Abstract
The MRI hypoxia marker trifluoro-misonidazole (TFMISO) [1-(2-nitro-1H-imidazol-1-yl)-3-(2,2,2-trifluoroethoxy)propan-2-ol] was successfully labeled with 18F to expand its role into a bimodal PET/MRI probe. 18F-Labeling was achieved via a 3-step procedure in which 2,2,2-[18F]trifluoroethyl p-toluenesulfonate prepared by 18F-19F exchange served as the [18F]trifluoroethylating agent. The O-[18F]trifluoroethylation reaction proceeded efficiently to give the intermediate 1,2-epoxy-3-(2,2,2-[18F]trifluoroethoxy)propane, with approximately 60% of 18F incorporated from the tosylate precursor, which was condensed with 2-nitroimidazole to yield [18F]TFMISO. Approximately 40% of the [18F]trifluoroethyl tosylate precursor was converted into the final product. In stark contrast, 2,2,2-[18F]trifluoroethyl iodide failed to produce [18F]TFMISO, giving instead 1,1-[18F]difluoro-2-iodoethoxy and 1-[18F]fluoro-2-iodovinyloxy analogs of [18F]TFMISO. Thus, this investigation has identified 2,2,2-[18F]trifluoroethyl tosylate as an excellent [18F]trifluoroethylating agent, which can convert efficiently an alcohol into the corresponding [18F]trifluoroethyl ether.
Keywords: 18F-labeled trifluoromisonidazole ([18F]TFMISO); bimodal MRI and PET hypoxia marker; 2,2,2-[18F]trifluoroethyl tosylate; O-[18F]trifluoroethylation
1. Introduction
Non-invasive imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) may be used to visualize the in vivo distribution of hypoxic regions within a tumor based on hypoxia-targeting tracers1,2. The extent of tumoral hypoxia provides information that may assist in predicting the response to cancer therapies2,3. In vivo MRI or PET imaging of hypoxia has also been suggested as a tool for determining radioresistant hypoxic sub-volumes within the tumor suitable for biologically-based intensity modulated radiotherapy (IMRT)4,5. For PET hypoxia imaging, the most frequently employed radiotracers are 2-nitroimidazole analogs labeled with the positron emitter 18F such as [18F]FMISO [1-(2-nitroimidazolyl)-3-[18F]fluoro-2-propanol] and [18F]FAZA [1-(5-[18F]fluoro-5-deoxy-ß-D-arabinofuranosyl)-2-nitroimidazole]. Several tri- and hexa-fluoro-2-nitroimidazole analogs have been developed as hypoxia probes for MRI, the localization of which in the living body can be detected and traced by 19F-magnetic resonance spectroscopy (MRS). These are being extensively tested and studied in animal tumor models and in humans6-15.
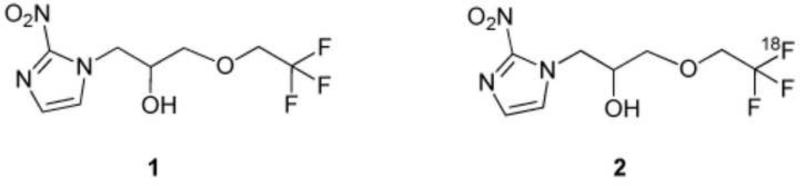
TFMISO [1-(2-nitro-1H-imidazol-1-yl)-3-(2,2,2-trifluoroethoxy)propan-2-ol] (1, Figure 1), is an FMISO analog which has three magnetically equivalent 19F atoms in the molecule and was originally developed as a radiosensitizer for cancer radiation therapy6-9. Chapman et al, who studied the in vivo behavior of 14C-labeled TFMISO in EMT-6 tumor-bearing mice, suggested the potential of TFMISO as an in vivo hypoxia marker for MRI as well as for PET if 18F-labeling of TFMISO could be done16: the three 19F atoms of the CF3 group in the molecule make it possible to detect the compound by MRS from outside of the body, and [18F]TFMISO (2, Figure 1) makes PET imaging feasible as well. The first in vivo 19F-MRS detection of TFMISO in a tumor was reported by Raleigh et al17. More recently, in vivo MR spectroscopic images of the TFMISO distribution have been acquired in two preclinical tumor models18,19. Labeling of the CF3 group in TFMISO with 18F transforms the MR spectroscopic imaging agent into a PET radiotracer and, thus, TFMISO becomes a bimodal probe for 19F-MRI as well as 18F-PET.
Figure 1.
Chemical structures of TFMISO and [18F]TFMISO
It is well recognized that labeling of a trifluoroalkyl group in a complex molecule with 18F is not easily achieved, and normally requires a multi-step procedure. In the past, 18F-labeling of the trifluoroethyl moiety in oxaquazepam, a benzodiazepine receptor antagonist, has been achieved using 2,2,2-[18F]trifluoroethyl triflate, which was prepared via a 3 step procedure20. The trifluoropropyl moiety of EF3, a hypoxia marker and a potential bimodal probe, has also been labeled with 18F via a 5-step method using [18F]-poly(hydrogen fluoride)pyridinium as the precursor21. Following the prior work reported in the literature, we attempted to label TFMISO with 18F via four approaches. Through our evolving approaches, which frequently gave unexpected outcomes, we have discovered a novel and versatile method for 18F-labeling of TFMISO.
2. Approaches
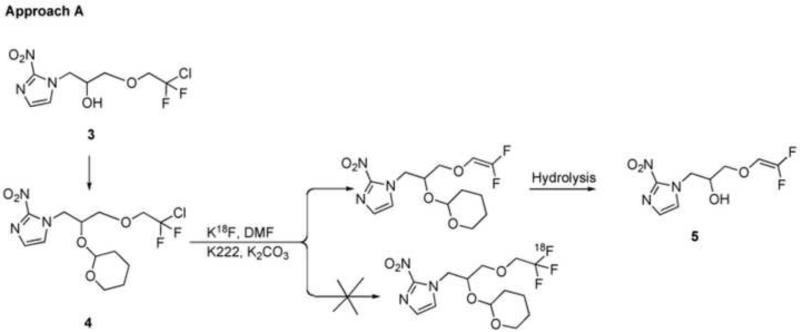
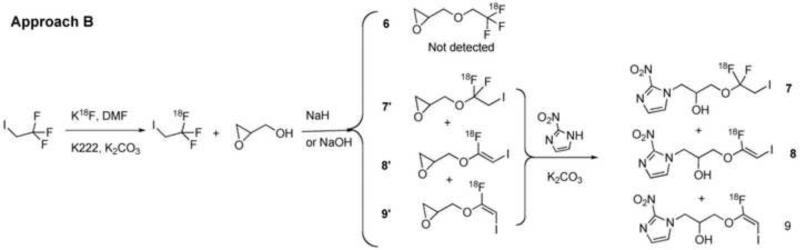
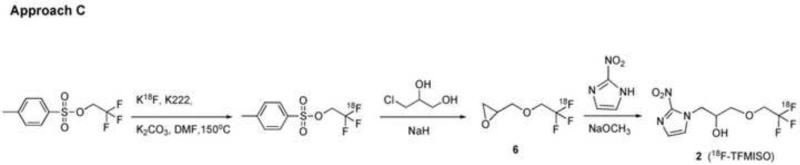
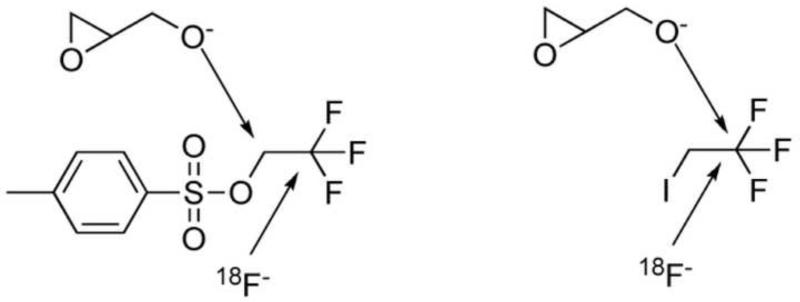
The first approach (Approach A, Scheme 1) is to convert a 2-halo-2,2-difluoroethoxy (XCF2CH O-, X=Cl/Br) analog of TFMISO (3) into [18F]TFMISO (2) via halogen-18F exchange. This approach seemed like an ideal one because it is simple and can be done in one pot. In addition, this is a no-carrier-added method, which can produce the PET tracer with high specific activity. The second and third approaches are based on O-[18F]trifluoroethylation of an alcohol, glycidol or 3-chloro-1,2-propanediol, with 2,2,2-[18F]trifluoroethyl iodide (Approach B, Scheme 2) or 2,2,2-[18F]trifluoroethyl p-toluenesulfonate (Approach C, Scheme 3). The resulting [18F]trifluoroethyl ether intermediate is then reacted with 2-nitroimidazole to produce [18F]TFMISO (2). The trifluoroethylating agents, the iodide and the tosylate, are labeled via 18F-19F exchange. The fourth approach (Approach D, Scheme 5) is designed to combine the second and third steps of Approach C, i.e. O-[18F]trifluoroethylation of the alcohol and the reaction between [18F]trifluoroethyl ether intermediate and 2-nitroimidazole, into one step by using 3-(2-nitroimidazolyl)-1,2-propanediol (10), the primary alcohol of which is the target of O-[18F]trifluoroethylation with 2,2,2-[18F]trifluoroethyl tosylate.
