Abstract
Urea solution is one of the most commonly employed protein denaturants for protease digestion in proteomic studies. However, it has long been recognized that urea solution can cause carbamylation at the N-termini of proteins/peptides and at the side chain amino groups of lysine and arginine residues. Protein/peptide carbamylation blocks protease digestion and affects protein identification and quantification in mass spectrometry analysis by blocking peptide amino groups from isotopic/isobaric labeling and changing peptide charge states, retention times and masses. In addition, protein carbamylation during sample preparation makes it difficult to study in vivo protein carbamylation. In this study, we compared the peptide carbamylation in urea solutions of different buffers and found that ammonium containing buffers were the most effective buffers to inhibit protein carbamylation in urea solution. The possible mechanism of carbamylation inhibition by ammonium containing buffers is discussed, and a revised procedure for the protease digestion of proteins in urea and ammonium containing buffers was developed to facilitate its application in proteomic research.
Keywords: Carbamylation, Urea, Ammonium containing buffer, Proteomics, Mass spectrometry
INTRODUCTION
Urea is the most widely used denaturant in proteomic studies, and it is used to increase protease efficiency in protein digestion. Urea can also solubilize proteins by preventing protein precipitation and aggregation [1; 2]. However, the digestion of proteins in urea solution causes the carbamylation of proteins/peptides. Although it has been used in proteomic quantification [3], carbamylation has several disadvantages for proteomic research. First, it blocks the N-termini of proteins and the side chain amino groups of lysine and arginine residues in proteins/peptides and prevents them from further use, such as coupling to solid-phase support or iTRAQ labeling [4; 5; 6]. Second, it prevents proteins from many enzymatic digestions, resulting in incompletely digested peptides. Third, it leads to unexpected chromatographic retention time in protein/peptide separation and unpredicted masses, thus increasing the complexity of samples [7]. Fourth, it affects peptide and protein identification and accurate quantification by reducing the ionization efficiency and signal intensity of the detected peaks [8]. Fifth, it affects the study of in vivo carbamylation [9], which has been recognized as an important protein modification associated with uremia, severe renal and cardiovascular disorders [10; 11; 12]. Therefore, reducing and preventing peptide carbamylation resulting from urea is necessary to improve the quality of quantitative proteomic data and the analysis of in vivo protein modification by carbamylation.
Carbamylation is caused by isocyanic acid (CNOH) derived from urea [13]. Urea spontaneously dissociates to form cyanate and ammonia in aqueous solutions [14]. Temperature, incubation time and pH are factors that are known to affect the rate of urea dissociation and the degree of protein/peptide carbamylation [14; 15]. Based on this knowledge, strategies have been employed to protect peptides from urea carbamylation by either reducing the generation of cyanates or removing active cyanates from solution. For example, most proteomic procedures involving the use of urea solution suggest that it should be freshly prepared and further deionized prior to use to reduce cyanate from the beginning of the sample preparation procedure. Some proteomic protocols indicate that either the urea needs to be removed from the sample before digestion, or the sample should be maintained at low temperature to reduce the decomposition rate of urea [14; 15]. Acidifying the sample is another method to drive the equilibrium to favor urea over the formation of isocyanic acid [14; 16]. These strategies, however, require a long handling time and may not be compatible with many enzymatic digestion procedures. Additionally, removing urea before protein digestion may result in the precipitation of many readily denatured proteins. Numerous amino-containing reagents, such as methylamine, ethanolamine, ethylenediamine, Tris-HCl and 1,2-ethylene diamine, have been suggested for use as cyanate scavengers for protein digestion in urea solution [9; 17]. These reagents work by competing with peptides for cyanates, thereby minimizing protein or peptide carbamylation. Still, protein/peptide carbamylation occurs in almost all conditions of commonly used sample preparation procedures involving urea [9].
In this paper, we report the use of ammonium containing buffers to inhibit protein/peptide carbamylation in urea solution. Ammonium bicarbonate (NH4HCO3) and two other ammonium containing buffers showed better carbamylation inhibition efficiency than phosphate buffer (PB) and Tris-HCl buffer. A high concentration of NH4HCO3 buffer (1M) inhibited almost all carbamylation on proteins/peptide and human serum with no effect on trypsin digestion. More importantly, NH4HCO3 solution is a commonly used buffer in many enzymatic digestion protocols and therefore is very applicable for proteomic research.
Materials and methods
Chemicals and reagents
Standard peptides angiotensin and neurotensin, standard protein bovine fetuin, human sera (frozen liquid), tris (2-Carboxyethyl) phosphine (TCEP), iodoacetamide, ammonium bicarbonate, ammonium acetate, triethylammonium bicarbonate buffers and Tris-HCl buffer (pH 7.6) were purchased from Sigma-Aldrich (St. Louis, Missouri,). Sequencing-grade urea, LC-MS-grade acetonitrile (ACN), trifluoroacetic acid (TFA) and formic acid (FA) were purchased from Thermo Fisher Scientific (Fair Lawn, NJ). Sequencing-grade modified trypsin was purchased from Promega Corp. (Madison, WI). The peptide standard kit for MALDI-TOF-MS was purchased from AB SCIEX (Framingham, MA). Sep-Pak C18 columns (1cc) were obtained from Waters (Milford, MA). C18 zip-tips were purchased from Millipore (Bedford, MA). Alpha-cyano-4-hydroxycinnamic acid (CHCA) matrix was purchased from Agilent (Palo Alto, CA).
