Abstract
The TNO intestinal model (TIM-1) of the human upper gastrointestinal tract was used to compare intestinal absorption/bioaccessibility of blueberry anthocyanins under different digestive conditions. Blueberry polyphenol-rich extract was delivered to TIM-1 in the absence or presence of a high-fat meal. HPLC analysis of seventeen anthocyanins showed that delphinidin-3-glucoside, delphinidin-3-galactoside, delphinidin-3-arabinoside and petunidin-3-arabinoside were twice as bioaccessible in fed state, whilst delphinidin-3-(6″-acetoyl)-glucoside and malvidin-3-arabinoside were twice as bioaccessible under fasted conditions, suggesting lipid-rich matrices selectively effect anthocyanin bioaccessibility. TIM-1 was fed blueberry juice (BBJ) or blueberry polyphenol-enriched defatted soybean flour (BB-DSF) containing equivalent amounts of free or DSF-sorbed anthocyanins, respectively. Anthocyanin bioaccessibility from BB-DSF (36.0 ± 10.4) was numerically, but not significantly, greater than that from BBJ (26.3 ± 10.3). Ileal efflux samples collected after digestion of BB-DSF contained 2.8-fold more anthocyanins than same from BBJ, suggesting that protein-rich DSF protects anthocyanins during transit through upper digestive tract for subsequent colonic delivery/metabolism.
Keywords: Blueberry, Anthocyanins, Bioavailability, Bioaccessibility, Gastrointestinal model, TIM-1, Soybean flour, Nutrition
1. Introduction
Despite their instability and low plasma bioavailability, the antioxidant anthocyanin pigments contained in fruits and vegetables have demonstrated biological efficacy for a variety of clinical targets related to cardiovascular disease (CVD), metabolic syndrome, cancer and age-related neurodegeneration (Spencer, 2010; Tsuda, 2008; Wallace, 2011; Wang & Stoner, 2008). Blueberries are a particularly rich source of anthocyanins (Wu et al., 2006) and contain a complex mixture of up to 27 different anthocyanins (Wu & Prior, 2005). Blueberry consumption has been associated with improved insulin sensitivity (Stull, Cash, Johnson, Champagne, & Cefalu, 2010) and decreased CVD factors (Basu et al., 2010) in men and women with metabolic syndrome, as well as lowered risk of type-2 diabetes (Wedick et al., 2012). When C57BL/6J mice were fed whole blueberry powder or purified blueberry anthocyanins, only the latter, which lacked sugars and lipid components, was effective in decreasing body weight gain, correcting dyslipidemia or lowering blood glucose (Grace et al., 2009; Prior et al., 2008, 2010). These data suggest that sugars or lipid components in whole blueberries may counteract the anti-diabetic effects of blueberry anthocyanins and other polyphenols. Protein-rich flours, such as defatted soy flour (DSF), can preferentially sorb mid-polarity range anthocyanins and other polyphenols from fruit juices, whilst polar sugars remain in the juice supernatant, yielding a stable and biologically active polyphenol-enriched DSF food ingredient (Roopchand, Grace et al., 2012; Roopchand, Kuhn et al., 2012). Compared to DSF alone, blueberry polyphenol-enriched DSF was shown to significantly reduce hyperglycemia, body weight gain and serum cholesterol in C57BL/6J mice (Roopchand, Kuhn, Rojo, Lila, & Raskin, 2013; Roopchand, Grace et al., 2012).
The high antioxidant capacity and ability to modulate molecular signalling pathways within tissue/cellular targets contribute to the therapeutic effects of anthocyanins; however, no definite mechanism(s) of action have been assigned for anthocyanin compounds (Fraga, Galleano, Verstraeten, & Oteiza, 2010; Williams, Spencer, & Rice-Evans, 2004). Mechanistic studies on anthocyanins have been complicated by their instability, uncertain metabolism and low bioavailability (D’Archivio, Filesi, Vari, Scazzocchio & Masella, 2010; Lila, 2004). Several definitions of bio-availability exist, but the most appropriate seems to be the fraction of ingested nutrient/compound that reaches the systemic circulation and the specific target tissue(s) where it can exert its biological action (Porrini & Riso, 2008). This definition implies release from the carrier matrix, intestinal absorption and tissue uptake. Anthocyanin bioavailability studies in animals and humans indicate that low levels are absorbed in the circulation and excreted in urine, whilst the highest levels are found in the gastrointestinal (GI) tract (McGhie & Walton, 2007). For example, three hours after radiolabeled cyanidin-3-glucoside was orally administered to mice, 87.9% of the radioactivity was recovered in the GI tract with 50.7% from the small intestine, whilst only 3.3% and 0.044% was recovered in urine and plasma, respectively (Felgines et al., 2010). Nevertheless, several reports indicate that unmetabolized anthocyanins can be detected in circulation (McGhie & Walton, 2007; Nurmi et al., 2009) and have bioactivity in vitro (Rojo et al., 2012; Roopchand et al., 2013) and in vivo (Grace et al., 2009). Low levels of anthocyanins (<4% of input) can be absorbed across human intestinal Caco-2 cell membranes, and malvidin-3-glucoside and anthocyanins with acetyl groups showed the highest transport efficiency (Yi, Akoh, Fischer, & Krewer, 2006b). Anthocyanins were shown to be incorporated into the membrane and cytosol of vascular endothelial cells, protecting them against oxidative stress (Youdim, Martin, & Joseph, 2000). Anthocyanins can be in the stable flavylium cation form in the acidic pH environment of the stomach, but are rapidly changed to the unstable hemiketal, chalcone and quinoidal forms in the more neutral pH environment of the small intestine and colon (McGhie & Walton, 2007). In vivo, anthocyanins may also undergo glucuronidation and methylation (Felgines et al., 2005; Wu, Cao, & Prior, 2002). Exposure to microbiota in the oral cavity and GI tract results in anthocyanin metabolism to unstable anthocyanidin forms and degradation to phenolic acids (Kamonpatana et al., 2012; Nurmi et al., 2009); however, breakdown products remain largely unknown. It remains unclear which form(s) of anthocyanins or which of the many metabolites are ultimately responsible for biological activities observed in vivo.
Little is known about the effects of the food matrix on the intestinal absorption/bioavailability of anthocyanins and the paradox of low anthocyanin bioavailability has made such analyses extremely challenging to perform in vivo. Anthocyanin bioavailability may be improved, hindered or unaltered when co-delivered with other foods rich in fat, protein, carbohydrate or fibre (Yang, Koo, Song, & Chun, 2011). To study intestinal absorption of blueberry anthocyanins in the presence or absence of a high fat meal, or when sorbed to protein-rich DSF, the TNO gastrointestinal Model (TIM-1) of the upper human GI tract (Minekus, Marteau, Havenaar, & Huis in’t Veld, 1995) was utilised and coupled with HPLC analysis of individual anthocyanins, or colourimetric quantification of total monomeric anthocyanins. TIM-1 is a dynamic,multi-compartment, computer-controlled system that simulates the in vivo conditions and kinetic events of the stomach and duodenum, jejunum and ileum compartments of the small intestine. TIM-1 provides information about nutrient/compound transit, release, stability and availability for intestinal absorption or bioaccessibility. In TIM-1, bioaccessibility is defined as the amount of compound released from a food matrix that can bypass simulated jejunal and ileal membranes reflecting availability for intestinal absorption in vivo. Depending on the biological mechanisms (e.g. specific transporters) present in vivo, bioaccessible compounds are not necessarily bioavailable; however, compounds that are not bioaccessible are generally not bioavailable. TIM-1 has been used to investigate bioaccessibility of phytochemicals (Blanquet-Diot, Soufi, Rambeau, Rock, &Alric, 2009; Lila et al., 2011; Mateo Anson, Van den Berg, Havenaar, Bast, & Haenen, 2009; Minekus et al., 2005), dietary nutrients (Haraldsson et al., 2005; Verwei, Freidig, Havenaar, & Groten, 2006; Verwei et al., 2003) and drug formulations (Blanquet et al., 2004; Tenjarla, Romasanta, Zeijdner, Villa, & Moro, 2007) under fed or fasted states.