Scheme 1.
Scheme 2.
Scheme 3.
Scheme 5.
3. Results
3.1 Approach A (Scheme 1)
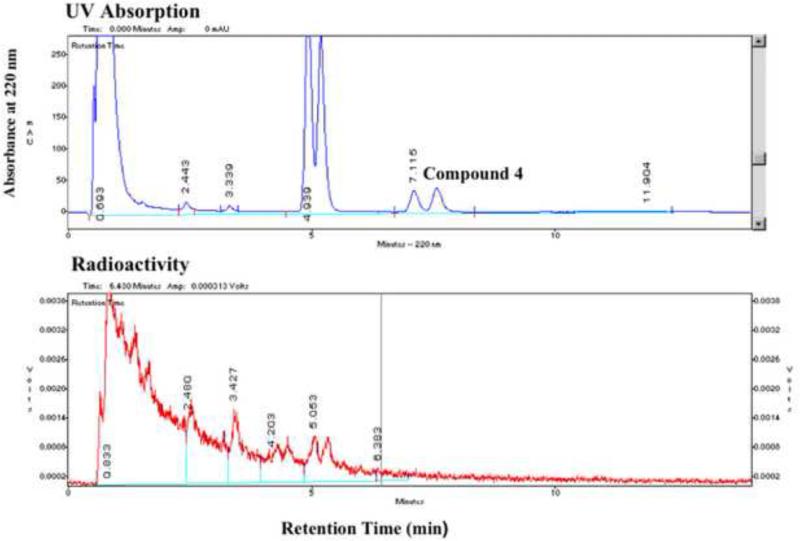
Analytical HPLC data of the Cl-18F substitution reaction showed that a) after heating at 80-130°C, most of the OH-protected ClCF2-precursor (4) was converted into a more hydrophilic compound and only a small portion of the precursor was left intact (Figure 2a), and b) after removal of the OH-protection, only a trace amount of [18F]TFMISO, the retention time of which agreed with that of authentic TFMISO by co-injection, was produced (Figure 2b). The major unlabeled by-product was isolated by semi-preparative HPLC and was found to be the elimination product (5) (Scheme 1) by mass spectrometry and 1H-/19F-NMR. 1H-NMR (CDCl3), δ 3.74 (dd, 5.5 Hz, 10.2 Hz, 1H), 3.85 (dd, 4.0 Hz, 10.2 Hz, 1H), 4.27 (m, 1H), 4.41 (dd, 7.9 Hz, 14.0 Hz), 4.74 (dd, 3.4 Hz, 14.0 Hz), 5.77 (dd, 3.2 Hz, 15.4 Hz, 1H), 7.15 (s, 1H), 7.21 (s, 1H); 19F-NMR (CDCl3), δ 98.67 (d, 80 Hz), 118.76 (d, 80 Hz); MS (ESI); 356 ([M+Na]+); 346 ([M+Cl]-). The use of the OH-protected BrCF2-precursor instead of 4 did not change the outcome. Compound 5 was also the major product of the reaction between the ClCF2-precursor and tetrebutylammonium fluoride.
Figure 2a.
HPLC analysis of a reaction between the OH-protected ClCF2-precursor (4, Approach A) with K18F/Kryptofix in DMF after heating at 150°C for 10 minutes. The analytical HPLC was performed using a C18 column and a mobile phase of acetonitrile and water (40/60) at a flow rate of 2 mL/min (for details see section 6.1. General Methods). Compound 4, which is a mixture of 2 diastereomers, appears as a double peak in the HPLC chromatogram. The major product of the reaction, which appears as a double peak at a retention time of approximately 5 min, is also a mixture of 2 diastereomers (see Scheme 1). (Vertical axes are in arbitrary units.)
Figure 2b.
HPLC analysis of the reaction mixture co-injected with authentic TFMISO after removal of the OH-protection group. The analytical HPLC was performed using a C18 column and a mobile phase of acetonitrile and water (20/80) at a flow rate of 2 mL/min (for details see section 6.1. General Methods). (Vertical axes are in arbitrary units.)
3.2 Approach B (Scheme 2)
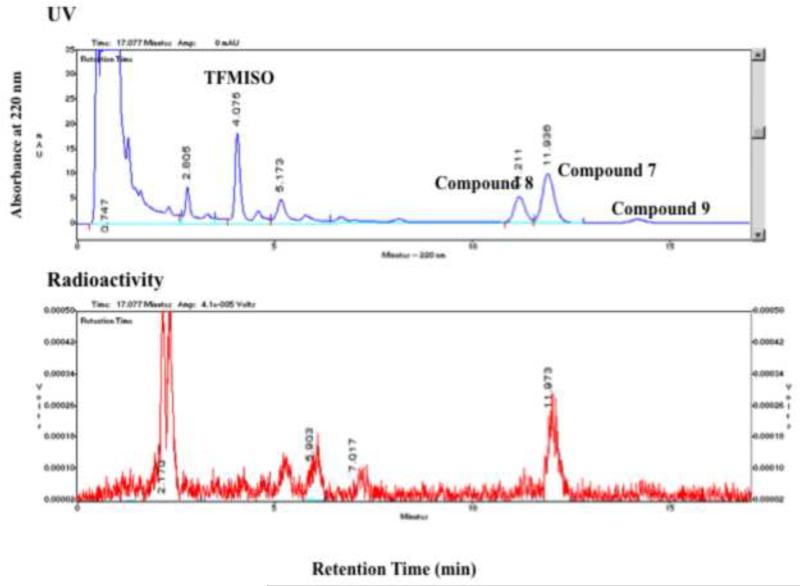
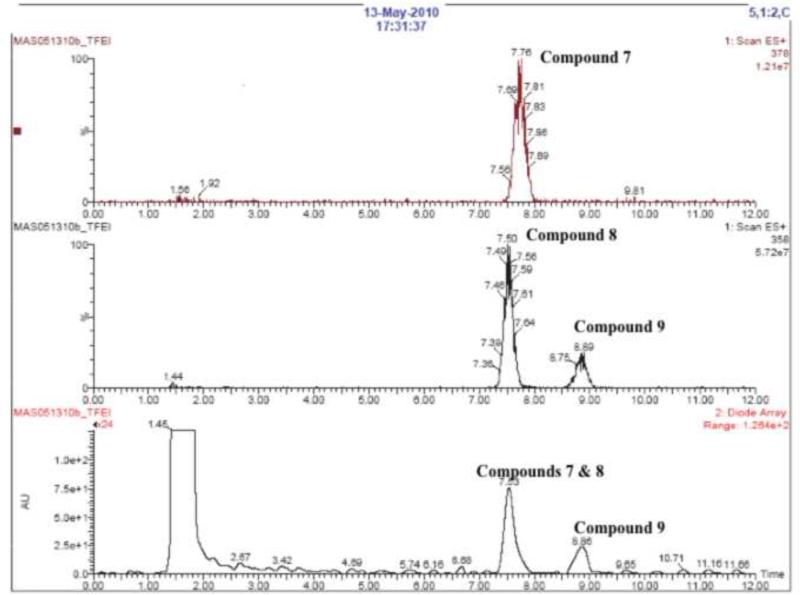
Labeling of 2,2,2-trifluoroethyl iodide with 18F via 18F-19F exchange proceeded in a labeling efficiency of approx 90-95% as reported in the literature22. After reacting 2,2,2-[18F]trifluoroethyl iodide with potassium (or sodium) glycidoxide, the intermediates were combined with 2-nitroimidazole, which resulted in 3 major products (Figure 3a). By co-injection with TFMISO, these products were found more lipophilic than TFMISO (Figure 3a). The reaction was repeated using non-radioactive 2,2,2-trifluoroethyl iodide and the final products analyzed by LC-MS. The LC-MS profile shown in Figure 3b indicates that one of the products has a mass of 378, and the other 2 have the same mass of 358, suggesting the latter 2 products to be isomers. These mass numbers correspond to these of 1-(1,1-difluoro-2-iodoethoxy)-3-(2-nitro-1H-imidazol-l-yl)propan-2-ol (7) and (E) and (Z)-1-(1-fluoro-2-iodovinyloxy)-3-(2-nitro-1H-imizdazol-1-yl)prpane-2-ol (8 and 9), respectively. These peaks were collected and further analyzed by 1H NMR and mass spectrometry. The analysis data confirmed the final products of Approach B as Compounds 7, 8 and 9. This result suggests an unusual nucleophilic substitution reaction in which the oxygen nucleophile derived from glycidol attacked on the electron-deficient carbon site of the CF3-group instead of the adjacent carbon bound to the iodine, yielding the intermediates 7’, 8’ and 9’, which led to the formation of Compounds 7, 8 and 9 as the final products. Compound 7: 1H NMR (DMF-d7): δ 3.83 (t, 10.4 Hz, 2H), 3.93 (dd, 5.2 Hz, 10.3 Hz, 1H), 3.98 (dd, 8.7 Hz, 13.9 Hz, 1H), 4.23 (m, 1H), 4.52 (dd, 8.5 Hz, 13.9 Hz, 1H), 4.79 (dd, 3.8 Hz, 13.8 Hz, 1H), 5.77 (d, 5.7 Hz, 1H), 7.21 (s, 1H), 7.67 (s, 1H). 19F NMR (CDCl3): 73.38 (d, 8.5 Hz); MS 378 ([M+H]+) and 400 (M+Na]+). Compound 8: 1H NMR (DMF-d7): δ 4.15 (dd, 5.3 Hz, 10.6 Hz, 1H), 4.19 (dd, 9.7 Hz, 10.6 Hz, 1H), 4.30 (m, 1H), 4.58 (dd, 8.8 Hz, 13.8 Hz, 1H), 4.84 (dd, 3.6 Hz, 13.8 Hz, 1H), 5.12 (d, 4.7 Hz, 1H), 5.83 (d, 5.7 Hz, 1H), 7.21 (s, 1H), 7.68 (s, 1H); 19F NMR (CDCl3): 74.60; MS 358 ([M+H]+) and 380 ([M+Na]+). Compound 9: 1H NMR (DMF-d7): δ 4.19 (dd, 5.7 Hz, 11.4 Hz, 1H), 4.20 (dd, 9.5 Hz, 11.4 Hz, 1H), 4.23 (m, 1H), 4.53 (dd, 8.2 Hz, 13.8 Hz, 1H), 4.77 (dd, 3.8 Hz, 13.9 Hz), 5.37 (t, 4.8 Hz, 1H), 5.77 (d, 5.8 Hz, 1H), 7.20 (s, 1H), 7.67 (s, 1H); 19F NMR (CDCl3): 74.40; MS 358 ([M+H]+) and 380 ([M+Na]+). The ratio between Compounds 7, 8 and 9 varied depending upon the base used for deprotonation of the alcohol.