Carbamylation on standard peptides
Five microliters of a 1mM equimolar solution of angiotensin and neurotensin was diluted ten times with 45 µL 1.6M urea in one of the following buffers: 0.1M phosphate buffer (PB), pH 8; 0.2M Tris-HCl, pH 7.6; 0.2M NH4HCO3; 0.1M NH4HCO3; 0.5M NH4HCO3; 1M NH4HCO3; 1M ammonium acetate and 1M triethylammonium bicarbonate (TEAB) buffers. After incubation at 37°C for 18 h, the samples were cooled down on ice and 1/10 of each sample was desalted by C18 zip-tip. Samples were eluted from the C18 tips with 1.5 µL 80%ACN/0.1%TFA and directly spotted onto a MALDI plate with 0.5µL CHCA matrix, according to the manufacturer’s recommendations. Samples were air dried and analyzed by a MALDI-TOF mass spectrometer (Applied Biosystems 4800, Framingham, MA).
Carbamylation on a standard protein
Bovine fetuin (40 µg) was denatured and incubated in 20 µL 8M urea in one of the following buffers: 0.1M PB, pH8; 0.2M Tris-HCl, pH7.6; 0.2M NH4HCO3; and 1M NH4HCO3 at room temperature for 30 min. The protein was reduced with 10 mM TCEP at 37°C for 1 h and alkylated using 15 mM iodoacetamide at room temperature for 30 min in the dark. The samples were diluted 5-fold with the respective buffers as mentioned above (final concentration of urea was 1.6M) and were digested via trypsin (protein: enzyme, 50:1, w/w) at 37 °C for 18 h. Then, 10% of each sample was desalted by C18 zip-tip prior to MALDI-TOF-MS analysis.
Carbamylation on human serum
Two microliters of human serum (Sigma) was diluted 10-fold with 8M urea in one of the following buffers: PB, pH8; 0.2M Tris-HCl; 0.2M NH4HCO3; and 1M NH4HCO3. After being reduced and alkylated as indicated above, the samples were diluted 5-fold with each of the buffers followed by digestion with trypsin (protein: enzyme, 50:1, w/w) at 37°C for 18 h. The samples were desalted by employing 1cc C18 columns and dried in a SpeedVac. The dried peptides were resuspended in 0.1%TFA for LC-MS/MS analysis.
MALDI-TOF-MS and LC-MS/MS analysis
For MS analysis, the peptides obtained above were analyzed by MALDI-TOF-MS (Applied Biosystems 4800). Spectra were acquired in the reflector mode. The relative carbamylation ratio using the peak area of the carbamylated peptide divided by the peak area of non-carbamylated peptides was used for quantification of the carbamylated peptides. For LC-MS/MS analysis, 1µg of tryptic peptides generated from human sera was analyzed by an LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific). Peptides were separated on a Dionex Ultimate 3000 RSLCnano system (Thermo Scientific) consisting of a 75 μm × 15 cm Acclaim PepMap100 separating column (Thermo Scientific) downstream of a 2 cm guard column (Thermo Scientific). The mobile phase flow rate was set to 300 nL/min and was comprised of 0.1% formic acid in water (A) and 0.1% formic acid in 95% acetonitrile (B). The gradient profile was established as follows: 4-35% B for 70 min, 35-95% B for 5 min, 95% B for 10 min and equilibration at 4% B for 15 min. MS analysis was performed using an Orbitrap Velos Pro mass spectrometer (Thermo Scientific). The spray voltage was set at 2.2 kV. Orbitrap MS1 spectra (AGC 1×106) were acquired from 400-1800 m/z at a resolution of 60K followed by data-dependent HCD MS/MS (resolution 7500, collision energy 45%, activation time 0.1 ms) of the ten most abundant ions using an isolation width of 2.0 Da. Charge state screening was enabled to reject unassigned and singly charged ions. A dynamic exclusion time of 35 sec was used to discriminate against previously selected ions.
Database search
The LC-MS/MS data was searched against an IPI human protein database (v3.87) via Sequest (Proteome Discoverer, Thermo Scientific). The database search parameters were set as follows: two missed protease cleavage sites were allowed for trypsin digests with 10 ppm precursor mass tolerance and 0.06 Da fragment mass tolerance. Carbamidomethylation (C; +57Da) was set as a static modification while oxidation (M; +16 Da) and carbamylation (N-terminal, K; +43Da) were set as dynamic modifications. 1% FDR was appointed as a filter for peptide identification.