2. Materials and methods
2.1. Chemicals
Pepsin A from porcine stomach mucosa (2500-3500 units/mg, P-7012), trypsin from bovine pancreas (7500 N-α-benzoyl-l-arginine ethyl ester (BAEE) units/mg, T9201), and α-Amylase Type II-A: from Bacillus species (1333 units/mg A-6380) were obtained from Sigma-Aldrich (Stockholm, Sweden). Fresh pig bile was obtained from TNO Zeist, Netherlands. Rhizopus lipase (150,000 units/mg F-AP-15) was from Amano Enzyme Inc. (Nagoya, Japan).
2.2. High fat meal
A standardised high fat meal was prepared as described in Guidance for Industry, December 2002, to meet U.S. Food and Drug Administration (FDA) and Center for Drug Evaluation and Research (CDER) requirements for experiments performed with drugs ingested with a high fat meal. The high fat meal included cooked eggs and bacon, whole cow’s milk, butter, white bread and margarine, which were weighed to required proportions, mashed, mixed, and divided into portions of 100 g that were stored frozen at −20 °C. This meal delivers 800–1000 kcal with approximately 50% of calories from fat, 20% from protein and 30% from carbohydrates. This is for adult human; in TIM (5× downscales) the meal (100 g) has 165 kcal.
2.3. Blueberry polyphenol-rich extract
Quick-frozen, whole lowbush blueberries (Vaccinium angustifolium Aiton) were obtained from the Wild Blueberry Association of North America (Old Town, ME, USA). The blueberries were a composite of fruits from all major growing sites including Prince Edward Island, New Brunswick, Québec, Nova Scotia and Maine. The composite was made in the fall of 2008, frozen by Cherryfield Foods, Inc. at −15 °C (Cherryfield, ME, USA), and subsequently stored at −80 °C until use. Whole frozen blueberries were blended (Waring, Inc., Torrington, CT, USA) with methanol acidified with 0.3% TFA (fruit to solvent ratio 1:2), and filtered first through multiple layers of muslin sheets, and then on Whatman filter paper # 4 (Florham Park, NJ, USA) with vacuum. Organic solvent in the collected hydro-alcohol extract was evaporated by rotary evaporation set at 40 °C. The concentrated aqueous extract was loaded onto an Amberlite XAD-7 column preconditioned with acidified water (0.3% TFA). The resin was washed thoroughly with acidified water (0.3% TFA, 3 l) to remove free sugars, pectins and organic acids. The polyphenolic mixture was then eluted with methanol, the methanol was evaporated and the aqueous eluate was freeze-dried to produce a blueberry polyphenol-rich extract.
2.4. Blueberry polyphenol-enriched DSF
Blueberry (Vaccinium corymbosum) juice concentrate (65 Brix; Fruit Smart, WA) produced from cultivated highbush blueberries was diluted 4× in water. Defatted soy flour (DSF; Hodgson Mill Inc., IL, USA) was added to the diluted juice at 30 g/l and mixed for 15 min. The blueberry juice–flour mixture (pH 3.7) was centrifuged for 10 min at 4000 rpm (Eppendorf, model 5810R) and the blueberry polyphenol-enriched DSF (BB-DSF) was collected after decanting the blueberry juice supernatants, freeze-dried and powdered. The concentration of total monomeric anthocyanins in the DSF matrix was quantified using the pH differential method (Lee, Durst, & Wrolstad, 2005) as previously described (Roopchand, Grace et al., 2012). Briefly, blueberry juice supernatants were passed through a 2 μm filter prior to quantification of blueberry anthocyanins. Total monomeric anthocyanins, calculated as cyanidin 3-O-glucoside equivalents, were measured in the blueberry juice supernatant and in the diluted concentrate using the pH differential method using a UV/Vis spectrophotometer (Synergy HT Multi-Detection Microplate Reader, BioTek). The amount of anthocyanins sorbed per gram of DSF was calculated by subtracting their concentration in the DSF-treated juice supernatants, from that measured in the untreated juice samples and dividing by the concentration of DSF used for sorption.
2.5. TIM-1
The dynamic, computer-controlled TIM-1 system has been described at length (Minekus et al., 1995). Briefly, TIM-1 consists of stomach, duodenum, jejunum and ileum compartments, each composed of a glass capsule encasing a flexible inner silicone jacket. Water heated to 37 ± 1 °C is pumped through the space between the glass and silicone to maintain body temperature, and mechanically compress and release the silicone jackets to imitate peristalsis and mixing of chyme. Secretions of amylase (saliva), gastric juice, bile and pancreatin/pancreatic juice (Larsson, Minekus, & Havenaar, 1997), are introduced via pumps connected to GI compartments. pH is regulated by secretion of hydrochloric acid in the stomach and sodium bicarbonate in intestinal compartments. Simulated gastric (pepsin and lipase), biliary (fresh pig bile) and pancreatic secretions are introduced into compartments by computer-controlled pumps. Gastric emptying and intestinal transit time are controlled by three peristaltic valves that move specific volumes of chyme with each open-close cycle, which is altered to simulate either the fasted state, corresponding to intake of compounds with water, or the fed state, for intake of compounds with a meal/food matrix. Connected to jejunal and ileal compartments are hollow fibre filtration devices, composed of semi-permeable membranes (0.05 μm pore size, Spectrum Milikros modules M80S-300-01P) that simulate absorption of released/digested water or fat soluble compounds less than 50 nm in size. During TIM-1 digestion, samples are collected hourly from fluids absorbed through jejunal and ileal filtration devices, as well as the ileal efflux, fluids that pass through the ileocaecal valve of the model and contain compounds that would theoretically be delivered to the colon.
2.6. TIM-1 experiments with blueberry polyphenol-rich extract in the absence or presence of a lipid-rich food matrix
Two independent experiments were performed for each condition. For the fasted state, 0.5 g of blueberry polyphenol-rich extract was mixed with artificial saliva, which consisted of 100 ml electrolyte solution, 30 ml citrate buffer and 11.5 mg amylase. For the fed state, 0.5 g of blueberry polyphenol-rich extract was mixed with 100 g of high fat meal matrix and artificial saliva. Double-distiled water was added to each mixture up to a final volume of 300 ml. The final mixture was introduced in the gastric compartment of TIM-1 and digestion was initiated. To mimic the physiological conditions of monogastric digestion for the fasted state, 100 ml of fresh porcine bile and 3.5 g of centrifuged pancreatin were continually secreted into the duodenum compartment, whilst 7.5 mg of lipase and 6 mg of pepsin were delivered into the stomach compartment based on the TIM-1 computer-controlled settings. Consistent with physiological events during food intake, greater concentrations of digestive secretions were used for fed state conditions. Specifically, 500 ml of bile and 17.5 g of pancreatin were secreted into the duodenum compartment whilst 37.5 mg lipase and 30 mg pepsin were delivered into the stomach compartment. Each TIM-1 digestion experiment was terminated at 240 min (4 h) when approximately 80% of the stomach contents had passed the ileocaecal valve of the model and become the ileal efflux. Bioaccessibility of blueberry anthocyanins was evaluated by collecting taking 40 mL of jejunum and ileum filtrate and ileal efflux samples collected in 1 h aliquots for 4 h after initiation of digestion. Residues, which comprised fluids remaining in the stomach, duodenum, jejunum and ileum compartments after the 4 h digestion period, were also collected and anthocyanin content was analysed.
