Abstract
The treatment of invasive candidiasis associated with growing numbers of immunocompromised patients remains a major challenge complicated by increasing drug resistance. A novel class of branched histidine-lysine (bHK) peptides has promising antifungal activity, and exhibits a mechanism similar to natural histatins, and thus may avoid drug resistance. The present studies evaluate ligand targeting of bHK peptides to fungal surface integrins by determining whether a cyclic RGD (cRGD) peptide with a large PEG linker could enhance bHK peptide antifungal activity. Whereas conjugates containing only the PEG linker reduced bHK peptide activity, conjugates with the cRGD-PEG ligand resulted in marked enhancement of activity against C. albicans. This study provides the first demonstration of benefit from ligand targeting of antifungal agents to fungal surface receptors.
Keywords: targeting, peptide, copolymer, conjugate, integrin, fungal infections
INTRODUCTION
Treatment of invasive candidiasis and other life-threatening disseminated invasive fungal infections remains a challenge, with a persistently high rate of mortality. Moreover, the number of immuno-compromised and immunosuppressed patients is increasing and as a result, the numbers of opportunistic infections including both superficial and invasive fungal infections are increasing [1–3]. Current therapeutics for invasive fungal infections involve three classes: polyenes, primarily amphotericin B [4]; azoles, including recently introduced voriconazole [4,5]; and echinocandins, such as caspofungin [6]. Nonetheless, use of these agents is limited by emerging resistance and/or serious side effects [5,7,8]. Thus, development of new and more effective antifungal agents, preferably with new modes of action, is urgently needed, particularly since invasive fungal infections affect multiple organs. Thus a major challenge has been achieving adequate biodistribution to achieve therapeutic levels at the tissues where the fungal infection reside.
Recent studies of pathogenic fungi have suggested that surface integrin-like receptors may play a role in attachment to endothelial cells, potentially an important contributor to disseminated invasive infections [9,10]. Furthermore, studies have shown that, like mammalian integrins, peptides with an RGD sequence inhibit fungal binding to endothelial cells [9,10]. Also, use of RGD peptides to target these fungal surface integrins is a potential strategy for targeted delivery of antifungal agents to enhance interaction and uptake in fungal-infected tissues, leading to improved efficacy.
Recently, antifungal activity was identified for several branched histidine-lysine (bHK) peptides, originally developed as gene delivery agents [11,12]. This novel bHK peptide class of macromolecules with non-natural branching, and capacity to incorporate non-natural amino acids via solid phase synthesis, offers a large range in biochemical structure to optimize anti-microbial activity. An initial set of bHK peptides was identified that have widespread antifungal activity against a variety of pathogenic fungal species, including candida, aspergillus, and cryptococcus [12,13], with a mode of action that appears to be similar to that of naturally occurring salivary histidine-rich histatins, with which microbial resistance has not been reported [14–16]. Most studies indicate that histatins are internalized and target either mitochondria or Trk.1 potassium channel, although one recent study indicates that histatins may act at the fungal cell surface [14–16]. Despite uncertainty in the precise target, both bHK peptides and histatins have very specific antifungal activity, in contrast to most linear antimicrobial peptides that have widespread antibacterial activity [17]. Interestingly, bHK peptides studied to date have significantly greater activity, as well as low mammalian cell cytotoxicity, making them particularly attractive for development of new treatments for disseminated invasive fungal infections. In addition, the non-natural bHK peptide branching increases the potential for conjugated ligands to act multivalently in binding to fungal surface receptors as a means to achieve targeting to widespread infections. Thus, the novel bHK peptide class has broad structural versatility for optimization and site specific ligand attachment at a molecular level.
This study investigated whether a cyclic RGD peptide ligand can facilitate delivery of bHK peptides to candida, and enhance antifungal activity. The small cyclic RGD pentapeptide studied, c(RGDfK), has been clinically validated for targeting a pair of mammalian integrins associated with endothelial cells at sites of neovasculature such as tumor angiogenesis with low levels of nonspecific tissue uptake [18]. The studies here evaluated whether conjugation of this ligand to antifungal bHK peptides using a large PEG-5000 linker could enhance antifungal activity, overcoming any potential adverse effects of conjugation. This study is the first to show enhanced antimicrobial activity by ligand targeting.