Figure 3a.
HPLC analysis of a reaction between 2,2,2-[18F]trifluoroethly iodide and sodium glycidoxide (Approach B) co-injected with authentic TFMISO. The analytical HPLC was performed using a C18 column with a mobile phase of acetonitril and water (20/80) at a flow rate of 2 mL/min (for details see section 6.1. General Methods). (Vertical axes are in arbitrary units.)
Figure 3b.
LC-MS analysis of the products from Approach B. Top row: Detection of Compound 7 at a mass of 378. Middle row: Detection of Compounds 8 and 9 at a mass of 358. Bottom row: Detection of Compounds 7, 8 and 9 with UV absorption at 320 nm (for details see section 6.1. General Methods). (Vertical axes are in arbitrary units.)
3.3 Approach C (Scheme 3)
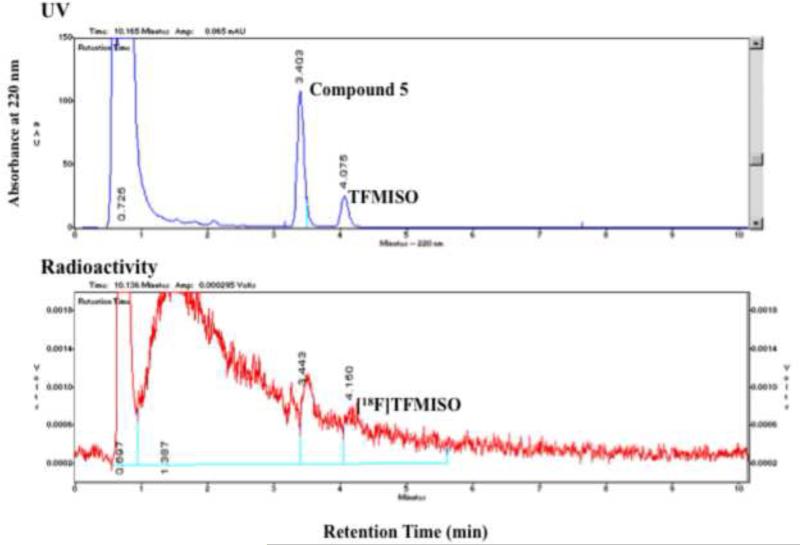
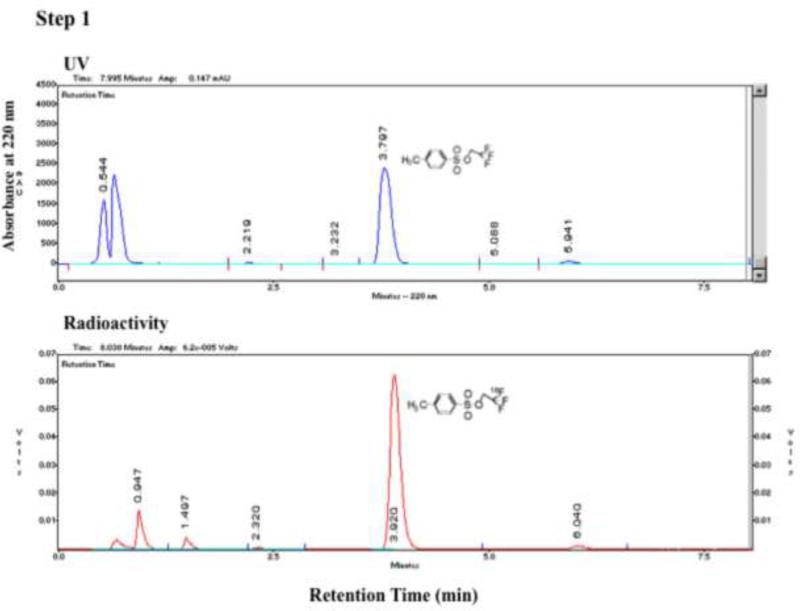
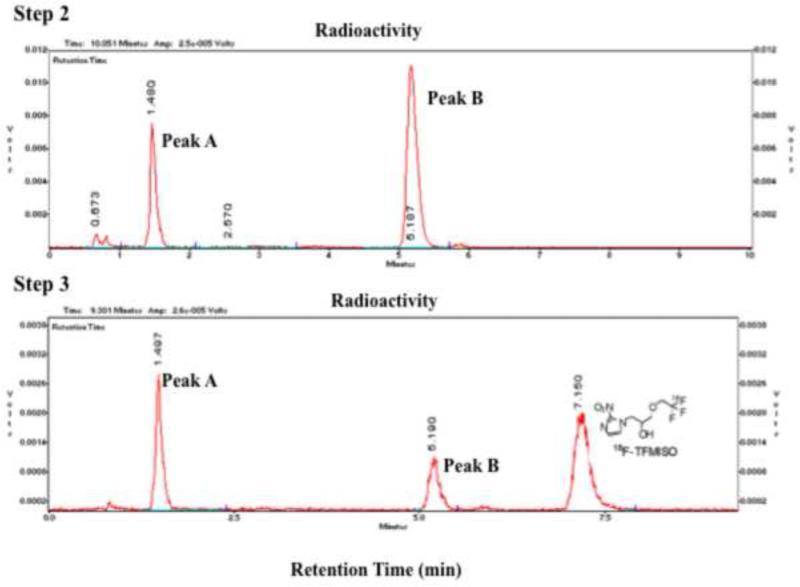
Heating 2,2,2-trifluoroethyl tosylate with 18F at 150°C in DMF in the presence of Kryptofix 222 and K2CO3 yielded 2,2,2-[18F]trifluoroethyl tosylate via 18F - 19 exchange in a surprisingly high labeling efficiency as indicated by analytical HPLC (Figure 4a). The 2,2,2-[18F]trifluoroethyl tosylate precursor formed in Step 1 was separated from the reaction mixture by extraction into ether. Approximately 30% (27±5%, n=16) of 18F was extracted into the organic layer as 2,2,2-[18F]trifluoroethyl tosylate while 40% (38±7%, n=15) remained in the aqueous layer. Step 2, in which 2,2,2-[18F]trifluoroethyl tosylate was reacted with 3-chloro-1,2-propanediol deprotonated in the presence of NaH, proceeded smoothly, and in 45-60 minutes, the 2,2,2-[18F]trifluoroethyl tosylate precursor was nearly completely converted into two 18F-labeled intermediates: one a [18F]trifluoroethoxy compound (Peak B in Figure 4b), which reacted with 2-nitroimidazole and gave [18F]TFMISO in Step 3 (Figure 4b), and the other a more hydrophilic compound (Peak A), which did not react with the imidazole. The average conversion rate from the 2,2,2-[18F]trifluoroethyl tosylate precursor into the [18F]trifluoroethoxy intermediate (Peak B) was 57±10% (n=17). In Step 3, in which the 18F-labeled intermediates from Step 2 were reacted with 2-nitroimidazole, approximately 70% (average: 66.5±16.1%, n=16) of the [18F]trifluoroethoxy intermediate (Peak B) was incorporated into [18F]TFMISO (Figure 4b). This means that approximately 40% (average: 39.6±12.3%, n=16) of the 2,2,2-[18F]trifluoroethyl tosylate precursor produced in Step 1 was converted into the desired final product (Figures 4a and 4b).
Figure 4a.
HPLC chromatograms of a Step 1 reaction of Approach C. The analytical HPLC was performed using a C18 column with a mobile phase of acetonitril and water (50/50) at a flow rate of 2 mL/min (for details see section 6.1. General Methods). (Vertical axes are in arbitrary units.)
Figure 4b.
HPLC chromatograms of Step 2 and 3 reactions of Approach C. The analytical HPLC was performed using a C18 column with a mobile phase of acetonitril and water (15/85) at a flow rate of 2 mL/min (for details see section 6.1. General Methods). (Vertical axes are in arbitrary units.)
