display greater levels of phosphorylated BCL-XL, however, Western analysis demonstrates that a greater amount of BCL-XL protein was immunoprecipitated from the C6-ceramide-treated sample. Densitometric analysis revealed that there was only a slight increase (~ 17 %) in phosphorylated BCL-XL compared to untreated cells when normalized to the amount of protein immunoprecipitated. This finding suggests that ceramide inaction of BCL-XL is likely not involved in C6-ceramide induced apoptosis in the K562 cells.
Discussion
Previous studies have demonstrated that ceramide generation induces apoptosis in other cell types but not in CML-derived K562 cells17-19, 41. Ceramide is a component of the lipid signaling pathway that is synthesized de novo or through the catabolism of sphingomyelin in response to cell stresses, including ionizing radiation, chemotherapeutical drugs, and cytokines17-19. Studies were performed to determine if short chain ceramide analogs like C6-ceramide could promote apoptosis in CML-derived K562 cells. C6-ceramide potently induces cell death in these cells and the mechanism of cell death is apoptosis. Moreover, the apoptotic process is mediated by activation of Caspases. Ceramide promotes PARP cleavage and activation of Caspase-3 (which is an effector Caspase for both intrinsic and extrinsic pathways). Ceramide-mediated apoptosis is Caspase dependent in K5632 cells as the specific Caspase-3 inhibitor Z-DEVDFMK protects K562 cells from apoptosis in response to treatment with C6-ceramide. Both initiator Caspase-8 (extrinsic, receptor mediated pathway) and Caspase-9 (intrinsic, mitochondrial pathway) were activated by C6-ceramide in K562 cells. However, only Caspase-8 proved to be essential for C6-ceramide induced apoptosis. These findings suggest that while C6-ceramide induced apoptosis, the mechanism of cell death relies on the extrinsic apoptotic pathway.
C6-ceramide was effective at killing another CML derived cell line, KBM5. KBM5-STI is an imatinib-resistant variant of KBM5 (resulting from mutation of the BCR-ABL kinase)40. C6-ceramide was able to promote apoptosis in KBM5-STI cells despite the presence of the imatinib-resistant BCR-ABL mutant; however, the sphingolipid was only half as effective at killing these cells compared to the imatinib-sensitive KBM5 cells. These findings suggest that BCR-ABL cannot fully block ceramide-induced apoptosis but may accord some protection to cells. Recent studies have demonstrated that BCR-ABL suppresses PP2A in CML cells36-39. A therapeutic strategy for the therapy of CML involves the activation of PP2A to promote proapoptotic signaling using the compound FTY72038. In the present study, ceramide was able to promote apoptosis in the absence of activation of PP2A. This finding suggests that activation of PP2A is not required for apoptosis (at least in K562 cells) and that activation of ceramide-mediated death pathways may provide alternative means to kill CML cells or other BCR-ABL positive cells such as Philadelphia positive ALL cells. This notion suggests that a potential therapeutic anti-CML strategy could involve the use of drugs that may promote ceramide production.
Previous studies have shown that direct elevation of intracellular ceramide levels by treatment of cells with ceramide analogs markedly activated JNK/SAPK cascade but not the ERK kinase cascade in acute myeloid leukemia cells52. In this study treatment of CML derived K562 cells with C6-ceramide did indeed induce JNK activation but also activated ERK over a short period of time. In K562 cells, C6-ceramide did not activate the p38 kinase. Perhaps the brief activation of ERK represents an attempt of the cell to protect itself during ceramide stress challenge. The role of ERK in ceramide-induced apoptosis in the K562 cells remains to be determined. Still, evidence is strong that C6-ceramide-induced cell death of K562 cells requires JNK.