MATERIALS AND METHODS
C. Albicans strains
C. albicans (ATCC 10231, MYA-576) were obtained from American Type Culture Collection (Manassas, VA).
Maintenance of C. Albicans
C. albicans were maintained in yeast-maltose (YM) medium (Becton Dickinson, Sparks, MD) containing 0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 1.0% glucose. Minimal inhibitory concentration (MIC), metabolic, and uptake experiments were done in RPMI medium (buffered by MOPS, pH-7.2).
Synthesis of HK Peptides
The biopolymer core facility at the University of Maryland synthesized the branched HK peptides on a Ranin Voyager solid phase synthesizer (PTI, Tucson, AZ) as previously described [11]. If the peptide purity was less than 95%, then the peptides were further purified on an HPLC column with System Gold operating software by using a Dynamax 21-4 × 250 mm C-18 reversed phase preparative column with a binary solvent system. Further analyses of the peptides were performed with a Voyager MALDI-TOF mass spectroscopy (Applied Biosystems, Foster City, CA) and amino acid analysis (AAA Laboratory Service, Boring, OR).
PEG-cRGD conjugates of HK peptides
The above HK peptides were then modified with the PEG and the cRGD targeting ligand as follows. cRGD with a sequence, cyclo (Arg-Gly-Asp-D-Phe-Lys), was obtained from Peptides International (Louisville, KY). Targeting ligand cRGD was conjugated to the bHK peptide through a PEG molecule. Ligand targeted bHK peptide conjugates were synthesized in a two-step procedure as described previously with the polyethyleneimine polymer [18]. In the first step, cRGD was conjugated to a 5-KD molecular weight polyethylene glycol (PEG) by using a heterobifunctional PEG, maleimide-PEG-succinimidyl carboxylmethyl (SCM), obtained from Creative PEG Works (Salem, NC). Molar equivalents of cRGD and maleimide-PEG-SCM were reacted in the presence of 1.5 equivalents of N,N-diisopropylethylamine (DIPEA) in dimethyl sulfoxide (DMSO). The reaction was completed in 1 h and the product was precipitated with dry ether. The resulting conjugate was characterized by mass spectrometry. In the second step, the Mal-PEG-cRGD was reacted with bHK peptide in DMSO at 4:1 molar ratio and in presence of 2 equivalents of DIPEA. The reaction mixture was stirred at room temperature for 24 h and the product was dialyzed in 25-KD MWCO dialysis tubing against 0.05% TFA/water for 48 h and was lyophilized and characterized by amino acid analysis.
Solid-phase αvβ3 binding assay
The binding of cRGD, H2K4b-PEG-cRGD and H3K(H)4b-PEG-cRGD to αvβ3 integrin was determined using a solid-phase competitive binding assay [19]. The cRGDfK(biotin-PEG-PEG)] peptide (Peptides International), together with the HK or control peptides, was used to detect binding to αvβ3 integrin. Microtiter 96-well vinyl assay plates (Corning, NY) were coated overnight at room temperature with 100 μl/well of a solution of purified human integrin αvβ3 in Triton X-100 (Millipore, Billerica, MA) at a concentration of 100 ng/ml in coating buffer (1 × TBS, pH 7.4, 2 mM MgCl2, 1.5 mM MgCl2) overnight at room temperature. The plates were then washed three times with binding buffer (0.1% Tween-20, 1% BSA in coating buffer). The wells were blocked for 1 h with 250 μl of blocking buffer (3% BSA in binding buffer). The plates were washed three times with binding buffer. After blocking, 50 μl of the competitors (peptide concentrations range from 1 × 10−4 to 0.5 mM) was first added into wells, and then 50 μl of c[RGDfK(biotin-PEG-PEG)] peptide were added into the wells with or without pre-added competitors. The plate was gently mixed and incubated for 1 h at 37°C. After washing away the unbound competitors and c[RGDfK(biotin-PEG-PEG)] peptide, the bound c[RGDfK(biotin-PEG-PEG)] peptide was detected with avidin-HRP (horseradish peroxidase) and TMB (3,3′,5,5′-tetramethylbenzidine) substrate with absorbance readings at 450 nm by using a microplate reader. Background reading was subtracted from all measurements. The wells without competitors were used as control (100% binding).