2.6.1. Quantification of individual anthocyanins in TIM-1 samples by HPLC
Each 40 ml jejunum, ileum, ileal efflux or residue sample was freeze-dried at −51 °C. Dried samples were extracted twice with 20 ml of acidified methanol (0.3% TFA), sonicated and centrifuged at 4 °C, 4000 rpm for 20 min. The supernatant was evaporated to dryness, weight was recorded and the dried extract was dissolved in 10 ml of methanol (0.3% TFA), filtered (0.2 μm PTFE filter) and subjected to HPLC analysis for quantification of anthocyanin content.
HPLC analyses were conducted using a 1200 HPLC (Agilent Technologies, Santa Clara, CA, USA) with a photodiode array (PDA) detector, and an autosampler with Chemstation software as a controller and for data processing. Anthocyanin separation was performed using a reversed phase Supelcosil-LC-18 column, 250 mm × 4.6 mm (L × I.D.); 5 μm particle size (Supelco, Bellefonte, PA, USA). The mobile phase consisted of 5% formic acid in H2O (A) and 100% methanol (B). The flow rate was constant during HPLC analysis at 1 ml/min with a step gradient of 10%, 15%, 20%, 25%, 30%, 60%, 10%, and 10% of solvent B at 0, 5, 15, 20, 25, 45, 47, and 60 min, respectively. Samples were injected (10 μl) onto the HPLC column with a constant temperature of 20 °C. Cyanidin-3-O-glucoside was prepared at 1.0, 0.5, and 0.25 mg/ml concentrations and 5 μl was injected as an external standard. Quantification of seventeen different anthocyanins was performed from the peak areas recorded at 520 nm, with reference to a calibration curve obtained with cyanidin-3-glucoside. Anthocyanins were identified by LC–MS as well as using the following available standards: cyanidin 3-glucoside, cyanidin 3-galactoside, delphinidin 3-glucoside, peonidin 3-glucoside, petunidin 3-glucoside and malvidin 3-glucoside (Polyphenols, Norway). All individual anthocyanins are reported as cyanidin-3-glucoside equivalents. Data presented are the mean and ranges (i.e. difference between largest and smallest values) for each set of duplicate experiments.
For fasted and fed state experiments, each of the seventeen anthocyanins quantified in jejunal and ileal samples at 1 through 4 h time points, were summed to obtain the total bioaccessibility amount (mg) over the 4 h digestion period. This bioaccessible amount of each anthocyanin was divided by the amount of corresponding anthocyanin in the original 500 mg of blueberry polyphenol-rich extract, to obtain the total bioaccessibility of each anthocyanin as a percentage of input. Similarly, amounts of anthocyanins measured in ileal efflux samples were expressed as a percentage of input to give the percentages of each anthocyanin that could be delivered to the colon. Anthocyanin amounts were also measured in the residues. The total recovery of anthocyanins or mass balance was calculated from adding the amounts of each anthocyanin in jejunum and ileum samples (representing total bioaccessibility), ileal efflux samples, and the residues collected after each experiment and then dividing by the total input of each anthocyanin in the extract.
2.7. TIM-1. experiments with blueberry juice and blueberry polyphenol-enriched DSF
Four independent experiments were performed with blueberry polyphenol-enriched DSF and three independent experiments were performed with blueberry juice using fed state conditions in each case. Blueberry polyphenol-enriched DSF (16 g) containing 156 mg of total monomeric anthocyanins was mixed with artificial saliva or a volume of blueberry juice concentrate diluted 3 times with water, and the same level of anthocyanins (156 mg) was mixed with artificial saliva. Double-distiled water was added to each mixture up to a final volume of 300 ml. The final mixture was introduced in the gastric compartment of TIM-1 and the digestion process was initiated; 500 ml of bile, 17.5 g of pancreatin, 37.5 mg of lipase and 30 mg of pepsin were added to the system. Experiments were terminated at 240 min. Bioaccessibility of blueberry anthocyanins was evaluated by collecting taking 40 mL of jejunum and ileum filtrate and ileal efflux samples collected in 1 h aliquots for 4 h after initiation of digestion. Anthocyanin content was also analysed in residues remaining in all compartments after the 4 h digestion period.
2.7.1. Quantification of total monomeric anthocyanins in TIM-1 samples by pH differential method
Each 40 mL jejunum and ileum filtrate or ileum efflux sample collected over the 4 h period was directly subjected to total monomeric anthocyanin quantification using the pH differential method (Lee et al., 2005). Briefly 200 μl of sample was added to 800 μl of pH 1 buffer or pH 4.5 buffer, samples were then vortexed, centrifuged and absorbance was read at 500 nm and 700 nm with an UV/Vis spectrophotometer (Shimadzu UV-2450 or Synergy HT Multi-Detection Microplate Reader, BioTek). The amounts of anthocyanins recovered at 1, 2, 3 and 4 h for the jejunum, ileum and ileal efflux samples were calculated and amounts of anthocyanins in the residues were determined. For each experiment the percentage of anthocyanins recovered in each compartment and the residue over the 4 h digestion period was calculated based on the input of 156 mg of anthocyanins. Data are presented as the mean and standard deviation for each set of experiments.
2.8. Statistics
Statistics were performed with STATISTICA v.10 (StatSoft). Unpaired, two-tailed t-tests were performed to compare data between independent groups.
3. Results
3.1. Bioaccessibility of blueberry anthocyanins in the absence or presence of a high fat meal
Blueberries possess one of the largest collections of anthocyanins in terms of anthocyanidin core structures, as well as conjugation to functional groups and sugars (Fig. 1). Blueberry polyphenol-rich extract (500 mg) was delivered to TIM-1 for digestion in the absence (fasted state) or presence (fed state) of a high fat meal matrix. Anthocyanins that bypassed the semi-permeable capillary membranes connected to the jejunum and ileum compartments represented anthocyanins that were absorbed/bioaccessible. Anthocyanins in the ileal efflux represented compounds that would theoretically be delivered to the colon for further metabolism and bioconversion by gut microbiota. Jejunal and ileal absorption samples and ileal efflux fluids were collected hourly for 4 h. The residues, which comprised the fluids that remained in all TIM-1 compartments after the 4 h experiment, were also collected and analysed for anthocyanins. In total, seventeen blueberry anthocyanins were putatively identified and quantified by HPLC in all samples collected over the 4 h period.
Fig. 1.
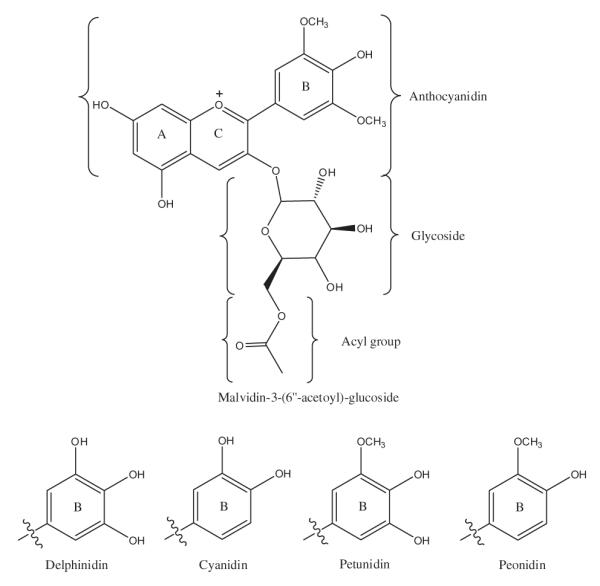
Chemical structures of blueberry anthocyanins. Top: Structure of malvidin-3-(6″-acetoyl)-glucoside showing A, B and C rings of anthocyanidin core and conjugation of glycoside (glucose, galactose or arabinose) and acyl groups. Bottom: Substitution of hydroxyl and methoxy groups on B-ring structures corresponding to delphinidin, cyanidin, petunidin and peonidin anthocyanidin cores.