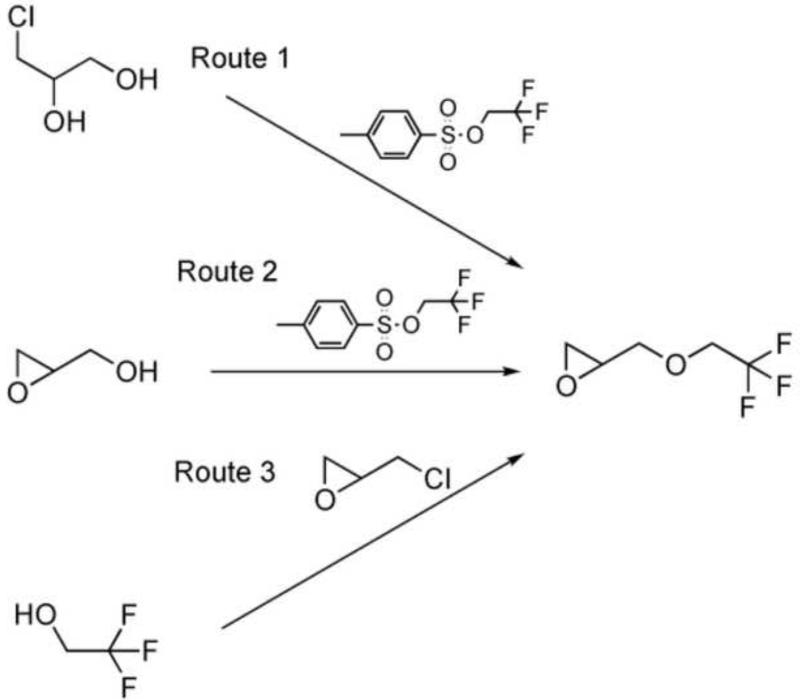
The key intermediate (Peak B), which led to the formation of [18F]TFMISO, was identified as 1,2-epoxy-3-(2,2,2-[18F]trifluoroethoxy)propane (6), based on the facts that a) the [18F]trifluoroethylation products of 3-chloro-1,2-propanediol, 3-bromo-1,2-propanediol and glycidol, appeared at the same HPLC retention time corresponding to Peak B and all afforded the same final product, [18F]TFMISO and b) 1H NMR spectra of the trifluoroethylation products of 3-chloro-1,2-propanediol and glycidol matched that of authentic 1,2-epoxy-3-(2,2,2-trifluoroethoxy)propane synthesized via a literature procedure (Scheme 4). Among the 3 alcohols, 3-chloro-1,2-propanediol appeared to give the key intermediate (6) in a higher 18F-labeling efficiency than the other two. It was also noted that the stoichiometric ratio between the diol and NaH did not significantly affect the yield of the epoxide 6: When the ratio, NaH/diol, was 1.2-1.3, 55.7±11.5 % (n=13) of 18F was incorporated into the epoxide, whereas with the ratio increased to 2, the 18F-incorporation was 61.5± 2.9 % (n=4). This suggests that the NaH promotes both O-[18F]trifluoroethylation of the diol and cyclization, and that these two events occur in succession.
Scheme 4.
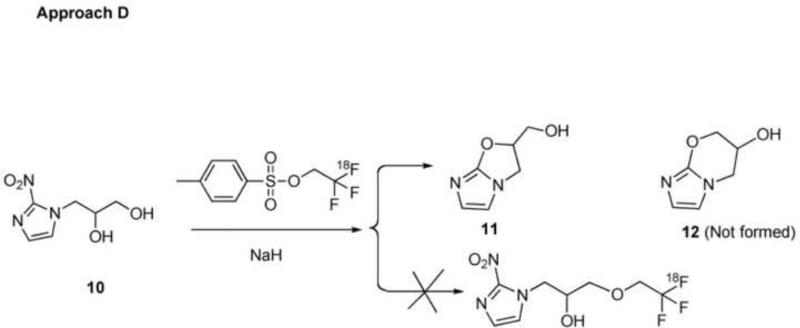
3.4 Approach D (Scheme 5)
The results of Approach C suggested the possibility of direct O-[18F]trifluoroethylation of the primary alcohol of 3-(2-nitroimidazolyl)-1,2-propanediol (10) with 2,2,2-[18F]trifluoroethyl tosylate to produce [18F]TFMISO as shown in Scheme 5, which reduces the number of the synthesis steps to 2 and the synthesis time by approximately 1.5 h. However, our attempt to [18F]trifluoroethylate the primary alcohol of the diol precursor in a similar fashion to Approach C was unsuccessful. It was noted that the UV absorption profile of the reaction mixture dramatically shifted after the addition of NaH suggesting loss of the nitro group of the 2-nitroimidazole ring, which has its major absorption at 320 nm. Proton NMR data strongly suggested that NaH prompted intramolecular nucleophilic substitution of the nitro group yielding the cyclized product 11. This indicates that the nucleophile derived from the secondary alcohol of the propanediol moiety intramolecularly attacks on the carbon at the 2 position of the imidazole ring and triggers the departure of the nitro group, which results in the 5-membered cyclic ether formation. The 5-membered cyclic ether 11 shows distinct 1H NMR spectra from those of the 6-membered one 12 including the signal at 5.3 ppm from the proton attached to the chiral carbon; in comparison, the chemical shift of the proton at the chiral carbon of Compound 12 is 3.9 ppm23 (both in CD3 OD). 1H NMR of the product: (DMF-d7) δ 3.78 (dd, 4.30 Hz, 12.4 Hz, 1H), 3.89 (dd 3.70 Hz, 12.4 Hz, 1H), 4.08 (t, 8.1 Hz, 1H), 4.27 (t, 9.1 Hz, 1H), 5.34 (m, 1H) 6.53 (2, 1H), 6.70 (d, 1.3 Hz); (CD3OD) δ 3.74 (dd, 4.25 Hz, 12.7 Hz, 1H), 3.90 (dd 3.30 Hz, 12.7 Hz, 1H), 4.04 (dd, 7.1 Hz, 9.3 Hz, 1H), 4.22 (t, 9.2 Hz, 1H), 5.32 (m, 1H) 6.53 (d, 1.3 Hz, 1H), 6.70 (d, 1.3 Hz); MS: 163 ([M+Na]+).
4. Discussion
We commenced this TFMISO radiolabeling project by examining a simple halogen-18F exchange method in which a 2-halo-2,2-difluoroethoxy (XCF2CH2O-, X=Cl/Br) analog of TFMISO (3) is used as the precursor, and the halogen (X) is replaced with [18F]-fluoride in a nucleophilic substitution reaction (Approach A, Scheme 1). Considering the relatively short half-life of 18F, 110 minutes, it is essential that the entire radiosynthesis be complete within a period of 1-2 half-lives of the radionuclide in order to obtain a sufficient amount of the final product for in vivo imaging. We expected reasonably good 18F incorporation into TFMISO molecules after heating the ClCF2-precursor (4) with K18F at 100-150°C for 10-30 minutes. Our expectation was based on a relatively large volume of literature describing the use of halogen-18F exchange for 18F-labeling of a variety of compounds24 including a few trifluoromethylated aromatic compounds such as α,α,α-trifluoro-toluene or trifluoromethyl-benzophenone which were labeled from their corresponding halo-difluoromethyl analog precursors24d,24e. However, contrary to our expectation, we found that the predominant mechanism governing the reaction between our OH-protected XCF2-precursor (4) and [18F]fluoride was elimination of the halogen rather than halogen-18F substitution, resulting in only a trace amount of [18F]TFMISO and a large amount of the unlabeled elimination product (5) (Figure 2b).
After our attempt of this simple one-pot method revealed unexpected outcomes, we switched our strategy to O-[18F]trifluoroethylation approaches using 2,2,2-[18F]trifluoroethyl halide or tosylate as a [18F]trifluoroethylating agent: Approaches B and C depicted in Schemes 2 and 3, respectively. Approach B was designed assuming that in parallel to the nucleophilic reactions between alkylhalides and glycidol which give glycidyl ethers25, a similar reaction between 2,2,2-[18F]trifluoroethyl iodide and glycidol would lead to the intermediate, 1,2-epoxy-3-(2,2,2-[18F]trifluoroethoxy)propane (6), which, condensed with 2-nitroimidazole, would produce [18F]TFMISO. 2,2,2-[18F]Trifluoroethyl iodide can be prepared via 18F-19F exchange in high radiochemical yield as reported in the literature22. However, contrary to our expectation, the oxygen nucleophile derived from glycidol did not replace the iodine of the trifluoroethyl iodide molecule. Instead, the nucleophile attacked on the carbon of the CF3 group resulting in 1,1-difluoro-2-iodoethoxy and 1-fluoro-2-iodovinyloxy analogs of TFMISO (Scheme 2, Figures 3a and 3b). In stark contrast, [18F]trifluoroethyl tosylate yielded the key intermediate (6) smoothly without complication, and, through this intermediate, the 2,2,2-[18F]trifluoroethyl group was successfully incorporated into the TFMISO molecule giving [18F]TFMISO (2) as the final product (Scheme 3, Figure 4b). 2,2,2-[18F]Trifluoroethyl tosylate was prepared via 18F-19F exchange in good radiochemical yield (Figure 4a), which was also contrary to our expectation that the overwhelmingly predominant mechanism underlying the reaction between the tosylate and [18F]fluoride would be nucleophilic 18F-tosyl substitution such that radiolabeling of 2,2,2-trifluoroethyl tosylate via 18F-19F exchange would be unlikely to occur.
