Summary
Pairing two animals in parabiosis to test for systemic or circulatory factors from one animal affecting the other animal has been used in scientific studies for at least 150 years. These studies have led to advances in fields as diverse as endocrinology, immunology, and oncology. A variation on the technique, heterochronic parabiosis, whereby two animals of different ages are joined to test for systemic regulators of aspects of aging or age-related diseases also has almost a century-long scientific history. In this review, we focus on the history of heterochronic parabiosis, methodological considerations and caveats, and the major advances that have emerged from those studies, including recent advances in our understanding of stem cell aging.
Keywords: aging, anti-aging, heterochronic studies, longevity, mice, parabiosis
Introduction
Parabiosis [from the Greek ‘para’ (next to) and ‘bios’ (life)] refers to the condition in which two entire living animals are joined surgically and develop a single, shared circulatory system. The procedure is essentially a more complete form of ‘transbiosis’, ranging from the transplantation of cells, tissues, or entire body parts such as limbs or other appendages from one organism to another. At the beginning of the 20th century, Alex Carrel performed daring experiments that merged the boundaries between parabiosis and transbiosis. Carrel, who received a Nobel Prize for developing a novel method of blood vessel connection, removed multiple internal organs from cats and dogs and artificially ventilated the lungs until the blood vessels of such a ‘visceral organism’ were surgically connected to another animal, which provided a blood supply to both sets of organs. Carrel performed such experiments in his quest to understand organ aging and prolong life (Carrel, 1913), and he wrote, ‘Since the survival of entire organs outside of the body would undoubtedly have important physiological uses, I began in June, 1912, to develop a technique by means of which a system of organs could be made to live and functionate when separated from the other organs’. While the ‘visceral organisms’ did not live very long and were subjects to immune rejection, these studies led to the development of organ transplants and without a doubt influenced later studies of heterochronic parabiosis.
Unlike transbiosis, there are no formal donor and host in parabiosis as each animal can be viewed as an equal partner in the pairing, each influencing the other parabiont. The analogy is often made between parabionts and human or nonhuman conjoined twins, where the latter are result of abnormal developmental processes. Whereas the physiology of conjoined twins may be informative with regard to certain experimental questions addressed by parabiosis, clearly the experimental methods offered by parabiosis, especially the joining of two animals that differ genetically or physiologically, vastly expand the types of biological phenomena related to the circulatory milieu that may be investigated.
With the advent of immunosuppressants, many transplantation paradigms became widely successful. Cross-species grafts became possible and have been extremely valuable for understanding many developmental, physiological, and pathological processes. In contrast, parabiosis in mammals has only been successful when involving genetically identical or inbred animals (typically short-lived rodents). The development of high-throughput genomics and proteomics has thrust parabiotic studies into a new era, where this procedure could pave the way to the improved understanding of the systemic regulation of organismal aging.
Heterochronic parabiosis
Heterochronic parabiosis, the parabiotic pairing of two animals of different ages, provides an experimental system to test for systemic effects on the process of cell and tissue aging, the development of age-related diseases, or other age-related parameters including organismal longevity. We will review the development of heterochronic parabiotic studies from a historical perspective, leading up to recent advances. We will also provide detailed methodological considerations, with specific attention to unique aspects and interpretations of heterochronic studies.
Heterochronic parabiosis: historical perspective
The application of heterochronic studies using parabiosis followed the general development of parabiosis in the study of various physiological and pathological phenomena. In fact, interest in studies of the effects of parabiosis on the processes of normal aging and longevity may have emerged as much from the observation that parabiosis to healthy animals could extend the lifespan of animals that would otherwise succumb earlier due to disease or lethal treatment of some sort. For example, the lethality of irradiation was shown to be rescued by parabiotic pairings, by allowing survival during recovery of the intestinal epithelium and the hematopoietic systems (Finerty et al., 1952; Binhammer et al., 1953; Warren et al., 1960; Carroll & Kimeldorf, 1967, 1969), the latter occurring by the seeding of the hematopoietic system with cells from the healthy parabiont and thus providing evidence in support of the basic notion of stem cells being responsible for maintenance of hematopoiesis (Nisbet, 1973). Parabiosis to a healthy partner was shown to extend lifespan of mice with a form of muscular dystrophy (Hall et al., 1959). These and other comparable studies, while not specifically addressing basic mechanisms of aging, may have stimulated interest in the possibility that the dysfunctions associated with normal aging might likewise be rescued by parabiosis to a ‘healthy’, that is younger, partner and that lifespan itself might be amenable to prolongation by heterochronic parabiosis. In the following sections, we detail some of the key historical advances in heterochronic parabiosis, century by century.