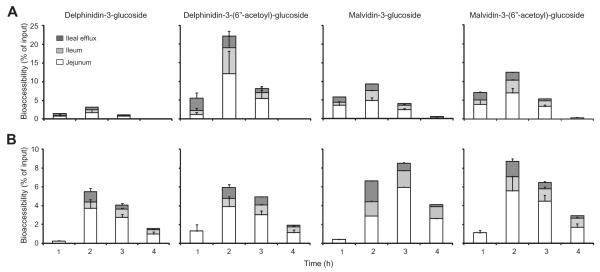
Intestinal absorption or bioaccessibility of individual anthocyanin species varied considerably. The average hourly bioaccessibility as a percentage of anthocyanin input for delphinidin-3-glucoside, delpinidin-3-(6″-acetoyl)-glucoside, malvidin-3-glucoside and malvidin-3-(6″-acetoyl)-glucoside, four common blueberry anthocyanins, is shown for duplicate experiments performed in fasted (Fig. 2A) and fed state (Fig. 2B) conditions. After 1 h of digestion in fasted state conditions, these anthocyanins had 0.1–3.8% bioaccessibility in jejunal or ileal samples. In the fasted state, the greatest absorption was observed at 2 h and 3 h where the bioaccessibility ranged from 0.8% to 12.1% in jejunal samples and 0.2–6.9% in ileal samples. Anthocyanins showed almost no absorption after 4 h, except for small amounts of malvidin-3-glucoside (0.8% in jejunum and 0.3% in ileal samples) and malvidin-3-(6″-acetoyl)-glucoside (0.3% in ileal samples) (Fig 2A). Across all time points for each of the four anthocyanins, the percentage of anthocyanins in the ileal efflux was 0–3.3%. In the fed state, small amounts of each anthocyanin were detected only in jejunal samples after 1 h (0.2–1.3%), due to the delayed gastric emptying in the fed protocol vs. the fasted state protocol parameters. In the fed state, the majority of anthocyanins were also absorbed at 2 h and 3 h, and bioaccessibility ranged from 2.8% to 5.9% for the jejunal compartment and 0.6–1.5% for the ileal compartment (Fig. 2B). Compared to the fasted state, more anthocyanins were absorbed at 4 h in the fed state and bioaccessibility ranged from 1% to 2.6% in jejunal samples and 0.5–1.3% in ileal samples. This again seems to be a result of the delayed gastric emptying in the fed protocol, suggesting that not all anthocyanins had passed through the model after 4 h (Fig. 2B).
Fig. 2.
Bioaccessibility of four major blueberry anthocyanins obtained in (A) fasted or (B) fed TIM-1 conditions. TIM-1 was fed 500 mg of polyphenol-enriched extract in (A) the absence of the high fat meal for fasted state or (B) with the high fat meal for fed state; the corresponding TIM-1 program for gastric emptying was used. Anthocyanins were measured by HPLC in samples collected from jejunum, ileum and ileal efflux at 1, 2, 3 and 4 h after start of digestion. Data are presented as stacked bar graphs and are the average of two independent experiments and error bars represent the range. Anthocyanin bioaccessibility as a percentage of anthocyanin input was determined in jejunum (white bars) and ileum (light grey bars) samples at each time point and represent the total bioaccessibility of each anthocyanin. Anthocyanins in the ileal efflux (dark grey bars) as a percentage of input were also measured and represent the percentage of compound that would theoretically be delivered to the colon.
Summarised in Tables 1 and 2 for fasted and fed state experiments, respectively, are the bioaccessible amounts of each anthocyanin (mg) over the 4 h digestion period, the total bioaccessibility of each anthocyanin as a percentage of input, the percentages of each anthocyanin that could be delivered to the colon and the anthocyanin recovery as a percentage of input. The total bioaccessibility of individual anthocyanins after TIM-1 digestion in the fasted state ranged from 4.3% to 36.9% (Table 1) and from 9.3% to 28.9% in the fed state (Table 2). For most anthocyanins the total bioavailability was comparable between fed and fasted states with some exceptions. Delphinidin-3-glucoside, delphinidin-3-galactoside, delphinidin-3-arabinoside and petunidin-3-arabinoside were twice as bioaccessible in the fed state, whilst delphinidin-3-(6″-acetoyl)-glucoside and malvidin-3-arabinoside were twice as bioaccessible in the fasted state (Tables 1 and 2). These data indicate that a lipid-rich meal matrix can selectively affect the bioaccessibility of anthocyanins.
Table 1.
Bioaccessibility of blueberry anthocyanins in fasted state conditions.
| Aglycone | Anthocyanin (ACN) | ACN input (mg) |
BA amt of ACN (mg) |
Total ACN BA as % of input |
ACNs in I.E. as % of input |
ACN recovery as % of input |
|---|---|---|---|---|---|---|
| Delphinidin (dp) |
dp-3-Galactoside | 13.1 | 0.7 ± 0.3 | 5.0 ± 2.2 | 1.5 ± 0.6 | 7.0 ± 2.8 |
| dp-3-Glucoside | 16.8 | 0.7 ± 0.3 | 4.3 ± 1.7 | 1.3 ± 0.5 | 6.1 ± 2.2 | |
| dp-3-Arabinoside | 8.9 | 0.4 ± 0.2 | 4.6 ± 1.7 | 1.5 ± 0.5 | 6.6 ± 2.2 | |
| dp-3-(6″-Acetoyl)-glucoside | 3.4 | 1.0 ± 0.8 | 28.3 ± 24.7 | 7.6 ± 6.5 | 36.3 ± 31.2 | |
| Subtotals/averages | 42.2 | 2.7 ± 1.6 | 6.5 ± 3.8 | 1.9 ± 1.0 | 9.4 ± 6.3 | |
| Cyanidin (cy) | cy-3-Galactoside | 6.7 | 1.1 ± 0.2 | 16.6 ± 2.9 | 3.9 ± 1.0 | 21.0 ± 3.9 |
| cy-3-Glucoside | 8.4 | 1.3 ± 0.1 | 15.8 ± 1.3 | 4.0 ± 1.0 | 20.5 ± 2.3 | |
| cy-3-Arabinoside | 7.9 | 1.0 ± 0.3 | 12.1 ± 3.3 | 2.8 ± 0.9 | 15.4 ± 4.2 | |
| cy-3-(6″-Acetoyl)-glucoside | 1.6 | 0.4 ± 0.1 | 22.4 ± 6.3 | 4.0 ± 1.2 | 27.0 ± 7.5 | |
| Subtotals/averages | 24.6 | 3.9 ± 0.7 | 15.9 ± 2.8 | 3.6 ± 1.0 | 20.6 ± 4.4 | |
| Malvidin (mal) | mal-3-Galactoside | 21.5 | 2.5 ± 1.1 | 11.7 ± 5.2 | 2.5 ± 1.0 | 14.9 ± 6.2 |
| mal-3-Glucoside | 23.6 | 3.7 ± 0.3 | 15.8 ± 1.4 | 3.6 ± 0.1 | 20.1 ± 1.5 | |
| mal-3-Arabinoside | 8.6 | 3.1 ± 1.7 | 36.9 ± 20.2 | 9.5 ± 6.1 | 47.0 ± 26.3 | |
| mal-3-(6″-Acetoyl)- galactoside |
2.2 | 0.5 ± 0.1 | 20.6 ± 3.3 | 4.8 ± 1.5 | 25.8 ± 4.8 | |
| mal-3-(6″-Acetoyl)- glucoside |
6.2 | 1.3 ± 0.1 | 20.4 ± 0.7 | 4.6 ± 0.8 | 25.5 ± 1.5 | |
| Subtotals/averages | 62.1 | 10.6 ± 3.3 | 17.1 ± 5.31 | 4.2 ± 1.3 | 22.6 ± 7.5 | |
| Petunidin (pt) pt-3-Galactoside | 13.2 | 1.5 ± 0.4 | 11.7 ± 2.9 | 3.3 ± 0.3 | 15.7 ± 3.2 | |
| pt-3-Arabinoside | 4.4 | 0.5 ± 0.2 | 11.9 ± 3.5 | 2.5 ± 0.8 | 14.8 ± 4.3 | |
| pt-3-(600-Acetoyl)-glucoside | 2.3 | 0.3 ± 0.1 | 11.5 ± 2.8 | 2.7 ± 1.1 | 14.4 ± 3.9 | |
| Subtotals/averages | 19.9 | 2.1 ± 0.7 | 10.4 ± 3.5 | 3.0 ± 0.5 | 14.6 ± 3.4 | |
| Peonidin (pn) | pn-3-Galactoside | 3.0 | 0.5 ± 0.1 | 16.7 ± 3.3 | 3.3 ± 1.0 | 19.6 ± 3.4 |
| Totals/cumulative averages | 152.0 t | 17.6 ± 0.5 t | 11.6 ± 0.3 a | 3.3 ± 0.8 a | 15.5 ± 1.1 a |
BA = bioaccessibility; I.E. = ileal efflux; ACN input = mg per 500 mg of blueberry polyphenol-rich extract; BA amt. of ACN = bioaccessible amount of ACN;
= total ACN input or total bioaccessible amount of ACN;
= Cumulative averages in respective columns. Data are expressed as the mean ± range from two TIM-1 experiments.