Results and discussion
Carbamylation of peptide in different buffers with urea
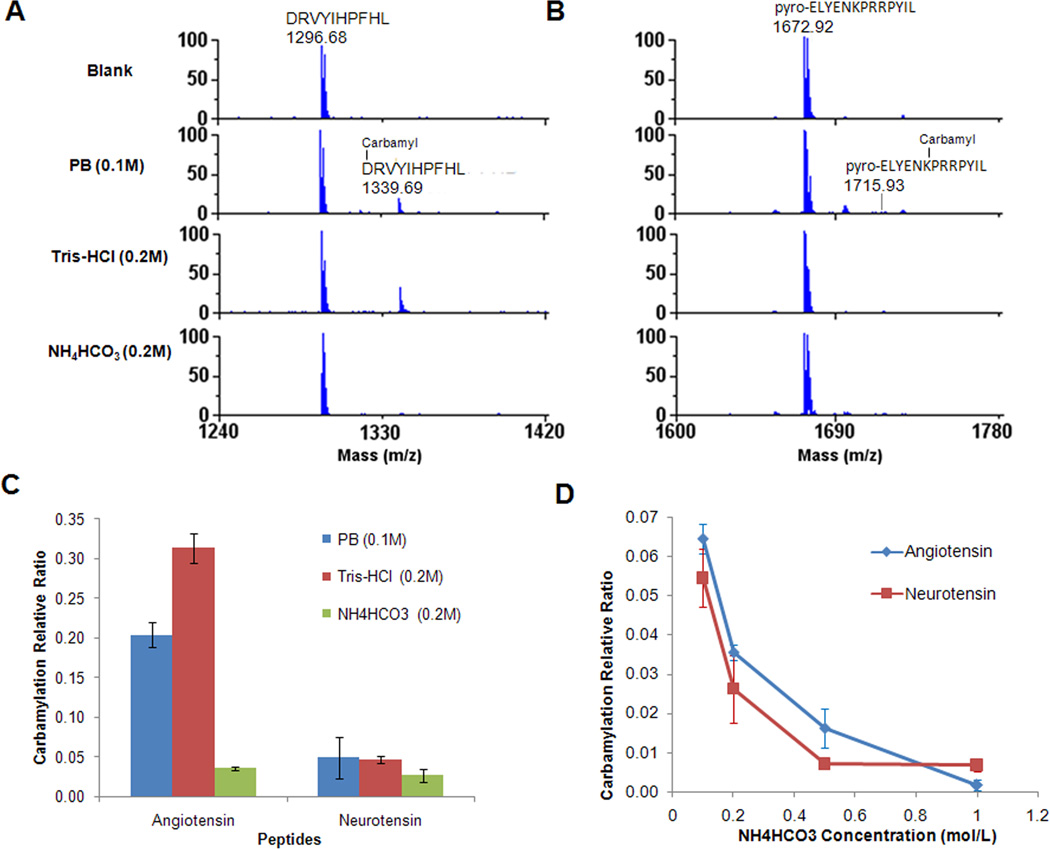
To determine the extent of carbamylation of the β-amino group at the N-terminal of peptides and the β-amino group at the side chain of lysine residues, we chose two standard peptides, angiotensin and neurotensin, and tested the extent of peptide carbamylation using urea solutions in different buffers (Table 1). Angiotensin contains one N-terminal β-amino group and one arginine residue that can potentially be carbamylated. However, because carbamylation only occurs sporadically at the arginine side chain [15], the potential carbamylation site on this peptide is its N-terminal residue. Neurotensin contains a pyroglutamic acid residue at its N-terminus; hence, it does not have an active amino group at its N-terminus that can be carbamylated. Although it contains one lysine residue and two arginine residues as potential carbamylation sites, carbamylation would mainly occur at the lysine residue. Both peptides were incubated in 1.6M urea solution at 37°C for 18 h (the commonly used condition for protein digestion) in one of the following three commonly used buffers: 0.1M PB (pH 8, no amino groups, served as a negative control), 0.2M Tris-HCl buffer (pH 7.6, containing active amino groups) and 0.2M NH4HCO3 buffer (containing NH4+ ion).
Table 1.
Standard peptides used for peptide carbamylation assay and the peptide masses of their unmodified and carbamylated form.
| Peptide | Sequencea | Unmodified mass (Da) | Carbamylated mass (Da)b | ||
|---|---|---|---|---|---|
| Angiotensin | D*R*VYIHPFHL | 1296.68 | 1339.69 | 1382.7 | |
| Neurotensin | pyro-ELYENK*PR*R*PYIL | 1672.92 | 1715.93 | 1758.94 | 1801.95 |
“*” highlighted the amino acid residues that can be carbamylated in urea solution. ‘Pyro-’ represents pyroglutamic acid.
The masses of peptides with 1, 2 or 3 amino acid residues carbamylated (+43 Da). All masses are monoisotopic masses [M+H]+.
To determine the extent of carbamylation of the N-terminal amino group and lysine amino group in urea with different buffers, we calculated the relative carbamylation ratio using the peak area of the carbamylated peptide divided by the peak area of non-carbamylated peptides. The relative ratio of carbamylation does not reflect the percent of carbamylation of a specific peptide since the ionization efficiencies of the carbamylated and non-carbamylated forms of the same peptide are different. However, the relative carbamylation ratio provides a convenient means of measuring the extent of carbamylation of the same peptide in different conditions. The relative carbamylation ratios of both peptides in different buffers are shown in Figure 1. For angiotensin, which contains an amino group at its N-terminus, the relative ratio of carbamylation is 0.20 and 0.31 in PB buffer and 0.2M Tris-HCl buffer, respectively, whereas the relative carbamylation ratio was dramatically reduced to 0.036 in 0.2M NH4HCO3 buffer (Fig. 1A and 1C). For neurotensin, which does not contain an amino group at its N-terminus, the carbamylation mainly occurs at the lysine residues with relative carbamylation ratios of 0.049 and 0.047 in PB buffer and 0.2M Tris-HCl buffer, respectively. The relative carbamylation ratio is reduced to 0.026 in 0.2M NH4HCO3 buffer (Fig. 1B and 1C). The overall trend was that carbamylation occurred more frequently at the β-amino groups of peptide/protein N-termini than at the β-amino groups of lysine side chains, which is consistent with previous reports [9; 16]. The NH4HCO3 buffer supplied the highest protection to peptides against carbamylation among all the buffers that we tested.
