Abstract
Both the serotonin and glutamate systems have been implicated in the pathophysiology of schizophrenia, as well as in the mechanism of action of antipsychotic drugs. Psychedelic drugs act through the serotonin 2A receptor (5-HT2AR), and elicit a head-twitch response (HTR) in mice, which directly correlates to 5-HT2AR activation and is absent in 5-HT2AR knockout mice. The precise mechanism of this response remains unclear, but both an intrinsic cortico-cortical pathway and a thalamo-cortical pathway involving glutamate release have been proposed. Here, we used a genetic model of NMDAR hypofunction, a serine racemase knockout (SRKO) mouse, to explore the role of glutamatergic transmission in regulating the 5-HT2AR-mediated cellular and behavioral responses. SRKO mice treated with the 5-HT2AR agonist (+/-)-2,5-dimethoxy-4-iodoamphetamine (DOI) showed a clearly diminished HTR and lower induction of c-fos mRNA. These altered functional responses in SRKO mice were not associated with changes in cortical or hippocampal 5-HT levels or in 5-HT2AR and metabotropic glutamate-2 receptor (mGluR2) mRNA and protein expression. Together, these findings suggest that D-serine-dependent NMDAR activity is involved in mediating the cellular and behavioral effects of 5-HT2AR activation.
Keywords: NMDA receptor, D-serine, serotonin, 5-HT2A receptor, metabotropic glutamate 2 receptor
Activation of serotonin 2A receptors (5-HT2ARs) by psychedelic drugs mediates their psychotomimetic effects, which include alterations of human consciousness, emotion and cognition [1]. In rodents, administration of hallucinogens results in an induction of immediate-early genes (IEGs), such as Arc, c-fos and Egr-2 in cortical brain regions and a characteristic head-twitch response (HTR) [2, 3]. These cellular and behavioral outputs are blocked by selective 5-HT2AR antagonists [4] and eliminated in 5-HT2AR-/- mice, but can be rescued by genetic restoration of 5-HT2AR to cortical pyramidal neurons [5]. Furthermore, only compounds, which cause hallucinations in humans induce HTR, suggesting that this response is an indicator of hallucinogenic action [5]. Thus, both the induction of cortical IEG expression and HTR can be utilized as a 5-HT2AR-specific measure of receptor efficiency.
Several studies suggest an interaction between 5-HT2ARs and the glutamate system. 5-HT2ARs are primarily expressed on apical dendrites of pyramidal neurons, particularly in cortical layer V [6]. Glutamate serves as the principal neurotransmitter of the pyramidal cells, and it is thought that cortico-cortical connections, which are mostly comprised of synaptic contacts at apical dendrites [7], are important in generating and shaping the neural activity that underlies consciousness [8].
Acute administration of the NMDA receptor (NMDAR) antagonists PCP and MK-801 to rodents induces hyperlocomotion and HTR, which are blocked by 5-HT2AR antagonists [9-11]. Also, atypical antipsychotics with 5-HT2AR antagonistic properties are able to reverse acute PCP-induced cellular disruptions in the prefrontal cortex (PFC) [12]. Together, these studies indicate a potential role of circuits working through the 5-HT2AR in mediating the behavioral responses induced by NMDAR antagonists.
Previous studies have proposed that cortical activation by DOI requires the activation of 5-HT2ARs expressed on thalamo-cortical axon terminals [13]. However, recent evidence suggests that the circuit involved in this effect is a cortico-cortical circuit and does not require activation of a thalamo-cortical pathway [5]. In fact, DOI does not induce Arc directly in 5-HT2AR expressing neurons, but in neurons containing AMPA and NMDA receptors [3]. Furthermore, activation of 5-HT2ARs in the PFC results in a robust increase in glutamate receptor activation, which suggests that certain actions of 5-HT2ARs in this region may be mediated by the release of glutamate [14].