Antifungal activity of bHKP and conjugates
Turbidity
Antifungal efficacy of bHK peptides was determined by measuring the viability of the cells (see next section) or by determining the growth of yeast cells in 96-well microtiter plates. Yeast cells were diluted between 2.5 × 103 and 5 × 103 cells/ml in RPMI1640-MOPS medium; 55 μl of the cell suspension were then added to each well of a 96-well plate containing 45 μl of bHK peptides with final concentrations of 0.1, 1, 2, 5, 10, 15, 20, and 25 μM. Peptide-free controls were also included. The microtiter plates were then incubated at room temperature or 37°C for 24–48 h. After incubation, the microplates were shaken vigorously on a plate shaker to disperse the cells into a uniform fungal suspension. Turbidity of the suspension, a measure of fungal growth, was measured at 595 nm with a microplate reader with each data point representing the mean and standard deviation. MIC was defined as the lowest concentration of the peptide that resulted in at least 95% inhibition from untreated control and no visible growth.
WST-1 Cell viability Assay
Fungal growth was also assayed using the WST-1 assay which determines the mitochondrial activity of the treated and control fungal cells. C. albicans cells were cultured in a micro-plate with 100 μl of RPMI-1640-MOPS containing increasing concentrations of bHK peptides (0.1, 1, 2, 5, 10, 15, 20 and 25 μM) for 48 h at 37°C. At the end of the treatment, 10 μl of WST-1 assay solution were added to each well. After gentle shaking, the plate was incubated for 30 min., and then shaken again for 1 min. WST-1 tetrazolium salt (Dojindi, Rockville, MD) was reduced to formazan by cellular dehydrogenases, generating a deep yellow colored formazan that was measured at 450 nm (650 nm as reference) in a Microplate reader. The color is directly correlated to cell number. Cells without bHK treatment were used as a control. Readings were then converted to percent absorbance, with control set at 100% and medium (negative control) at 0%.
Fluorescent Binding and Uptake of H2K4b and H2K4b-PEG-cRGD
C. albicans (ATCC 10231; 1 × 105 cells/ml) were added to each well of a four-chamber culture slide (BD Sciences, Bedford, MA) containing RPMI1640 with 20 mM MOPS (300 μl). After 4 h, fluorescently-labeled (CF750) peptides, H2K4b and H2K4b-PEG-cRGD, (final concentration, 100 ug/ml) were incubated with the cells for an additional 4 h at room temperature. The cells were then fixed with 4% paraformaldehyde and counterstained with cyto 9 (Invitrogen, Grand island, NY). After the coverslips were placed on the slides and sealed with cytoseal-60 (Richard-Allan scientific, Kalamazoo, MI), cell images were captured with a fluorescent Olympus IX81 microscope, fitted with a Hamamatsu Photonics C9100-02 EMCCD camera.
RESULTS
H2K4b-PEG-cRGD conjugates
Previous studies had shown that antifungal activity of bHK peptide was greater with increasing branching, up to the maximum of four branches. One of the most active species identified had a branch sequence that contained five repeats of a tripeptide with two histidines and one lysine, called H2K4b. The structures and sequences of H2K4b and H3K(H)4b peptides are shown in Figure 1. While these two anti-fungal bHK peptides have branches with several lysine residues, the vastly greater histidine content results in a macromolecule with physicochemical characteristics unlike the cationic anti-microbial peptides which bind and destabilize cell membranes with low specificity. However, the lysine content provides numerous primary amines for random conjugation by use of methodology we developed to generate cyclic RGD (cRGD) peptide ligand conjugates via large PEG linkers of polycation nanoparticle carriers of nucleic acids [18]. Conjugates of H2K4b and H3K(H)4b were prepared using this random attachment method with approximately four PEG-cRGD per macromolecule, labeled H2K4b-PEG-cRGD and H3K(H)4b-PEG-cRGD, respectively. Also, a control conjugate was prepared with only a PEG linker, labeled H2K4b-PEG.
Figure 1.
