Abstract
Corneal wound healing studies have a long history and rich literature that describes the data obtained over the past 70 years using many different species of animals and methods of injury. These studies have lead to reduced suffering and provided clues to treatments that are now helping patients live more productive lives. In spite of the progress made, further research is required since blindness and reduced quality of life due to corneal scarring still happens. The purpose of this review is to summarize what is known about different types of wound and animal models used to study corneal wound healing. The subject of corneal wound healing is broad and includes chemical and mechanical wound models. This review focuses on mechanical injury models involving debridement and keratectomy wounds to reflect the authors’ expertise.
Keywords: cornea, wound healing, animal models, mouse strain, keratectomy, debridement
Introduction
Despite the research and efforts made in corneal wound healing over the last 70 years, according to the World Health Organization, corneal scarring secondary to corneal infection remains a major cause of preventable blindness. Corneal scarring caused by trachoma is responsible for blindness in 6 million people mostly in sub-Saharin Africa and at any one time there are 146 million people infected with this parasite (Roodhooft, 2002). The worms invade the cornea and scarring results from the immune response of the cornea to infection. Unfortunately, after infections are cured with anti-helminthics, the cornea remains cloudy and scarred. At present, only corneal transplants, which are neither available nor affordable in these countries, can restore vision in these patients. Therefore, preventative diagnosis and treatments that promote resolution of corneal scars are the best options for these patients. In developed countries, traumatic corneal abrasions are the leading cause of visits to the emergency room (Jackson, 1960; Jones et al., 1986; Laibson, 2010; Reidy et al., 2000) and can lead to permanent scarring and increase the risk of recurrent epithelial erosions (Reidy et al., 2000). Corneal infections due to bacteria (Staphylococcus aureus and Pseudomonas aeruginosa), acanthamoeba, fungi, and/or viruses (Herpes simplex, adenovirus, Epstein-Barr, cytomegalovirus) also can cause corneal scars that can persist for months or years (Bearer et al., 2000; Chodosh et al., 2000; Chodosh et al., 1998; Dart et al., 2008; Green et al., 2008; Koizumi et al., 2008; Sheridan et al., 2009). If those scars are in the central visual axis, they will impair the quality of life for these patients.
The goal of providing better treatments for patients blinded by corneal scarring can best be achieved through a more complete understanding of corneal biology obtained through utilizing the most appropriate in vivo and ex vivo animal models. Numerous different wound types and species have been used to gain insight into how the cornea heals and there are many choices that need to be made once a researcher decides to study corneal wound healing. Some models are better for studying corneal scarring and others for recurrent erosions and basement membrane defects. The purpose of this review is to describe the different types of in vivo mechanical corneal injury models and highlight the features that make a given species and wound type ideal for specific questions and analyses with the goal of minimizing the number of animals used and maximizing acquisition of data relevant to clinicians. While the main focus will be on in vivo studies, we will also touch briefly on organ culture and in vitro studies performed using cultured primary cells and cell lines. It is outside the scope of this review to discuss the mechanistic insights gained using the techniques we describe. Those interested in corneal wound healing mechanisms will want to read articles by our colleagues (Bazan, 2005; Fini and Stramer, 2005; Klenkler et al., 2007; McLaughlin et al., 2010; Sassani et al., 2003; Sosne et al., 2010; Yu et al., 2010).
Variations in Wound Types
Circular debridement wound
The presence or absence of the basement membrane has defined debridement compared to keratectomy wounds. Debridement wounds leave the basement membrane on the stroma whereas keratectomy wounds remove it. To preserve the basement membrane, a dulled blade held by a blade breaker is used to remove the epithelium from within an area marked with a trephine. This method was used extensively by Ilene Gipson and her colleagues in the early 1980’s to show for the first time that corneal epithelial wound healing occurs via sheet –movement (Gipson and Kiorpes, 1982). They characterized the cytoskeletal components that are involved in mediating epithelial cell migration (Gipson et al., 1982) and showed differences between dulled blade debridement and deeper keratectomy wounds (Fujikawa et al., 1984). Figure 1 shows the dulled blade and blade breaker and another popular tool used to create corneal wounds, the rotating burr, which will be described in the next section. The edge of a device called a crescent knife or spoon can also be used instead of a dulled blade to create debridement wounds. After wounding, the epithelial tissue on the tip of the dulled blade can be frozen in liquid nitrogen and the blade tip released for subsequent RNA or protein studies making the dulled blade a superior choice compared to spoon or crescent knife.
Figure 1. The mouse cornea can be wounded with a dulled blade or rotating burr (AlgerbrushII).
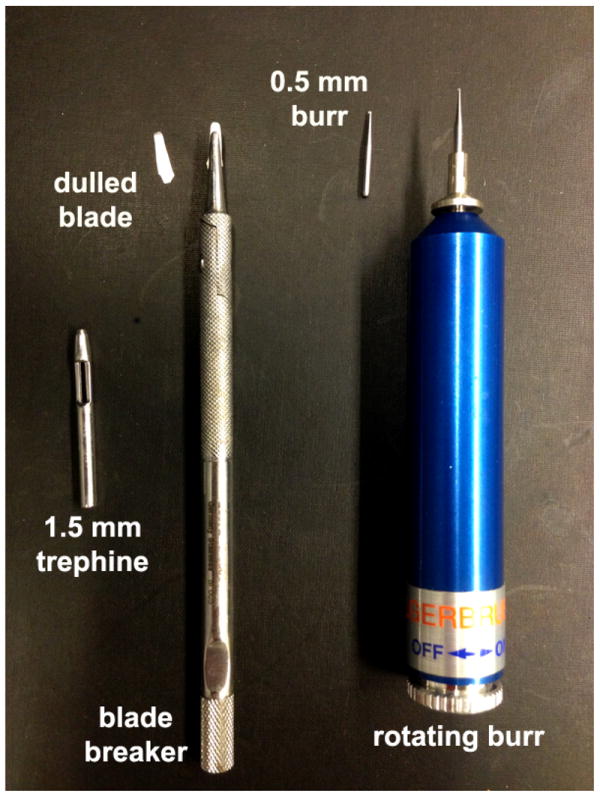
Typically, a 1.5 mm trephine is used to demarcate a circular area at the center of the cornea and the edge of the dulled blade is used to remove epithelial cells. The dulled blade is held by a blade breaker. Alternatively, a roating burr equipped with a 0.5mm burr can be used. After wounding, the epithelial tissue removed can be frozen in liquid nitrogen and used for biochemical or molecular analyses. Trephine sizes can by varied to create smaller (1.0 mm) or larger (2.0 or 2.5 mm) wounds in the mouse. For the rat, 3.0 mm to 4.0 mm trephines are used.
We confirmed that the basement membrane was left behind after dulled blade debridement wounding in mice by performing TEM studies (Sta Iglesia and Stepp, 2000). Those studies showed that not only the basement membrane but also the basal surface of basal cells and the β4 integrin ectodomain were left behind with the basal cells sheered above the plane of the basement membrane. Interestingly, debridement wounds made in the rat using the dulled blade show that the epitope recognized by a cytoplasmic domain directed antibody against β4 integrin is removed (Stepp et al., 1993). To confirm the presence or absence of the basement membrane after debridement wounds in the mouse and rat, cross sections obtained from wounded corneas can be stained with antibodies against laminin 332 and/or type VII collagen. Staining for α6β4 is also informative; the β4 ectodomain is left behind embedded in the basement membrane zone (BMZ) whereas the cytoplasmic domain may or may not be left behind on the bare stroma. We find it most informative to stain whole corneas to avoid missing focal sites lacking the BMZ. When focal sites lacking LN332 and type VII collagen are present, the wound should be considered a partial debridement wound.
Circular debridement wounds are ideal for a number of different types of analyses including quantifying re-epithelialization, cell proliferation rates, re-innervation, and innate immune responses (Table 1). A typical study might involve the use of a transgenic or tissue specific gene targeted mouse to investigate whether the change in protein expression or function impacts corneal wound healing. Care needs to be taken before beginning such studies to determine whether the unwounded corneas of the genetically modified mice are normal using standard histological stains and by examining the expression profile of corneal and limbal specific proteins. The limbal vasculature, corneal epithelial and stromal thickness, resident immune cell numbers and morphology, and cell proliferation rates should be compared to the appropriate littermate or strain-specific controls. If these assessments show significant differences in some of these parameters, the wound response can still be assessed by comparing wounded corneas against unwounded strain matched controls with care taken to assure that injury creates the same amount of damage and that the cornea does not rupture as it heals.
Table 1. Types of analyses done using different wound models.
| DB 1.5 mm | RB 1.5 mm | SMK/PRK 1.5 mm | Tissue paper | Incisional | Suture/Pocket Assay | DB 2.5 mm | Organ culture 1.5 mm DB/RB | |
|---|---|---|---|---|---|---|---|---|
| E. Cell migration | + | + | + | − | − | − | + | + |
| E. Cell proliferation | + | + | + | + | − | − | + | + |
| E. Cell differentiation | − | − | − | − | − | + | + | − |
| S. Cell differentiation | − | − | + | − | + | + | + | − |
| E. Barrier function | − | + | + | + | + | − | + | − |
| Scarring | − | + | + | − | + | − | − | − |
| Reinnervation | + | + | + | + | + | − | + | − |
| Inflammation | + | + | + | + | + | + | + | − |
| Angiogenesis/Lymphangiogenesis | − | − | − | − | + | + | + | − |
DB=dulled blade, RB=rotating burr, SMK/PRK= superficial manual keratectomy/photorefractive keratectomy
In the mouse, debridement wounds re-epithelialize rapidly but may also lead to recurrent erosions via mechanisms that involve upregulated matrix metalloproteinase-9 (MMP9) within the corneal epithelial cells (Pal-Ghosh et al., 2011a). Our data indicate that MMP9 degrades α6β4 integrin within the basal membrane of the corneal epithelial basal cell layer adjacent to the erosion and sensory nerve reinnervation is disorganized or absent (Pal-Ghosh et al., 2011a). While excess MMP9 contributes to erosion formation, it is critical for efficient corneal wound healing since mice lacking MMP9 heal poorly (Mohan et al., 2002). Interestingly, the majority of the erosions that arise spontaneously beginning 2 weeks after injury form not over the central cornea where the wound was made but in the nasal quadrant as shown in Figure 2; this suggests that anatomical differences contribute to the sites where erosions form. By placing a small suture in the sclera adjacent to the limbus in the temporal quadrant after sacrifice and before enucleation, the orientation of each eye can be successfully determined (Pajoohesh-Ganji et al., 2006). Clinical data and studies done in rats and rabbits indicate that erosion formation after debridement wounds that leave the basement membrane intact are rare; most debridement wounds resolve fully in corneas of larger mammals. These differences make the mouse a good model to study recurrent erosions after simple debridement wounding and are a reminder that important differences in corneal wound healing responses between species are common. This topic is discussed in more detail in a later section.
Figure 2. After 1.5 and 2.5 mm debridement wounds made using the dulled blade, the mouse cornea spontaneously develops erosions most often located in the nasal quadrant.
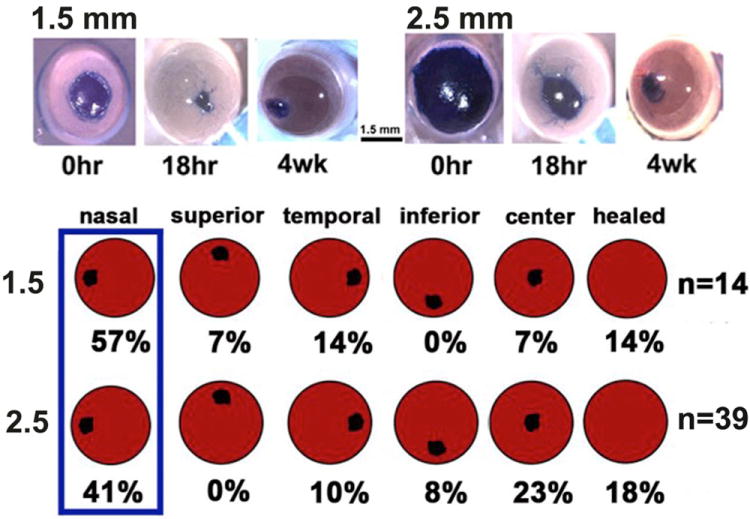
The images on top show mouse corneas with a 1.5 or a 2.5 mm debridement wound stained either immediately with the vital dye Richardson stain or allowed to heal for 18 hr or 4 weeks. Note that wounds close rapidly after both wound types and small erosions are present 4 weeks after wounding. To determine the sites where erosions are most likely to form, the corneas of BALB/c mice were wounded and allowed to heal for 4 weeks. At the time of sacrifice, eyes were stained Richardstain stain to reveal exposed basement membrane and the locations of the erosions determined by quadrant. Corneas lacking erosions are considered as healed. Although there was a greater tendancy after large wounds for erosions to be in the center compared to small wounds, more erosions were present at the nasal quardrant of the cornea after 1.5 and 2.5 mm wounds.