To study the involvement of the glutamate system in mediating the cellular and behavioral responses to 5-HT2AR activation, we used an animal model of NMDAR hypofunction, the constitutive serine racemase knockout (SRKO) mouse. Activation of NMDARs requires the binding of either glycine or D-serine to the glycine modulatory site (GMS) of the GluN1 subunit. SRKO mice, which lack the ability to convert L-serine to D-serine, have an 85% reduction in cortical D-serine [15]. D-serine is enriched in corticolimbic regions of the brain, where its localization closely parallels that of NMDARs [16]. Thus, SRKO mice exhibit reduced forebrain NMDAR-mediated neurotransmission [15, 17] that is associated with cognitive impairments dependent on the PFC [18] and hippocampus [17]. In the present study, we tested whether the functional outputs following 5-HT2AR activation were altered due to decreased NMDAR activity.
SR −/− mice were generated as previously described [15]. Mice with a serine racemase null mutation resulting from targeted deletion of the first coding exon were backcrossed for over 10 generations onto a C57BL/6J background. SR+/− sires and dams were bred to produce wild-type (WT), as well as SR−/−; offspring. Adult male and female mice were used for all the experiments in this study. Animals were housed in groups of four in polycarbonate cages and maintained on a 12:12 h light/dark cycle in a temperature (22 °C) and humidity controlled vivarium. Animals were given access to food and water ad libitum. All the animal procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Head-twitch response (HTR) scoring was performed as previously reported [19], with minor modifications. In one cohort, WT and SR-/- mice (n = 6 per group) were injected with either (±)-2,5-dimethoxy-4-iodoamphetamine hydrocloride (DOI: synthesized at the Department of Medicinal Chemistry, Faculty of Pharmaceutical Sciences, Copenhagen University; 2 mg/kg, 10 ml/kg, i.p.) or a corresponding volume of saline (10 ml/kg). In a second cohort, WT and SR-/- mice (n = 5-6 per group) were injected with either DOI (0.5 mg/kg, 10 ml/kg, i.p.) or a corresponding volume of saline (10 ml/kg). After drug administration the mice were immediately moved to a standard cage (15 × 24 cm) without enrichment material and videotaped for 25 min. Subsequently, the total number of head-twitches was scored in the interval between 5 and 25 minutes after drug administration by an observer not aware of the treatment of the animal. For the first cohort, the experiment was repeated one week later with a crossover design so that animals receiving DOI on the first test day were given saline on the second test day and vice versa. The crossover design was not implemented for the second cohort, as separate groups of WT and SR-/- mice received either DOI or saline.
For mRNA expression analysis, animals (n = 6-9 per group) were injected with either DOI (2 mg/kg, 10 ml/kg, i.p.) or a corresponding volume of saline and returned to their home-cage. After 60 minutes, the mice were killed by cervical dislocation, and their brains quickly removed and frozen in powdered dry ice. Total RNA from cortical samples was isolated with Trizol Reagent (Sigma-Aldrich). cDNA for each RNA sample was generated using the High Capacity cDNA Reverse transcription kit (Applied Biosystems; Foster City, CA) according to the manufacturer’s instructions. The real-time qPCR reactions were performed by adding the sample cDNA to a reaction mixture consisting of 1× Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA) and 15 pmol of each primer. Specific primers were as follows: GAPDH primers, Forward: 5’-CATCAAGAAGGTGGTGAAGCA-3’, Reverse: 5’-CTGTTGAAGTCACAGGAGACA-3’; c-fos primers, Forward: 5’- CAAAGTAGAGCAGCTATCTCC-3’, Reverse: 5’-CTCGTCTTCAAGTTGATCTGT-3’; 5-HT2AR primers, Forward: 5’- CCGCTTCAACTCCAGAAC CAAAGC-3’, Reverse: 5’-CTTCGAATCATCCTGTACCCGAA-3’; 5-mGluR2 primers, Forward: 5’- CCATCTTCTACGTCACCTCC-3’, Reverse: 5’-AGGAACAAGCTGGGATCCAG-3’. Data were collected using a 48-well MJ Minioption Personal thermal cycler (BioRad; Hercules, CA). Each sample was assayed in triplicate. For relative quantification of mRNA expression, geometric means were calculated using the comparative 2−ΔΔCt method, with the housekeeping gene GAPDH used as the endogenous reference.