Fig. 1.
Inhibition of peptide carbamylation by different buffers. Angiotensin and neurotensin were incubated at 37°C for 18h in different buffers (0.1M PB, 0.2M Tris-HCl and 0.2M NH4HCO3) with 1.6M urea solution. A) Carbamylation of angiotensin peptide at its N-terminus in different buffers with urea. B) Carbamylation of neurotensin at the side chain of Lys in different buffers with urea. C) Relative carbamylation of angiotensin and neurotensin in different buffers with urea. D) Inhibition of peptide carbamylation by different concentrations of NH4HCO3.
To further reduce peptide carbamylation in NH4HCO3 buffer with urea, different concentrations of NH4HCO3 buffer with 1.6M urea were evaluated. The two standard peptides, antiotensin and neurotensin, were incubated at 37°C for 18 h in the 1.6M urea solution with NH4HCO3 at concentrations of 0.1M, 0.2M, 0.5M, and 1M. The results of relative carbamylation ratio are shown in Figure 1D. The general tendency observed for both peptides was that the relative ratio of carbamylation decreased with increased NH4HCO3 concentrations. The relative peptide carbamylation ratio was only 0.0016 for angiotensin and 0.0067 for neurotensin when they were incubated in 1M NH4HCO3 protecting buffer with 1.6M urea for 18h at 37°C.
The effect of temperature and urea concentration on carbamylation inhibition by NH4HCO3 solution was also investigated. The results indicated that although the NH4HCO3 buffer showed effective protection on peptides compared to PB buffer even at 95°C in 1 h, the relative carbamylation ratio dramatically increased compared to lower temperature (Table S1). For example, 1M NH4HCO3 could still almost completely prevent any N-terminal peptide carbamylation with incubation at 60°C for 1h. However, the relative carbamylation ratio of angiotensin and neurotensin reached 12.2 and 3.7 at 95°C in 1h, respectively. The urea concentration was another very important factor that affected the carbamylation level of peptide. The data showed a general trend that the carbamylation of peptides would continue increasing with increased urea concentrations, even in the presence of 0.2M NH4HCO3 buffer (Fig. S1). Considering all the factors listed above, it was concluded that the carbamylation at both the N-termini and lysine side chains of peptides could be avoided in 1M NH4HCO3 buffer with 1.6M urea solution at 37°C for as long as 18h.
Carbamylation during protein digestion in urea solution
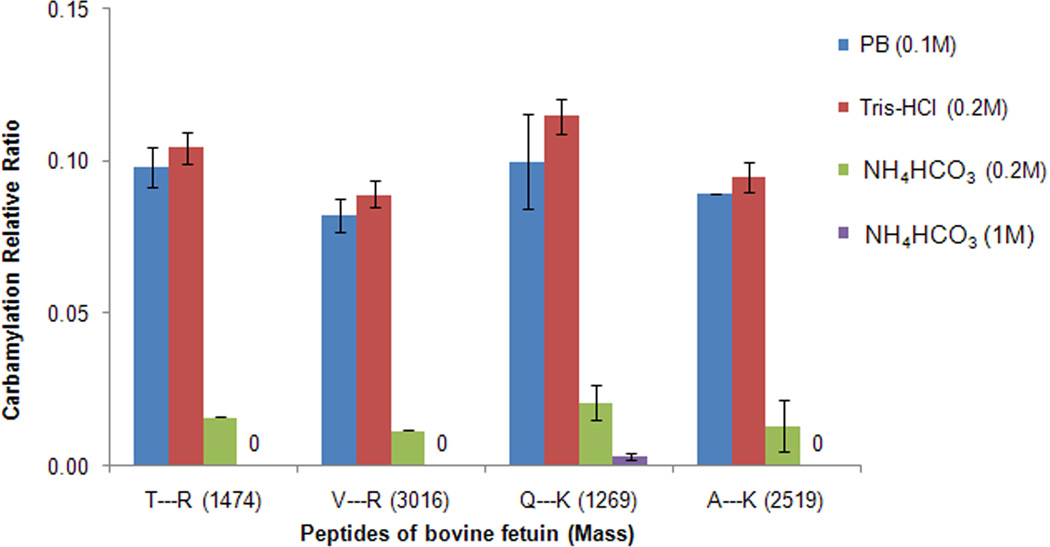
To investigate the effect of different buffers on protein digestion, a standard protein bovine fetuin was selected for trypsin digestion and carbamylation measurement. Fetuin was denatured (room temperature for 30 min), reduced (37°C for 1 h), and alkylated (room temperature for 30 min) in 0.1M PB, pH8; 0.2M Tris-HCl, pH7.6; 0.2M NH4HCO3 or 1M NH4HCO3 buffer with 8 M urea followed by 5-fold dilution with the corresponding buffer (the final urea concentration was 1.6M and the buffer concentration was unchanged) and trypsin was added for digestion at 37 °C for 18h. The results showed that 16 out of the 21 theoretical tryptic peptides (peptide masses between 500 and 5000 Da) were detected from digestion in all the buffers by MALDI-TOF-MS (Table S2). The undetected peptides could contain glycosylation (2 peptides) or other unknown modifications (3 peptides). In addition, the profiles and intensity of the MALDI-TOF-MS spectra between the samples in different buffers were very similar (data not shown). These data indicated that high concentration of NH4HCO3 buffer (1M) did not affect the trypsin digestion of fetuin.