Table 2.
Bioaccessibility of blueberry anthocyanins in fed state conditions.
| Aglcyone | Anthocyanin (ACN) | ACN input (mg) |
ACN BA (mg) |
Total ACN BA as % of input | ACNs in I.E. as % of input | ACN recovery as % of input |
|---|---|---|---|---|---|---|
| Delphinidin (dp) |
dp-3-Galactoside | 13.1 | 1.3 ± 0.5 | 9.7 ± 3.6 | 1.6 ± 1.1 | 12.5 ± 4.9 |
| dp-3-Glucoside | 16.8 | 1.6 ± 0.6 | 9.7 ± 3.5 | 1.6 ± 1.1 | 12.4 ± 4.9 | |
| dp-3-Arabinoside | 8.9 | 0.8 ± 0.3 | 9.3 ± 3.4 | 1.6 ± 1.2 | 12.05 ± 4.8 | |
| dp-3-(6″-Acetoyl)-glucoside | 3.4 | 0.4 ± 0.2 | 11.9 ± 5.3 | 2.2 ± 0.8 | 15.2 ± 8.6 | |
| Subtotals/averages | 42.2 | 4.1 ± 1.5 | 9.7 ± 3.6 | 1.7 ± 1.1 | 12.5 ± 5.0 | |
| Cyanidin (cy) | cy-3-Galactoside | 6.7 | 0.9 ± 0.4 | 14.1 ± 5.3 | 2.3 ± 0.7 | 17.7 ± 6.1 |
| cy-3-Glucoside | 8.4 | 1.2 ± 0.5 | 14.6 ± 5.5 | 2.4 ± 0.7 | 18.4 ± 6.4 | |
| cy-3-Arabinoside | 8.0 | 1.0 ± 0.4 | 12.8 ± 4.8 | 2.1 ± 0.8 | 16.1 ± 6.0 | |
| cy-3-(6″-Acetoyl)-glucoside | 1.6 | 0.3 ± 0.2 | 17.3 ± 9.9 | 2.2 ± 1.1 | 20.2 ± 11.3 | |
| Subtotals/averages | 24.8 | 3.4 ± 1.5 | 13.9 ± 6.0 | 2.3 ± 0.9 | 17.4 ± 7.1 | |
| Malvidin (mal) | mal-3-Gal | 21.5 | 3.7 ± 1.3 | 17.0 ± 5.8 | 2.6 ± 0.8 | 21.0 ± 8.0 |
| mal-3-Glu | 23.6 | 3.9 ± 0.1 | 16.4 ± 0.0 | 3.2 ± 0.1 | 21.4 ± 0.4 | |
| mal-3-ara | 8.6 | 1.3 ± 0.5 | 15.0 ± 6.0 | 2.6 ± 0.4 | 18.9 ± 6.6 | |
| mal-3-(6″-Acetoyl)- galactoside |
2.2 | 0.4 ± 0.2 | 17.1 ± 8.3 | 2.4 ± 0.9 | 21.7 ± 9.9 | |
| mal-3-(6″-Acetoyl)-glucoside | 6.2 | 1.0 ± 0.4 | 16.6 ± 6.3 | 2.6 ± 0.8 | 20.6 ± 7.4 | |
| Subtotals/averages | 62.1 | 10.3 ± 2.4 | 16.6 ± 3.9 | 2.8 ± 0.5 | 21.0 ± 4.7 | |
| Petunidin (pt) | pt-3-Gal | 13.2 | 1.3 ± 1.0 | 9.7 ± 7.8 | 1.7 ± 1.2 | 12.2 ± 9.2 |
| pt-3-Ara | 4.4 | 1.3 ± 0.5 | 28.9 ± 10.7 | 4.5 ± 1.5 | 36.3 ± 13.7 | |
| pt-3-(6″-Acetoyl)-glucoside | 2.3 | 0.3 ± 0.2 | 13.5 ± 6.6 | 1.6 ± 0.6 | 15.9 ± 6.6 | |
| Subtotals/Averages | 19.9 | 2.9 ± 1.7 | 14.6 ± 8.5 | 2.4 ± 1.2 | 18.5 ± 10.6 | |
| Peonidin (pn) | pn-3-Galactoside | 3.0 | 0.6 ± 0.1 | 18.4 ± 2.7 | 3.0 ± 1.5 | 23.9 ± 5.4 |
| Totals/cumulative Averages | 152.0 t | 23.1 ± 5.5 t | 15.2 ± 3.6 a | 2.3 ± 0.8 a | 18.8 ± 4.7 a | |
BA = bioaccessibility; I.E. = ileal efflux; ACN input = mg per 500 mg of blueberry polyphenol-rich extract; BA amt. of ACN = bioaccessible amount of ACN;
= total ACN input or total bioaccessible amount of ACN;
= Cumulative averages in respective columns. Data are expressed as the mean ± range from two TIM-1 experiments.
Bioaccessibility of individual anthocyanins did not correlate to the absolute amount of anthocyanin in the extract. For example, in the fasted state, malvidin-3-arabinoside was two times more bioaccessible (36.9%) than the most abundant anthocyanin in the extract, malvidin-3-glucoside (15.8%), even though it was present at about one third the concentration of the latter (8.6 mg vs. 23.6 mg in 0.5 g of extract). Similarly, cyanidin-3-(6″-acetoyl)-glucoside was present at low levels in the extract (1.6 mg per 0.5 g extract), but had relatively high levels of bioaccessibility; 22.4% in fasted state and 17.3% in fed state.
The levels of anthocyanins detected in the ileal efflux ranged from 1.3% to 9.5% in fasted state (Table 1) and 1.6–4.5% in fed state (Table 2) and the cumulative average percentage of anthocyanins in the ileal efflux was 3.3% and 2.3% for fasted and fed states, respectively. This indicates that low amounts of intact anthocyanins are available for colonic metabolism or bioconversion in either state. Total anthocyanin recovery ranged from 6.1% to 47.0% of input in the fasted state and 12.4–36.3% of input in the fed state (Tables 1 and 2), and cumulative average percentage of anthocyanins recovered were similar for fasted (15.5%) and fed (18.8%) states.
When considered by group, the malvidin-containing anthocyanins had the highest relative bioaccessibility on average (17.1% of intake), followed by cyanidin (15.9%), petunidin (10.4%) and delphinidin-containing anthocyanins (6.5%). This same ordering of anthocyanin groups was observed for the percentages of anthocyanins in the ileal efflux and the anthocyanins recovered as percentage of input. The malvidins were the most bioaccessible group with the greatest absolute anthocyanin amount (62 mg); however, the recovery of the delphinidin anthocyanins (9.4%) was similar to the recovery of the petunidin anthocyanins (14.6%) even though the delphinidins were present at greater than twice the amount in the original extract. The cumulative average percentage of all bioaccessible anthocyanins was similar for fasted (11.6%) and fed (15.2%) states.