The unusual chemistry observed in Approaches A-C was derived from remarkable electron-withdrawing effects of the fluorine atom. Base-promoted dehydrochlorination of 2-chloro-2,2-difluoro-1-phenylethane (ClF2CCH2C6H5) with sodium ethoxide occurs 50 times quicker than that of 2-chloro-1-phenylethane (ClC H 26,27, and the difference in the rate of dehydrobromination between 1,2-dibromo-2,2-difluoro-1-phenylethane (BrF2CCHBrC6H5) and 1,2-dibromo-1-phenylethane (BrCH2CHBrC6H5) is 250 times28, which suggest that the 2 electron-withdrawing fluorine atoms of the XCF2 (X = Cl/Br) group greatly facilitate the departure of the halogen leaving group. Compared to these accelerated halogen elimination reactions, substitution of the halogen (X) of the XCF2 moiety with a nucleophile such as fluoride or alkoxide appears much slower27,29. Thus, it is most likely that in our reaction system, the halogen elimination promoted by the basic environment created by the Kryptofix-K2CO3 complex occurred overwhelmingly rapidly compared to the Cl-(or Br-)18F exchange reaction. As a consequence, only a trace amount of [18F]TFMISO was produced while most of the ClCF2-precursor was converted into the 2,2,-difluorovinyloxy-analog of TFMISO (5). Although iodide is a better leaving group, use of the ICF2-precursor as an alternative might not be helpful because the rate of I-elimination could be several orders of magnitude larger than that of Cl-elimination27 and would likely dominate the 18F-I substitution reaction.
The electronegativity of fluorine also affects greatly the reactivity of the halogen leaving group on the carbon adjacent to a fluoro-methyl group. The reactivity of the iodide leaving group of 2-fluoroethyl iodide toward a nucleophile such as sodium thiophenoxide dropped exponentially with increasing number of fluorine atoms attached to the position 2–carbon30. Bodor et al.31 reported that because of the high electronegativity of fluorine, replacement of one of the 3 hydrogen atoms of the CH3 group of ethyl iodide with a fluorine atom changes the charge on the methyl carbon center from negative to positive, and with additional two fluorine atoms replacing the remaining two hydrogen atoms, which converts the CFH2 moiety into a CF3 group, the same carbon center becomes 24 times more positive, while the charge on the CH2I carbon remains negative. Because of deactivation of the halide leaving group in trifluoroethyl halide by the 3 electron-withdrawing fluorine atoms of the CF3 group, Satter et al. observed that a nucleophile such as [18F]fluoride replaces one of the 3 fluorine atoms instead of the halogen (Cl, Br or I) resulting in 2,2,2-[18F]trifluoroethyl halide instead of 1,1,1,2-[18F]tetrafluoroerthane in remarkably high radiochemical yields22, which we also confirmed in Approach B. Presumably by a similar mechanism31, 2,2,2-[18F]trifluoroethyl tosylate was produced via 18F-19F exchange (Approach C, Scheme 3) in a surprisingly high labeling efficiency. Aigbirhio et al.32 produced 1,1,1,2-[18F]tetrafluoroethane from 2,2,2-trifluoroethyl tosylate via nucleophilic substitution with 18F using a mildly pressurized reaction vessel in a radiochemical yield of 50% but also reported that due to the 18F-19F exchange reaction simultaneously occurring and competing with the tosyl-18F substitution reaction, the specific activity of the substitution product 1,1,1,2-[18F]tetrafluoroethane was 2-3 orders of magnitude lower than expected. Thus, two competing mechanisms, i.e. fluorine-fluorine exchange, which produces 2,2,2-[18F]trifluoroethyl tosylate, and nucleophilic substitution of the tosyl leaving group, which produces 1,1,1,2-[18F]tetrafluoroethane, appear to underlie the reaction between the nucleophile fluoride and 2,2,2-trifluoroethyl tosylate.
There were striking differences between 2,2,2-[18F]trifluoroethyl tosylate and iodide as a [18F]trifluoroethylating agent. [18F]Trifluoroethylation of an alcohol, such as 3-chloro-1,2-propanediol, 3-bromo-1,2-propanediol or glycidol, with 2,2,2-[18F]trifluoroethyl tosylate proceeds smoothly affording a [18F]trifluoroethylether such as the key intermediate for the [18F]TFMISO production: 1,2-epoxy-3-(2,2,2-[18F]trifluoroethoxy)propane (6) (Schemes 3 and 4). By contrast, our attempt of O-[18F]trifluoroethylation of the same alcohol using 2,2,2-[18F]trifluoroethyl iodide failed since unusual substitution reactions occurred in which the iodide leaving group was not replaced with the same oxygen nucleophile. Instead one or two of the 3 fluorine atoms of the CF3- group were replaced, resulting in the formation of 1-iodo-2,2-[18F]difluoroethyl and 1-iodo-2-[18F]fluoro-vinyl ethers. As a consequence, the reaction between 2,2,2-[18F]trifluoroethyl iodide and glycidoxide led to the production of 1-iodo-2,2-[18F]difluoroethoxy and 1-iodo-2-[18F]fluoro-vinyloxy analogs of [18F]TFMISO (Scheme 2). Obviously, the same oxygen nucleophile which reacts on the carbon attached to the tosyl group of 2,2,2-trifluoroethyl tosylate and replaced the tosyl leaving group attacks on the CF3 site of 2,2,2-trifluoroethyl iodide, replacing one or two of the fluorine atoms instead of the iodide leaving group. Similar complications have been observed in nucleophilic substitution reactions with 2,2,2-trifluoroethyl halides33. The differences observed in this investigation between trifluoroethyl halide and tosylate as a trifluoroethylaing agent are in part similar to the differences between alkyl tosylates and halides. Alkyl tosylates are considered to be “harder” electrophiles than the counterpart halides, which makes tosylates more reactive in nucleophilic substitution reactions and less reactive in accompanying and competing side reactions. Depuy et al. observed that the SN2 reactivity of phenylethyl tosylate toward sodium ethoxide is 4 times higher than that of the counterpart iodide whereas the accompanying elimination reaction was 68 times slower with the tosylate than with the iodide, as a result of which the yield of the nucleophilic substitution product, phenylethyl ether, was approximately 20-30 times higher with the tosylate than the iodide26,34. Similar differences in SN2 and E2 reactivity between ethyltosylate and ethylbromide have also been reported35. In the case of O-[18F]trifluoroethylation reported here, the differences between [18F]trifluoroethyl tosylate and iodide as an electrophile appeared to be more pronounced. Besides the general differences as electrophiles between tosylates and halides, the most striking difference which makes [18F]trifluoroethyl tosylate a superior [18F]trifluoroethylating agent is that the two different nucleophiles, [18F]fluoride and an alkoxide such as glycidoxide, react on the 2 different carbon sites of the trifluoroethyl moiety of the tosylate, i.e. [18F]fluoride on C-2 and glycidoxide on C-1, whereas, in contrast, these nucleophiles both attack on the same carbon of trifluoroethyl iodide (Scheme 6). Further empirical and theoretical investigations are needed to elucidate the mechanisms by which trifluoroethyl halide and trifluoroethyl tosylate react with a nucleophile differently.
Scheme 6.
After [18F]TFMISO (2) was successfully produced via the 3-step procedure (Scheme 3), we attempted direct O-[18F]trifluoroethylation of the primary alcohol of 3-(2-nitroimidazolyl)-1,2-propanediol (10) (Scheme 5) using the same [18F]trifluoroethylating agent to produce [18F]TFMISO. However, this approach was unsuccessful because of intramolecular nucleophilic substitution of the nitro group of the nitroimidazole moiety, which gave a 5-membered cyclic ether (11). It was unexpected that the nucleophile derived from the secondary alcohol, instead of the primary alcohol, of the propanediol moiety attacks intramolecularly on the carbon at the 2 position of the imidazole ring and triggers the departure of the nitro group, resulting in the 5-membered cyclic ether formation. The formation of the 6-membered cyclic ether (12) has been reported to occur when the cyclization is prompted by removal of the primary OH protection of the derivative of Compound 9 in which primary and secondary hydroxyl groups are both protected23. Similar to our finding depicted in Scheme 5, Fekner et al. observed that, in a reaction of 3-(2-(2-nitrophenyl)-benzoimizadolyl)-propane-1,2-diol with NaH, a cyclic ether was formed from intramolecular nucleophilic substitution of the nitro group with the secondary alkoxide of the propanediol moiety while no cyclization occurred with the primary alkoxide36. They suggested that the cyclization with the secondary alkoxide is kinetically more favored. This result of our attempt to directly [18F]trifluoroethylate 3-(2-nitroimidazolyl)-1,2-propanediol (10) suggests that it is essential to avoid the intramolecular nucleophilic substitution of the nitro group by choosing a base which deprotonates the primary alcohol but prevent the alkoxide from eliminating the nitro group.