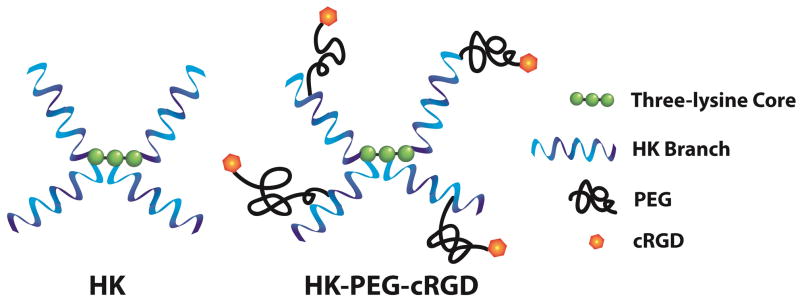
Schematic structure of unmodified and modified bHKP. The unmodified peptides consist of four histidine-lysine branches originating from the lysine core. The HK branches contained KHKHHKHHKHHKHHKHHKHK for H2K4b or KHHHKHHHKHHHHKHHHK for H3K(H)4b. Modified HK were similar except that PEG-cRGD conjugates were added randomly to the branches.
bHK-PEG-cRGD binding to αvβ3 integrins
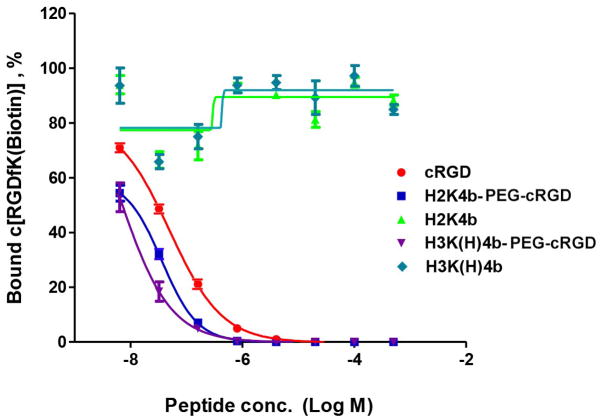
Competitive binding assays were used to assess the effect of the cRGD ligand conjugate on binding to immobilized αvβ3 integrin, shown in Figure 2. Whereas the non-ligand bHK peptides (H2K4b and H3K(H)4b) did not bind immobilized integrin, the ligand-conjugated H2K4b-PEG-cRGD and H3K(H)4b-PEG-cRGD showed higher affinity than free cRGD pentapeptide. Multivalency of cRGD ligands on the bHK peptides likely accounts for higher affinity for integrin compared to the free cRGD. Moreover, on the basis of data obtained from untargeted bHK peptides that show no competitive binding, cationic charge associated with the bHK-PEG-cRGD peptides had little contribution to integrin binding with this assay.
Figure 2.
Competitive inhibition of biotinylated RGD peptide binding to immobilized integrin αvβ3 by cRGD-conjugated and unconjugated HK peptides. Competitive binding curves were obtained from the interactions between immobilized αvβ3 and the commercially available c[RGDfK(Biotin-PEG-PEG)] peptide in the presence of the cRGD conjugates. Binding in the absence of competitor was set at 100%. Bound c[RGDfK(Biotin-PEG-PEG)] was detected by avidin-HRP and TMB substrate.
H2K4b-PEG-cRGD antifungal activity
The antifungal activity of these ligand-conjugates against two strains of C. albicans was determined and compared with that of the H2K4b parent macromolecule. In conditions favoring cell growth of candida shown in Figures 3 and 4, PEGylation of H2K4b resulted in complete loss of antifungal activity, whereas the cRGD-PEG conjugate showed increased potency(MIC:H2K4b-PEG-cRGD, 1.5×10−5 M; H2K4b>2.5×10−5 M). These effects were observed by both turbidity and WST-1 metabolic assays. Compared to unmodified H2K4b, the ligand conjugate exhibited an altered dose response curve for metabolic inhibition, with a sigmoid shape expected for a binding mediated effect, shown in Figure 4. These effects of ligand conjugation on fungal growth inhibition were the same for both fluconazole-sensitive (10231) and refractory (MYA-576) strains. Notably, the cRGD peptide had no inhibitory effect on the growth of C. albicans (data not shown).
Figure 3.