The diameter of circular wounds can be varied using different size trephines to demarcate the area within which tissue is removed. Trephines are very sharp and are designed to penetrate tissues. To prevent penetrating the stroma when demarcating the wound site to make debridement wounds, trephines need to be dulled on an Arkansas stone or similar tool with care taken to dull the device uniformly. Wounds larger than 2 mm in the mouse induce morphological changes within the corneal epithelial cells that are similar to those seen in corneal stem cell deficiency 2-4 weeks after injury (Pal-Ghosh et al., 2004). Limbal epithelial cell keratins begin to be expressed by epithelial cells covering the cornea, goblet cells appear, and the cornea becomes vascularized (Pajoohesh-Ganji et al., 2012). Regardless of the device used to create the wound (dulled blade or rotating burr), erosions will develop once goblet cells are resident on the cornea. Figure 2 also shows the anatomical distribution of erosions after large wounds that induce goblet cells on the central cornea.
In the mouse, 1.5mm size debridement wounds are commonly used and do not induce differentiation or morphological changes in the corneal epithelial cells. By 24 hours, this size debridement wound made in an adult wild-type mouse is closed. Within hours after wounding, resident leukocytes become activated and recruited leukocytes enter the stroma from the limbal vasculature (Byeseda et al., 2009; Li et al., 2011a; Li et al., 2011b; Li et al., 2006; Petrescu et al., 2007). Larger, poorly centered wounds induce a more robust immune response since more leukocytes are recruited into the cornea when wounds are closer to the limbus. Failure to center the wound adds variability to the results obtained. In order to assess reepithelialization rates, a number of time points between 3 and 24 hours are required after 1.5 mm wounds.
In the rabbit the typical wound size used is 5 or 6 mm; in the rat, it is 3 mm. Despite the larger size, a 3 mm rat debridement wound closes within 24 hr which is the same time frame seen for wound closure after a 1.5 mm wound to the mouse cornea (Stepp et al., 1993). When harvesting epithelial tissue after migration in response to a 3 mm central debridement injury in the rat, the trephine can be repositioned and the epithelial cells that have migrated into the wound site harvested. In the mouse, this is not possible because of its small corneal size; instead, limbal to limbal debridement using a dulled blade is performed for biochemical and gene expression studies at various time points. Thus, differences in protein or RNA expression in actively migrating cells can be more readily demonstrated in the rat.
In 1944, Friedenwald and Buschke inhibited proliferation during the healing of small wounds in the rat cornea and showed that it did not delay closure rates (Friedenwald and Buschke, 1944). Likewise in the mouse, as long as wounds leave 60-70% of the epithelium intact, the corneal epithelial cells do not divide at significantly higher rates until after re-epithelialization (Stepp et al., 2002). Although cell proliferation rates increase after larger wounds that remove more of the epithelium, proliferating cells are generally located behind the leading edge. Re-epithelialization occurs via sheet-movement that involves dramatic changes in cell shape (Gipson et al., 1984; Zhao et al., 1996). These changes place significant forces on cell:cell and cell:substrate adhesions as the sheet slides over the exposed wound bed (Theveneau and Mayor, 2013). The leading edge is often only 1-2 cell layers thick and hemidesmosomes are disassembled during migration. When epithelial cells within a sheet divide, the number of desmosomes between cells is reduced (Baker and Garrod, 1993; Simpson et al., 2011). Delaying cell proliferation until after sheet-movement is complete we hypothesize helps maintain sheet integrity during migration.
While circular corneal debridement wounding is ideal for short term studies of cell migration rate and recurrent erosions in mice, other types of wounds are better suited for studies of epithelial barrier reformation and corneal haze as discussed below. Recurrent erosions occur frequently beginning 2-4 weeks after debridement wounds in mice (Pal-Ghosh et al., 2011a). As a result, the ability of the corneal epithelium to function as a barrier is disrupted. Debridement wounds do not activate expression of αSMA in stromal fibroblasts in the mouse (Hutcheon et al., 2005). Deeper wounds that disrupt the basement membrane, such as keratectomy and full thickness incisions must be used to assess myofibroblast formation.
Rotating Burr Circular Wounds
Most reports on mechanical corneal wounding done in mice, rats, and rabbits, use a rotating burr(AlgerbrushII), shown in Figure 1, to create wounds (Chinnery et al., 2012; Ferrington et al., 2013; Pal-Ghosh et al., 2011a; Terai et al., 2011; Yang et al., 2013). This tool is similar to the diamond burr used frequently in the clinic to clean up the area surrounding a cornea abrasion and/or to remove surface irregularities at wound sites (Ramamurthi et al., 2006; Sridhar et al., 2002). Although it is user friendly and carries little risk of rupturing the cornea, there is disagreement regarding whether it removes the basement membrane. In the mouse cornea, in our hands, the rotating burr removes most of the basement membrane (Pal-Ghosh et al., 2011b). Figure 3 compares the localization of LN332 and type VII collagen after debridement and rotating burr wounds in the mouse and rat corneas. Dulled blade debridement wounds to the mouse and rat leave the basement membrane behind. The rotating burr completely removes the BMZ at the center of the mouse cornea but only partially removes it in the center of the rat cornea; some regions appear denuded of the BMZ completely but others show it remaining. Using cross sections, others have shown an intact basement membrane in the mouse after use of the rotating burr (Boote et al., 2012). Factors such as the rotational speed of the burr, the force applied to the corneal surface, the angle of the burr relative to the ocular surface, and the residence time of the burr on the cornea all impact the removal of the basement membrane.
Figure 3. Rotating-burr wounds remove basement membrane components LN332 and type VII collagen at the center of the wounded mouse cornea but leave patches of both proteins behind on the rat cornea.
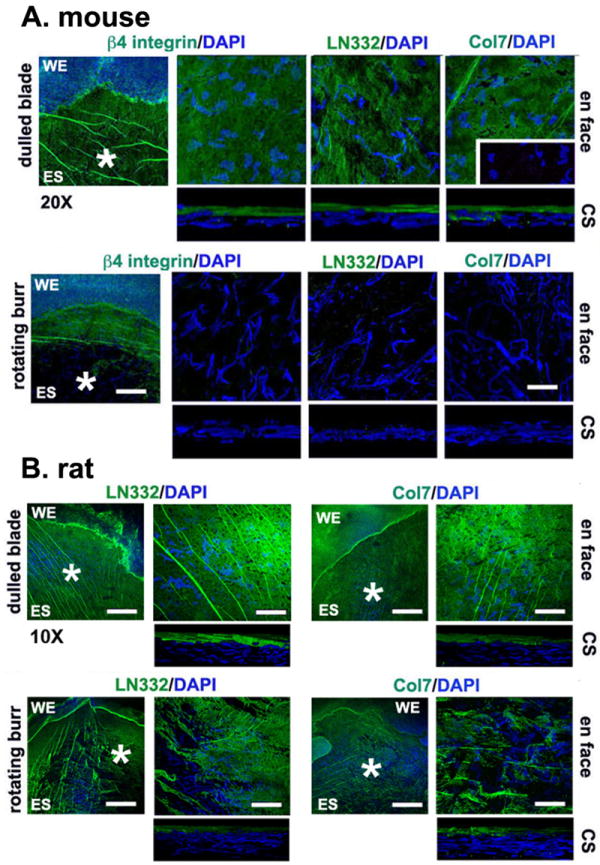
Representative images showing the status of the BMZ immediately after dulled blade and rotating burr wounds in the mouse (A) and rat (B). On the right are en face 63x images of the regions indicated by the asterisks on the 20x (mouse) or 10x (rat) images shown on the left to highlight the localization of two basement membrane components, LN332 and type VII collagen, shown in green and nuclei stained with DAPI shown in blue. For the mouse, the en face 63x confocal images were merged images obtained from 18 confocal layers taken at 1 mm intervals; CS images are 3D reconstructions obtained by rotating 18 layers at a 90° angle to obtain cross sections. For the rat, the en face confocal images were merged images obtained from 40 confocal layers taken at 1 mm intervals; CS images are 3D reconstructions obtained by rotating 40 layers at a 90° angle to obtain cross sections. Note the presence of an intact basement membrane after dulled-blade wounding in both mouse and rat corneas and its disappearance in the mouse and disruption in the rat after rotating-burr wounding. The mouse data shown above were taken from Pal-Ghosh et al., 2011b; the rat data were generated using ithe same methods and antibodies. WE = wound edge; ES = exposed stroma. Bar in 20x image = 50 μm; Bar in 10x image = 100 μm; and bar in 63x image = 7 μm.
Because the rotating burr can remove the basement membrane in the mouse, it can be used to study the de novo reformation of the basement membrane after wounding. We have compared the thickness of the mouse stroma in corneas fixed immediately after dulled blade and rotating burr injuries and found that the difference was less than 1 μm (Pal-Ghosh et al., 2011b). Dulled blade wounds are superior to rotating burr if a study is designed to evaluate short-term responses, such as rate of re-epithelialization. This is because the rotating burr makes it hard to remove the epithelial cells at the edges of the wound adjacent to the trephine site without inadvertently enlarging the wound. Cells pushed aside by the burr will cover the wound site once the mouse resumes blinking which makes it difficult to determine the wound size. If a study involves long-term healing over several weeks, the rotating burr results in superior wound resolution. Neither the dulled blade nor the rotating burr allows for the assessment of corneal scarring in mice.
Manual Superficial Keratectomy and Photorefractive Keratectomy Wounds
The manual superficial keratectomy (MSK) has been the wound type of choice to study corneal scarring. Clinically oriented scientists use the excimer laser to generate photorefractive keratectomy wounds (PRK) in rabbits, rats, and mice (Azar et al., 1998; Kato et al., 2003; Mohan et al., 2008; Singh et al., 2011). To create a MSK, a sharp trephine is rotated with slightly greater pressure applied to the cornea in order to penetrate the superficial anterior stroma. A sharp jeweler’s forceps is used to remove a corneal button that can be frozen and used for RNA or biochemical analyses. This type of wound is made possible by the lamellar structure of the corneal stromal matrix. A limitation of this procedure is the variable depth of the injury during trephination. The trephine used for MSK wounds are not be the same as those used for debridement wounds since the latter require a dulled trephine to prevent stromal injury. Due to the thinness of the mouse cornea, MSK wounds can be difficult to create and the risk of rupturing the cornea is higher than debridement wounds. However, with practice, the procedure is reproducible and has been used successfully to study mouse corneal wound healing (Blanco-Mezquita et al., 2011). To reduce variability at the time of injury, researchers can perform PRK using the excimer laser; ablated tissues will not be available for subsequent analysis after PRK. Results for the two approaches are believed to be similar.
The wound healing that occurs after MSK or PRK includes re-epithelialization, basement membrane reassembly, re-innervation, and corneal scarring. The αSMA positive cells that are generated by these types of wounds are generally transient. They remain for several weeks before disappearing (Netto et al., 2005; Ruberti and Zieske, 2008). While scars generated by MSK and PRK can remain for months, recent studies (see below) suggest that these models may not be as useful to study the types of chronic scars that can lead to blindness in patients as incisional wounds.
Incisional Wounds
Incisional wounds can also be studied using the cornea (Blanco-Mezquita et al., 2013; Kato et al., 2003; Saika et al., 2013; Stramer et al., 2003; Vij et al., 2005). This type of wound models the corneal wound healing response to incisions made during surgeries performed on the cornea (refractive surgery and corneal transplants) including procedures where access to the lens and retina is obtained by incisions through the peripheral cornea near the limbus. Incisional wounds induce myofibroblasts and corneal scarring. The myofibroblasts induced by incisional wounds remain over time; this distinguishes them from the myofibroblasts that form after manual keratectomy wounds. A 1.5 mm trephine is used to define the length of the incision and a central 1.5 mm nasal-temporal orientated full-thickness penetrating incision is created in the center of the cornea with a sharp surgical blade. Prior to injury, atropine is instilled to make sure that the iris is not inadvertently injured. Failure to use atropine results in the iris sticking to the posterior cornea; pigmented cells from the iris then migrate into the wound site and complicate assessment of myofibroblasts and scarring (Blanco-Mezquita et al., 2013). This model can be used to assess re-epithelialization and barrier reformation but is generally used when studying stromal activation in response to injury.
Pocket Assays and Suture Wounds
The corneal pocket assay can be used to study angiogenesis in wild-type and genetically engineered mice. A partial thickness incision is made at a given distance from the limbus and a small pellet containing a growth factor like VEGF or FGF is inserted under the epithelium within the stroma (Kojima et al., 2007b). Deletions or mutations in specific receptors will prevent or reduce angiogenesis in response to a given growth factor. Uniform placement of the pocket at a standard distance from the limbus is critical for these studies to be successful. Pellets soaked in an inert protein are used to control for increased angiogenesis due to the incision.