As proven here, the use of 2,2,2-[18F]trifluoroethyl tosylate as a [18F]trifluoroethylating agent is a highly efficient way to label a compound containing a trifluoroethyl group with 18F. However, there is a limitation of this method, which is the low specific activity of the final product. The average specific activity of [18F]TFMISO produced via Approach C was 80±7μCi/μmol at EOS (n=12) and is anticipated to increase to approximately 0.2 mCi/μmol if the above-mentioned approach (Approach D), where the diol precursor (10) is directly O-[18F]trifluoroethylated with 2,2,2-[18F]trifluoroethyl tosylate, is improved and successfully implemented. Low specific activity does not seem to be an issue when the [18F]trifluoethylated final product is used as a hypoxia marker as is the case for [18F]TFMISO. As Wyss et al. have demonstrated using nanoPET, the amount of unlabeled FMISO contained in a [18F]FMISO injection does not influence quality of the tumor hypoxia image up to 300 mg/kg37. Likewise, for in vivo MR imaging of hypoxia with TFMISO, a dose greater than or equal to 75 mg/kg was used18. However, when high specific activity is required, this 18F-19F exchange-based method is disadvantageous compared to a no-carrier-added approach such as the one we tried in Approach A (Scheme 1) if a) elimination of the leaving group does not overwhelm its substitution with 18F and b) no isotopic dilution occurs. It may be noteworthy that in labeling of a trifluoroalkyl group in a complex molecule with 18F, which often involves unusual chemistry as reported here, even a no-carrier-added approach does not necessarily result in a high specific activity radiotracer20.
[18F]TFMISO resulting from Approach C is a racemate of the 2 enantiomers. By using the S or R form of 3-chloro-1,2-propanediol or glycidol in Step 2, [18F]TFMISO of high enantiopurity can be synthesized. However, although enantiomeric isomers of a drug often exhibit distinct biological behavior and potency, it is unlikely that one enantiomer of [18F]TFMISO shows better properties as a hypoxia marker than the other or the racemate: this issue has been investigated in the past using TFMISO analog hypoxia markers such as FMISO or pimonidazole38,39, and no differences were found between an enantiopure isomer and the racemic mixture in pharmacokinetics, biodistribution, toxicity or metabolism of the hypoxia marker.
5. Conclusions
This investigation has identified 2,2,2-[18F]trifluoroethyl tosylate as an excellent [18F]trifluoroethylating agent, which can convert an alcohol into the corresponding [18F]trifluoroethyl ether in a high efficiency. 2,2,2-[18F]Trifluoroethyl tosylate can be produced via 18F-19F exchange in good radiochemical yield. Unlike [18F]trifluoroethylation with 2,2,2-[18F]trifluoroethyl halide, which promotes unusual nucleophilic substitution reactions, [18F]trifluoroethylation with 2,2,2-[18F]trifluoroethyl tosylate proceeds smoothly without complication. Using 2,2,2-[18F]trifluoroethyl tosylate as the [18F]trifluoroethylating agent, the bimodal hypoxia marker TFMISO (1) was successfully labeled with 18F (2). In addition to O-[18F]trifluoroethylation, an amine or a thiol can also be [18F]trifluoroethylated with the same [18F]trifluoroethylating agent. Thus the [18F]trifluoroethylation method using [18F]trifluoroethyl tosylate as described here is a novel and versatile tool for 18F-radiolabeling of a wide variety of pharmaceuticals containing a O-, N- or S-trifluoroethyl group in their molecules.
6. Experimental
6.1. General Methods
TFMISO was purchased from SynChem OHG Laboratories (Altenburg, Germany). Reagents were used as received from commercial sources. Flash chromatography was carried out using silica gel 170-400 mesh (Fisher, Pittsburgh, PA). Proton (1H) and fluorine (19F) NMR spectra were recorded at 500 MHz using a Bruker DRX spectrometer. Mass spectra were obtained with a PE SCIEX API100 mass spectometer. Radiochemical reactions were carried out in sealed 5 mL Reacti-vials (Pierce, Rockford, IL). Analytical HPLC was performed using a C18 Nova-Pak column (4 μm, 4.6 × 150mm) (Waters, Milford, MA)) with a mobile phase of acetonitrile and water at a flow rate of 2 mL/min. Semi-preparative HPLC was performed using a C18 Nova-Pak column (6 μm, 7.8 × 300mm) with a mobile phase of acetonitrile and water (15/85) at a flow rate of 5 mL/min. The HPLC output was monitored using a Diode Array Detector (SPD-M10AVP) (Shimadzu, Columbia, MD) and a radiation detector (Bioscan Flow-Count, Washington D.C.).
6.2. Preparation of the OH-protected ClCF2-precursor (4, Scheme 1)
The ClCF2-precursor (3) for Approach A was synthesized by reacting 2-epoxy-3-(2-chloro-2,2-difluoroethoxy)propane with 2-nitroimidazole according to the literature procedure6. 2-Epoxy-3-(2-chloro-2,2-difluoroethoxy)propane was prepared following the procedure reported by Brey et al.40 with modifications. Briefly 3.6 mL of 10% sodium hydroxide solution was added to a mixture of 1.0 g of 2-chloro-2,2-difluoroethanol (Matrix, Columbia, SC) and 0.78 g of epichlorohydrin (Fluka, Buchs, Switzerland) and the mixture stirred at room temperature overnight. The organic layer was separated and used without further purification. 1H NMR (CDCl3); δ 2.65 (dd 2.7 Hz, 4.7 Hz, 1H), 2.83 (dd 4.70 Hz, 4.75 Hz, 1H), 3.19 (m, 1H), 3.58 (dd 5.85 Hz, 11.8 Hz, 1H), 3.95-4.07 (m, 3H). 2-Epoxy-3-(2-chloro-2,2-difluoroethoxy)propane (740 mg) prepared as above was reacted with 243 mg of 2-nitroimidazole (Aldrich, St Louis, MO) in ethanol (20 mL) at 110°C for 2.5 hours in the presence of 68 mg of K2CO3. After evaporation of ethanol, the product 3-(2-nitroimidazolyl)-1-(2-chloro-2,2-difluoroethoxy)-propane-2-ol (3), was isolated by silica gel column chromatography using a solvent of ethylacetate-hexane (3:1). 1H NMR: (CD3OD) δ 3.59 (dd 5.2 Hz, 10.9 Hz, 1H), 3.64 (dd, 5.1 Hz, 10.5 Hz, 1H), 4.00 (dt, 1.8 Hz, 11.4 Hz, 2H), 4.00 (m, 1H), 4.32 (dd, 8.4 Hz, 13.9 Hz, 1H), 4.64 (dd, 3.6 Hz, 13.9 Hz), 7.03 (d, 0.95 Hz, 1H), 7.34 (d, 0.90 Hz, 1H); 19F NMR (CDCl3): δ 63.04; MS (ESI): 286 ([M+H]+), 308 ([M+Na]+), 320 ([M+Cl]-). The hydroxyl group of the ClCF2-precursor (3) was tetrahydropyranylated in THF (20 mL) using 2,3-dihydro-4H-pyran (2 mL) (Aldrich) and pyridinium p-toluenesufonate (0.6 g) (Aldrich) as reported by Miyashita et al.41. The reaction mixture was heated at 80°C for 3.5 hours and the progress of the reaction was monitored by analytical HPLC with a mobile phase of acetonitrile and water (40/60) as the double peak corresponding to the 2 diastereoisomers of the OH-protected product grew. The OH-protected ClCF2-precursor (4) was separated by silica gel column chromatography using a solvent of ethylacetate-hexane (1:1). Yield 43%; 1H NMR (CDCl3), (mixture of diastereoisomers): δ 1.44-1.68 (m, 12H), 3.20-3.24 (m, 1H), 3.29-3.34 (m, 1H), 3.43-3.47 (m, 1H), 3.71-3.74 (m, 2H), 3.77 (dd, 4.6 Hz, 10.6 Hz, 1H), 3.81-3.83 (m, 1H), 3.86 (dd, 3.7 Hz, 10.3 Hz, 1H), 3.91-4.06 (m, 4H), 4.07-4.10 (m, 1H), 4.24 (sextet, 4.2 Hz, 1H), 4.29-4.31 (m, 1H), 4.44 (dd, 7.5 Hz, 14.1 Hz, 1H) 4.54 (dd, 8.1 Hz, 14.0 Hz, 1H), 4.66 (m, 1H), 4.77 (dd, 3.8 Hz, 14.1 Hz, 1H), 4.87 (dd, 3.8 Hz, 14.1 Hz, 1H), 7.13 (d, 0.8 Hz, 1H), 7.14 (s, 2H), 7.20 (d, 0.65 Hz, 1H); 19F NMR (CDCl3): δ 61.41, 61.49; MS (ESI): 370 ([M+H]+), 392 ([M+Na]+).
6.3. Preparation of 3-(2-nitroimidazolyl)-1,2-propanediol (10, Scheme 5)
The diol precursor for Approach D (10) was synthesized following the method of Beaman et al.6. Briefly, 400 mg of 2-nitroimidazole and 530 mg of glycidol (Acros, Geel, Belgium) were reacted in 30 mL of ethanol in the presence of 40 mg of K2CO3. When the exothermic reaction started, the heater was shut off, but stirring continued for 40 minutes. After unreacted glycidol and the solvent were evaporated under vacuum, the final product (10) was separated by silica gel column chromatography using a solvent of ethylacetate-methanol (3:1). Yield 45%; 1H NMR (CD3OD) δ 3.54 (dd, 5.5 Hz, 11.35Hz, 1H), 3.57 (dd, 5.5 Hz, 11.35 Hz, 1H), 3.94 (m, 1H), 4.36 (dd, 8.6 Hz, 13.9 Hz), 4.74 (dd, 3.4 Hz, 13.9 Hz), 7.12 (s, 1H), 7.44 (s, 1H); MS (ESI): 210 ([M+Na]+), 397 ([2M+Na]+).