19th century
Although experimentation with animal grafting may well have extended back into medieval if not ancient times, the most widely attributed earliest publication describing parabiotic pairings is the study, published in French and titled ‘Expériences et Considérations Sur la Greffe Animale’, by Bert (1864). This monograph was the publication of the author’s thesis work (De la Greffe Animale) for a Doctorate of Medicine for the Faculté de Médecine de Paris in 1863 and contains such intriguing historical citations as ‘An account of two successful operations for restoring a lost nose’ by an author named Carpue in London in 1816. In his 1864 monograph, Bert introduces the parabiotic surgery:
‘The experiments called by comparison ‘grafts by approach’ (‘Greffe par approche’), aimed and resulted in attaching one animal to the other through their skin, so as to create an exchange of nutrients by establishing a common circulatory system, and so that a more or less extended physiological and pathological connection results from the vascular connection.
The process is one the simplest: a strip of skin is removed along the opposite flanks of the two experimental animals; stitches and others handling systems that I described in my memoirs, maintain the animals attached and prevent frictions. Now, let’s see the main results’.
He then went on to show that fluid injected into a vein of one animal passed to a vein of the other animal, and he performed numerous autopsies and reported that vascular channels developed between the two animals. For his work, Bert was awarded the prize in Experimental Physiology by the French Academy of Science in 1866. Dr. Bert died on November 11, 1868, and an obituary published in the Boston Medical and Surgical Journal refers specifically to his work on grafting as well as his studies of high-altitude physiology and his colorful political career (Anonymous, 1886). Following on the studies of Bert, parabiosis was explored in the later decades of the 19th century and in the 20th century for the study of conditions ranging from cancer to dental caries (Finerty, 1952), but not specifically with regard to aging until the middle of the 20th century.
20th century
There are few reports of studies using parabiosis after the work of Bert until 1908 when Sauerbruch and Heyde reported successful parabiotic pairings (and coined the term ‘parabiosis’) (Sauerbruch & Heyde, 1908). The early decades of the 20th century saw a dissemination of the procedure and its application to numerous physiological studies (Schmidt, 1922).
To our knowledge, the earliest reported studies that used heterochronic parabiosis to study the regulation of lifespan were published in the late 1950s and early 1960s (Pope et al., 1956; McCay et al., 1957; Lunsford et al., 1963). These studies, while of limited statistical power and largely anecdotal, provided evidence of the benefit to the older parabiont in terms of both longevity and tissue function. These studies also included parabiosis of animals that had been subjected to caloric restriction (to the point of growth retardation), with animals fed ad libitum, and demonstrated a beneficial effect on lifespan extension. In 1971, it was reported that heterochronic parabiosis improved cholesterol metabolism of the older parabiont (Hruza, 1971). This was followed in 1972 by the first systematic study the effect of heterochronic parabiosis on lifespan (Ludwig & Elashoff, 1972). This report provided evidence of an extension of lifespan, particularly for female pairings, of the older parabiont in heterochronic pairings compared with unpaired or isochronically paired animals. In the latter part of the 20th century, heterochronic parabiosis was used to study aspects of the physiology of aging, published largely in the Russian literature (Butenko & Gubrii, 1980, 1981; Sidorenko et al., 1986; Gubrii et al., 1987; Butenko, 1990; Frol’kis et al., 1996).
21st century
In the early 21st century, we resurrected the use of heterochronic parabiosis to ask specific questions about the aging of somatic stem cells (Conboy et al., 2005). We assessed the function of the stem cells based on their ability to sustain or regenerate tissues, a process that universally declines with age in mammals. Specifically, we were interested to determine whether the decline in regenerative potential was due to irreversible, age-related changes in the stem cells themselves or whether stem cell functionality was instead primarily influence by the environment in which they resided. The results of these studies unequivocally pointed to the aged environment contributing substantially to the impaired regenerative potential of older individuals (Conboy et al., 2005). When exposed to youthful influences, aged stem cells adopted a more youthful potential, and when exposed to the influences of an aged systemic milieu, young stem cells lost regenerative potential (Conboy et al., 2005; Brack et al., 2007; Villeda et al., 2011). These studies also definitively confirmed, using genetic lineage tracing, that the tissue regeneration phenotype was due to the resident stem cells, not due to those that could potentially migrate to the tissue from the partner parabiont.