Alternatively, sutures can be placed in the central cornea. This also induces an immune response and leads to an induction of angiogenesis (Amescua et al., 2008; Dana and Streilein, 1996). Mice with defects in their ability to induce angiogenesis or an immune response will have fewer and/or smaller blood vessels induced in the cornea. The amount of suture material in each suture can be varied; a more robust response is induced if there are more knots in the suture. This technique is used to study corneal transplant rejection (Dana and Streilein, 1996). Sutures are placed in the central cornea and new vessels are recruited into the cornea. Two weeks later, these corneas serve as hosts for corneal transplants from donor mouse corneal buttons. The presence of blood vessels in the peripheral cornea of the host increases the risk that the donor corneal button will be rejected (Dana and Streilein, 1996). Whether a pellet with a growth factor or a suture is placed in the cornea, blood vessels will begin migrating from the limbus into the corneal stroma beginning 1-2 days after the procedure; these studies generally last 7-14 days. After sacrifice, blood vessels on the whole corneas are stained using vascular endothelial cell surface markers and the size and number of vessels present inside the limbal arcade are measured using Image J. Data are generally expressed as a percent of the total corneal area occupied by blood vessels (Chung et al., 2009; Kojima et al., 2007a; Singh et al., 2006).
Filter Paper or Impression Injury
When a filter paper is applied to the ocular surface, superficial cells are removed and the barrier is disrupted. This method, called impression cytology, has been used for diagnosing corneal stem cell deficiency (Calonge et al., 2004; Singh et al., 2005). Chemical injuries to the eye have been studied for many years using filter papers to deliver chemicals to the cornea. These studies provide insight and have improved treatment for patients whose corneas have been injured by chemicals (Fish and Davidson, 2010; Javadi et al., 2005; Khaw et al., 2004; Saika, 2007; Sosne et al., 2002). In addition, studies are now being carried out to better understand and treat the corneal pathology seen in response to nerve gas treatment (Gordon et al., 2010; McNutt et al., 2012). The most frequently used model to study the corneas response to chemical injury involves soaking circular filters in 0.15M NaOH (Bargagna-Mohan et al., 2012; Lee et al., 2013). Alkali wounds induce a rapid and intense immune response. Pressing a dry filter paper on the cornea creates injuries equivalent to tape-striping injuries studied in skin wound healing (Stepp et al., 2002). Superficial cells are removed from the epithelium; basal cells are left alive on the basement membrane. The cornea will respond by increasing epithelial cell proliferation and the synthesis of proteins needed to assemble new functional tight junctions at the apical aspect of the corneal epithelium. This method could be used to study the ability of the epithelium to reform an intact epithelial barrier by assessing penetration of the stroma using topically applied fluorescein or Rose-Bengal stain (Argueso et al., 2009; Reichl et al., 2004; Sasaki et al., 1999). Filter paper injury could also be useful on the corneas of transgenic mice with thin, fragile corneas that cannot be safely injured by debridement. It is always useful to evaluate the extent of the injury right after any procedure on the genetically-modified mouse cornea. Fragile, thin corneas may sustain more serious injuries initially which can lead to delayed barrier reformation secondary to a greater initial wound defect.
Ex vivo Experimental Options
Organ Culture Studies
The use of organ culture to study corneal responses to drugs and various injuries has significantly reduced the number of procedures performed on live animals. Organ culture studies have generated insight into the basic science aspects of epithelial cell migration (Gipson and Keezer, 1982; Gipson et al., 1982; Trinkaus-Randall and Gipson, 1984; Zieske and Gipson, 1986) and have allowed investigation of the impact of addition of signaling and growth factors (Carrington and Boulton, 2005; Ma et al., 2011), drugs (Yamada et al., 2004), bacteria (Spurr-Michaud et al., 1988; Wagoner et al., 1984), and cells (Wagoner et al., 1984) on re-epithelialization of corneal debridement wounds. Building on the solid background of research done using organ cultures, an in vitro organ culture system was recently described to study corneal scarring using donor human corneas (Janin-Manificat et al., 2012). Much attention has been given to optimizing conditions to allow using primate and human corneas in organ culture models (He et al., 2010; Janin-Manificat et al., 2012; Saghizadeh et al., 2010). The cornea derives its nutrients primarily from the aqueous humour that contains low levels of serum-derived proteins and growth factors. The media used for organ culture studies include those that have defined components and are serum–free, or have reduced serum (Stepp et al., 1993; Zheng et al., 2013; He et al., 2010; Pipparelli et al., 2013; Saghizadeh et al., 2010; Saghizadeh et al., 2011) although some groups use 8% FCS in their media (Kryczka et al., 2012). Most protocols maintain corneas at an air-liquid interface which is achieved by maintaining the height of the media in the dish at the limbal border and gently rocking it so that media intermittently moistens the surface of the cornea.
During the storage of corneas to be used for transplantation, endothelial cell density has been found to decrease over time (Frueh and Bohnke, 2000; Lindstrom et al., 1992). Recent exciting studies on the ability of a Rho-associated protein kinase (ROCK) inhibitor to reduce corneal endothelial cell loss in stored corneas have renewed interest in corneal organ culture studies (Hirata-Tominaga et al., 2013; Nejepinska et al., 2010; Pipparelli et al., 2013). Growth factors, function blocking antibodies, and various activators and inhibitors can be added to organ cultures to assess their impact on cell migration, proliferation, and/or scarring. Transfection studies, using human corneas, have been performed in organ culture for times up to several weeks (He et al., 2010; Jessup et al., 2005; Saghizadeh et al., 2010; Saghizadeh et al., 2011).
Rat, rabbit, porcine, and human corneas from eye banks are most commonly used for studies of corneal wound healing using organ cultures, (Notara et al., 2011; Yin and Yu, 2010). Since porcine skin is often used for studies of skin wound healing (Lademann et al., 2011; Sullivan et al., 2001; Vukelic et al., 2011), using their corneas allows scientist to confirm that results obtained studying skin wounds are relevant to the cornea. Typical sizes for the circular wounds for rabbit, porcine, and human corneas in organ culture are 5 or 6 mm although sizes up to 8.5 mm can be used. Although the size of the mouse eye makes it difficult to perform organ culture studies, evaluating the rate of re-epithelialization after debridement, rotating burr, PRK, or MSK wounding can still be done in organ culture over a 24-48 hour time frame. Corneas are wounded after sacrifice and prior to enucleation and then dissected leaving a limbal rim. After dissection, corneas are inverted to form a cup and an agar- or collagen-containing media is added to maintain the curvature of the cornea. Corneas are then inverted again so the gel is in contact with the culture dish, media is added, and cultured corneas are maintained at an air-liquid interface (Zheng et al., 2013). Because of its small size, for short term mouse organ culture studies, the intact eye can be incubated in media without dissecting the cornea (Singh et al., 2009). However, with practice, the same method used for large corneas can be adapted successfully to the mouse.
Enucleating the eye cuts the nerves and the blood and lymphatic vessels that project towards the cornea and encircle the limbus. The sensory nerves that innervate the cornea come primarily from the trigeminal ganglia (Muller et al., 2003). The nerve fibers (axons) in the cornea begin to disassemble when placed in organ culture (Stepp et al., 1993). Figure 4 shows images of unwounded mouse corneas fixed immediately after sacrifice or at 1, 3, and 6 hours after being placed in organ culture stained to show neuron specific class III β–tubulin and keratin-8. In the center of the cornea fixed immediately after sacrifice (Figure 4), note the high density of fine varicose fibers and the swirling pattern of the sub-basal nerves that terminate in at the center of the cornea. The K8+ clusters shown in Fig 4B define the border between cornea and limbus (Pajoohesh-Ganji et al., 2012). At the corneal periphery, bundles or leashes of fibers emerge from the thicker nerve bundles that circle the corneal periphery and radiate towards the center of the cornea. Within 1 hour of placement in organ culture, thinner sub-basal nerve fibers at the corneal center and periphery begin to disappear (Figure 4C and D) and by 3 hours nerves are missing at some sites (Figure 4E and F). By 6 hours (Figure 4G and H), the sub-basal nerves and some larger nerves at the periphery are difficult to detect.
Figure 4. Sub-basal nerves and deeper stromal nerves become disassembled within hours after enucleation in organ culture.
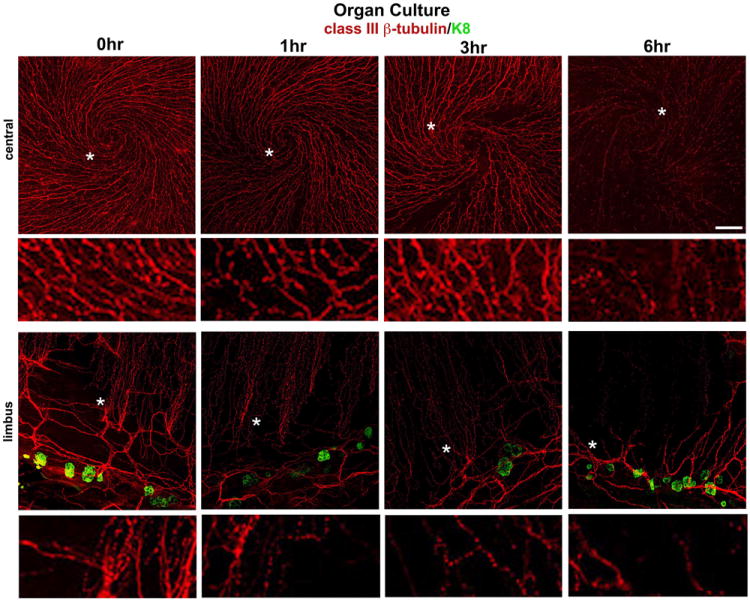
Shown here are whole mount confocal images obtained after staining mouse corneas with the classIII βtubulin antibody (TuJ1) in red and keratin-8 in green with the central cornea shown above and the limbal region shown below. K8+ goblet cells define the the corneal:limbal junction (Pajoohesh-Ganji et al., 2012). Corneas were either fixed immediately after sacrifice (0 hr) or placed in organ culture for 1, 3, or 6 hrs prior to fixing and staining. A minimum of 3 corneas each were used for these studies and the method and antibodies used for these studies have been described (Pal-Ghosh et al., 2011b). The sub-basal nerves form distinct swirling patterns and appear beaded and varicose. The sites indicated by the white asterisks have been digitally magnified 4-fold and are shown below lower magnification images. The thinnest nerve fibers become less distinct 1 and 3 hrs after corneas are placed in organ culture. By 6 hours the sub-basal nerves are barely detected in the central cornea and only the larger nerves can be seen at the limbus. Bar = 20 μm.
If sensory nerves regulate an event being studied, then aspects of corneal wound healing seen in organ culture will not recapitulate what takes place in vivo. Yet, we know that re-epithelialization takes place in organ culture over a similar time frame as it does in vivo. Therefore, we can conclude that sheet-movement takes place in the absence of functional sensory nerves (Trinkaus-Randall and Gipson, 1984; Zieske and Gipson, 1986). Transient denervation of the cornea can occur in response to neurological trauma such as stroke and after LASIK procedures and can result in neurotrophic keratitis. The organ-cultured cornea could be used to study the impact of denervation on corneal epithelial cell biology.
Recent studies by Saghizadeh and colleagues (Saghizadeh et al., 2011) have helped to improve confidence in the ability to draw meaningful conclusions from organ culture studies. They showed that abnormal localization of stem cell markers in human diabetic corneas can be reverted by overexpressing c-met. Based on these studies, and the fact that using organ cultures reduces the use of living animals, expanding our use of organ cultures for corneal wound healing studies can be justified. Studies involving co-culture with trigeminal ganglial cells and organ cultured corneas could pave the way for an improved understanding of the factors that delay and prevent reinnervation not only in the cornea but throughout the peripheral nervous system.
Another difference in organ cultured corneas is the lack of a functional vascular system at the limbus. The blood vessels that carry blood to the limbus and the lymphatic vessels that function to remove excess fluid from the stroma are located at the limbal rim, which is left intact when corneas are placed in organ culture. Although these structures are present in organ cultured corneas, they are no longer have fluid flowing through them. In vivo, recruited immune cells play important roles in mediating corneal wound healing responses. While organ cultured corneas have numerous different classes of resident leukocytes, whether they respond to injury in a similar manner in organ culture as they do in vivo is not clear. Long term studies lasting several weeks and involving events in the stroma could well be affected by changes in the limbal vasculature in vitro.