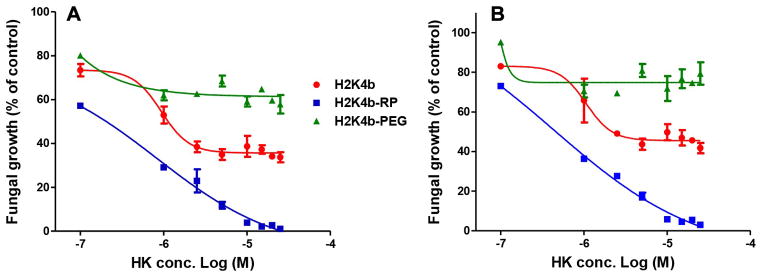
In vitro antifungal activities of H2K4b and conjugates against two different strains of C. albicans (A, 10231; B, MYA-576). C. albicans cells were cultured in a microplate with 100 μl of RPMI-1640 containing increasing concentrations of bHK peptides (0.1–25 μM) for 48h at room temperature. At the end of treatment, fungal growth was measured at 595 nm. Cells without peptide treatment were used as a control. H2K4b-RP represents H2K4b-PEG-cRGD.
Figure 4.
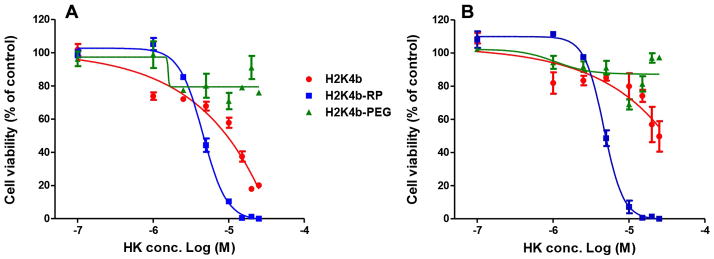
WST-1 cell viability assay. C. albicans cells (A, 10231; B, MYA-576) were cultured in a microplate with 100 μl of RPMI-1640 containing varying concentrations of bHK peptides for 48h at room temperature. At the end of the treatment, 10 μl of WST-1 assay solution were added to each well, and after 30 mins, the reduced formazan was measured at 450 nm (650nm as reference). Cells without peptide were used as control. H2K4b-RP represents H2K4b-PEG-cRGD.
In addition to the antifungal assays, we examined the antifungal effects of H2K4b-PEG-cRGD versus H2K4b with light microscopy. After overnight incubation of C. albicans at 37°C, which enables both hyphae and cell growth, there were marked differences observed between the H2K4b and H2K4b-PEG-cRGD treatment groups. Representative results, shown in Figure 5, illustrate the increased potency by ligand-targeting of H2K4b, as well as a shift in the effects on cells vs. hyphae. Whereas H2K4b significantly reduced the number of cells and hyphae, their growth in “nests” was still apparent. In contrast, there were only a few scattered hyphae in the H2K4b-PEG-cRGD treated group.
Figure 5.
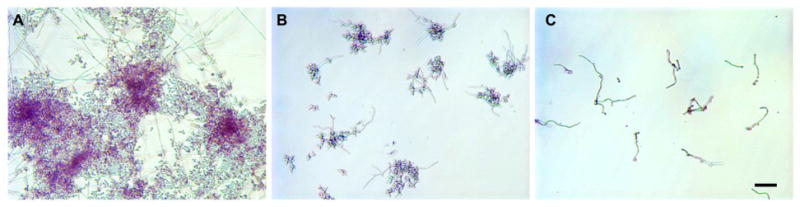
Effects of bHK peptides and conjugates on the growth of MYA576, a fluconazole-resistant C. albicans. Fungal cells were incubated with 20 μM of H2K4b or conjugates at 37°C for overnight. (A) untreated; (B) 25 μM of H2K4b; and (C) 25 μM of H2K4b-PEG-cRGD. Bar represents 20 μm.
Comparison of binding and uptake into C. albicans of targeted and non-targeted H2K4b
We compared the binding of fluorescently-labeled H2K4b and H2K4b-PEG-cRGD to the C. albicans. As shown in Figure 6(B) and (E), binding and uptake of H2K4b-PEG-cRGD to C. albicans were much greater than binding and uptake of H2K4b, consistent with greater potency of the targeted bHKP at this concentration.