To further assess the protective effect of NH4HCO3 buffer on peptide carbamylation during protein digestion, six tryptic peptides from fetuin were selected for further analysis to determine the relative carbamylation ratio in different buffers based on N-terminal carbamylation, lysine carbamylation of peptides after tryptic digestion, and lysine carbamylation at the protein level causing a missed cleavage of tryptic peptides at the carbamylated lysine (Table 2). Two of the tryptic peptides contain internal N-terminal amino groups and arginine residues at their C-termini (peptide #1 and #2 in Table 2), another two contain internal N-terminal amino groups and lysine residues at their C-termini (peptide #3 and #4 in Table 2), while the remaining two contain carbamylated lysine residues at the missed cleaved lysine residues, internal N-terminal amino groups, and arginine residues at the C-termini of the tryptic peptides (peptide #5 and #6 in Table 2). The relative carbamylation ratios at the N-terminal of peptide #1 and #2 were 0.098 and 0.082 in PB, 0.11 and 0.089 in 0.2M Tris-HCl buffer, 0.016 and 0.012 in 0.2M NH4HCO3, and no carbamylation in 1M NH4HCO3, respectively (Fig. 2). However, the total carbamylation ratios of the double carbamylation tryptic peptides containing both lysine residues at their C-terminus and internal N-terminal amino groups (peptide #3 and #4) were 0.10 and 0.089 in PB, 0.12 and 0.095 in 0.2M Tris-HCl buffer, 0.021 and 0.013 in 0.2M NH4HCO3 buffer, and 0.003 and 0 in 1M NH4HCO3 buffer, respectively (Fig. 2). These data indicated that the carbamylation at both N-terminal and lysine residues of peptides could be efficiently inhibited in 1M NH4HCO3 buffer. The two selected peptides with internal missed cleaved lysines were not detected by MALDI-TOF-MS, which suggested that the carbamylation that occurs on the protein level during the denaturation, reduction and alkylation processes was negligible, and the trypsin digestion was equally complete in all four buffers. The results showed that NH4HCO3 buffer provided the best protection against carbamylation on both protein and peptide levels, and 1M NH4HCO3 almost completely inhibited protein and peptide carbamylation.
Table 2.
Six bovine fetuin tryptic peptides that were selected for peptide carbamylation analysis.
| No. | Peptide sequencea | Unmodified peakb | Carbamylated peak |
|---|---|---|---|
| 1 | T*PIVGQPSIPGGPVR | √ | √ |
| 2 | V*VHAVEVALATFNAESNGSYLQLVEISR | √ | √ |
| 3 | Q*DGQFSVLFTK* | √ | √ |
| 4 | A*QFVPLPVSVSVEFAVAATDCIAK* | √ | √ |
| 5 | G*SVIQK*ALGGEDVR | - | - |
| 6 | H*TLNQIDSVK*VWPR | - | - |
“*” highlighted the amino acid residues that can be carbamylated in urea solution.
“√” represents the unmodified peptides or carbamylated peptide of fetuin were detected by MALDI-TOF-MS. “-” represents the unmodified peptides or carbamylated peptide of fetuin were not detected.
Fig. 2.
Relative carbamylation by protein digestion in different buffers with urea. Bovine fetuin was denatured, reduced, alkylated, and digested in different buffers (0.1M PB, 0.2M Tris-HCl, 0.2M NH4HCO3 and 1M NH4HCO3) with urea solution.
Carbamylation on human serum proteins
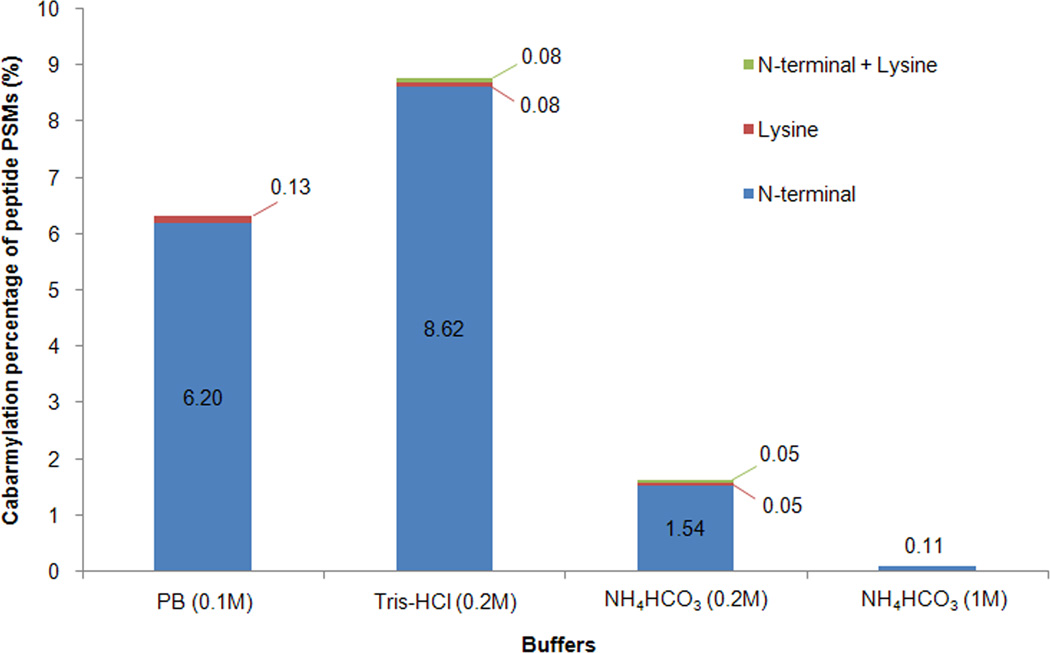