In vitro cell culture
In order to sort out specific mechanisms suggested by in vivo studies, an in vitro model is often needed. Both 2D (cells grown on uncoated or ECM coated tissue culture plastic or cells grown on feeder layers) and 3D (cells growing within gels and/or bioengineered scaffolds) culture models have been adapted and used for studies of corneal wound healing. 3D cultures are newer, more expensive, and more difficult to manipulate: much more research has been done using 2D cultures. Investigators have used corneal stromal cells derived from a variety of species for these types of studies (Dreier et al., 2013; Kim et al., 2010; Lakshman and Petroll, 2012; Micera et al., 2006; Yanez-Soto et al., 2013).
Corneal epithelial cells have generally been studied in 2D cultures. Discarded limbal rims from corneal transplants or eye banks are often a source for primary human corneal epithelial cells (de Paiva et al., 2005; Kahn et al., 1993; Kim et al., 2012; Nakamura et al., 2004). Rabbit (Cha et al., 2004; Fini and Girard, 1990), rat (Boesch et al., 1996; Marshall and Hanrahan, 1991), and mouse (Kawakita et al., 2004; Li et al., 2010) primary corneal epithelial cells have also been generated and used successfully. Due to its small size, it is more difficult to generate primary corneal epithelial cells from the mouse. While primary cell cultures derived from the mouse and rat cornea have been generated by numerous groups, their properties remain poorly understood compared to those of primary human corneal epithelial cells and mouse epidermal keratinocytes. Primary corneal epithelial cells include stem-like cells from the limbal basal epithelium and corneal epithelial cells derived from the basal epithelial cell layer. Because corneal epithelial cells differentiate over time in vitro, limbal basal derived cells become the major cell type present over several days in culture. Due to the variability present in primary corneal epithelial cell cultures and the fact that they can only be maintained for several population doublings, corneal epithelial cell lines are popular for in vitro studies of corneal epithelial cells.
There are numerous human corneal epithelial cell lines that have been described but three are used more frequently than others: 1) SV40 transfected (Araki-Sasaki et al., 1995), 2) HCLE (Gipson et al., 2003), and 3) hTCEpi (Robertson et al., 2005). Both HCLE and hTCEpi lines were generated using telomerase transfection. To our knowledge, there have been no studies directly comparing the three different cell lines. When possible, data obtained using cell lines should be confirmed using primary cells. Telomerase and SV40 both allow cells to grow for prolonged times in culture and cell lines derived using these approaches have many properties similar to primary cells such as Ca2+ mobilization, cell adhesion, and migration. However, the regulation of their proliferation and differentiation are altered by transformation. The advantages of cell lines are the lower cost and their ability to give more reproducible results than primary cells. To our knowledge, there are no well-characterized cell lines for the rat or mouse corneal epithelium.
Since corneal stromal cells normally exist within a 3D matrix, 3D cultures where cells are studied while growing within a collagen-based matrix are considered preferable for experiments involving stromal cells (Kim et al., 2010; Lakshman and Petroll, 2012). These cultures have been used to study the ability of cells to migrate within a 3D matrix (Lakshman and Petroll, 2012). With the right imaging system, excellent data can be obtained using 3D cell cultures. Because proteins extracted from cells in 3D culture are contaminated with proteins from the collagen gels they are suspended in, performing biochemical studies are more challenging.
Corneal stromal cells are derived from the corneal stroma following collgenase digestion and are often used after 2-3 passages. One popular method used to study quiescent corneal stromal cells involves growing the cells in serum free defined medium in order to maintain a dendritic phenotype similar to keratocytes in vivo (Beales et al., 1999; Jester et al., 1994). Because resident leukocytes also adhere and have similar phenotypes, these cultures are likely a mixture of keratocytes and different classes of resident immune cells. Passaging these stromal cells generates a more homogeneous cell population but induces a phenotype similar to that of a fibroblast or activated myofibroblast. Corneal stromal cells are initially αSMA negative but they rapidly acquire αSMA expression in vitro when grown under proliferating conditions (Bernstein et al., 2007; Jester et al., 1995; Masur et al., 1996)
Success has also been achieved using heterotypical 3D cell culture models that incorporate several different cell types into a single construct. In 1994, one of the first 3D organotypic cell culture systems was described which incorporated the three major corneal cell types: endothelial cells, fibroblasts, and epithelial cells (Zieske et al., 1994). Building on those observations, 3D culture systems have been developed involving bioengineered scaffolds, to gain insight into collagen biosynthesis and scarring (Dreier et al., 2013; Karamichos et al., 2010; Ren et al., 2008; Ruberti and Zieske, 2008; Saeidi et al., 2012; Yanez-Soto et al., 2013). Other groups have used these types of models to study corneal stem cells (Papini et al., 2005). The ultimate objective for some researchers is to develop an artificial cornea that could be used for transplantation (Polisetti et al., 2013).
Table 1 summarizes the above discussion by listing analyses best suited for the different types of mechanical injury models described.
Animal Species for in vivo studies
Corneal wound healing research has made use of several vertebrates, including the chicken, rat, rabbit, mouse, dog, and cat, recognizing that each has pros and cons. The research done thus far on the corneas in lower vertebrates including xenopus and zebrafish has primarily focused on corneal and anterior segment development. In the 1960’s, it was found that the Xenopus cornea can regenerate the lens (Freeman, 1963; Overton, 1965) and this developmental event is still being studied today (Day and Beck, 2011; Fukui and Henry, 2011; Hu et al., 2013). The main drawback of using Xenopus to study the adult cornea is that it takes several months for the tadpole cornea to mature to the adult stage.
Studying the factors that mediate the ability of corneal epithelial cells to differentiate into other cell types will give us insight into corneal pathologies that involve changes in cell differentiation such as corneal stem cell deficiency. In 2006, a paper describing the development and structure of the zebrafish cornea was published (Zhao et al., 2006). By inserting genes for proteins mutated in various corneal dystrophies into the zebrafish genome, the roles of these proteins in normal development and the mechanisms underlying the structural changes taking place in the corneas of patients with corneal dystrophies are being revealed (Bibliowicz et al., 2011; Boisset et al., 2008). Zebrafish have also provided insight into how the cornea’s anti-angiogenic privilege is maintained (Norrby, 2006). While no corneal epithelial wound healing studies have yet been published using zebrafish, a report has appeared involving studies of corneal endothelial proliferation (Heur et al., 2013). Advantages of zebrafish for studies of the cornea are the relatively low cost, the speed with which genetic studies can be performed, and the rapid maturation of the immature zebrafish to an adult. Tables 2 and 3 list a number of important studies published using different species of animals with the studies categorized by their major focus. Table 2 focuses on different vertebrate models excluding mice and Table 3 focuses exclusively on mice. The discussion that follows is intended as a broad overview of this topic.
Table 2.
Corneal Studies Performed Using Vertebrate Animals Excluding Mice
Table 3.
Corneal Studies Performed Using Mice
Chickens
Studies of the chick cornea not only have contributed to a better understanding of wound healing, it has also helped foster improvements in the basic science of the extracellular matrix and development of new microscopic imaging technologies impacting many different realms of biomedical science. Elizabeth Hay began studying the chicken cornea because its organization allowed high resolution TEM imaging to be obtained (Bard and Hay, 1975; Dodson and Hay, 1974; Meier and Hay, 1974). The developing chick cornea is large and inexpensive to obtain. The ability to image the corneal epithelial basement membrane and collagen molecules in the chick corneal stroma lead directly to improved understanding of collagen and glycosaminoglycan biosynthesis and matrix assembly and highlighted the importance of the basement membrane in development and pathology (Conrad, 1970; Conrad et al., 1977; Svoboda et al., 1988; Trelstad, 1973). The history of important studies using the chick cornea continues with recent studies to understand how innervation of the tissue by sensory nerves is regulated during development (Kubilus and Linsenmayer, 2010a, b; Schwend et al., 2012).
Rats
In 1944, Friedenwald and Buschke examined cell proliferation and cell cycle progression in the epithelium of unwounded and debridement wounded rat corneas (Friedenwald and Buschke, 1944). This is one of the first of thousands of studies published focusing on corneal wound healing. It showed that the rate of epithelial cell proliferation in the cornea was acutely sensitive to corneal injury. The study used the Sprague-Dawley rat strain that remains the most commonly used rat strain 69 years later. Advantages of using rats include the size of the cornea (~5mm diameter) which permits precise wound sizes and provides adequate tissue for biochemical and molecular analyses as well as data from studies already done. Also, inhalant anesthesia can be used reducing mortality rates and recovery times.
Studies using rats from the 1970-1990’s gave important insight into the proteins and molecules that regulate corneal epithelial cell metabolism, adhesion, and migration (Anderson, 1977; Gipson and Kiorpes, 1982; Gipson et al., 1993; Murakami et al., 1992; Stepp et al., 1993; Zieske and Gipson, 1986). Models developed in the rat to study the mechanisms leading to diabetic retinopathy and to test potential treatments have also been used to study corneal homeostasis and wound healing in diabetic rats (Azar et al., 1992; Friend et al., 1982; Xu and Yu, 2011). Although now there are several strains of rats that develop diabetes spontaneously due to genetic mutations (Li et al., 2007), the injection of Sprague-Dawley or Wistar rats with streptozotocin remains the most well-studied diabetic model in use (Inoguchi et al., 1992; Shimomura et al., 1999). There has also been significant progress made in understanding the role of opioid signaling in corneal wound healing using diabetic rats (Klocek et al., 2009; McLaughlin et al., 2010; Zagon et al., 2009). With streptozotocin treated rats, Kloecek and colleagues (Klocek et al., 2009) showed that the opioid antagonist naltrexone accelerated corneal reepithelialization after debridement wounding. Researchers are also studying Sprague-Dawley or Wistar rats placed on high-fat diets to better understand and improve treatments for obesity-induced pathologies (Qin et al., 2004; Srinivasan et al., 2005; Thaler et al., 2012).
Rabbits
Rabbits have been used to study corneal wound healing for many decades and offer advantages for translational research. The rabbit cornea is similar in size to the human cornea; this allows clinicians to use the same instruments and methods of evaluation as used in patients. Rabbit models were critical in the studies that lead to the clinical use of PRK and LASIK procedures (Munnerlyn et al., 1988; Pallikaris et al., 1990). The strain used most often is an albino one: New Zealand White. The rabbit cornea was found to respond similarly to the human cornea in terms of the timing, the extent of scarring, and myofibroblast formation (Helena et al., 1998; Imanishi et al., 2000; Mohan et al., 2003; Netto et al., 2006; Wilson, 2002). Experiments using cultured rabbit stromal cells confirmed in vivo data (Jester et al., 1995; Masur et al., 1996). Currently, the rabbit cornea is being used to study gene transfer procedures (Mohan et al., 2013). A recent study showed that gold nanoparticles coated with BMP7 can reduce corneal fibrosis in the rabbit (Tandon et al., 2013). Disadvantages of using rabbits include higher cost for purchase and maintenance than rats, restricted availability of polyclonal antibodies against various proteins of interest, and reduced availability of genetically diverse strains and transgenic animals.
Domestic Companion Animals: Dogs and Cats
Veterinary medicine has been on the forefront of developing better treatments for ocular surface defects. Boxers, a popular canine companion animal breed, are prone to developing spontaneously occurring recurrent erosions that lead to pain and eventually corneal scarring. Studies aimed at improving treatment options for dogs with corneal defects have been carried out at various different academic medical centers over the past 20 years (Acheampong et al., 1999; Bentley et al., 2001; Gosling et al., 2013; Morgan and Abrams, 1991; Murphy et al., 2001). At the same time, academic researchers have sought to determine the genetics underlying these conditions (Gosling et al., 2013). These studies have informed and improved treatments for humans. For example, dry eye disease was being treated with cyclosporine in dogs long before being used in people (Stern et al., 1998).
Domestic cats do not suffer from recurrent erosions as frequently as dogs but have been studied to determine the best way to reduce corneal scarring after ocular herpes simplex virus (HSV) infections (Gould, 2011). HSV is a major cause of corneal scarring in human populations (Kaye and Choudhary, 2006) and studies done by veterinarians treating cats are likely to extend and improve treatment options available for humans. Currently antiviral medications require long treatment times and are quite expensive. Additional research has focused on cat corneal endothelial cells (Huang et al., 1989; Petroll et al., 1999; Rotatori et al., 1994). Corneal scarring in response to corneal epithelial wound healing is also being studied in cats (Buhren et al., 2009; Huxlin et al., 2013). Cats, like rabbits, respond similarly to humans in terms of corneal scarring (Nagy et al., 2007). Disadvantages of using companion animals for corneal wound healing research include their large size, high cost, and their genetic heterogeneity.
Mice
The development of genetically engineered mice is the primary reason researchers began to utilize mouse models in the 1980’s and 1990’s (Adams et al., 1985; Cramer et al., 2003; Hanahan, 1985; Rudnicki et al., 1993). Other factors that contributed to a shift from rats and rabbits to mice included improved biochemical methods requiring less tissue and negative publicity regarding the use of rabbits in cosmetic testing. Despite the present mouse-centric era, research on specific pathologies is still done in rabbits, rats, dogs, and chickens.