A number of JNK targets have been recently identified including many members of the BCL2 family (e.g. BCL-2, BCL-XL, Bim, MCL-1). Recent studies have suggested that multi-site phosphorylation of BCL-XL results in the functional inactivation of the anti-apoptotic protein51. K562 cells exhibited basal levels of phosphorylated BCL-XL and C6-ceramide did not augment this phosphorylation suggesting that inactivation of BCL-XL anti-apoptotic function by phosphorylation is not involved in ceramide-mediated apoptosis in the K562 cells. JNK is a MCL-1 kinase30. JNK-mediated phosphorylation of MCL-1 at Thr-163 promotes Glycogen Synthase Kinase 3 (GSK3) phosphorylation of the protein at Ser 159 which results in proteasome-mediated degradation of the anti-apoptotic protein31, 32. It should be noted that JNK may support cell survival function of MCL-1 by phosphorylating the protein at Ser 64 though Cyclin Dependent Kinase 2 and Cyclin Dependent Kinase 3 appear to be responsible for this function33. In the present study, C6-ceramide appears to promote cell death in conjunction with MCL-1 phosphorylation by a mechanism that involves JNK. This finding suggests that in K562 cells, JNK phosphorylation of MCL-1 would inactivate the anti-apoptotic protein. A better understanding of this mechanism will occur when the MCL-1 sites that are phosphorylated in response to ceramide are identified. Still, that ceramide promotes JNK-mediated phosphorylation of MCL-1 is consistent with a mechanism whereby the anti-apoptotic agent is inactivated by the stress kinase during programmed cell death. Further studies will be necessary to unravel the role of MCL-1 phosphorylation in ceramide-induced apoptosis in CML cells.
In conclusion, in the present study we showed that C6 ceramide induces apoptosis mediated by the extrinsic apoptotic pathway (through Caspase-8), in the absence of PP2A activation, and in the presence of JNK/SAPK cascade activation. JNK participates in MCL-1 phosphorylation which may inactivate the anti-apoptotic function of this BCL2 family protein. A better understanding of how JNK regulates apoptosis in CML cells may lead to improved strategies for the treatment of CML.
Acknowledgments
This work was supported by funds from the Hormel Foundation (PR) and a grant from the National Cancer Institute (PO1 CA55164 to MA). Current address for Alina Felicia Nica is: Investigational Cancer Therapeutics, M.D. Anderson Cancer Center, Division of Cancer Medicine, 1515 Holcombe Blvd., FC 6.3015, Unit 455, Houston, TX 77030. Current address for Chun Chui Tsao is: GU Medical Oncology Dept., M.D. Anderson Cancer Center, Division of Cancer Medicine, 1515 Holcombe Blvd., Box 1374, Houston, TX 77030. Current address for Paul Jurasz is: St. Michael’s Hospital, 30 Bond Street, Queen Wing, 8-030, Toronto, Ont., Canada M5B 1W8
REFERENCES
- 1.Cortes JE, Talpaz M, Kantarjian H. Chronic myelogenous leukemia: a review. Am J Med. 1996;100:555–570. doi: 10.1016/s0002-9343(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle. 2007;6:550–566. doi: 10.4161/cc.6.5.3919. [DOI] [PubMed] [Google Scholar]
- 3.Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103:4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- 4.Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 5.Kurzrock R, Gutterman JU, Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988;319:990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- 6.Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233:212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 8.Puil L, Liu J, Gish G, Mbamalu G, Bowtell D, Pelicci PG, Arlinghaus R, Pawson T. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J. 1994;13:764–773. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deininger MW, Vieira S, Mendiola R, Schultheis B, Goldman JM, Melo JV. BCR-ABL tyrosine kinase activity regulates the expression of multiple genes implicated in the pathogenesis of chronic myeloid leukemia. Cancer Res. 2000;60:2049–2055. [PubMed] [Google Scholar]
- 10.Deininger MW, Druker BJ. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol Rev. 2003;55:401–423. doi: 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- 11.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 12.Thiesing JT, Ohno-Jones S, Kolibaba KS, Druker BJ. Efficacy of STI571, an abl tyrosine kinase inhibitor, in conjunction with other antileukemic agents against bcr-abl-positive cells. Blood. 2000;96:3195–3199. [PubMed] [Google Scholar]