Figure 6.
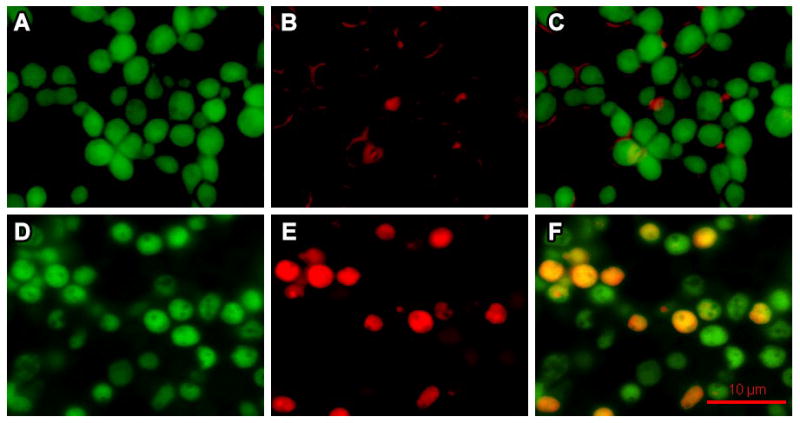
Comparison of binding and uptake into C. albicans with targeted and untargeted H2K4b. After fluorescent-labeled (CF750 - red) peptides, H2K4b and H2K4b-PEG-cRGD, (final concentration, 10 μM) were incubated with C. albicans for 4h at RT, the cells were fixed and counterstained with cyto 9. Cell images were then captured with a fluorescent Olympus IX81 microscope. (A, B, C), H2K4b; (D, E, F), H2K4b-PEG-cRGD; (A, D), cyto 9 counterstain; (B, E), labeled H2K4b and H2K4b-PEG-cRGD peptides; (C, F), merged images of labeled peptides with counterstain. The bar represents 10 μm.
H3K(H)4b-PEG-cRGD
Given that the cRGD-ligand enhanced the potency of H2K4b, we examined H3K(H)4b, which has a similar structure but higher content of histidine and has been used in studies with RNAi nanoparticles [20]. The results also showed an increase in antifungal activity of H3K(H)4b by ligand conjugation with the cRGD pentapeptide in both fluoconazole-sensitive and resistant strains, shown in supplementary Figure S1.
Discussion
The present study was designed to determine whether the biological antifungal activity of an emerging class of branched antimicrobial macromolecules composed of histidines and lysines could be enhanced by ligand-mediated targeting. Previous investigations have found αvβ3 integrin-like receptors on the surface of several pathogenic fungi, including C. albicans [9,10]. These integrins are up-regulated on cell surfaces and hyphae of candida species and appeared to have an important role in binding of candida to endothelial cells and invading tissues. We found that the ligand conjugated bHK peptides that targeted the integrins on the surface of C. albicans resulted in marked growth inhibition of the fungus. Although in this report we did not examine whether our ligand-targeted peptides reduced binding of the fungus to activated endothelial cells, it will be of interest to determine if the H2K4b-PEG-cRGD has a dual mechanism of action in reducing growth of C. albicans in vivo: a direct inhibitory or killing action as described here; and/or an indirect action, preventing C. albicans from interaction with vitronectin [10].