Heterochronic parabiosis: experimental design, methodology, and interpretations
Experimental design and methodology
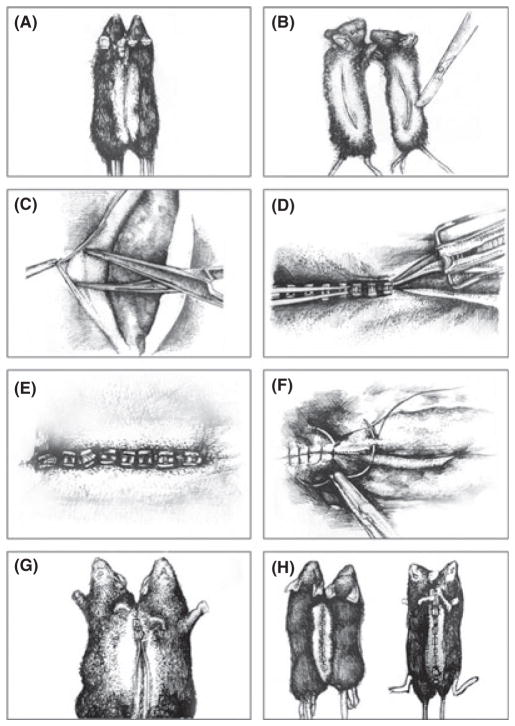
The techniques of establishing the parabiotic state are the same for heterochronic parabionts as for isochronic parabionts. These methodologies have evolved over time and have involved the surgical connection of different body parts. For example, in the studies of parabiosis by Andresen and colleagues, rabbits were joined parabiotically at the ears (Andresen et al., 1957). However, the most widely used approach is to connect two animals along their flanks as first described by Bert and refined by Bunster & Meyer (1933). The detailed description of Bunster and Meyer remains that basis of protocols used to this day. We have included an appendix to provide an example of a current protocol (Conboy & Conboy, 2009). The appendix contains a detailed description of the preparation, surgery (Fig. 1), and postoperative monitoring. We hope that this may be valuable for investigators considering using this technique to address fundamental questions of the biology of aging and longevity.
Fig. 1.
Illustrations of stages of the parabiosis surgery. Following all the requisite surgical preparations and shaving of the skin along the flanks of mice to be joined (A), a skin incision is made along the opposing flanks of each mouse (B). The skin is freed from the underlying peritoneal lining of each skin flap (C), being careful not to damage the peritoneum. With the mice side-by-side in a prone position, the dorsal skin flaps from the two mice are pinched together and stapled in a rostral-to-caudal fashion (D), until the dorsal flaps are stably joined (E). The connection is secured by suturing the corresponding joints (elbows and knees), using a suture passed through the soft tissues of each joint while avoiding passing through the joints themselves (F). With the pair flipped over to reveal the ventral skin flaps (G), the process of stapling the skin and suturing the joints is repeated, resulting in the generation of stable parabionts (H) (illustrations are adapted from photographs in Conboy & Conboy, 2009).
The general experimental design of studies of heterochronic parabiosis is to pair a young animal with an old animal, the specific ages determined by the parameters and definition of the study. While there are no agreed-upon ages that define ‘young’ or ‘old’ for any mammal, it is important to consider what parameters will be assessed in the study and characterize the trajectory of those parameters in a given tissue during distinct life phases. In particular, it would be important to avoid the confounding feature an ongoing, postnatal developmental changes still occurring in the ‘young’ parabiont. Ideally, whatever physiological parameters to be studied will have reached an adult steady state, from which age-related changes can be assessed. Likewise, there is no specific age that defines a border between adulthood and old age for any species. Heterochronic parabionts can be established between animals of any age, of course, but the interpretation of the results will vary depending on which phase each parabiont is in at the time of pairing. For ease of interpretation, it is probably best to choose ages that are not confounded by the profound changes that occur during postnatal development or that occur toward the end of life. Regardless, the controls for such experiments are isochronic parabiotic parings with animals of the then-defined ‘young’ and ‘old’ cohorts.