To determine receptor binding, animals (n = 8 per group) were decapitated and brains were quickly removed and stored at ÷80 °C until sectioning. Brains were cut in 12 μm coronal sections and mounted on Super Frost Plus slides and stored at -80 °C until further processing. 5-HT2AR autoradiography was performed using [3H]-MDL100907 [R(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorphenyl)-ethyl]-4-piperidin-methanol] (specific activity; 64 Ci/mmol, a gift from Professor Halldin, Karolinska Institute, Stockholm), and non-specific binding was determined using 10 μM ketanserin tartrate (Sigma). For mGluR2/3 autoradiography, [3H]-LY341495 (GE Healthcare, UK) was used and non-specific binding was measured with 10 μM glutamate (Sigma). Briefly, sections were allowed to thaw for 1 h at RT and then pre-incubated with 50 mM Tris–HCl (Sigma), pH 7.4 for 30 min at RT under constant gentle shaking. Sections were then incubated for 60 min at RT using the same buffer containing either 2 nM [3H]-MDL100907 or 2 nM [3H]-LY341495 with or without the respective cold ligand. Following incubation, slides were washed for 2×20 s in ice-cold 50 mM Tris–HCl, pH 7.4, for 20 s in ice-cold H2O, and dried for 1 h under a gentle stream of air. All sections were placed at 4 °C overnight in a fixator containing paraformaldehyde vapor and then put in an excitator box for 3 h before slides, together with [3H]-microscales (GE Healthcare, UK), were exposed to a BAS-TR2040 Imaging Plate (Science Imaging Scandinavia AB, Nacka, Sweden) for 3-14 days at 4 °C. Finally, the imaging plate was scanned on a BAS-2500 phosphoimage scanner (Fujifilm Europe GmbH, Düsseldorf, Germany). Specific and non-specific binding were determined at two brain levels where the following regions were analyzed (i) bregma 2.34 mm: total M2/CG1/PrL (motor/cingulate/prelimbic) cortex, (ii) bregma –2.06 mm: hippocampus and S1 cortex.
Head-twitch behavior and c-fos mRNA expression in cortical areas are reliably and robustly elicited by hallucinogenic 5-HT2AR agonists in rodents. We first assayed the HTR induced by DOI in wild-type and SRKO mice (Fig. 1A). Two-way ANOVA showed a significant effect of treatment [F(1, 19) = 57.16 ; p < 0.001] in animals treated with 0.5 mg/kg DOI or saline, as well as a significant interaction between treatment [F(1,19) = 131.4; p < 0.0001] and genotype [F(1,19) = 10.06; p < 0.0050] in animals treated with 2 mg/kg DOI or saline. Further, Tukey’s post hoc analysis revealed that DOI induced significantly fewer head-twitches in SRKO mice (p = 0.0054). Next we assessed c-fos mRNA expression in S1 cortex following DOI treatment. Again, a two-way ANOVA showed an interaction between the effects of treatment [Fig. 1B; F(1,26) = 19.81; p = 0.0001] and genotype [F(1,26) = 4.651; p = 0.0405]. Tukey’s post hoc analysis revealed that DOI induced c-fos mRNA to a lesser extent in SRKO mice compared to wild-type mice (p = 0.0054).
Fig. 1.