We also examined whether the random PEGylation conjugation method used to couple the peptide ligand to the antifungal bHK peptides affected the anti-fungal activity of this molecule observed in our previous study [12], by interfering with interactions between bHK peptides and the surfaces of fungi. PEGylation of proteins is well established to enhance pharmacology for therapeutic application, such as minimizing cytokine induction, reducing interaction with cell surfaces such as the cells of the reticuloendothelial system, thereby increasing the circulating half-life of the PEGylated proteins in the blood stream. Since it is possible that PEGylation might interfere with the activity of H2K4b, we synthesized and tested two different conjugates of this peptide, one with PEG alone (H2K4b-PEG) and the other with cRGD attached to H2K4b through the PEG (H2K4b-PEG-cRGD). Indeed, we observed that the PEG-5000 linker had a PEGylation effect when no cRGD targeting ligand was present, significantly diminishing the observed anti-fungal activity of H2K4b. Interestingly, the addition of ligand to the end of PEG linker enhanced its antifungal activity: the H2K4b-PEG-cRGD peptide exhibited more potent and more consistent anti-fungal activity than did the unmodified form. Differences in activity and binding suggest quantitative and perhaps qualitative differences in the inhibition of fungi, most likely through the integrin-like receptors reported in previous studies [9,10]. Moreover, we think that the most likely explanation for the opposing results is that the PEGylation interferes with the binding mechanism of unmodified bHK peptide interaction with the fungi whereas the cRGD ligand conjugate provides a new mechanism of interaction through the αvβ3 integrins. In support of this interpretation is the observation that fluorescently labelled cRGD conjugate exhibited far higher levels of uptake into fungal cells throughout the region stained with a nucleic acid selective dye. Importantly, another antifungal bHK peptide also showed a similar increase in potency and shift in antifungal activity by the integrin binding ligand conjugation. Thus, the judicious addition of cRGD-PEG conjugates to bHK peptides augmented their antifungal activity, and indicates that targeting to surface expressed fungal integrin homologs when PEG linkers are utilized is promising for enhanced pharmacology including enhanced localization at sites of fungal infection that is expected to enhance efficacy and therapeutic index. Although our studies demonstrated that the ligand-mediated antifungal activity was enhanced when using a relatively large PEG-5000 linker, future studies are required to determine whether PEG linkers of different molecular weights will further augment activity in vitro and in vivo.
In addition to the cRGD peptide, a sufficient cationic charge on the HK peptides is likely essential to maintain its antifungal activity. Random addition of –PEG-cRGD with the conjugation method used reduces the effective cationic charge on HK, and creates a steric barrier, which was found to reduce antifungal activity. Therefore, alternative conjugation approaches may provide better retention of antifungal activity, such as site-specific addition of the –PEG-cRGD conjugate or intracellular cleavage. The addition of a PEG-ligand to specific locations could be done by adding reactive amino acids (e.g., cysteine) within the peptide that would not be likely to affect the antifungal activity (e.g., cysteine attached to the C-terminal end of the lysine core and PEG is conjugated to the sulfhydryl group of cysteine). Our group modified several bHK peptides by this method so that technical problems are unlikely [20]. Alternatively, the fungal binding by conjugated bHK peptides could be maintained by adding more lysines at the N-terminal ends of the branches. Exploring several methods to add ligands to different bHK peptides may be important because of differences in their activity, lysine content, and safety profile.
Conclusions
This study investigated whether a small cyclic RGD peptide ligand selective for mammalian αvβ3 and αvβ5 integrins can facilitate delivery and antifungal activity of bHK peptides via integrins on the surface of C. albicans. The studies demonstrated conjugation of this ligand to bHK peptides could enhance antifungal activity, overcoming any adverse effects of the conjugation on the bHK peptide antifungal activity. This study is the first to show enhanced antimicrobial activity by ligand targeting, and opens the door to what may be an important new direction for addressing difficult to treat infections. Specifically for bHK peptide antifungal therapeutic candidates, on the basis of these and our previous results we anticipate that ligand-mediated targeting will provide a potent broad spectrum therapeutic for treatment of life threatening invasive candidiasis [12,13], and potentially many other invasive fungal infections that require parenteral treatment. Nonetheless further studies of this advance are needed to explore more fully the therapeutic potential. In addition to ligands targeting candida integrin, different ligands may be considered to enhance targeting and activity toward a broad range of pathogenic fungi [21], adding to the versatility of this approach.
Supplementary Material
Acknowledgments
The authors thank P. Talalay for careful reading and invaluable editing assistance of this manuscript. This work was supported by the National Institutes of Health (R01-CA136938).