The ability to protect proteins/peptides from carbamylation by NH4HCO3 buffer was applied to the proteomic analysis of human serum proteins. As described above and shown in Figure 2, the 1M NH4HCO3 buffer provides the best protection against protein and peptide carbamylation during protease digestion. When 1M NH4HCO3 buffer with urea was used to denature, reduce, alkylate and digest serum proteins, followed by mass spectrometric analysis of tryptic peptides, the database search results showed that carbamylation was almost completely avoided (only one peptide was identified in its carbamylated form). In contrast, when serum proteins were digested in other buffers, 6.3% and 8.7% of the peptide spectrum matches (PSMs) (9.3% and 11.3% of the unique peptides) were identified in their carbamylated forms in PB and Tris-HCl buffer, respectively, but only 1.6% of PSMs (3.3% of unique peptides) were identified in their carbamylated forms in 0.2M NH4HCO3 buffer. The results from the proteomic analysis of tryptic peptides by tandem mass spectrometry further verified that the majority of the carbamylation occurred at the N-terminal amino groups of peptides (Fig. 3). The NH4HCO3 buffer, high concentrations of NH4HCO3 buffer in particular, effectively protected proteins and peptides in urea solution from carbamylation. The 1M NH4HCO3 buffer also did not show any adverse effects on trypsin digestion based on the number of identified peptides and missed cleavage sites information (Table S3). The only observed potential drawback of using a high concentration of NH4HCO3 buffer (0.5-1M) was the extensive formation of bubbles during pH adjustment of the tryptic peptide sample before C18 cleanup. However, this issue can be resolved by using a sample tube with a volume that is preferably 10 times larger than that of the sample.
Fig. 3.
Percentage of carbamylated peptides from serum proteins digested in different buffers with urea. Human serum proteins were denatured, reduced, alkylated, and digested in different buffers (0.1M PB, 0.2M Tris-HCl, 0.2M NH4HCO3 and 1M NH4HCO3) with urea. The tryptic peptides were analyzed by LC-MS/MS and spectra were assigned by database search. The percentage of peptides assigned to different forms of carbamylated peptides was determined based on spectral counts.
Possible mechanism of carbamylation inhibition by ammonium containing buffer
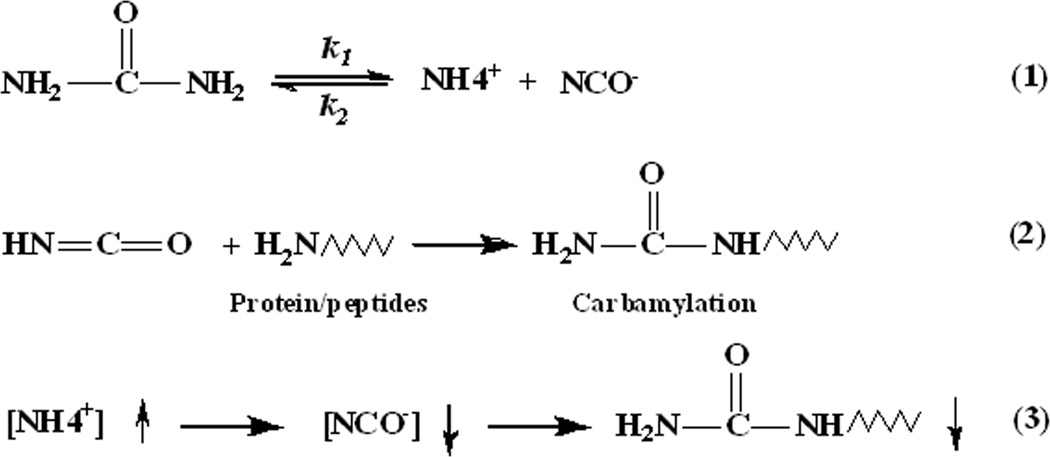
The possible mechanism of carbamylation inhibition by ammonium bicarbonate is as follows (Fig. 4): Ammonium bicarbonate exists as NH4+ and HCO3− ions in aqueous solution. Since the dissociation of urea to cyanate and ammonia is reversible, the NH4+ ion from NH4HCO3 drives the equilibrium of the urea dissociation reaction in solution to favor urea over isocyanic acid [13] (Equation #1), which reacts with the amino groups of proteins or peptides to produce carbamylated forms (Equation #2). When the temperature and the concentration of urea solution are fixed, the concentration of cyanate has an inverse ratio with the concentration of NH4+ ions. According to previous reports, cyanate formation (and NH4+) from urea can increase to an equilibrium value of approximately 5-10 mM in 2M urea solutions at neutral or basic pH values [14; 17]. Therefore, the cyanate concentration should have decreased at least 100-fold in the 1.6M urea solution when the 1M NH4HCO3 buffer was used, which could explain why 1M NH4HCO3 nearly completely prevented peptide carbamylation in the 1.6M urea solution (Equation #3).