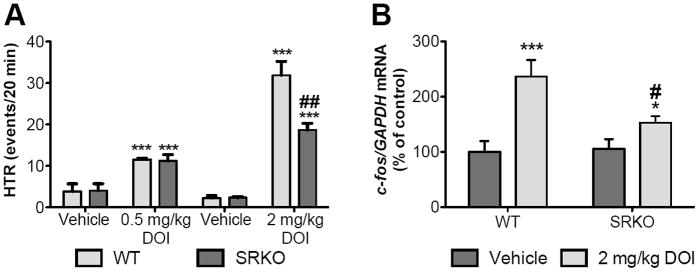
DOI-induced HTR and c-fos mRNA expression are reduced in SRKO mice. (A) Two-way ANOVA showed a significant effect of treatment [F(1, 19) = 57.16 ; p < 0.001] in animals treated with 0.5 mg/kg DOI or saline. For both WT (n = 5-6; light gray bars) and SRKO (n = 5-6; dark gray bars) mice, 0.5 mg/kg DOI highly induced head-twitches (HTR; ***p < 0.001). In addition, two-way ANOVA showed a significant interaction between treatment [F(1,19) = 131.4; p < 0.0001] and genotype [F(1,19) = 10.06; p < 0.0050] in animals treated with 2 mg/kg DOI or saline. For both WT (n = 6; light gray bars) and SRKO (n = 6; dark gray bars) animals, 2 mg/kg DOI highly induced head-twitches (HTR; ***p < 0.001). Post hoc analysis showed that SRKO mice displayed a significantly lower induction of HTR compared to WT mice (##p < 0.005). (B) Two-way ANOVA showed an interaction between the effects of treatment [Fig. 2; F(1,26) = 19.81; *** p = 0.0001] and genotype [F(1,26) = 4.651; * p = 0.0405]. Data are expressed as geometric means ± SEM of individual expression values normalized to the housekeeping gene GAPDH using the comparative 2 −ΔΔCt method. DOI (light gray bars) induced c-fos mRNA in both WT (n = 9) and SRKO (n = 9) mice. Post hoc analysis revealed that DOI induced c-fos mRNA to a lesser extent in SRKO mice compared to wild-type mice (# p = 0.005). All values represent the mean ± SEM.
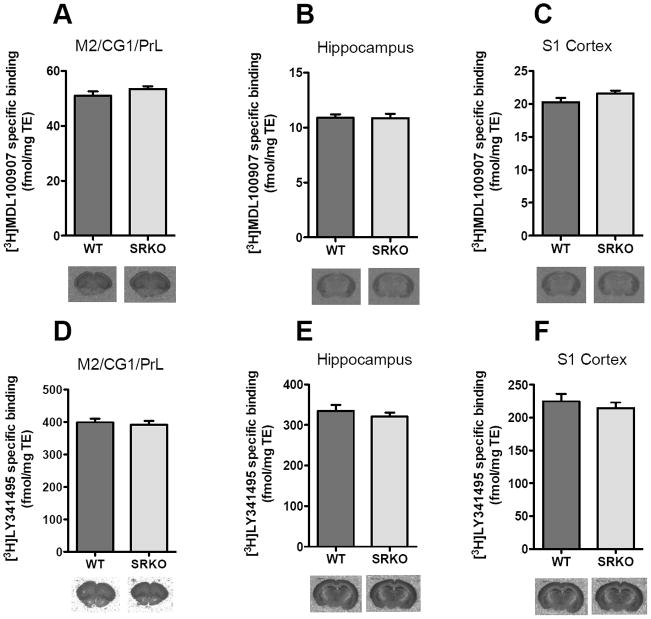
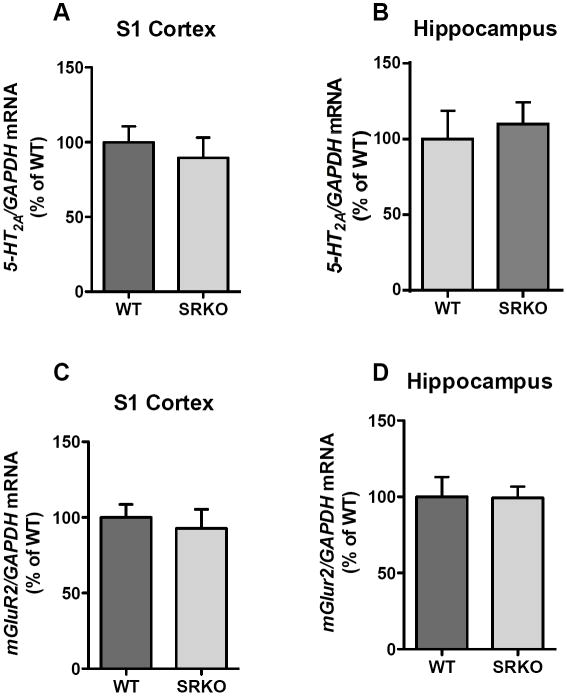
The decreased functional responses in SRKO mice following administration of DOI led us to examine the level of expression of 5-HT2A and mGluR2/3 receptors. The densities of 5-HT2A and mGluR2/3 receptors in M2/CG1/PrL cortex, S1 cortex and hippocampus were determined using quantitative in vitro receptor autoradiography. No differences in [3H]-MDL100907 (Fig 2A-C) or [3H]-LY341495 (Fig. 2D-F) binding were observed between WT and SRKO mice. Further, the mRNA expression of 5-HT2A (Fig. 3A-B) and mGluR2/3 (Fig. 3C-D) receptors in the S1 cortex and hippocampus was unaltered in SRKO mice compared to WT controls.