Strain
An important concern for researchers beginning to study the cornea using mice is the choice of the mouse strain. Albino strains are optimal for imaging of whole flat mounts of the cornea because the cells from the iris, which stick to the outer surface of the cornea during dissection and processing, are not pigmented and do not interfere with imaging. Using pigmented strains reduces the risk of confusion caused by pigmented cells from the iris invading the cornea in response to incisional injuries. We found that the rate of re-epithelialization, frequency of recurrent erosions, and extent of pathology resulting from wounds made close to the limbus varied between two commonly used mouse strains: BALB/c and C57BL6 (Pal-Ghosh et al., 2008). While we did not see any significant difference in cell proliferation in BALB/c and C57BL6 mice during late stages of re-epithelialization, we did not investigate cell proliferation after wounds had closed. It is likely that differences in the timing and extent of the increase in cell proliferation rates will be seen when experiments are performed at multiple time points and in several different strains after corneal wounding. Different strains of mice have corneas that scar more easily than others and these differences also should be taken into consideration when planning experiments. A study by Singh and colleagues showed that DBA/2J mice have more αSMA-positive myofibroblasts, cells whose presence is associated with active corneal scarring, than BALB/c, C57BL6, or C3H mice after irregular phototherapeutic keratectomy with an excimer laser (Singh et al., 2013). In general, corneal scarring is less severe in mice compared to rats and rabbits.
Colleagues from Jackson Labs recently genotyped over 1000 strains of mice to determine which carried gene defects that induce blindness (Chang et al., 2013). They found that 204 strains carry founder mutations in one or more of three genes that lead to blindness: Pde6b, Crb1, and Gnat2. Unfortunately for those who study the retina, these include C57BL6/N, FVB, and C3H mouse strains. This issue has been a major source of frustration for those studying retinal development and function in mice. Most ES cell lines used from 1990-2000 were derived from FVB mice (Taketo et al., 1991); as a result, the majority of conditionally null and transgenic strains carry these mutations.
The most popular mouse strain in use today is the C57BL6 mouse; the N substrain and its derivatives, which is maintained at the NIH and marketed by Taconic and Charles River has mutations in the Crb1rd8 gene that lead to blindness whereas the J substrain, maintained at the Jackson Labs, does not (Chang et al., 2013). Electroretinogram (ERG) testing can be performed to determine whether mice are blind due to retinal defects (Pardue et al., 1998; Peachey et al., 1993). The ARVO Statement for the Use of Animals in Ophthalmic and Visual Research says that “visually disabling procedures should not be performed bilaterally.” To avoid engaging in debates over whether corneal injury impairs vision, most who conduct corneal wound healing experiments have used one eye per mouse. Yet, if corneal researchers use strains of mice congenitally blind due to retinal defects, ethical concerns related to performing bilateral corneal procedures are no longer valid reasons for using more animals than necessary to obtain statistically significant results.
Reporter Mice
Mice expressing the reporter gene β-galactosidase under the control of promoters for various different genes have been generated (Soriano, 1999). These mice allow scientists to follow the expression of a given protein within tissues by staining for β-galactosidase activity and/or the expression of the β-galactosidase protein after wounding. When there is no reliable antibody for a specific protein these types of mice are useful. Much has been learned about the role of the Wnt pathway in epithelial cell differentiation using TOPGAL mice which express β-galactosidase under the control of LEF-1/TCF promoter (DasGupta and Fuchs, 1999). The expression of this transcription factor is turned on when β-catenin is activated during Wnt mediated signal transduction. Another reporter mouse strain that has made significant contributions to epithelial and neuronal cell biology is the Gli-1lz reporter mouse which is used to study Hedgehog signaling pathway (Blaess et al., 2011).
Null and Transgenic Mice
Mice lacking expression of specific proteins (knock-out or null mice) and transgenic mice expressing novel or mutated forms of specific proteins in all of their tissues have been generated for numerous proteins of interest to those studying corneal wound healing. Transgenic and null mice may not develop, die at birth, or survive but have a poorly developed cornea. Other proteins may compensate completely or partially for the targeted protein masking its function. To get around these issues, tissue specific null and transgenic mice are generated (see below). Despite these caveats, complete null and transgenic mice have been very useful for corneal wound healing studies. To cite all studies done using these types of mice would be impossible; we cite here only those that the coauthors contributed (Blanco-Mezquita et al., 2011; Blanco-Mezquita et al., 2013; Mayo et al., 2008; Sta Iglesia et al., 2000; Stepp et al., 2002).
Tissue Specific Gene Alterations in Mice
Tissue specific null and transgenic mice make use of promoters that can drive expression of proteins within specific tissues or cell types. For studies of corneal epithelial wound healing, the most commonly used promoter for tissue specific gene expression has been keratin 12 (Chikama et al., 2005; Kao et al., 1996). Keratins are epithelial intermediate filament proteins that function in pairs and are differentially expressed in various epithelial tissues throughout the body. The mature cornea of most mammals expresses the K3K12 pair; the mouse and rat genomes have a pseudogene for K3 (Lu et al., 2006) making it necessary that K12 pair with another keratin (K4 or K5) in these mammals. Because the conjunctiva and limbal epithelial cells do not express K12, they would be normal in the corneas of mice generated using K12 promoters. Since expression of K12 increases overtime after eyelid opening (Kao et al., 1996), phenotypes for mice generated using K12 promoters can take several weeks before being detected. These features have been exploited in numerous studies that have lead to a much deeper understanding of corneal structure and the functions of specific proteins (Chikama et al., 2008; Kao, 2006; Yuan et al., 2013).
The corneal, limbal, conjunctival, and epidermal epithelia express several of the same keratins. All four epithelia express K5 and K14 and researchers in the field of skin biology have used these promoters to generate mice to study skin, hair development, and skin cancer. Unlike K12, K5 and K14 are expressed very early in epithelial differentiation. Because the corneal, limbal, and conjunctival epithelia all express these keratins, these tissues will be affected by K5- or K14-promoter driven alterations in protein expression. Researchers interested in corneal development and wound healing have used mice generated using K5 (Cascallana et al., 2005; Yoshioka et al., 2010) and K14 (Lu et al., 2012; Singh et al., 2009; Xie et al., 1999; Xu et al., 2007) specific promoters. To modify protein expression in the mouse corneal stroma, stromal cell specific promoters are used. Success has been achieved using keratocan and lumican as promoters to drive gene expression in the corneal stroma (Hayashi et al., 2005; Meij et al., 2007).
Inducible Tissue Specific Gene Alterations in Mice
When the proteins encoded by genes essential to corneal development are eliminated or mutated early in corneal development, corneal and/or conjunctival epithelial tissues may fail to differentiate from the epidermis making it impossible to study the function of the protein in the cornea. To get around this problem, mouse genetic engineers developed inducible systems to temporally regulate gene deletions and/or transgenic gene expression. These types of models allow specific proteins to be deleted or expressed at critical times during corneal development or in the adult cornea after a normal cornea has developed. A thorough description of the different types of genetically engineered mice available today is beyond the scope of this review but can be found on line from Jackson Laboratories at http://research.jax.org/mousegenetics/index.html and from The National Cancer Institute’s Frederick Mouse Repository at http://mouse.ncifcrf.gov/. At present, only a few studies have been published on corneal development using inducible tissue specific genetically engineered mice (Kao, 2006; Ouyang et al., 2006). These types of studies will no doubt begin to be more common; they can elegantly address questions of when and where a protein is functioning in corneal homeostasis and in wound healing.
Differences in mouse strain also impact results obtained from corneal wound healing studies using null, transgenic, tissue-specific, and/or inducible mice. When possible, mice should be backcrossed 7-10 generations onto a pure (C57BL6, BALB/c, FVB, C3H) strain and then results from null mice compared to those from wild-type mice from the parental strain. For tissue specific and inducible engineered mice this is not an option; litter-mate controls determined through careful genotyping must be utilized. Because these types of mice are generated using mice on mixed genetic backgrounds, increased variability from mouse to mouse should be expected compared to studies done using pure strains.
While there are many advantages in the use of genetically engineered mice for corneal wound healing studies, there are also some disadvantages. The most significant drawbacks are time and costs required to breed the mice. Time and money have to be invested knowing that there might be no phenotype in the corneas of these mice. When performing biochemical studies of protein expression, the small size of the mouse cornea requires more animals per experiment compared to similar experiments done using rats. The need to use injectable anesthesia means that there is always a risk of lethal overdose. Variability in wound responses between different mouse strains complicates our ability to compare data between groups using different strains. Finally, no animal, including the mouse, reproduces with 100% accuracy the events that take place in the human cornea after wounding.
Summary
The data from corneal wound healing studies will help us understand how the cornea responds to injury. Each wound healing technique described can be used to address a number of different experimental questions but some methods are more appropriate for looking at, for example, cell proliferation rather than cell migration or scarring. Table 1 lists the different types of mechanical wounds discussed in this review and the most appropriate experimental analyses that should be done with each wound type; Tables 2 and 3 summarize some of the many different types of corneal studies done over the years using various different animal models. This review provides insight into the rationale involved in deciding to use a specific animal model to ask precise research questions related to corneal wound healing. Our hope is to enhance progression of results obtained studying corneal wound healing into the clinic where patients suffering from corneal and other surface defects can be treated successfully.
Highlights.
-
*
We show that studies of corneal wound healing contribute knowledge in the basic sciences and result in improved treatments for patients.
-
*
We describe the different options available for mechanically wounding the cornea
-
*
We offer guidance for researchers on how to decide which vertebrate cornea to use.
-
*
We summarize issues relevant to using the mouse to study the cornea.