ABBREVIATIONS
- bHK
branched histidine-lysine
- cRGD
cyclic peptide containing arginine-glycine-aspartic acid motif
- DIPEA
N,N-diisopropylethylamine
- DMSO
dimethyl sulfoxide
- HK
generic term for histidine-lysine peptides
- H3K(H)4b and H2K4b
two unmodified four-branched peptides that differ in their histidine and lysine content
- HRP
horseradish peroxidase
- Mal-PEG-SCM
maleimide-PEG-succinimidyl carboxylmethyl
- PEG
polyethylene glycol
- PEG-cRGD
conjugate of PEG and cRGD attached to a branched HK peptide
- TFA
trifluoroacetate
- TMB
3,3′,5,5′-tetramethylbenzidine
Footnotes
Declaration of Interest
PVS, YL, and MCW have financial interest and equity in Aparna Biosciences. PVS, YL, MCW, and AJM are interested in commercialization of the biomacromacromolecules in this manuscript. QL and S-TC declare no conflict of interest.
References
- 1.Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010;28:197–201. doi: 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez JA. Invasive fungal infections in the intensive care unit. Semin Respir Crit Care Med. 2010;31:79–86. doi: 10.1055/s-0029-1246289. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 5.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 6.Cappelletty D, Eiselstein-McKitrick K. The echinocandins. Pharmacotherapy. 2007;27:369–388. doi: 10.1592/phco.27.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Dockrell DH. Salvage therapy for invasive aspergillosis. J Antimicrob Chemother. 2008;61(Suppl 1):i41–44. doi: 10.1093/jac/dkm426. [DOI] [PubMed] [Google Scholar]
- 8.Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, et al. Non-albicans Candida species in blood stream infections in a tertiary care hospital at New Delhi, India. Indian J Med Res. 2013;136:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 9.Santoni G, Spreghini E, Lucciarini R, Amantini C, Piccoli M. Involvement of alpha(v)beta3 integrin-like receptor and glycosaminoglycans in Candida albicans germ tube adhesion to vitronectin and to a human endothelial cell line. Microb Pathog. 2001;31:159–172. doi: 10.1006/mpat.2001.0459. [DOI] [PubMed] [Google Scholar]
- 10.Spreghini E, Gismondi A, Piccoli M, Santoni G. Evidence for alphavbeta3 and alphavbeta5 integrin-like vitronectin (VN) receptors in Candida albicans and their involvement in yeast cell adhesion to VN. J Infect Dis. 1999;180:156–166. doi: 10.1086/314822. [DOI] [PubMed] [Google Scholar]
- 11.Leng Q, Mixson AJ. Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 2005;33:e40. doi: 10.1093/nar/gni040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Luther PW, Leng Q, Mixson AJ. Synthetic histidine-rich peptides inhibit Candida species and other fungi in vitro: role of endocytosis and treatment implications. Antimicrob Agents Chemother. 2006;50:2797–2805. doi: 10.1128/AAC.00411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verwer PE, Woodle MC, Boekhout T, Hagen F, Bakker-Woudenberg IA, et al. Cryptococcus and Trichosporon spp. are susceptible in vitro to branched histidine- and lysine-rich peptides (BHKPs) J Antimicrob Chemother. 2011;66:1649–1652. doi: 10.1093/jac/dkr175. [DOI] [PubMed] [Google Scholar]
- 14.Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, et al. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh K, Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol. 2004;56:285–289. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 16.Mochon AB, Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008;4:e1000190. doi: 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matejuk A, Leng Q, Begum MD, Woodle MC, Scaria P, et al. Peptide-based Antifungal Therapies against Emerging Infections. Drugs Future. 2010;35:197–210. doi: 10.1358/dof.2010.035.03.1452077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarem R, Newham P, Askari JA, Green LJ, Clements J, et al. Competitive binding of vascular cell adhesion molecule-1 and the HepII/IIICS domain of fibronectin to the integrin alpha 4 beta 1. J Biol Chem. 1994;269:4005–4011. [PubMed] [Google Scholar]
- 20.Chou ST, Leng Q, Scaria P, Kahn JD, Tricoli LJ, et al. Surface-Modified HK:siRNA Nanoplexes with Enhanced Pharmacokinetics and Tumor Growth Inhibition. Biomacromolecules. 2013;14:752–760. doi: 10.1021/bm3018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lionakis MS, Lahdenranta J, Sun J, Liu W, Lewis RE, et al. Development of a ligand-directed approach to study the pathogenesis of invasive aspergillosis. Infect Immun. 2005;73:7747–7758. doi: 10.1128/IAI.73.11.7747-7758.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.