The bioaccessibility data for anthocyanins was regrouped and analysed in terms of the type of sugar conjugation (Table 3). Anthocyanins containing acetyl groups had the highest level of bioaccessibility in both the fasted (20.1%) and fed states (15.3%). In comparison, galactosides had the lowest levels bioaccessibility (10.3% in fasted and 7% in fed states), whilst glucosides (11.9% in fasted and 13.8% in fed states) and arabinosides (16.2% in fasted and 14.7% in fed states) showed intermediate levels (Table 3). Hydrolysis of the sugars from anthocyanins results in unstable anthocyanidins that are degraded to protocatechuic, syringic, vanillic and 4-hydroxybenzoic acids (Fleschhut, Kratzer, Rechkemmer, & Kulling, 2006). These phenolic acids were detected in TIM-1 samples, but amounts were small and not quantified.
Table 3.
Bioaccessibility of blueberry anthocyanins grouped by sugar.
| Sugar | ACN input (mg) | ACN BA (mg) |
ACN BA as % of input |
||
|---|---|---|---|---|---|
| FASTED | FED | FASTED | FED | ||
| Glucosides | 48.9 | 5.8 ± 0.7 | 6.8 ± 1.2 | 11.9 ± 1.5 | 13.8 ± 2.4 |
| Galactosides | 57.6 | 5.9 ± 2.8 | 4.1 ± 3.1 | 10.3 ± 4.8 | 7.0 ± 5.4 |
| Arabinosides | 29.9 | 4.8 ± 2.3 | 4.4 ± 1.7 | 16.2 ± 7.6 | 14.7 ± 5.6 |
| Acyl-sugars | 15.7 | 3.3 ± 1.1 | 2.4 ± 1.1 | 20.1 ± 7.1 | 15.3 ± 6.8 |
ACN = anthocyanins; BA = bioaccessibility; ACN input = 500 mg of blueberry polyphenol-rich extract. Data are expressed as the mean ± range from duplicate TIM-1 experiments performed in fasted or fed conditions.
3.2. Comparison of anthocyanin bioaccessibility from blueberry juice and blueberry polyphenol-enriched DSF
The bioaccessibility of anthocyanins were compared after feeding TIM-1 blueberry juice (n = 3 independent TIM-1 runs) or blueberry polyphenol-enriched DSF (n = 4 independent TIM-1 runs), each delivering 156 mg of total monomeric anthocyanins. Analysis of the data obtained for the jejunum and ileum compartments as well as the ileal efflux at the four time points, showed that mean anthocyanin bioaccessibility as percentage of input was comparable for blueberry juice and blueberry polyphenol-enriched DSF (Fig. 3A and B, top), except at 3 h the percentage of anthocyanins in the ileum (t-test, p = 0.024) and ileal efflux (t-test, p = 0.006) was significantly higher for blueberry polyphenol-enriched DSF (Fig. 3B, top).
Fig. 3.
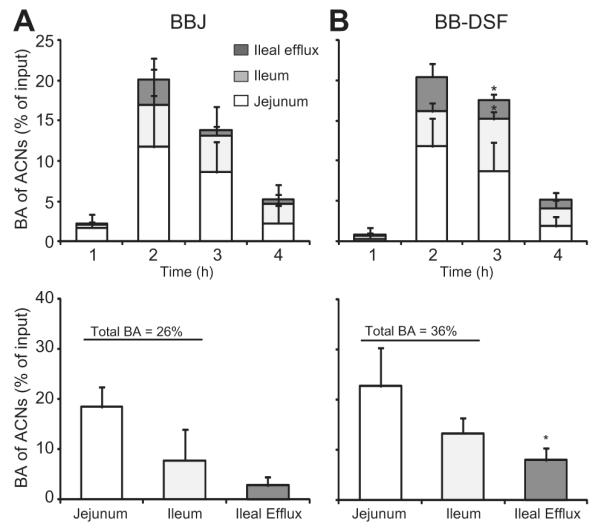
Comparison of anthocyanin bioaccessibility from blueberry juice (BBJ) or blueberry polyphenol-enriched DSF (BB-DSF). TIM-1 was fed BBJ (n = 3 experiments) or BB-DSF (n = 4 experiments) each delivering 156 mg of anthocyanins (ACNs). The pH differential method was used to quantify total monomeric anthocyanins in samples collected from jejunum, ileum and ileal efflux at 1, 2, 3 and 4 h after start of digestion. Anthocyanin bioaccessibility (BA) as a percentage of anthocyanin input was determined in jejunum (white bars) and ileum (light grey bars) samples at all time points and represented total BA. Anthocyanins in the ileal efflux (dark grey bars) as a percentage of input were also measured and represent the percentage of compound that could be delivered to the colon. A. and B. (top): Mean BA of ACNs (% of input) for each compartment and standard deviation (SD) are presented as stacked bar graphs for each time point. A. and B. (bottom): Mean BA of ACNs (% of input) over the 4 hour time period for each compartment. t-Tests were performed to compare BA of ACNs from BBJ and BB-DSF in corresponding compartments; *p = 0.025.
Anthocyanin bioaccessibility as a percentage of input was calculated across the four time points for the jejunal and ileal compartments (Fig. 3A and B, bottom). Anthocyanin bioaccessibility in jejunal samples was 22.7 ± 7.7% for blueberry polyphenol-enriched DSF compared to 18.4 ± 4.1% for blueberry juice (t-test, p = 0.43). In ileal samples, anthocyanin bioaccessibility was 13.3 ± 3.1% for blueberry polyphenol-enriched DSF and 7.9 ± 6.2% for blueberry juice (t-test, p = 0.18). Total bioaccessibility of anthocyanins was 36.0 ± 10.4% for digested blueberry polyphenol-enriched DSF and 26.3 ± 10.3% for blueberry juice (t-test, p = 0.27).
A 2.8-fold greater percentage of anthocyanins (t-test, p = 0.025) were present in the ileal efflux of blueberry polyphenol-enriched DSF (8.0 ± 2.4%) compared to blueberry juice (2.8 ± 1.7%), indicating that when sorbed to a protein-rich matrix, greater amounts of anthocyanins may be delivered to the colon for further biotrans-formation/metabolism. The mean percentage of anthocyanins measured in residues was 15.6 ± 8.3% for blueberry polyphenol-enriched DSF and 7.6 ± 0.8% for blueberry juice (t-test, p = 0.17). The total percentage of anthocyanin recovered or mass balance was calculated to be 63.4 ± 20.4% for blueberry polyphenol-enriched DSF and 36.7 ± 11.2% for blueberry juice (t-test, p = 0.12). Whilst not significantly different at p < 0.05, the higher percentage of recovery obtained for anthocyanins sorbed to DSF suggest that anthocyanins bound to a high protein matrix are protected during transit through the upper GI tract allowing greater amounts to be available for delivery to the colon.
4. Discussion
TIM-1 simulates many features of the human GI system and is useful to track stability and intestinal absorption/bioaccessibility of ingested compounds; however, it does have limitations. For example, the dialysis/filtering in TIM-1 model does not fully capture complex molecular events that occur in the endothelial cells lining the human intestinal tract, nor does the model contain the microorganisms present in the upper GI tract. Nevertheless, TIM-1 enabled the assessment of the impact of different food matrices on anthocyanin bioaccessibility between initial ingestion and prior to colonic delivery, studies difficult to perform in vivo.