Fig. 2.
Regional protein expression of 5-HT2A and mGluR2/3 receptors. No difference was observed in the 5-HT2AR antagonist [3H]-MDL100907 binding in (A) M2/CG1/PrL (motor/cingulate/prelimbic) cortex, (B) hippocampus and (C) S1 cortex between WT (dark gray; n = 8) and SRKO (light gray; n = 8) animals. (D-F) No difference was observed in mGluR2/3 antagonist [3H]-LY341495 binding in (D) M2/CG1/PrL cortex, (E) hippocampus and (F) S1 cortex WT (dark gray; n = 8) and SRKO (light gray; n = 8) animals. Unpaired Student’s t-test. All values represent the mean ± SEM.
Fig. 3.
mRNA expression of 5-HT2A and mGluR2 receptors in S1 cortex and hippocampus. No difference was observed in 5-HT2AR mRNA expression between WT (dark gray; n = 8) and SRKO (light gray; n = 8) animals in (A) S1 cortex and (B) hippocampus. No difference was observed in mGluR2 mRNA expression in (C) S1 cortex and (D) hippocampus. Unpaired Student’s t-test. All values represent the mean ± SEM.
Using high pressure liquid chromatography (HPLC) analysis [20], we measured the levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) to assess whether the changes in 5-HT2AR-mediated responses were mediated by changes in cortical serotonin content. We found no differences between genotypes in 5-HT or 5-HIAA tissue content in the frontal cortex (Fig. 4A) and S1 cortex (Fig. 4B).
Fig. 4.
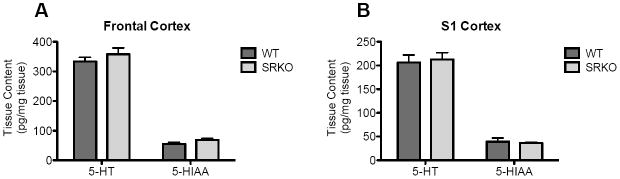
Tissue levels of 5-HT or 5-HIAA in the frontal cortex and S1 cortex. No differences were observed in the tissue levels of 5-HT or 5-HIAA in the (A) frontal cortex or (B) S1 cortex of WT (dark gray) and SRKO (light gray) animals. Unpaired Student’s t-test. n = 5-6. All values represent the mean ± SEM.
In the present study, we show that NMDAR hypofuction leads to attenuated functional responses to 5-HT2AR activation. We found that both cortical c-fos mRNA induction and HTR after DOI treatment were significantly reduced in SRKO mice, suggesting that glutamate signaling is necessary for at least some of the cellular and behavioral responses induced by 5-HT2AR activation. This is consistent with previous reports showing that 5-HT2AR receptor activation by DOI significantly increases glutamate release and glutamatergic pyramidal neuronal activity in the medial PFC layer V [13, 14, 21]. Results from both in vitro and in vivo studies in rats show that DOI increases spontaneous and evoked excitatory currents in layer V pyramidal cells (EPSCs) [22, 23] and increases the firing rate of a majority of neurons [24]. Further, DOI-mediated induction of Arc mRNA in rat PFC can be blocked by the NDMAR antagonist MK-801 [3], suggesting that the cellular effects of DOI are mediated by glutamate release. However, it should be noted that in rats [25] and certain mouse strains [26, 27], acute MK-801 pretreatment enhances the DOI-induced HTR. Future experiments will be needed to determine the behavioral consequence of MK-801 pretreatment on the HTR in C57BL/6 mice.