Acknowledgments
We could not cite all of the studies done over the years that have moved this field forward. We ask our colleagues to understand that we did our best. We especially want to acknowledge our (MAS, VTR, and JDZ) shared mentor, Ilene K. Gipson, and the Schepens Eye Research Institute for getting us started studying the cornea. We are also grateful for our students, trainees, and staff over the years whose dedication and enthusiasm have kept us engaged and our labs productive. Funding for this work comes from the National Eye Institute. EY008512, EY021784, and EY023106 for MAS; EY06000 and EYO6000S for VTR; EY005665, EY020886, EY003790 for JDZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheampong AA, Shackleton M, Tang-Liu DD, Ding S, Stern ME, Decker R. Distribution of cyclosporin A in ocular tissues after topical administration to albino rabbits and beagle dogs. Current eye research. 1999;18:91–103. doi: 10.1076/ceyr.18.2.91.5381. [DOI] [PubMed] [Google Scholar]
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Amescua G, Collings F, Sidani A, Bonfield TL, Rodriguez JP, Galor A, Medina C, Yang X, Perez VL. Effect of CXCL-1/KC production in high risk vascularized corneal allografts on T cell recruitment and graft rejection. Transplantation. 2008;85:615–625. doi: 10.1097/TP.0b013e3181636d9d. [DOI] [PubMed] [Google Scholar]
- Anderson RA. Actin filaments in normal and migrating corneal epithelial cells. Investigative ophthalmology & visual science. 1977;16:161–166. [PubMed] [Google Scholar]
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investigative ophthalmology & visual science. 1995;36:614–621. [PubMed] [Google Scholar]
- Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. The Journal of biological chemistry. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar DT, Pluznik D, Jain S, Khoury JM. Gelatinase B and A expression after laser in situ keratomileusis and photorefractive keratectomy. Archives of ophthalmology. 1998;116:1206–1208. doi: 10.1001/archopht.116.9.1206. [DOI] [PubMed] [Google Scholar]
- Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Archives of ophthalmology. 1992;110:537–540. doi: 10.1001/archopht.1992.01080160115045. [DOI] [PubMed] [Google Scholar]
- Baker J, Garrod D. Epithelial cells retain junctions during mitosis. Journal of cell science. 1993;104(Pt 2):415–425. doi: 10.1242/jcs.104.2.415. [DOI] [PubMed] [Google Scholar]
- Bard JB, Hay ED. The behavior of fibroblasts from the developing avian cornea. Morphology and movement in situ and in vitro. The Journal of cell biology. 1975;67:400–418. doi: 10.1083/jcb.67.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Paranthan RR, Hamza A, Zhan CG, Lee DM, Kim KB, Lau DL, Srinivasan C, Nakayama K, Nakayama KI, Herrmann H, Mohan R. Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin. The Journal of biological chemistry. 2012;287:989–1006. doi: 10.1074/jbc.M111.297150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan HE. Cellular and molecular events in corneal wound healing: significance of lipid signalling. Experimental eye research. 2005;80:453–463. doi: 10.1016/j.exer.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Investigative ophthalmology & visual science. 1999;40:1658–1663. [PubMed] [Google Scholar]
- Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8146–8150. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley E, Abrams GA, Covitz D, Cook CS, Fischer CA, Hacker D, Stuhr CM, Reid TW, Murphy CJ. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Investigative ophthalmology & visual science. 2001;42:2262–2269. [PubMed] [Google Scholar]
- Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Molecular biology of the cell. 2007;18:2716–2727. doi: 10.1091/mbc.E06-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibliowicz J, Tittle RK, Gross JM. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Progress in molecular biology and translational science. 2011;100:287–330. doi: 10.1016/B978-0-12-384878-9.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess S, Bodea GO, Kabanova A, Chanet S, Mugniery E, Derouiche A, Stephen D, Joyner AL. Temporal-spatial changes in Sonic Hedgehog expression and signaling reveal different potentials of ventral mesencephalic progenitors to populate distinct ventral midbrain nuclei. Neural development. 2011;6:29. doi: 10.1186/1749-8104-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AE, Stepp MA, Zieske JD. alphaVbeta6 integrin promotes corneal wound healing. Investigative ophthalmology & visual science. 2011;52:8505–8513. doi: 10.1167/iovs.11-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AE, Zieske JD. Role of thrombospondin-1 in repair of penetrating corneal wounds. Investigative ophthalmology & visual science. 2013;54:6262–6268. doi: 10.1167/iovs.13-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch JS, Lee C, Lindahl RG. Constitutive expression of class 3 aldehyde dehydrogenase in cultured rat corneal epithelium. The Journal of biological chemistry. 1996;271:5150–5157. doi: 10.1074/jbc.271.9.5150. [DOI] [PubMed] [Google Scholar]
- Boisset G, Polok BK, Schorderet DF. Characterization of pip5k3 fleck corneal dystrophy-linked gene in zebrafish. Gene expression patterns : GEP. 2008;8:404–410. doi: 10.1016/j.gep.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Boote C, Du Y, Morgan S, Harris J, Kamma-Lorger CS, Hayes S, Lathrop KL, Roh DS, Burrow MK, Hiller J, Terrill NJ, Funderburgh JL, Meek KM. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Investigative ophthalmology & visual science. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhren J, Nagy L, Swanton JN, Kenner S, MacRae S, Phipps RP, Huxlin KR. Optical effects of anti-TGFbeta treatment after photorefractive keratectomy in a cat model. Investigative ophthalmology & visual science. 2009;50:634–643. doi: 10.1167/iovs.08-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeseda SE, Burns AR, Dieffenbaugher S, Rumbaut RE, Smith CW, Li Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. The American journal of pathology. 2009;175:571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonge M, Diebold Y, Saez V, Enriquez de Salamanca A, Garcia-Vazquez C, Corrales RM, Herreras JM. Impression cytology of the ocular surface: a review. Experimental eye research. 2004;78:457–472. doi: 10.1016/j.exer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Carrington LM, Boulton M. Hepatocyte growth factor and keratinocyte growth factor regulation of epithelial and stromal corneal wound healing. Journal of cataract and refractive surgery. 2005;31:412–423. doi: 10.1016/j.jcrs.2004.04.072. [DOI] [PubMed] [Google Scholar]
- Cascallana JL, Bravo A, Donet E, Leis H, Lara MF, Paramio JM, Jorcano JL, Perez P. Ectoderm-targeted overexpression of the glucocorticoid receptor induces hypohidrotic ectodermal dysplasia. Endocrinology. 2005;146:2629–2638. doi: 10.1210/en.2004-1246. [DOI] [PubMed] [Google Scholar]
- Cha SH, Lee JS, Oum BS, Kim CD. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clinical & experimental ophthalmology. 2004;32:180–184. doi: 10.1111/j.1442-9071.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- Chang B, Hurd R, Wang J, Nishina P. Survey of common eye diseases in laboratory mouse strains. Investigative ophthalmology & visual science. 2013;54:4974–4981. doi: 10.1167/iovs.13-12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama T, Hayashi Y, Liu CY, Terai N, Terai K, Kao CW, Wang L, Hayashi M, Nishida T, Sanford P, Doestchman T, Kao WW. Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Investigative ophthalmology & visual science. 2005;46:1966–1972. doi: 10.1167/iovs.04-1464. [DOI] [PubMed] [Google Scholar]
- Chikama T, Liu CY, Meij JT, Hayashi Y, Wang IJ, Yang L, Nishida T, Kao WW. Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. The American journal of pathology. 2008;172:638–649. doi: 10.2353/ajpath.2008.070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, McLenachan S, Binz N, Sun Y, Forrester JV, Degli-Esposti MA, Pearlman E, McMenamin PG. TLR9 ligand CpG-ODN applied to the injured mouse cornea elicits retinal inflammation. The American journal of pathology. 2012;180:209–220. doi: 10.1016/j.ajpath.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J, Astley RA, Butler MG, Kennedy RC. Adenovirus keratitis: a role for interleukin-8. Investigative ophthalmology & visual science. 2000;41:783–789. [PubMed] [Google Scholar]
- Chodosh J, Holder VP, Gan YJ, Belgaumi A, Sample J, Sixbey JW. Eradication of latent Epstein-Barr virus by hydroxyurea alters the growth-transformed cell phenotype. The Journal of infectious diseases. 1998;177:1194–1201. doi: 10.1086/515290. [DOI] [PubMed] [Google Scholar]
- Chung ES, Saban DR, Chauhan SK, Dana R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Investigative ophthalmology & visual science. 2009;50:1613–1618. doi: 10.1167/iovs.08-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad GW. Collagen and mucopolysaccharide biosynthesis in mass cultures and clones of chick corneal fibroblasts in vitro. Developmental biology. 1970;21:611–635. doi: 10.1016/0012-1606(70)90080-1. [DOI] [PubMed] [Google Scholar]
- Conrad GW, Hamilton C, Haynes E. Differences in glycosaminoglycans synthesized by fibroblast-like cells from chick cornea, heart, and skin. The Journal of biological chemistry. 1977;252:6861–6870. [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Investigative ophthalmology & visual science. 1996;37:2485–2494. [PubMed] [Google Scholar]
- Dart JK, Radford CF, Minassian D, Verma S, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115:1647–1654. 1654 e1641–1643. doi: 10.1016/j.ophtha.2008.05.003. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Day RC, Beck CW. Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC developmental biology. 2011;11:54. doi: 10.1186/1471-213X-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson JW, Hay ED. Secretion of collagen by corneal epithelium. II. Effect of the underlying substratum on secretion and polymerization of epithelial products. The Journal of experimental zoology. 1974;189:51–72. doi: 10.1002/jez.1401890106. [DOI] [PubMed] [Google Scholar]
- Dreier B, Thomasy SM, Mendonsa R, Raghunathan VK, Russell P, Murphy CJ. Substratum compliance modulates corneal fibroblast to myofibroblast transformation. Investigative ophthalmology & visual science. 2013;54:5901–5907. doi: 10.1167/iovs.12-11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Roehrich H, Chang AA, Huang CW, Maldonado M, Bratten W, Rageh AA, Heuss ND, Gregerson DS, Nelson EF, Yuan C. Corneal wound healing is compromised by immunoproteasome deficiency. PloS one. 2013;8:e54347. doi: 10.1371/journal.pone.0054347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Girard MT. Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Investigative ophthalmology & visual science. 1990;31:1779–1788. [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Current opinion in ophthalmology. 2010;21:317–321. doi: 10.1097/ICU.0b013e32833a8da2. [DOI] [PubMed] [Google Scholar]
- Freeman G. Lens Regeneration from the Cornea in Xenopus Laevis. The Journal of experimental zoology. 1963;154:39–65. doi: 10.1002/jez.1401540105. [DOI] [PubMed] [Google Scholar]
- Friedenwald JS, Buschke W. Some Factors Concerned in the Mitotic and Wound-Healing Activities of the Corneal Epithelium. Transactions of the American Ophthalmological Society. 1944;42:371–383. [PMC free article] [PubMed] [Google Scholar]
- Friend J, Ishii Y, Thoft RA. Corneal epithelial changes in diabetic rats. Ophthalmic research. 1982;14:269–278. doi: 10.1159/000265202. [DOI] [PubMed] [Google Scholar]
- Frueh BE, Bohnke M. Prospective, randomized clinical evaluation of Optisol vs organ culture corneal storage media. Archives of ophthalmology. 2000;118:757–760. doi: 10.1001/archopht.118.6.757. [DOI] [PubMed] [Google Scholar]
- Fujikawa LS, Foster CS, Gipson IK, Colvin RB. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. The Journal of cell biology. 1984;98:128–138. doi: 10.1083/jcb.98.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui L, Henry JJ. FGF signaling is required for lens regeneration in Xenopus laevis. The Biological bulletin. 2011;221:137–145. doi: 10.1086/BBLv221n1p137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Keezer L. Effects of cytochalasins and colchicine on the ultrastructure of migrating corneal epithelium. Investigative ophthalmology & visual science. 1982;22:643–650. [PubMed] [Google Scholar]
- Gipson IK, Kiorpes TC. Epithelial sheet movement: protein and glycoprotein synthesis. Developmental biology. 1982;92:259–262. doi: 10.1016/0012-1606(82)90170-1. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Kiorpes TC, Brennan SJ. Epithelial sheet movement: effects of tunicamycin on migration and glycoprotein synthesis. Developmental biology. 1984;101:212–220. doi: 10.1016/0012-1606(84)90131-3. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Investigative ophthalmology & visual science. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Elwell J, Stepp MA. Redistribution of the hemidesmosome components alpha 6 beta 4 integrin and bullous pemphigoid antigens during epithelial wound healing. Experimental cell research. 1993;207:86–98. doi: 10.1006/excr.1993.1166. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Westcott MJ, Brooksby NG. Effects of cytochalasins B and D and colchicine on migration of the corneal epithelium. Investigative ophthalmology & visual science. 1982;22:633–642. [PubMed] [Google Scholar]
- Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, Anumolu SS, Schlager JJ, Gallo MA, Gerecke DR, Heindel ND, Svoboda KK, Babin MC, Sinko PJ. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling AA, Labelle AL, Breaux CB. Management of spontaneous chronic corneal epithelial defects (SCCEDs) in dogs with diamond burr debridement and placement of a bandage contact lens. Veterinary ophthalmology. 2013;16:83–88. doi: 10.1111/j.1463-5224.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- Gould D. Feline herpesvirus-1: ocular manifestations, diagnosis and treatment options. Journal of feline medicine and surgery. 2011;13:333–346. doi: 10.1016/j.jfms.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Hayashi Y, Liu CY, Tichelaar JW, Kao WW. Over expression of FGF7 enhances cell proliferation but fails to cause pathology in corneal epithelium of Kerapr-rtTA/FGF7 bitransgenic mice. Molecular vision. 2005;11:201–207. [PubMed] [Google Scholar]
- He Z, Pipparelli A, Manissolle C, Acquart S, Garraud O, Gain P, Thuret G. Ex vivo gene electrotransfer to the endothelium of organ cultured human corneas. Ophthalmic research. 2010;43:43–55. doi: 10.1159/000246577. [DOI] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Investigative ophthalmology & visual science. 1998;39:276–283. [PubMed] [Google Scholar]