Compared to the fasting state conditions, the presence of a high fat meal selectively altered the bioaccessibility of six out of seventeen blueberry anthocyanins quantified by HPLC, but did not have any substantial impact on the amount of anthocyanins in the ileal efflux. Bioaccessibility of malvidin-3-glucoside was ~16% in both fasted and fed states, whilst for delphinidin-3-glucoside bioaccessibility was 4.3% and 9.7% in fasted and fed states, respectively (Tables 1 and 2). The lower bioaccessibility of delphinidin-3-glucoside is consistent with studies that demonstrated its lower bioactivity, urine and plasma concentrations and transport efficiency across cell membranes. Orally administered malvidin-3-glucoside, but not delphinidin-3-glucoside, was shown to be hypoglycemic in mice with diet-induced diabetes (Grace et al., 2009). Delphinidin-3-glucoside had lower transport efficiency in Caco-2 human intestinal cells compared to malvidin-3-glucoside (Yi et al., 2006). Investigation of urinary excretion of anthocyanin glycosides from four different berries showed lower levels of delphinidins than malvidins (McGhie, Ainge, Barnett, Cooney, & Jensen, 2003), and in a study of bilberry anthocyanin bioavailability in rats, plasma clearance of malvidin anthocyanins was slower than delphinidins (Ichiyanagi, Shida, Rahman, Hatano, & Konishi, 2006). It has been suggested that the higher number of free hydroxyl groups on delphinidin-3-glucoside may contribute to its lower bioavailability. Delphinidins have 5 free hydroxyl units contained on the primary ring structure making them more prone to hydrolysis when compared to malvidins, which only contain 3 hydroxyl units (Fig. 1). Malvidins also contain 2 methoxy units that are not present in delphinidins, which may contribute to their enhanced stability and protection from hydrolysis.
In addition to anthocyanidin structure, sugar conjugation affects anthocyanin absorption and excretion in vivo. Regardless of the presence of the high fat meal, anthocyanins with acyl-sugars groups had the highest bioaccessibility, consistent with acylated anthocyanins being stable at both acidic and neutral pH, followed by arabinosides, glucosides and galactosides (Table 3). This correlates with previous studies showing that anthocyanins with glucose groups to have higher transport efficiency in Caco-2 cells than those having galactose groups (Yi et al., 2006). Anthocyanins conjugated with arabinose had similar or higher levels of TIM-1 bioaccessibility compared to other sugar conjugates; however, in vivo studies showed that recovery of arabinose conjugates in urine was lower than anthocyanins conjugated with other sugars (McGhie et al., 2003). In rats orally administered bilberry extract, the anthocyanins conjugated with galactose (galactosides) attained the highest concentration in plasma whilst arabinosides had the lowest (Ichiyanagi et al., 2006). TIM-1 data for anthocyanins conjugated with arabinose did not correlate with their metabolic fate in vivo, indicating arabinose conjugates have reduced bioavailability or greater chemical reactivity in vivo.
Direct evidence of the absorption of anthocyanins from the stomach has been demonstrated in rats (Passamonti, Vrhovsek, Vanzo, & Mattivi, 2003), which cannot be addressed using the TIM-1 since the model is based on the human example where very few compounds, such as uncharged lipid soluble drugs, have shown limited absorption in the stomach (Hogben, Tocco, Brodie, & Schanker, 1959). Absorption of intact anthocyanins in humans is thought more likely to occur in the small intestine than in the stomach, which has a thicker mucosa than that of rats. However, in vitro studies demonstrated anthocyanin glycosides and diglycosides to be competitive inhibitors and possible substrates of bilitranslocase transporters present in epithelial cells of gastric mucosa of humans, providing a possible mechanism of anthocyanin absorption from the stomach (Passamonti et al., 2009).
Polyphenols have a natural affinity for proteins (Bandyopadhyay, Ghosh, & Ghosh, 2012) and protein-rich food matrices, such as defatted soy flour (DSF) can sorb, concentrate and stabilize anthocyanins and other polyphenols from berry juices (Roopchand, Grace et al., 2012; Roopchand, Kuhn et al., 2012; Roopchand et al., 2013). Oral administration of blueberry or grape polyphenol-enriched DSF had a hypoglycemic effect in mice (Roopchand, Grace et al., 2012; Roopchand, Kuhn et al., 2012; Roopchand et al., 2013) indicating that the polyphenols complexed to proteins are bioactive; however, anthocyanin release from the protein-rich food matrix after entering the physiochemical environments of the GI tract has not previously been investigated. TIM-1 digestion experiments with blueberry juice or blueberry polyphenol-enriched DSF delivering equivalent amounts of anthocyanins (156 mg) demonstrated that anthocyanins trended towards being more bioaccessible when bound to the protein matrix (36% of input) compared to anthocyanins in blueberry juice (26% of input; t-test, p = 0.27). The ileal efflux obtained from blueberry polyphenol-enriched DSF digestion contained almost three times the level of anthocyanins than the same samples from blueberry juice therefore a greater amount of anthocyanins would theoretically be delivered to the colon for biotransformation. A higher percentage of anthocyanins were recovered from the TIM-1 system when sorbed to DSF (63%) compared to when unbound in blueberry juice (36%). These data suggest that sorption to DSF protected anthocyanins during transit through the upper GI tract allowing greater amounts to be available for delivery to the colon, although the difference was statistically significant only at p = 0.12. Further in vivo experimentation will be needed to determine whether the higher TIM-1 bioaccessibility of anthocyanins complexed to protein translates to higher in vivo anthocyanin bio-availability. Quercetin plasma concentrations were five times higher when quercetin was consumed as quercetin-enriched cereal bars compared to capsules containing equivalent amounts of quercetin (130 mg), indicating that the food matrix can enhance polyphenol bioavailability in human subjects (Egert et al., 2012). Understanding anthocyanin bioaccessibility/absorption, metabolism and bioavailability as well as the impact of different food matrix components on these factors will enable the development of dietary recommendations and food products that provide optimal therapeutic bioactivity.
Acknowledgments
DR designed polyphenol extract TIM-1 experiments, assisted with TIM-1 experiments, analysed data and wrote the manuscript. DER designed BBJ/BB-DSF TIM-1 experiments, assisted with TIM-1 experiments, did ACN quantification, performed statistical analysis and wrote the manuscript. AO performed TIM-1 experiments. MG prepared the blueberry polyphenol-rich extract and performed HPLC analysis and quantification of individual anthocyanins. AP did HPLC analysis. RH assisted with data analysis and interpretation. IR and MAL are the principal investigators of the laboratories where the research was performed. All authors read and approved the final manuscript. This work was supported in part by Botanical Research Center grant P50AT002776-01 and NIH training grant T32 AT004094 (supporting DER) from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements.
Abbreviations
- BA
bioaccessibility
- ACN
anthocyanin
- DSF
defatted soybean flour
- TIM-1
TNO gastrointestinal model
Footnotes
Disclosure statement DER, MAL and IR have equity in Nutrasorb LLC, which has interest in developing polyphenol sorption technology.