The mechanism behind HTR is more complex, but likely also involves glutamatergic signaling. Both systemic and direct injection of 5-HT2AR agonists into the PFC produce HTR [28], which is dependent exclusively on 5-HT2AR in the pyramidal neurons in the cerebral cortex, because region-specific rescue of 5-HT2AR expression in these particular cells in transgenic animals is sufficient to restore induction of transcriptional factors after systemic administration of DOI [5]. In rodents, hyperlocomotion and HTR induced by acute PCP can be blocked by 5-HT2AR antagonists [9-11], and 5-HT2AR antagonists are able to reverse acute PCP-induced cellular disruptions in PFC [12]. These findings indicate that the cellular and behavioral responses induced by acute PCP are mediated through circuits that are modulated by 5-HT2AR.
To complicate matters, it was recently demonstrated that the mGluR2 receptor interacts with the 5-HT2AR, forming a heteromeric complex in which mGluR2 activation attenuates both 5-HT2AR-mediated c-fos mRNA expression and HTR [29]. However, signaling through 5-HT2AR is also dependent on mGluR2, as mGluR2 receptor knockout mice are insensitive to the HTR-inducing effects of DOI (and LSD) [30]. Although the neuropharmacological mechanisms underlying these interactions have not been delineated, these data suggest a prominent role for glutamate receptor systems in the behavioral effects of DOI, and a strong interaction between glutamatergic and 5-HT2AR systems.
The decreased c-fos mRNA induction and HTR following administration of DOI led us to examine the level of expression of 5-HT2A and mGluR2/3 receptors in the SRKO mice. We found no changes in the binding of [3H]-MDL100907 and [3H]-LY341495 – measuring 5-HT2AR and mGluR2/3, respectively, in M2/CG1/PrL cortex, S1 cortex or hippocampus. Further, there was no difference between WT and SRKO mice in the mRNA expression of 5-HT2AR and mGluR2. These results suggest that the functional deficits observed in SRKO mice are not caused directly by changes in receptor density, but could be due to decreased secondary activation of post-synaptic NMDARs and/or altered G-protein coupling. Furthermore, in the PFC and S1 cortex, the brain regions responsible for the cellular and behavioral effects of 5-HT2AR activation [5], SRKO mice have pyramidal neurons with reduced dendritic complexity and spine density, as well as reduced BDNF protein [18, 31]. Future experiments will be needed to determine whether the altered response to DOI in SRKO mice is due to impaired connectivity in the cortex.
Taken together our study demonstrates a role for D-serine mediated NMDAR activity in regulating particular behavioral and cellular responses to 5-HT2AR activation, although given the constitutive nature of the SR deletion, future work will be needed to directly link impaired D-serine dependent NMDAR hypofunction to the blunted DOI responses in SRKO mice. Furthermore, we show that 5-HT2AR-mediated signaling can be altered without correlation to receptor binding.
D-serine deficiency diminishes head twitch response to a 5-HT2A receptor agonist.
D-serine deficiency diminishes c-fos induction to a 5-HT2A receptor agonist.
D-serine deficiency does not alter 5-HT2A and mGluR2receptor expression
Acknowledgments
We thank Jiamin Feng for animal colony maintenance and genotyping and Dr. Michael Benneyworth for helpful discussions. An Andrew P. Merrill Research Fellowship, and Phyllis & Jerome Lyle Rappaport Mental Health Research Scholars Award (DTB), as well as National Institutes of Health grants R01MH05190 and P50MH0G0450 (JTC) supported this work.