Fig. 4.
Possible mechanism of carbamylation inhibition by ammonium containing buffer. (1) The decomposition of urea in aqueous solutions, where k1 and k2 represent the decomposition constant and formation constant of urea, respectively. (2) Carbamylation of proteins or peptides by cyanate. (3) The relationship among ammonium ion and cyanate concentrations and protein carbamylation under conditions of fixed urea solution concentrations and temperature.
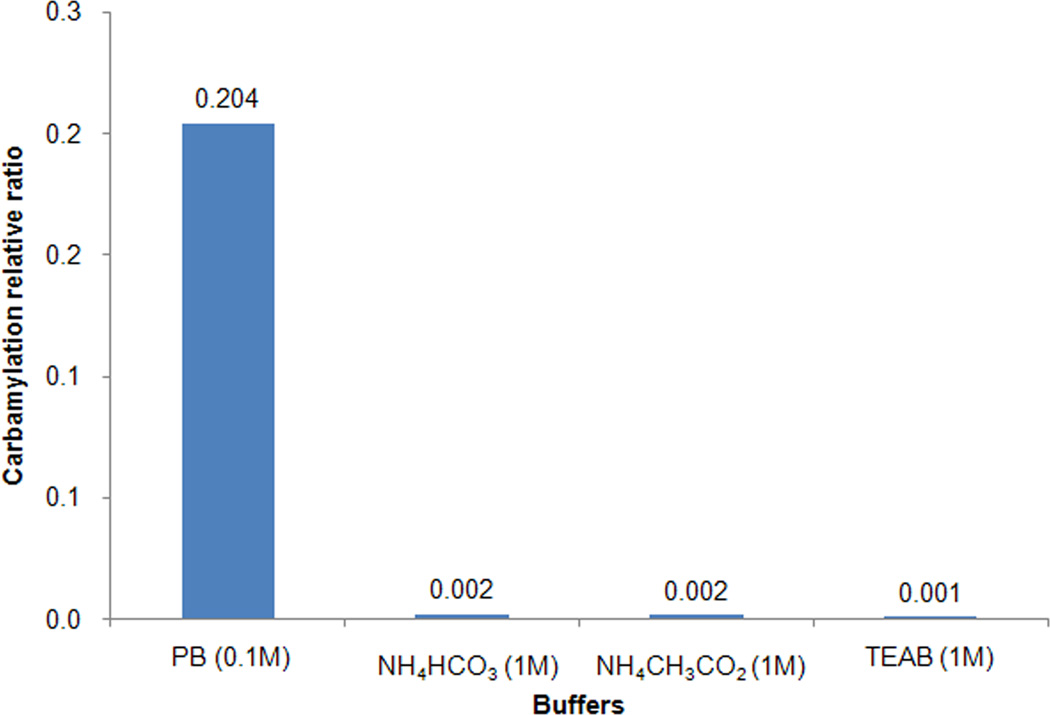
To test this hypothesis and determine the capability of other ammonium containing reagents in inhibition of carbamylation, we further tested ammonium acetate buffer (also contains NH4+ ion in solution) on inhibiting peptide carbamylation. After incubating angiotensin with 1.6M urea solution at 37°C for 18 h in 1M ammonium acetate buffer (pH8.5), the relative carbamylation ratio was calculated as described above. Our results showed that the same concentration of ammonium acetate buffer had almost the same inhibition efficiency on peptide carbamylation as ammonium bicarbonate buffer (Fig. 5). In quantitative proteomics experiments using isotopic/isobaric labeling, triethylammonium bicarbonate (TEAB) is recommended buffer instead of ammonium bicarbonate mainly to avoid primary amines which will react with the labeling reagents, e.g. iTRAQ. The TEAB buffer contains triethylammonium ions which have chemical properties similar to ammonium ions. Our results showed TEAB buffer also had almost the same inhibition efficiency on peptide carbamylation as the same concentration of ammonium bicarbonate buffer (Fig. 5). These data indicated that other ammonium (or tertiary ammonium) ion containing buffers could also be used for effective carbamylation inhibition during protein digestion in urea solution.
Fig. 5.
The carbamylation inhibition of standard peptide by other ammonium containing buffers. Angiotensin was incubated 18h at 37°C with 1.6M urea solution in 0.1M phosphate buffer (PB), pH 8; 1M NH4HCO3 buffer; 1M ammonium acetate (NH4CH3CO2) buffer and 1M triethylammonium bicarbonate (TEAB) buffer, respectively.
Revised procedure for trypsin digestion to eliminate carbamylation
Besides protein denaturation and protein digestion, urea is also widely used to extract proteins from tissues and cell samples. Although only one β-amino group is present at the N-terminal of each protein chain, the carbamylation may still occur at the N-terminal of the protein and in reduced degree at β-amino groups of lysine residues in proteins based on the results we showed in this study, and it may reach a relatively high level if the protein extraction is performed in a high concentration of urea solution (for example, 8M urea for protein extraction and denaturation), at the high temperature (which might be caused by sonication), high pH, or with a long incubation time. Therefore, 0.5-1M ammonium containing buffer should also be very helpful when urea is employed for protein extraction.