Specific challenges, caveats, and limitations of heterochronic parabiosis
Many of the technical and conceptual challenges of heterochronic parabiosis are identical to those of parabiosis in general, but accentuated primarily because the use of old animals has inherent challenges associated with aging in general and the increased risk of mortality. There are, however, certain issues that are unique to the heterochronic approach, and these are given due consideration.
Technical challenges
One of the major challenges of parabiotic experimentation is the well-described operative and perioperative mortality (McCay et al., 1957; Ludwig & Elashoff, 1972). The survival of parabionts has improved with better anesthesia and postoperative monitoring to the point where more than 90% of pairs recover from the procedure (our observation).
The other mortality risk, that is independent of the operation itself but central to the challenge of parabiotic pairing of animals, is the so-called ‘parabiotic disease’, originally termed ‘parabiotic intoxication’ (Finerty & Panos, 1951) that leads to death one to two weeks after surgery and coincides with the time that the vascular anastomoses are maturing to produce a single, shared circulatory system. For pairs that survive the postoperative period, the incidence of parabiotic disease can still be as high as 20–30%, even in highly inbred strains of mice and rats (Finerty, 1952). In parabiotic disease, one parabiont tends to become pale, anemic, and shriveled with the other parabiont appears swollen and plethoric (Finerty & Panos, 1951). Parabiotic disease may reflect the kinds of pathological processes that underlie graft-versus-host disease associated with organ transplantation, where the rejected ‘organ’ is the vascular anastomoses. Hilgard compared parabiotic intoxication between pairs of parental and F1 hybrid mice and found that the F1 typically developed the anemia (Hilgard et al., 1964). Lethal irradiation of the parental strain animal abrogated the parabiotic disease, indicating that the immune system of the plethoric partner was attacking the anemic partner, and also suggesting that the immune system found novel antigens in the F1. In our experience, parabiotic disease is at least as prominent in heterochronic parabionts as in isochronic parabionts, but surprisingly, it is the younger parabiont that is most commonly affected and, as described, becomes pale, shriveled, and anemic. We have not collected any quantitative data on this phenomenon in heterochronic parabiotic pairs, but ongoing studies will examine the immunopathology of mice succumbing to parabiotic disease, sacrificed when it is clear that the pairs will not survive.
Kinetic considerations
Multiple different surgical approaches have been used over the years to establish the parabiotic state but share the common feature of creating a state of tissue apposition between parabionts such that spontaneous vascular anastomoses develop at those points of tissue contact. Therefore, the ‘on-rate’ for any parabiotic effect is confounded by the fact that it takes 1–2 weeks for sufficient vascular connections to develop such that there is a common circulatory system as judged by chimerism of the blood cells, and that this rate appears to be similar in heterochronic parabionts as in isochronic parabionts (Conboy et al., 2005).
The study of the kinetics of the actual parabiotic effect depends of course on the specific physiological or pathological processes under investigation, whether in isochronic pairs or in heterochronic pairs, but the nature of the types of questions for which heterochronic parabiosis might address may generally involve more chronic processes. At the extreme is the use of heterochronic parabiosis to study lifespan extension, in which case the kinetics pose unique challenges, namely that both parabionts are aging, so the ‘effector’ parabiont age is not controlled. For example, it would be challenging to design experiments in which the ‘test parabiont’, that is, the animal whose lifespan was being measured, would undergo sequential parabiotic pairings to a series of young animals so as to provide a more continual exposure to a youthful systemic environment. Such an experimental design would suffer sequential risks of parabiotic intoxication, and it would be difficult to establish the appropriate nonaged, yet sequentially parabiosed, controls. However, given the suggestive extension of lifespan from one round of parabiosis (Ludwig & Elashoff, 1972), it is intriguing to consider how one might perform lifespan studies using heterochronic parabiotic techniques.