6.4. Preparation of Kryptofix-K18F complex
[18F]Fluoride was produced via the 18O(p,n)18F nuclear reaction using a biomedical cyclotron TR19/9 (EBCO Technologies, British Columbia, Canada). Fifty to 90 mCi of [18F]fluoride in 100-200 μL of H218O was added to a Reacti-vial containing acetonitrile (300 μL), water (50-100 μL), Kryptofix 222 (10-13 mg) and K2CO3 (2-3 mg). The solvents were evaporated azeotropically by repeatedly adding 500 μL acetonitrile until dry Kryptofix-K18F complex was obtained.
6.5. Radiolabeling of TFMISO with 18F via Cl-18F substitution (Approach A, Scheme 1)
To the dried Kryptofix-K18F complex prepared as above, 6-8 mg of the OH-protected ClCF2-precursor (4) dissolved in DMF (200 μL) was added, and the reaction mixture heated at 80-150°C for 10 minutes. And then the OH-protection was removed with 200 μL of 0.1N HCl at 80°C. The progress of the reaction was monitored by injecting an aliquot of the reaction mixture into analytical HPLC running with a mobile phase of acetonitrile and water (40/60 before hydrolysis or 20/80 after hydrolysis). After hydrolysis, the formation of [18F]TFMISO was examined by co-injecting the reaction mixture with authentic TFMISO. The most prominent unlabeled by-product was isolated by semi-preparative HPLC and characterized by mass spectrometry and 1H/19F NMR.
6.6. 18F-Labeling of TFMISO via O-[18F]trifluoroethylation with 2,2,2-[18F]trifluoroethyl iodide (Approach B, Scheme 2)
To the dried Kryptofix-K18F complex prepared as above, 5-10 μL of 2,2,2-trifluoroethyl iodide (Aldrich) in DMF (200 μL) was added, and the reaction mixture heated at 130°C for 5 minutes. Incorporation of 18F into CF3CH2I was monitored by injecting an aliquot of the reaction mixture into analytical HPLC running with a mobile phase of acetonitrile and water (40/60). [18F]CF3CH2I was distilled into a second vial containing 8 mg of glycidol and 10 mg of powdered KOH (or 6 mg of NaH) in 200 μL of anhydrous DMF, and the reaction stirred at 80°C (KOH) or room temperature (NaH) for 30 minutes. This reaction mixture was transferred into a third vial containing 12-13 mg of 2-nitroimidazole and 1-2 mg of K2CO3 in 200 μL of ethanol and heated at 100°C for 30 minutes. The progress of the reaction was monitored by analytical HPLC using a mobile phase of acetonitrile and water (20/80). In order to characterize the final products, similar reactions were carried out starting with non-radioactive CF3CH2I and the final products isolated using LC-MS equipped with a C18 preparative column (XBridge, Waters), a photodiode array detector (Model 2998, Waters) and a mass detector (Model 3100, Waters) with a mobile phase of acetonitrile-water (30/70) containing 0.1% of trifluoroacetic acid. The purified products were analyzed by 1H/19F NMR and mass spectrometry.
6.7. 18F-Labeling of TFMISO via O-[18F]trifluoroethylation with 2,2,2-[18F]trifluoroethyl tosylate (Approach C, Scheme 3)
Step 1: Preparation of 2,2,2-[18F]trifluoroethyl tosylate
To the dried Kryptofix-K18F complex prepared as above, 10-20 mg of 2,2,2-trifluoroethyl ptoluenesulfonate (Fluka) in DMF (200 μL) was added, and the reaction mixture heated at 150°C for 10 minutes. Incorporation of 18F into the tosylate precursor was monitored by analytical HPLC using a mobile phase of acetonitrile-water (50/50). From the reaction mixture, 2,2,2-[18F]trifluoroethyl tosylate was extracted into ether (1 mL) and the organic layer washed with brine (0.1 mL) followed by water (0.1 ml), and dried over sodium sulfate. After evaporation of ether, 2,2,2-[18F]trifluoroethyl tosylate was dissolved in anhydrous DMF.
Step 2: O-[18F]Trifluoroethylation of 3-chloro-1,2-propanediol
To a Reacti-vial containing 2-8 mg of NaH (60% in mineral oil) suspended in 100 μL of dry DMF, 4-15 mg of 3-chloro-1,2-propanediol in anhydrous DMF was added at 0°C followed by the [18F]trifluoroethyl tosylate solution obtained from Step 1, and stirred at room temperature for 45-60 minutes. An aliquot of the reaction mixture was analyzed by analytical HPLC running with a mobile phase of acetonitrile-water (15/85).
Step 3: Reaction of the intermediate products from Step 2 with 2-nitroimidazole
To a Reacti-vial containing 10-13 mg of 2-nitroimdazole in 100 μL of DMF, an equivalent amount of sodium methoxide in methanol was added and heated at 150°C for 3 minutes, the reaction mixture let cool to 120°C, and then the Step 2 reaction mixture added through a 0.45 μm membrane filter (Supelco, PA). The vial was heated at 120°C for 30 minutes. This procedure was adopted from Beaman et al.6 and used with modifications. Alternatively, the reaction mixture of Step 2 was added to a vial containing 2-nitroimidazole and K2CO3 in 0.3 mL of ethanol and the vial heated at 100°C for 30 minutes. The progress of the reaction was monitored by analytical HPLC running with a mobile phase of acetonitrile-water (15/85). The 18F-labeled product which eluted at the retention time corresponding to that of authentic TFMISO was isolated by semi-preparative HPLC, concentrated by solid phase extraction using a tC18 SepPak Plus cartridge (Waters) and eluted with 2 mL of methanol and the solvent evaporated. The final product was confirmed as [18F]TFMISO by HPLC and mass spectrometry. MS (ESI): 292 ([M+Na]+), 304 ([M+Cl]-. The radiochemical and chemical purity of the purified final product were determined by analytical HPLC and found to be 100% and greater than 98%, respectively. The specific activity of [18F]TFMISO was determined by dividing the radioactivity of the radiotracer by its mass, which was calculated by comparing the area under its HPLC UV peak at 320 nm with that of a standard TFMISO solution of known concentration.
6.8. Identification of the [18F]trifluoroethoxy intermediate produced in Step 2 of Approach C as Compound 6 (Figure 4b, Schemes 3 and 4)
The [18F]trifluoroethoxy intermediate produced in Step 2 of Approach C (Peak B in Figure 4b), which led to [18F]TFMISO formation in Step 3 (Figure 4b & Scheme 3) was identified as follows. In addition to 3-chloro-1,2-propanediol, two other alcohols, 3-bromo-1,2-propanediol and glycidol, were treated with NaH and reacted with 2,2,2-[18F]trifluoroethyl tosylate in a similar fashion to 3-chloro-1,2-propanediol and the products analyzed by analytical HPLC. These 18F-labeled intermediates were further reacted with 2-nitroimidazole in a similar fashion to the procedure with 3-chloro-1,2-propanediol, and the reactions monitored by analytical HPLC. The formation of the epoxide intermediate (6) was confirmed by repeating the O-trifluoroethylation reactions using non-radioactive 2,2,2-trifluoroethyl tosylate and the two alcohol, 3-chloro-1,2-propanediol and glycidol (Routes 1 and 2, Scheme 4) and comparing the 1H NMR spectra of the trifluoroethoxy products with that of the reference compound 1,2-epoxy-3-(2,2,2-trifluoroethoxy)propane. The reference compound was synthesized from 2,2,2-trifluoroethanol and epichlorohydrin following the method of Brey et al.40 (Route 3, Scheme 4). 1H NMR (DMF-d7) δ 2.63 (dd, 2.8 Hz, 5.2 Hz, 1H), 2.80 (t, 4.4 Hz, 1H), 3.20 (m, 1H), 3.52 (dd, 6.6 Hz, 11.7 Hz, 1H), 4.01 (dd, 2.5 Hz, 11.8 Hz, 1H), 4.17 (q, 9.3 Hz, 2H). The reactions between 3-chloro-1,2-propanediol (20 mg, 0.18 mmole)) and non-radioactive 2,2,2-trifluoroethyl tosylate (22 mg, 0.09 mmole) (Route 1) and between glycidol (15 mg, 0.19 mmole) and the non-radioactive tosylate (24 mg, 0.09 mmole) (Route 2) were carried out in DMF-d7 in the presence of NaH (60%, washed with pentane and dried under a stream of Ar) in a similar fashion as described above (Approach C).
6.9. 18F-Labeling of TFMISO using 3-(2-nitroimidazolyl)-1,2-propanediol (10) as the precursor (Approach D, Scheme 5)
[18F]Trifluoroethyl tosylate produced as described above was added to a DMF solution containing 8 mg of the diol precursor (10) and 3 mg of 60% NaH and the reaction mixture stirred at room temperature for 45 minutes. The progress of the reaction was examined by analytical HPLC with a mobile phase of acetonitrile-water (15/85). The deprotonation reaction of the diol precursor (10) with NaH was further examined by NMR as follows. To a Reacti-vial containing NaH (60%, 3.2 mg) washed with pentane and dried, the same precursor 10 (10 mg) dissolved in 200 μL of DMF-d7 was added at 0°C and the reaction stirred at room temperature for 5 minutes. The reaction mixture was analyzed by 1H NMR and mass spectrometry. In addition, the solvent was evaporated under vacuum and the residue dissolved in CD3OD for 1H NMR.