- Heur M, Jiao S, Schindler S, Crump JG. Regenerative potential of the zebrafish corneal endothelium. Experimental eye research. 2013;106:1–4. doi: 10.1016/j.exer.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata-Tominaga K, Nakamura T, Okumura N, Kawasaki S, Kay EP, Barrandon Y, Koizumi N, Kinoshita S. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem cells. 2013;31:1396–1407. doi: 10.1002/stem.1390. [DOI] [PubMed] [Google Scholar]
- Hu W, Haamedi N, Lee J, Kinoshita T, Ohnuma SI. The structure and development of Xenopus laevis cornea. Experimental eye research. 2013;116C:109–128. doi: 10.1016/j.exer.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Huang PT, Nelson LR, Bourne WM. The morphology and function of healing cat corneal endothelium. Investigative ophthalmology & visual science. 1989;30:1794–1801. [PubMed] [Google Scholar]
- Hutcheon AE, Guo XQ, Stepp MA, Simon KJ, Weinreb PH, Violette SM, Zieske JD. Effect of wound type on Smad 2 and 4 translocation. Investigative ophthalmology & visual science. 2005;46:2362–2368. doi: 10.1167/iovs.04-0759. [DOI] [PubMed] [Google Scholar]
- Huxlin KR, Hindman HB, Jeon KI, Buhren J, Macrae S, Demagistris M, Ciufo D, Sime PJ, Phipps RP. Topical rosiglitazone is an effective anti-scarring agent in the cornea. PloS one. 2013;8:e70785. doi: 10.1371/journal.pone.0070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Progress in retinal and eye research. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H. Effect of eye-pads on healing of simple corneal abrasions. British medical journal. 1960;2:713. doi: 10.1136/bmj.2.5200.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin-Manificat H, Rovere MR, Galiacy SD, Malecaze F, Hulmes DJ, Moali C, Damour O. Development of ex vivo organ culture models to mimic human corneal scarring. Molecular vision. 2012;18:2896–2908. [PMC free article] [PubMed] [Google Scholar]
- Javadi MA, Yazdani S, Sajjadi H, Jadidi K, Karimian F, Einollahi B, Ja’farinasab MR, Zare M. Chronic and delayed-onset mustard gas keratitis: report of 48 patients and review of literature. Ophthalmology. 2005;112:617–625. doi: 10.1016/j.ophtha.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Jessup CF, Brereton HM, Coster DJ, Williams KA. In vitro adenovirus mediated gene transfer to the human cornea. The British journal of ophthalmology. 2005;89:658–661. doi: 10.1136/bjo.2004.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Investigative ophthalmology & visual science. 1994;35:730–743. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Investigative ophthalmology & visual science. 1995;36:809–819. [PubMed] [Google Scholar]
- Jones NP, Hayward JM, Khaw PT, Claoue CM, Elkington AR. Function of an ophthalmic “accident and emergency” department: results of a six month survey. Br Med J (Clin Res Ed) 1986;292:188–190. doi: 10.1136/bmj.292.6514.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn CR, Young E, Lee IH, Rhim JS. Human corneal epithelial primary cultures and cell lines with extended life span: in vitro model for ocular studies. Investigative ophthalmology & visual science. 1993;34:3429–3441. [PubMed] [Google Scholar]
- Kao WW. Ocular surface tissue morphogenesis in normal and disease states revealed by genetically modified mice. Cornea. 2006;25:S7–S19. doi: 10.1097/01.ico.0000247207.55520.a4. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY, Converse RL, Shiraishi A, Kao CW, Ishizaki M, Doetschman T, Duffy J. Keratin 12-deficient mice have fragile corneal epithelia. Investigative ophthalmology & visual science. 1996;37:2572–2584. [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Human corneal fibrosis: an in vitro model. Investigative ophthalmology & visual science. 2010;51:1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Chang JH, Azar DT. Expression of type XVIII collagen during healing of corneal incisions and keratectomy wounds. Investigative ophthalmology & visual science. 2003;44:78–85. doi: 10.1167/iovs.01-1257. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Investigative ophthalmology & visual science. 2004;45:3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- Kaye S, Choudhary A. Herpes simplex keratitis. Progress in retinal and eye research. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Khaw PT, Shah P, Elkington AR. Injury to the eye. Bmj. 2004;328:36–38. doi: 10.1136/bmj.328.7430.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Lakshman N, Karamichos D, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Investigative ophthalmology & visual science. 2010;51:864–875. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Seo KY, Rhim T, Kim EK. Effect of retinoic acid on epithelial differentiation and mucin expression in primary human corneal limbal epithelial cells. Current eye research. 2012;37:33–42. doi: 10.3109/02713683.2011.620728. [DOI] [PubMed] [Google Scholar]
- Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. The ocular surface. 2007;5:228–239. doi: 10.1016/s1542-0124(12)70613-4. [DOI] [PubMed] [Google Scholar]
- Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Naltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type I diabetic rats. Experimental eye research. 2009;89:686–692. doi: 10.1016/j.exer.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Suzuki T, Uno T, Chihara H, Shiraishi A, Hara Y, Inatomi T, Sotozono C, Kawasaki S, Yamasaki K, Mochida C, Ohashi Y, Kinoshita S. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology. 2008;115:292–297 e293. doi: 10.1016/j.ophtha.2007.04.053. [DOI] [PubMed] [Google Scholar]
- Kojima T, Chang JH, Azar DT. Proangiogenic role of ephrinB1/EphB1 in basic fibroblast growth factor-induced corneal angiogenesis. The American journal of pathology. 2007a;170:764–773. doi: 10.2353/ajpath.2007.060487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Chung TY, Chang JH, Sayegh R, Casanova FH, Azar DT. Comparison of EphA receptor tyrosine kinases and ephrinA ligand expression to EphB-ephrinB in vascularized corneas. Cornea. 2007b;26:569–578. doi: 10.1097/ICO.0b013e3180335526. [DOI] [PubMed] [Google Scholar]
- Kryczka T, Ehlers N, Nielsen K, Midelfart A. Impact of organ culturing on metabolic profile of human corneas: preliminary results. Acta ophthalmologica. 2012;90:761–767. doi: 10.1111/j.1755-3768.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Investigative ophthalmology & visual science. 2010a;51:782–789. doi: 10.1167/iovs.09-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF. Developmental guidance of embryonic corneal innervation: roles of Semaphorin3A and Slit2. Developmental biology. 2010b;344:172–184. doi: 10.1016/j.ydbio.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lademann O, Kramer A, Richter H, Patzelt A, Meinke MC, Czaika V, Weltmann KD, Hartmann B, Koch S. Skin disinfection by plasma-tissue interaction: comparison of the effectivity of tissue-tolerable plasma and a standard antiseptic. Skin pharmacology and physiology. 2011;24:284–288. doi: 10.1159/000329913. [DOI] [PubMed] [Google Scholar]
- Laibson PR. Recurrent corneal erosions and epithelial basement membrane dystrophy. Eye & contact lens. 2010;36:315–317. doi: 10.1097/ICL.0b013e3181f18ff7. [DOI] [PubMed] [Google Scholar]
- Lakshman N, Petroll WM. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Investigative ophthalmology & visual science. 2012;53:1077–1086. doi: 10.1167/iovs.11-8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Lee JY, Lee SH, Choi TH. Accelerating repaired basement membrane after bevacizumab treatment on alkali-burned mouse cornea. BMB reports. 2013;46:195–200. doi: 10.5483/BMBRep.2013.46.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sun X, Wang Z, Li R, Li L. The effect of nerve growth factor on differentiation of corneal limbal epithelial cells to conjunctival goblet cells in vitro. Molecular vision. 2010;16:2739–2744. [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. The American journal of pathology. 2011a;178:1106–1116. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011b;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Investigative ophthalmology & visual science. 2006;47:1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- Lindstrom RL, Kaufman HE, Skelnik DL, Laing RA, Lass JH, Musch DC, Trousdale MD, Reinhart WJ, Burris TE, Sugar A, et al. Optisol corneal storage medium. American journal of ophthalmology. 1992;114:345–356. doi: 10.1016/s0002-9394(14)71803-3. [DOI] [PubMed] [Google Scholar]
- Lu H, Lu Q, Zheng Y, Li Q. Notch signaling promotes the corneal epithelium wound healing. Molecular vision. 2012;18:403–411. [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zimek A, Chen J, Hesse M, Bussow H, Weber K, Magin TM. Keratin 5 knockout mice reveal plasticity of keratin expression in the corneal epithelium. European journal of cell biology. 2006;85:803–811. doi: 10.1016/j.ejcb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ma A, Zhao B, Boulton M, Albon J. A role for Notch signaling in corneal wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:98–106. doi: 10.1111/j.1524-475X.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WS, Hanrahan JW. Anion channels in the apical membrane of mammalian corneal epithelium primary cultures. Investigative ophthalmology & visual science. 1991;32:1562–1568. [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo C, Ren R, Rich C, Stepp MA, Trinkaus-Randall V. Regulation by P2X7: epithelial migration and stromal organization in the cornea. Investigative ophthalmology & visual science. 2008;49:4384–4391. doi: 10.1167/iovs.08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Sassani JW, Klocek MS, Zagon IS. Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF)-OGF receptor (OGFr) axis with naltrexone: a review. Brain research bulletin. 2010;81:236–247. doi: 10.1016/j.brainresbull.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt P, Hamilton T, Nelson M, Adkins A, Swartz A, Lawrence R, Milhorn D. Pathogenesis of acute and delayed corneal lesions after ocular exposure to sulfur mustard vapor. Cornea. 2012;31:280–290. doi: 10.1097/ICO.0B013E31823D02CD. [DOI] [PubMed] [Google Scholar]
- Meier S, Hay ED. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Developmental biology. 1974;38:249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Meij JT, Carlson EC, Wang L, Liu CY, Jester JV, Birk DE, Kao WW. Targeted expression of a lumican transgene rescues corneal deficiencies in lumican-null mice. Molecular vision. 2007;13:2012–2018. [PubMed] [Google Scholar]
- Micera A, Lambiase A, Puxeddu I, Aloe L, Stampachiacchiere B, Levi-Schaffer F, Bonini S, Bonini S. Nerve growth factor effect on human primary fibroblastic-keratocytes: possible mechanism during corneal healing. Experimental eye research. 2006;83:747–757. doi: 10.1016/j.exer.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. The Journal of biological chemistry. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Experimental eye research. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Rodier JT, Sharma A. Corneal gene therapy: basic science and translational perspective. The ocular surface. 2013;11:150–164. doi: 10.1016/j.jtos.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Experimental eye research. 2008;86:235–240. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RV, Abrams KL. Topical administration of cyclosporine for treatment of keratoconjunctivitis sicca in dogs. Journal of the American Veterinary Medical Association. 1991;199:1043–1046. [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Experimental eye research. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. Journal of cataract and refractive surgery. 1988;14:46–52. doi: 10.1016/s0886-3350(88)80063-4. [DOI] [PubMed] [Google Scholar]
- Murakami J, Nishida T, Otori T. Coordinated appearance of beta 1 integrins and fibronectin during corneal wound healing. The Journal of laboratory and clinical medicine. 1992;120:86–93. [PubMed] [Google Scholar]
- Murphy CJ, Marfurt CF, McDermott A, Bentley E, Abrams GA, Reid TW, Campbell S. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: clinical features, innervation, and effect of topical SP, with or without IGF-1. Investigative ophthalmology & visual science. 2001;42:2252–2261. [PubMed] [Google Scholar]
- Nagy LJ, MacRae S, Yoon G, Wyble M, Wang J, Cox I, Huxlin KR. Photorefractive keratectomy in the cat eye: biological and optical outcomes. Journal of cataract and refractive surgery. 2007;33:1051–1064. doi: 10.1016/j.jcrs.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Inatomi T, Sotozono C, Koizumi N, Kinoshita S. Successful primary culture and autologous transplantation of corneal limbal epithelial cells from minimal biopsy for unilateral severe ocular surface disease. Acta ophthalmologica Scandinavica. 2004;82:468–471. doi: 10.1111/j.1395-3907.2004.00285.x. [DOI] [PubMed] [Google Scholar]
- Nejepinska J, Juklova K, Jirsova K. Organ culture, but not hypothermic storage, facilitates the repair of the corneal endothelium following mechanical damage. Acta ophthalmologica. 2010;88:413–419. doi: 10.1111/j.1755-3768.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Experimental eye research. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby K. In vivo models of angiogenesis. Journal of cellular and molecular medicine. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notara M, Schrader S, Daniels JT. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue engineering Part A. 2011;17:741–750. doi: 10.1089/ten.TEA.2010.0343. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Shen YC, Yeh LK, Li W, Coyle BM, Liu CY, Fini ME. Pax6 overexpression suppresses cell proliferation and retards the cell cycle in corneal epithelial cells. Investigative ophthalmology & visual science. 2006;47:2397–2407. doi: 10.1167/iovs.05-1083. [DOI] [PubMed] [Google Scholar]
- Overton J. Changes in Cell Fine Structure during Lens Regeneration in Xenopus Laevis. The Journal of cell biology. 1965;24:211–222. doi: 10.1083/jcb.24.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem cells. 2006;24:1075–1086. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem cells. 2012;30:2032–2043. doi: 10.1002/stem.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Blanco T, Tadvalkar G, Pajoohesh-Ganji A, Parthasarathy A, Zieske JD, Stepp MA. MMP9 cleavage of the beta4 integrin ectodomain leads to recurrent epithelial erosions in mice. Journal of cell science. 2011a;124:2666–2675. doi: 10.1242/jcs.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA. A mouse model for the study of recurrent corneal epithelial erosions: alpha9beta1 integrin implicated in progression of the disease. Investigative ophthalmology & visual science. 2004;45:1775–1788. doi: 10.1167/iovs.03-1194. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Stepp MA. Removal of the basement membrane enhances corneal wound healing. Experimental eye research. 2011b;93:927–936. doi: 10.1016/j.exer.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Tadvalkar G, Jurjus RA, Zieske JD, Stepp MA. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Experimental eye research. 2008;87:478–486. doi: 10.1016/j.exer.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikaris IG, Papatzanaki ME, Stathi EZ, Frenschock O, Georgiadis A. Laser in situ keratomileusis. Lasers in surgery and medicine. 1990;10:463–468. doi: 10.1002/lsm.1900100511. [DOI] [PubMed] [Google Scholar]