Future perspectives
It is clear that the application of parabiotic studies in the form of heterochronic parabiosis has been instrumental in addressing some of the fundamental questions about the systemic regulation of cell and tissue aging. Recent advances have opened new avenues of research in this area, most importantly the identification of factors that are carried in the circulation that can have ‘pro-aging’ or ‘anti-aging’ effects on cells and tissues. These have included effectors of the Wnt and TGF-β signaling pathways (Brack et al., 2007; Carlson et al., 2008), as well as cytokines with direct actions on stem cell populations (Villeda et al., 2011). Application of heterochronic parabiotic technology using genetically altered mouse strains with alterations in these pathways would allow for direct tests of these pathways and networks in regulating cell and tissue aging. Furthermore, the finding that heterochronic serum studies and blood transfusions can mimic aspects of heterochronic parabiosis in vitro and in vivo, respectively, is highly promising that the combinatorial factors in the circulation that regulate cell, tissue, and perhaps organismal aging are identifiable.
Conversely, studies of heterochronic parabiosis have provided a basis for understanding the epigenetic regulation of the cellular state that defines a cell as being ‘young’ or ‘old’. The isolation of cells exposed, in vivo, to heterochronic influences has revealed clear molecular changes that persist, at least for a time, following removal from these influences (Conboy et al., 2005; Brack et al., 2007). This clearly points to a kind of reprogramming, with a certain amount of ‘epigenetic memory’, that occurs in vivo in response to systemic influences. However, unlike the kind of reprogramming that occurs during induced pluripotent stem cell formation and also results in a ‘resetting of the aging clock’ (Rando & Chang, 2012), that which occurs during heterochronic parabiosis does not involve the loss of cellular differentiation characteristics. The cells continue to be of the same lineages as before parabiosis, but their regenerative performance becomes rejuvenated by the young blood milieu and aged by the old circulatory environment. Therefore, heterochronic parabiosis allows for the dissociation of ‘dedifferentiation’ from ‘rejuvenation’ and may provide an experimental system in which epigenetic features of aging and the maintenance of aging phenotypes can be investigated (Rando & Chang, 2012). Characterization of epigenetic profiles, including genome-wide DNA methylation patterns and chromatin-wide histone modification patterns, of cells exposed to heterochronic influences may allow for true molecular definitions of cellular age based on epigenetic states.
In summary, the development of heterochronic parabiosis to explore mechanisms and manifestations of the aging process has a long and distinguished history. As a powerful experimental system to probe aging at a cellular and molecular level, heterochronic parabiosis and related heterochronic methodologies are likely to continue to expand our knowledge and understanding of the basic mechanisms of aging, particularly related to the epigenetics of aging and rejuvenation.
APPENDIX. PARABIOSIS SURGERY
Institutional approval for parabiosis surgery
Submitting the protocol for parabiosis, and even more so for heterochronic parabiosis, for approval requires the inclusion of all the standard animal care, anesthetic, analgesic, surgical, and postsurgical methodologies plus considerable attention to the history and challenges of parabiosis surgery. The mortality rate is much higher than for standard survival surgeries, and this should be addressed. Evidence of training or other experience relevant to the surgical procedure is often very important. A clear outline of the historical use and uniqueness of the procedure to examine biological processes are essential for the justification of the procedure.
General methodology
This protocol is written for adult mice weighing 30–40 g. For aged animals, particularly if fed ad libitum all their lives, it will be necessary to adjust the specific details if they are considerably larger. The time to complete the surgery depends on the experience of the surgeon and can be as short as approximately 30 min. However, it generally requires up to twice as long for inexperienced surgeons, which is further reason to gain experience or training prior to initiating the protocol. The protocol described below is a variation of methods that have been described in detail previously, for both isochronic parabiosis and heterochronic parabiosis (McCay et al., 1957; Bunster & Meyer, 1933).
Anesthesia
Inhalation anesthetics (e.g., isoflurane) are desirable, with a separate nose cone for each animal, for more controlled anesthesia and more rapid recovery after surgery. Alternatively, standard injectable anesthetic anesthetics may be used (e.g., ketamine/xylazine).
Surgical preparation
Standard aseptic surgical procedures are used. The animals should be kept warm with a heating pad or with a carefully monitored heating lamp. Following the induction of anesthesia, the surgical site should be cleaned, and the hair should be shaved with an electric razor. Use of a commercial depilatory can also be used, followed by thorough rinsing with warm water. The exposed skin should then be disinfected.