Acknowledgments
We would like to thank Dr. Jacek Koziorowski of Herlev University Hospital in Denmark for fruitful discussions. We thank Ms. Chuyan (Alice) Cao of Organic Synthesis Core Facility for her assistance in LC-MS purification of Compounds 7 and 8. We also thank Dr. Jason Lewis, Mr. Howard Sheh, and Mr. Calvin Lom of the MSKCC Cyclotron Core Facility for 18F production essential for this work. The authors also thank Dr. Cynthia Jung for valuable assistance with the preparation of the manuscript. This work was supported by NIH grant P01 CA115675 (P.I. John Humm/Clifton Ling), and in part P30 CA 008748-43 (Cancer Center Support Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virkam DS, Zweier JL, Kuppusamy P. Antioxid Redox Signal. 2007;9:1745. doi: 10.1089/ars.2007.1717. [DOI] [PubMed] [Google Scholar]
- 2.Bache M, Kappler M, Said HM, Staab A, Vordermrk D. Curr Med Chem. 2008;15:322. doi: 10.2174/092986708783497391. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel P, Mayer A. Cancer Metastasis Rev. 2007;26:225. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 4.Ling CC, Humm J, Larson S, Amols H, Fuks Z, Leibel S, Koutcher JA. Int J Radiat Oncol Biol Phys. 2000;47:551–60. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 5.Schinagl DAX, Kaanders JHAM, Oyen WJG. Cancer Imaging. 2006;6:S107–16. doi: 10.1102/1470-7330.2006.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaman AG, Caldwell N, Duschinsky R, Monclair NJ, Tautz WP. 1970 U.S. Patent 3549653.
- 7.Chapman JD, Urtasun RC. Cancer. 1977;40:484. doi: 10.1002/1097-0142(197707)40:1+<484::aid-cncr2820400713>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Rauth AM, Chin J, Marchow L, Paciga J. Br J Can Suppl. 1978;3:202. [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson RF, Patel KB. Br J Cancer. 1979;39:705. doi: 10.1038/bjc.1979.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwock L, Gill M, McMurry HL, Beckman W, Raleigh JA, Joseph AP. Radiat Res. 1992;129:71. [PubMed] [Google Scholar]
- 11.Raleigh JA, Franko AJ, Treiber EO, Lunt JA, Allen PS. Int J Radiat Oncol Biol Phys. 1989;12:1243. doi: 10.1016/0360-3016(86)90268-3. [DOI] [PubMed] [Google Scholar]
- 12.Tracy M, Kelson AB, Workman P, Lewis A, Aboagye E. 1996 WO9604249A1.
- 13.Seddon BM, Maxwell RJ, Homess DJ, Grimshaw R, Raynaud F, Tozer GM, Workman P. Clin Can Res. 2002;8:2323. [PubMed] [Google Scholar]
- 14.Seddon BM, Payne GS, Simons L, Ruddle R, Grimshaw R, Tan S, Turnor A, Raynaud F, Halbert G, Leach MO, Judson I, Workman P. Clin Can Res. 2003;9:5101. [PubMed] [Google Scholar]
- 15.Papadopoulou MV, Ji M, Bloomer WD. Antican Res. 2006;26:3253. [PubMed] [Google Scholar]
- 16.Chapman JD, Lee J, Meeker BE. Int J Radiat Oncol Biol Phys. 1989;16:911. doi: 10.1016/0360-3016(89)90886-9. [DOI] [PubMed] [Google Scholar]
- 17.Raleigh JA, Franko AJ, Kelly DA, Trimble LA, Allen PS. Mag Res Med. 1991;22:451. doi: 10.1002/mrm.1910220253. [DOI] [PubMed] [Google Scholar]
- 18.Procissi D, Claus F, Burgman P, Koziorowski J, Chapman JD, Thakur SB, Matei C, Ling CC, Koutcher JA. Clin Cancer Res. 2007;13:3738. doi: 10.1158/1078-0432.CCR-06-1563. [DOI] [PubMed] [Google Scholar]
- 19.Ackerstaff E, Coman M, Carlin S, Burke S, Zakian K, Kotedia K, O'Donoghue J, Ling C, Koutcher J. Proceedings of 17th Scientific Meeting. International Society for Magnetic Resonance in Medicine; Honolulu, Hawaii: 2009. p. 196. [Google Scholar]
- 20.Johnström P, Stone-Elander S. J Labelled Compd Pharm. 1995;36:537. [Google Scholar]
- 21.Josse O, Labar D, Georges B, Gregoire V, Marchand-Brynaert J. Bioorg Med Chem. 2001;9:665. doi: 10.1016/s0968-0896(00)00279-0. [DOI] [PubMed] [Google Scholar]
- 22.Satter MR, Martin CC, Oakes TR, Christian BF, Nickles RJ. Appl Radiat Isot. 1994;45:1093. doi: 10.1016/0969-8043(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim P, Zhang L, Manjunatha UH, Singh R, Patel S, Jiricek J, Keller TH, Boshoff HI, Barry CE, III, Dowd CS. J Med Chem. 2009;52:1317. doi: 10.1021/jm801246z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a Berridge MS, Sayre LM, Arora PK, Terris AH, Riachi NJ, Harik SI. J Med Chem. 1993;36:1284. doi: 10.1021/jm00061a021. [DOI] [PubMed] [Google Scholar]; b Mathews WB, Ravert HT, Musachio JL, Frank RA, Rinaldi-Carmona M, Barth F, Dannals FR. J Labelled Comds Radiopharm. 1999;42:589. [Google Scholar]; c Hoyte RM, Zhang J-X, Lerum R, Oluyemi A, Persaud P, O'Connor C, Labaree DC, Hochberg RB. J Med Chem. 2002;45:5397. doi: 10.1021/jm0202775. [DOI] [PubMed] [Google Scholar]; d Angelini G, Speraza M, Shiue C-Y, Wolf AP. J Chem Soc Chem Commun. 1986:924. [Google Scholar]; e Kilbourn MR, Pavia MR, Gregor VE. Appl Radiat Isot. 1990;41:823. doi: 10.1016/0883-2889(90)90059-p. [DOI] [PubMed] [Google Scholar]
- 25.Itoh H, Nitta A, Tanaka T, Kamio H, Tsuboi K. 1985 European Patent 0150842A1.
- 26.DePuy CH, Bishop CA. J Amer Chem Soc. 1960;82:2532. [Google Scholar]
- 27.Koch HF, Tumas W, Knoll R. J Amer Chem Soc. 1981;103:5423. [Google Scholar]
- 28.Koch HF, Dahlberg DB, McEntee MF, Klecha CJ. J Amer Chem Soc. 1976;98:1060. [Google Scholar]
- 29.Miller WT, Jr, Fainberg AH. J Amer Cehm Soc. 1957;79:4164. [Google Scholar]
- 30.Hine J, Ghirardell RG. J Org Chem. 1958;23:1550. [Google Scholar]
- 31.Bodor N, Huang M-J. Tetrahedron. 1992;48:5823. [Google Scholar]
- 32.a Aigbirhio FI, Pike VW, Waters SL, Tanner RJN. J Fluorine Chem. 1995;70:279. [Google Scholar]; b Pike VW, Waters SL, Aigbirhio FI, Makepeace J, Tanner RJN. Org. Mass Spectrometry. 1994;29:499. [Google Scholar]
- 33.a Nakai T, Tanaka K, Ishikawa N. J Fluorine Chem. 1977;9:89. [Google Scholar]; b Tarrant P, Young JA. J Amer Chem. Soc. 1953;75:932. [Google Scholar]; c Wu K, Chen Q-Y. Tetrahedron. 2002;58:4077. [Google Scholar]
- 34.Depuy CH, Froemsdorf DM. J Amer Chem Soc. 1957;79:3710. [Google Scholar]
- 35.Fraser GM, Hoffman HMR, Ramsay W, Forster R. J Chem Soc (B) 1967:425. [Google Scholar]
- 36.Fekner T, Gallucci J, Chan MK. Org Lett. 2003;5:4795. doi: 10.1021/ol035761w. [DOI] [PubMed] [Google Scholar]
- 37.Wyss MT, Honer M, Schubiger PA, Ametamey SM. Eur J Nucl Med Mol Imaging. 2006;33:311. doi: 10.1007/s00259-005-1951-4. [DOI] [PubMed] [Google Scholar]
- 38.Peterson l. M., Muzi M, Adamsen TCH, Link JM, Rajendran JG, Spence AM, Scharnhorst J, Krohn KA. J Labelled Compd Radiopharm. 2007;50:S423. [Google Scholar]
- 39.Workman P, Newman HFV, Beechen NM, Ward R, Smithen C, Cancer Chemother Pharmacol E. 1991;28:118. doi: 10.1007/BF00689700. [DOI] [PubMed] [Google Scholar]
- 40.Brey ML, Torrant P. J Amer Chem Soc. 1957;79:6533. [Google Scholar]
- 41.Miyashita N, Yoshikoshi A, Grieco PA. J Org Chem. 1977;42:3772. [Google Scholar]