- 13.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 14.Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood. 2008;111:1039–1043. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 15.Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle. 2006;5:2862–2866. doi: 10.4161/cc.5.24.3573. [DOI] [PubMed] [Google Scholar]
- 16.Ruvolo PP. Ceramide regulates cellular homeostasis via diverse stress signaling pathways. Leukemia. 2001;15:1153–1160. doi: 10.1038/sj.leu.2402197. [DOI] [PubMed] [Google Scholar]
- 17.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 18.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 19.Morales A, Lee H, Goñi FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 22.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 23.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 24.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu A, You SA, Haldar S. Regulation of Bcl2 phosphorylation by stress response kinase pathway. Int J Oncol. 2000;16:497–500. doi: 10.3892/ijo.16.3.497. [DOI] [PubMed] [Google Scholar]
- 26.Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS. Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- 27.Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]
- 28.Zhou P, Qian L, Kozopas KM, Craig RW. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89:630–643. [PubMed] [Google Scholar]
- 29.Le Gouill S, Podar K, Harousseau JL, Anderson KC. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle. 2004;3:1259–1262. doi: 10.4161/cc.3.10.1196. [DOI] [PubMed] [Google Scholar]
- 30.Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, Umezawa A, Ichijo H. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 31.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 32.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi S, Lee SH, Meng XW, Mott JL, Bronk SF, Werneburg NW, et al. Serine 64 phosphorylation enhances the antiapoptotic function of Mcl-1. J Biol Chem. 2007;282:18407–18417. doi: 10.1074/jbc.M610010200. [DOI] [PubMed] [Google Scholar]
- 34.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 35.Xie P, Shen YF, Shi YP, Ge SM, Gu ZH, Wang J, Mu HJ, et al. Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leuk Res. 2008;32:475–480. doi: 10.1016/j.leukres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Perrotti D, Neviani P. ReSETting PP2A tumour suppressor activity in blast crisis and imatinib-resistant chronic myelogenous leukaemia. Br J Cancer. 2006;95:775–781. doi: 10.1038/sj.bjc.6603317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- 40.Ricci C, Scappini B, Divoky V, Gatto S, Onida F, Verstovsek S, Kantarjian HM, Beran M. Mutation in the ATP-binding pocket of the ABL kinase domain in an STI571-resistant BCR/ABL-positive cell line. Cancer Res. 2002;62:5995–5998. [PubMed] [Google Scholar]
- 41.Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood. 1998;91:1700–1705. [PubMed] [Google Scholar]
- 42.Thomas RL, Jr, Matsko CM, Lotze MT, Amoscato AA. Mass spectrometric identification of increased C16 ceramide levels during apoptosis. J Biol Chem. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- 43.Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, Birbes H, et al. Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J. Biol. Chem. 2001;276:24901–24910. doi: 10.1074/jbc.M100314200. [DOI] [PubMed] [Google Scholar]
- 44.Flamigni F, Faenza I, Marmiroli S, Stanic I, Giaccari A, Muscari C, et al. Inhibition of the expression of ornithine decarboxylase and c-Myc by cell-permeant ceramide in difluoromethylornithine-resistant leukaemia cells. Biochem J. 1997;324:783–789. doi: 10.1042/bj3240783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dbaibo GS, Hannun YA. Signal transduction and the regulation of apoptosis: roles of ceramide. Apoptosis. 1998;3:317–334. doi: 10.1023/a:1009668802718. [DOI] [PubMed] [Google Scholar]
- 46.Mathias S, Peña LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin CF, Chen CL, Lin YS. Ceramide in apoptotic signaling and anticancer therapy. Curr Med Chem. 2006;13:1609–1616. doi: 10.2174/092986706777441986. [DOI] [PubMed] [Google Scholar]
- 48.Obeid LM, Lindardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 49.Mumby MC, Walter G. Protein serine/threonine phosphatases: Structure, regulation, and function in cell growth. Physiological Reviews. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 50.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 51.Basu A, Haldar S. Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol-induced apoptosis. FEBS Lett. 2003;538:41–47. doi: 10.1016/s0014-5793(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 52.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]