- Papini S, Rosellini A, Nardi M, Giannarini C, Revoltella RP. Selective growth and expansion of human corneal epithelial basal stem cells in a three-dimensional-organ culture. Differentiation; research in biological diversity. 2005;73:61–68. doi: 10.1111/j.1432-0436.2005.07302006.x. [DOI] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally occurring mouse model of X-linked congenital stationary night blindness. Investigative ophthalmology & visual science. 1998;39:2443–2449. [PubMed] [Google Scholar]
- Peachey NS, Goto Y, al-Ubaidi MR, Naash MI. Properties of the mouse cone-mediated electroretinogram during light adaptation. Neuroscience letters. 1993;162:9–11. doi: 10.1016/0304-3940(93)90547-x. [DOI] [PubMed] [Google Scholar]
- Petrescu MS, Larry CL, Bowden RA, Williams GW, Gagen D, Li Z, Smith CW, Burns AR. Neutrophil interactions with keratocytes during corneal epithelial wound healing: a role for CD18 integrins. Investigative ophthalmology & visual science. 2007;48:5023–5029. doi: 10.1167/iovs.07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Jester JV, Bean J, Cavanagh HD. Labeling of cycling corneal endothelial cells during healing with a monoclonal antibody to the Ki67 antigen (MIB-1) Cornea. 1999;18:98–108. [PubMed] [Google Scholar]
- Pipparelli A, Arsenijevic Y, Thuret G, Gain P, Nicolas M, Majo F. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PloS one. 2013;8:e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisetti N, Islam MM, Griffith M. The artificial cornea. Methods in molecular biology. 2013;1014:45–52. doi: 10.1007/978-1-62703-432-6_2. [DOI] [PubMed] [Google Scholar]
- Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract prevents the insulin resistance induced by a high-fructose diet. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2004;36:119–125. doi: 10.1055/s-2004-814223. [DOI] [PubMed] [Google Scholar]
- Ramamurthi S, Rahman MQ, Dutton GN, Ramaesh K. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye. 2006;20:635–644. doi: 10.1038/sj.eye.6702005. [DOI] [PubMed] [Google Scholar]
- Reichl S, Bednarz J, Muller-Goymann CC. Human corneal equivalent as cell culture model for in vitro drug permeation studies. The British journal of ophthalmology. 2004;88:560–565. doi: 10.1136/bjo.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy JJ, Paulus MP, Gona S. Recurrent erosions of the cornea: epidemiology and treatment. Cornea. 2000;19:767–771. doi: 10.1097/00003226-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Ren R, Hutcheon AE, Guo XQ, Saeidi N, Melotti SA, Ruberti JW, Zieske JD, Trinkaus-Randall V. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:2705–2715. doi: 10.1002/dvdy.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investigative ophthalmology & visual science. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- Roodhooft JM. Leading causes of blindness worldwide. Bulletin de la Societe belge d’ophtalmologie. 2002:19–25. [PubMed] [Google Scholar]
- Rotatori DS, Kerr NC, Raphael B, McLaughlin BJ, Shimizu R, Stern GA, Schultz GS. Elevation of transforming growth factor alpha in cat aqueous humor after corneal endothelial injury. Investigative ophthalmology & visual science. 1994;35:143–149. [PubMed] [Google Scholar]
- Ruberti JW, Zieske JD. Prelude to corneal tissue engineering - gaining control of collagen organization. Progress in retinal and eye research. 2008;27:549–577. doi: 10.1016/j.preteyeres.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Saeidi N, Guo X, Hutcheon AE, Sander EA, Bale SS, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Disorganized collagen scaffold interferes with fibroblast mediated deposition of organized extracellular matrix in vitro. Biotechnology and bioengineering. 2012;109:2683–2698. doi: 10.1002/bit.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Investigative ophthalmology & visual science. 2010;51:1970–1980. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Molecular vision. 2011;17:2177–2190. [PMC free article] [PubMed] [Google Scholar]
- Saika S. Yin and yang in cytokine regulation of corneal wound healing: roles of TNF-alpha. Cornea. 2007;26:S70–74. doi: 10.1097/ICO.0b013e31812f6d14. [DOI] [PubMed] [Google Scholar]
- Saika S, Sumioka T, Okada Y, Yamanaka O, Kitano A, Miyamoto T, Shirai K, Kokado H. Wakayama symposium: modulation of wound healing response in the corneal stroma by osteopontin and tenascin-C. The ocular surface. 2013;11:12–15. doi: 10.1016/j.jtos.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, Ichikawa M. Enhancement of ocular drug penetration. Critical reviews in therapeutic drug carrier systems. 1999;16:85–146. [PubMed] [Google Scholar]
- Sassani JW, Zagon IS, McLaughlin PJ. Opioid growth factor modulation of corneal epithelium: uppers and downers. Current eye research. 2003;26:249–262. doi: 10.1076/ceyr.26.4.249.15427. [DOI] [PubMed] [Google Scholar]
- Schwend T, Lwigale PY, Conrad GW. Nerve repulsion by the lens and cornea during cornea innervation is dependent on Robo-Slit signaling and diminishes with neuron age. Developmental biology. 2012;363:115–127. doi: 10.1016/j.ydbio.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. Journal of virology. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nature reviews Molecular cell biology. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Jani PD, Suthar T, Amin S, Ambati BK. Flt-1 intraceptor induces the unfolded protein response, apoptotic factors, and regression of murine injury-induced corneal neovascularization. Investigative ophthalmology & visual science. 2006;47:4787–4793. doi: 10.1167/iovs.06-0419. [DOI] [PubMed] [Google Scholar]
- Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. The Journal of investigative dermatology. 2009;129:217–228. doi: 10.1038/jid.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. The British journal of ophthalmology. 2005;89:1655–1659. doi: 10.1136/bjo.2005.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Singh N, Ambati BK, Wilson SE. Effect of TGFbeta and PDGF-B blockade on corneal myofibroblast development in mice. Experimental eye research. 2011;93:810–817. doi: 10.1016/j.exer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Torricelli AA, Nayeb-Hashemi N, Agrawal V, Wilson SE. Mouse strain variation in SMA(+) myofibroblast development after corneal injury. Experimental eye research. 2013;115:27–30. doi: 10.1016/j.exer.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sosne G, Qiu P, Kurpakus-Wheater M, Matthew H. Thymosin beta4 and corneal wound healing: visions of the future. Annals of the New York Academy of Sciences. 2010;1194:190–198. doi: 10.1111/j.1749-6632.2010.05472.x. [DOI] [PubMed] [Google Scholar]
- Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Experimental eye research. 2002;74:293–299. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud SJ, Barza M, Gipson IK. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Investigative ophthalmology & visual science. 1988;29:379–386. [PubMed] [Google Scholar]
- Sridhar MS, Rapuano CJ, Cosar CB, Cohen EJ, Laibson PR. Phototherapeutic keratectomy versus diamond burr polishing of Bowman’s membrane in the treatment of recurrent corneal erosions associated with anterior basement membrane dystrophy. Ophthalmology. 2002;109:674–679. doi: 10.1016/s0161-6420(01)01027-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacological research : the official journal of the Italian Pharmacological Society. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Sta Iglesia DD, Gala PH, Qiu T, Stepp MA. Integrin expression during epithelial migration and restratification in the tenascin-C-deficient mouse cornea. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2000;48:363–376. doi: 10.1177/002215540004800306. [DOI] [PubMed] [Google Scholar]
- Sta Iglesia DD, Stepp MA. Disruption of the basement membrane after corneal debridement. Investigative ophthalmology & visual science. 2000;41:1045–1053. [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. Journal of cell science. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Investigative ophthalmology & visual science. 1993;34:1829–1844. [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Investigative ophthalmology & visual science. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- Svoboda KK, Nishimura I, Sugrue SP, Ninomiya Y, Olsen BR. Embryonic chicken cornea and cartilage synthesize type IX collagen molecules with different amino-terminal domains. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7496–7500. doi: 10.1073/pnas.85.20.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PloS one. 2013;8:e66434. doi: 10.1371/journal.pone.0066434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai K, Call MK, Liu H, Saika S, Liu CY, Hayashi Y, Chikama T, Zhang J, Terai N, Kao CW, Kao WW. Crosstalk between TGF-beta and MAPK signaling during corneal wound healing. Investigative ophthalmology & visual science. 2011;52:8208–8215. doi: 10.1167/iovs.11-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. The Journal of clinical investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cellular and molecular life sciences : CMLS. 2013;70:3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad RL. The developmental biology of vertebrate collagens. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1973;21:521–528. doi: 10.1177/21.6.521. [DOI] [PubMed] [Google Scholar]
- Trinkaus-Randall V, Gipson IK. Role of calcium and calmodulin in hemidesmosome formation in vitro. The Journal of cell biology. 1984;98:1565–1571. doi: 10.1083/jcb.98.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Investigative ophthalmology & visual science. 2005;46:88–95. doi: 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. The Journal of biological chemistry. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner MD, Kenyon KR, Gipson IK, Hanninen LA, Seng WL. Polymorphonuclear neutrophils delay corneal epithelial wound healing in vitro. Investigative ophthalmology & visual science. 1984;25:1217–1220. [PubMed] [Google Scholar]
- Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Transactions of the American Ophthalmological Society. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- Xie W, Chow LT, Paterson AJ, Chin E, Kudlow JE. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene. 1999;18:3593–3607. doi: 10.1038/sj.onc.1202673. [DOI] [PubMed] [Google Scholar]
- Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Investigative ophthalmology & visual science. 2011;52:3301–3308. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Mannik J, Kudryavtseva E, Lin KK, Flanagan LA, Spencer J, Soto A, Wang N, Lu Z, Yu Z, Monuki ES, Andersen B. Co-factors of LIM domains (Clims/Ldb/Nli) regulate corneal homeostasis and maintenance of hair follicle stem cells. Developmental biology. 2007;312:484–500. doi: 10.1016/j.ydbio.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Yanai R, Nakamura M, Inui M, Nishida T. Role of the C domain of IGFs in synergistic promotion, with a substance P-derived peptide, of rabbit corneal epithelial wound healing. Investigative ophthalmology & visual science. 2004;45:1125–1131. doi: 10.1167/iovs.03-0626. [DOI] [PubMed] [Google Scholar]
- Yanez-Soto B, Liliensiek SJ, Murphy CJ, Nealey PF. Biochemically and topographically engineered poly(ethylene glycol) diacrylate hydrogels with biomimetic characteristics as substrates for human corneal epithelial cells. Journal of biomedical materials research Part A. 2013;101:1184–1194. doi: 10.1002/jbm.a.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang H, Wang Z, Varadaraj K, Kumari SS, Mergler S, Okada Y, Saika S, Kingsley PJ, Marnett LJ, Reinach PS. Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury. Cellular signalling. 2013;25:501–511. doi: 10.1016/j.cellsig.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Investigative ophthalmology & visual science. 2010;51:1891–1897. doi: 10.1167/iovs.09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka R, Shiraishi A, Kobayashi T, Morita S, Hayashi Y, Higashiyama S, Ohashi Y. Corneal epithelial wound healing impaired in keratinocyte-specific HB-EGF-deficient mice in vivo and in vitro. Investigative ophthalmology & visual science. 2010;51:5630–5639. doi: 10.1167/iovs.10-5158. [DOI] [PubMed] [Google Scholar]
- Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain research bulletin. 2010;81:229–235. doi: 10.1016/j.brainresbull.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Yeh LK, Liu H, Yamanaka O, Hardie WD, Kao WW, Liu CY. Targeted overexpression of TGF-alpha in the corneal epithelium of adult transgenic mice induces changes in anterior segment morphology and activates noncanonical Wnt signaling. Investigative ophthalmology & visual science. 2013;54:1829–1837. doi: 10.1167/iovs.12-11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Dry eye reversal and corneal sensation restoration with topical naltrexone in diabetes mellitus. Archives of ophthalmology. 2009;127:1468–1473. doi: 10.1001/archophthalmol.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Agius-Fernandez A, Forrester JV, McCaig CD. Directed migration of corneal epithelial sheets in physiological electric fields. Investigative ophthalmology & visual science. 1996;37:2548–2558. [PubMed] [Google Scholar]
- Zhao XC, Yee RW, Norcom E, Burgess H, Avanesov AS, Barrish JP, Malicki J. The zebrafish cornea: structure and development. Investigative ophthalmology & visual science. 2006;47:4341–4348. doi: 10.1167/iovs.05-1611. [DOI] [PubMed] [Google Scholar]
- Zheng R, Po I, Mishin V, Black AT, Heck DE, Laskin DL, Sinko PJ, Gerecke DR, Gordon MK, Laskin JD. The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicology and applied pharmacology. 2013;272:345–355. doi: 10.1016/j.taap.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Gipson IK. Protein synthesis during corneal epithelial wound healing. Investigative ophthalmology & visual science. 1986;27:1–7. [PubMed] [Google Scholar]
- Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJ, Fukai N, Olsen BR, Parenteau NL. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Experimental cell research. 1994;214:621–633. doi: 10.1006/excr.1994.1300. [DOI] [PubMed] [Google Scholar]