Abbreviations
- DOI
(±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- 5-HT2ARs
serotonin 2A receptors
- HTR
head twitch response
- mGluR2
metabotropic glutamate receptor 2/3
- M2
motor cortex
- CG1
cingulate cortex
- PrL
prelimbic cortex
- NMDAR
NMDA receptor
- PFC
prefrontal cortex
- PCP
phencyclidine
- S1 cortex
primary somatosensory cortex
- SRKO
serine racemase knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:8836–43. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pei Q, Tordera R, Sprakes M, Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004;46:331–9. doi: 10.1016/j.neuropharm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology. 2010;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:735–40. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spratling MW. Cortical region interactions and the functional role of apical dendrites. Behavioral and cognitive neuroscience reviews. 2002;1:219–28. doi: 10.1177/1534582302001003003. [DOI] [PubMed] [Google Scholar]
- 8.Tononi G, Edelman GM. Consciousness and the integration of information in the brain. Advances in neurology. 1998;77:245–79. discussion 79-80. [PubMed] [Google Scholar]
- 9.Kitaichi K, Yamada K, Hasegawa T, Furukawa H, Nabeshima T. Effects of risperidone on phencyclidine-induced behaviors: comparison with haloperidol and ritanserin. Japanese journal of pharmacology. 1994;66:181–9. doi: 10.1254/jjp.66.181. [DOI] [PubMed] [Google Scholar]
- 10.Nabeshima T, Ishikawa K, Yamaguchi K, Furukawa H, Kameyama T. Phencyclidine-induced head-twitch responses as 5-HT2 receptor-mediated behavior in rats. Neuroscience letters. 1987;76:335–8. doi: 10.1016/0304-3940(87)90425-3. [DOI] [PubMed] [Google Scholar]
- 11.O’Neill MF, Hicks CA, Shaw G, Parameswaran T, Cardwell GP, O’Neill MJ. Effects of 5-hydroxytryptamine2 receptor antagonism on the behavioral activation and immediate early gene expression induced by dizocilpine. The Journal of pharmacology and experimental therapeutics. 1998;287:839–46. [PubMed] [Google Scholar]
- 12.Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14843–8. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–92. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 14.Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9870–5. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Molecular psychiatry. 2009;14:719–27. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3948–52. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, et al. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2400–9. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVito LM, Balu DT, Kanter BR, Lykken C, Basu AC, Coyle JT, et al. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 2011;10:210–22. doi: 10.1111/j.1601-183X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacology, biochemistry, and behavior. 2001;68:311–7. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- 20.Balu DT, Turner JR, Brookshire BR, Hill-Smith TE, Blendy JA, Lucki I. Brain monoamines and antidepressant-like responses in MRL/MpJ versus C57BL/6J mice. Neuropharmacology. 2013;67:503–10. doi: 10.1016/j.neuropharm.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celada P, Puig MV, Diaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biological psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–99. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 23.Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain research. 1999;825:161–71. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- 24.Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–82. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Marek GJ. AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:62–71. doi: 10.1016/j.pnpbp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Park IS, Park WK. NMDA receptor antagonists enhance 5-HT2 receptor-mediated behavior, head-twitch response, in mice. Life sciences. 1998;63:2305–11. doi: 10.1016/s0024-3205(98)00519-0. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Park IS, Lim HK, Choi HS. NMDA receptor antagonists enhance 5-HT2 receptor-mediated behavior, head-twitch response, in PCPA-treated mice. Archives of pharmacal research. 1999;22:113–8. doi: 10.1007/BF02976533. [DOI] [PubMed] [Google Scholar]
- 28.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. The Journal of pharmacology and experimental therapeutics. 1997;282:699–706. [PubMed] [Google Scholar]
- 29.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–7. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neuroscience letters. 2011;493:76–9. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balu DT, Basu AC, Corradi JP, Cacace AM, Coyle JT. The NMDA receptor co-agonists, d-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiology of disease. 2012;45:671–82. doi: 10.1016/j.nbd.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]