Based on the results and discussion described above, we propose a new procedure for the trypsin digestion of proteins from body fluids, cell cultures and solid tissues to revise our previously established protocol [18]. First, 8M urea solution in 1M NH4HCO3 buffer (or other ammonium containing buffer) is used to denature proteins from body fluids, lysed cells or solid tissues (after being sliced into small pieces) using sonication for 1-2 min (10-20s each time) until a clear solution is observed. The proteins are then reduced with 10 mM TCEP at 37°C for 1h and alkylated with 15 mM iodoacetamide at room temperature for 30min in the dark. The samples are diluted 5-fold using 1M NH4HCO3 buffer (or other ammonium containing buffer) and trypsin is added to digest proteins overnight at 37°C with shaking. Following digestion, the pH is adjusted to <3 using 100% TFA (~10% of the total sample volume), and the peptides are purified by a C18 column before proteomic analysis by mass spectrometry.
Conclusions
NH4HCO3 buffer showed more effective protection for proteins and peptides against carbamylation in urea solution than PB and Tris-HCl buffer. The inhibition efficiency increased with increased NH4HCO3 concentrations. The 1M NH4HCO3 buffer nearly completely prevented carbamylation on proteins and peptides during the process of trypsin digestion in urea solution, and it exhibited a negligible effect on the efficiency of trypsin digestion. Two other ammonium (or tertiary ammonium) containing buffers also effectively inhibited protein carbamylation during protein digestion in urea solution. NH4HCO3 is a commonly used buffer that is compatible with trypsin and many other enzymes, thus making it an ideal buffer for applications that entail the proteomic analysis of complex samples.
Supplementary Material
Acknowledgment
This work was partially supported by the National Institutes of Health, National Cancer Institute, Clinical Proteomic Tumor Analysis Consortium (CPTAC, U24CA160036) and the Early Detection Research Network (EDRN, U01CA152813).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabilloud T. Solubilization of proteins for electrophoretic analyses. Electrophoresis. 1996;17:813–829. doi: 10.1002/elps.1150170503. [DOI] [PubMed] [Google Scholar]
- 2.Herbert B. Advances in protein solubilisation for two-dimensional electrophoresis. Electrophoresis. 1999;20:660–663. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<660::AID-ELPS660>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Angel PM, Orlando R. Quantitative carbamylation as a stable isotopic labeling method for comparative proteomics. Rapid Commun. Mass Sp. 2007;21:1623–1634. doi: 10.1002/rcm.2990. [DOI] [PubMed] [Google Scholar]
- 4.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Mol. Cell. Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Shah P, Yang S, Sun S, Aiyetan P, Yarema KJ, Zhang H. Mass Spectrometric Analysis of Sialylated Glycans with Use of Solid-Phase Labeling of Sialic Acids. Anal. Chem. 2013;85:3606–3613. doi: 10.1021/ac3033867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Li Y, Shah P, Zhang H. Glycomic Analysis Using Glycoprotein Immobilization for Glycan Extraction. Anal. Chem. 2013 doi: 10.1021/ac400761e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole EG, Mecham DK. Cyanate formation and electrophoretic behavior of proteins in gels containing urea. Anal. Biochem. 1966;14:215–222. doi: 10.1016/0003-2697(66)90129-1. [DOI] [PubMed] [Google Scholar]
- 8.Tenga MJ, Lazar IM. Impact of Peptide Modifications on the Isobaric Tags for Relative and Absolute Quantitation Method Accuracy. Anal. Chem. 2011;83:701–707. doi: 10.1021/ac100775s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollipara L, Zahedi RP. Protein carbamylation: In vivo modification or in vitro artefact? Proteomics. 2013;13:941–944. doi: 10.1002/pmic.201200452. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 11.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68:173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 12.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic Uremia Stimulates LDL Carbamylation and Atherosclerosis. J. Am. Soc. Nephrol. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stark GR, Stein WH, Moore S. Reactions of the Cyanate Present in Aqueous Urea with Amino Acids and Proteins. J. Biol. Chem. 1960;235:3177–3181. [Google Scholar]
- 14.Marier JR, Rose D. Determination of cyanate, and a study of its accumulation in aqueous solutions of urea. Anal. Biochem. 1964;7:304–314. doi: 10.1016/0003-2697(64)90135-6. [DOI] [PubMed] [Google Scholar]
- 15.Hagel P, Gerding JJT, Fieggen W, Bloemendal H. Cyanate formation in solutions of urea: I. Calculation of cyanate concentrations at different temperature and pH. Biochimica et Biophysica Acta (BBA) - Protein Structure. 1971;243:366–373. doi: 10.1016/0005-2795(71)90003-1. [DOI] [PubMed] [Google Scholar]
- 16.Stark GR. Reactions of Cyanate with Functional Groups of Proteins. III. Reactions with Amino and Carboxyl Groups*. Biochemistry. 1965;4:1030–1036. doi: 10.1021/bi00882a008. [DOI] [PubMed] [Google Scholar]
- 17.Lin M-F, Williams C, Murray MV, Conn G, Ropp PA. Ion chromatographic quantification of cyanate in urea solutions: estimation of the efficiency of cyanate scavengers for use in recombinant protein manufacturing. J. Chromatogr. B. 2004;803:353–362. doi: 10.1016/j.jchromb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Tian YA, Zhou Y, Elliott S, Aebersold R, Zhang H. Solid-phase extraction of N-linked glycopeptides. Nat. Protoc. 2007;2:334–339. doi: 10.1038/nprot.2007.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.