References
- Bandyopadhyay P, Ghosh AK, Ghosh C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food & Function. 2012;3(6):592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. Journal of Nutrition. 2010;140(9):1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet S, Zeijdner E, Beyssac E, Meunier JP, Denis S, Havenaar R, et al. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharmaceutical Research. 2004;21(4):585–591. doi: 10.1023/b:pham.0000022404.70478.4b. [DOI] [PubMed] [Google Scholar]
- Blanquet-Diot S, Soufi M, Rambeau M, Rock E, Alric M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. Journal of Nutrition. 2009;139(5):876–883. doi: 10.3945/jn.108.103655. [DOI] [PubMed] [Google Scholar]
- D’Archivio M, Filesi C, Vari R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: Status and controversies. International Journal of Molecular Sciences. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert S, Wolffram S, Schulze B, Langguth P, Hubbermann EM, Schwarz K, et al. Enriched cereal bars are more effective in increasing plasma quercetin compared with quercetin from powder-filled hard capsules. British Journal of Nutrition. 2012;107(4):539–546. doi: 10.1017/S0007114511003242. [DOI] [PubMed] [Google Scholar]
- Felgines C, Krisa S, Mauray A, Besson C, Lamaison JL, Scalbert A, et al. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. British Journal of Nutrition. 2010;103(12):1738–1745. doi: 10.1017/S0007114510000061. [DOI] [PubMed] [Google Scholar]
- Felgines C, Talavera S, Texier O, Gil-Izquierdo A, Lamaison JL, Remesy C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. Journal of Agricultural and Food Chemistry. 2005;53(20):7721–7727. doi: 10.1021/jf051092k. [DOI] [PubMed] [Google Scholar]
- Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE. Stability and biotransformation of various dietary anthocyanins in vitro. European Journal of Nutrition. 2006;45(1):7–18. doi: 10.1007/s00394-005-0557-8. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular Aspects of Medicine. 2010;31(6):435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, et al. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsson A-K, Rimsten L, Alminger M, Andersson R, Aman P, Sandberg A-S. Digestion of barley porridges in a gastrointestinal model: Iron dialysability, iron uptake by Caco-2 cells and degradation of β-glucan. Journal of Cereal Science. 2005;42(2):243–254. [Google Scholar]
- Hogben CAM, Tocco DJ, Brodie BB, Schanker LS. On the mechanism of intestinal absorption of drugs. Journal of Pharmacology and Experimental Therapeutics. 1959;125(4):275–282. [PubMed] [Google Scholar]
- Ichiyanagi T, Shida Y, Rahman MM, Hatano Y, Konishi T. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. Journal of Agricultural and Food Chemistry. 2006;54(18):6578–6587. doi: 10.1021/jf0602370. [DOI] [PubMed] [Google Scholar]
- Kamonpatana K, Giusti MM, Chitchumroonchokchai C, MorenoCruz M, Riedl KM, Kumar P, et al. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chemistry. 2012;135(2):738–747. doi: 10.1016/j.foodchem.2012.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Minekus M, Havenaar R. Estimation of the bio-availability of iron and phosphorus in cereals using a dynamic in vitro gastrointestinal model. Journal of the Science of Food and Agriculture. 1997;73:99–106. [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colourants, and wines by the pH differential method: collaborative study. Journal of AOAC International. 2005;88(5):1269–1278. [PubMed] [Google Scholar]
- Lila MA. Anthocyanins and human health: An in vitro investigative approach. Journal of Biomedicine and Biotechnology. 2004;2004(5):306–313. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lila MA, Ribnicky DM, Rojo LE, Rojas-Silva P, Oren A, Havenaar R, et al. Complementary approaches to gauge the bioavailability and distribution of ingested berry polyphenolics. Journal of Agricultural and Food Chemistry. 2011;60:5763–5771. doi: 10.1021/jf203526h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Anson N, Van den Berg R, Havenaar R, Bast A, Haenen G. Bioavailability of ferulic acid is determined by its bioaccessibility. Journal of Cereal Science. 2009;49(2):296–300. [Google Scholar]
- McGhie TK, Ainge GD, Barnett LE, Cooney JM, Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. Journal of Agricultural and Food Chemistry. 2003;51(16):4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: Towards a better understanding. Molecular Nutrition & Food Research. 2007;51(6):702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- Minekus M, Jelier M, Xiao JZ, Kondo S, Iwatsuki K, Kokubo S, et al. Effect of partially hydrolyzed guar gum (PHGG) on the bioaccessibility of fat and cholesterol. Bioscience, Biotechnology, and Biochemistry. 2005;69(5):932–938. doi: 10.1271/bbb.69.932. [DOI] [PubMed] [Google Scholar]
- Minekus M, Marteau P, Havenaar R, Huis in’t Veld JHJ. A multi compartmental dynamic computer-controlled model simulating the stomach and small intestine. Alternatives to Laboratory Animals (ATLA) 1995;23:197–209. [Google Scholar]
- Nurmi T, Mursu J, Heinonen M, Nurmi A, Hiltunen R, Voutilainen S. Metabolism of berry anthocyanins to phenolic acids in humans. Journal of Agricultural and Food Chemistry. 2009;57(6):2274–2281. doi: 10.1021/jf8035116. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Terdoslavich M, Franca R, Vanzo A, Tramer F, Braidot E, et al. Bioavailability of flavonoids: A review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Current Drug Metabolism. 2009;10:369–394. doi: 10.2174/138920009788498950. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. The stomach as a site for anthocyanins absorption from food. FEBS Letters. 2003;544(1–3):210–213. doi: 10.1016/s0014-5793(03)00504-0. [DOI] [PubMed] [Google Scholar]
- Porrini M, Riso P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutrition, Metabolism & Cardiovascular Diseases. 2008;18(10):647–650. doi: 10.1016/j.numecd.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wilkes S, Rogers T, Khanal RC, Wu X, Hager TJ, et al. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. Journal of Agricultural and Food Chemistry. 2010;58(7):3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. Journal of Agricultural and Food Chemistry. 2008;56(3):647–653. doi: 10.1021/jf071993o. [DOI] [PubMed] [Google Scholar]
- Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, et al. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis) Food Chemistry. 2012;151(2):387–396. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Pouleva A, et al. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chemistry. 2012;131:1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand DE, Kuhn P, Poulev A, Oren A, Lila MA, Fridlender B, et al. Biochemical analysis and in vivo hypoglycemic activity of a grape polyphenol–soybean flour complex. Journal of Agricultural and Food Chemistry. 2012;60(36):8860–8865. doi: 10.1021/jf300232h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand DE, Kuhn P, Rojo LE, Lila MA, Raskin I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacological Research. 2013;68(1):59–67. doi: 10.1016/j.phrs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP. The impact of fruit flavonoids on memory and cognition. British Journal of Nutrition. 2010;104(Suppl. 3):S40–S47. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. Journal of Nutrition. 2010;140(10):1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenjarla S, Romasanta V, Zeijdner E, Villa R, Moro L. Release of 5-aminosalicylate from an MMX mesalamine tablet during transit through a simulated gastrointestinal tract system. Advances in Therapy. 2007;24(4):826–840. doi: 10.1007/BF02849976. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. Journal of Agricultural and Food Chemistry. 2008;56(3):642–646. doi: 10.1021/jf073113b. [DOI] [PubMed] [Google Scholar]
- Verwei M, Arkbage K, Havenaar R, van den Berg H, Witthoft C, Schaafsma G. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. Journal of Nutrition. 2003;133(7):2377–2383. doi: 10.1093/jn/133.7.2377. [DOI] [PubMed] [Google Scholar]
- Verwei M, Freidig AP, Havenaar R, Groten JP. Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. Journal of Nutrition. 2006;136(12):3074–3078. doi: 10.1093/jn/136.12.3074. [DOI] [PubMed] [Google Scholar]
- Wallace TC. Anthocyanins in cardiovascular disease. Advances in Nutrition. 2011;2(1):1–7. doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Letters. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. American Journal of Clinical Nutrition. 2012;95(4):925–933. doi: 10.3945/ajcn.111.028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radical Biology and Medicine. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. Journal of Agricultural and Food Chemistry. 2006;54(11):4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- Wu X, Cao G, Prior RL. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. Journal of Nutrition. 2002;132(7):1865–1871. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- Wu XL, Prior RL. Systematic identification and characterization of anthocyanins by HPLC–ESI-MS/MS in common foods in the United States: Fruits and berries. Journal of Agricultural and Food Chemistry. 2005;53(7):2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- Yang M, Koo SI, Song WO, Chun OK. Food matrix affecting anthocyanin bioavailability: Review. Current Medicinal Chemistry. 2011;18(2):291–300. doi: 10.2174/092986711794088380. [DOI] [PubMed] [Google Scholar]
- Yi W, Akoh CC, Fischer J, Krewer G. Absorption of anthocyanins from blueberry extracts by caco-2 human intestinal cell monolayers. Journal of Agricultural and Food Chemistry. 2006;54(15):5651–5658. doi: 10.1021/jf0531959. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Martin A, Joseph JA. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radical Biology and Medicine. 2000;29(1):51–60. doi: 10.1016/s0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]