Surgery
Approach
The decision as to which parabiotic surgical approach to use depends on the specific needs and experimental studies, as numerous approaches have been described. In particular, the decisions whether to join the peritoneums and how extensively to join the limbs are important because they can influence the duration of surgery and the stability of the pairings. We will describe a more extensive version for the purpose of detailing the various aspects of the surgical procedure.
Surgical procedure
Ophthalmic ointment is applied to the eyes to prevent drying during the procedure. A skin incision along the left side of one animal is made from lateral aspect of the elbow, along the flank, and to the lateral aspect of the knee. Free the skin from the subcutaneous fascia, keeping the tissues moist. The same incision is then made along the right side of the second animal. Place the animals in a supine position, side-by-side. Approximate the skin flaps and staple together using 7- or 9-mm wound clips, working from the middle outward and spacing the clips with less than a clip length between them. Carefully lift the pair and place them in a prone position. Join the limbs at the elbow and knee joints using 4–0 nylon monofilament suture with a curved tapered needle, placing the sutures slightly proximal to the joints and avoiding any joint constriction as the sutures are tied off. To join the peritoneal cavities, make small (~1 cm) incisions in the lateral peritoneum of each mouse, taking great care not to damage any viscera. The incisions should be just below the rib cage in the lateral abdomen. Using a tight spiral suture (6–0 absorbable vicryl or silk suture with a curved tapered needle), join the two peritoneal linings to prevent any visceral herniation. Now close the dorsal skin incision as with the ventral skin incision, using would clips to secure the pairing. For any incised areas of skin not well closed by the wound clips, close using 4–0 nylon or braided silk suture with a curved cutting needle. Clean surgical site gently.
Postsurgical care
Postsurgical survival
As noted in the text, the survival rate for parabionts can be very low, with as few as 50% of pairs surviving beyond 2–3 weeks because of operative and postoperative complications, most notably ‘parabiotic disease’ (see main text). Still, survival can be enhanced by careful postoperative monitoring.
Recovery from anesthesia
Mice should be monitored carefully to assure temperature regulation while recovering from anesthesia. Hydration may be provided by intraperitoneal injections of warm saline.
Infection control
A broad spectrum antibiotic (e.g., Baytril) should be administered once postoperatively. Pairs should then be monitored daily for any signs of infection, and antibiotics should be administered as needed. In our experience, surgical site infections are uncommon.
Analgesia
A single dose of a long-lasting analgesic (e.g., Buprenex) should be administered postoperatively following the full recovery from anesthesia. Subsequently, analgesics should be administered for any signs of distress, as per animal care facility guidelines.
Housing
Parabionts should be housed one pair per cage. Food, water, and hydrating gel should be made available on the floor of the cage for the first several days until the mice have returned to normal activity levels in terms of mobility and are clearly able to access food and water from the standard sources.
Removing the staples
When stable parabiosis appears to have been achieved approximately 2 weeks postoperatively, we generally remove the staples at that point. Parabionts are anesthetized as above, and the surgical area is cleaned and disinfected. The area should be examined for any signs of incomplete healing and, if necessary, should be sutured and perhaps restapled. Otherwise, if the area appears to be healed, all accessible staples should be removed, and stitches can also be removed at this time. Following recovery from anesthesia, parabionts are returned to their cage and monitored for return to normal activity.
Reversing the parabiotic pairings
For some applications and scientific questions, it may be necessary to reverse the parabiosis to observe the changes that occur (or those that persist) following parabiotic pairings. Also separating a pair with parabiotic disease will typically allow at least one partner to survive. This of course requires separate institutional approval as it constitutes a second survival surgery for each animal. The general considerations are the same as those described above for the parabiosis surgery, and below, we provide details of the surgery itself.
Anesthesia and surgical preparation are as described above. When full anesthesia of both mice has been achieved, the surgical site is cleaned, disinfected, and shaved. Remove any remaining staples. Make incisions along the dorsal skin and then the ventral skin where joined. Cut and remove sutures at the elbow and knee if they were connected. Carefully separate the peritoneal connection, if joined, and suture closed with 5–0 or 6–0 vicryl or silk suture, using a tapered needle as described above. Remove any scarred edges to the skin incisions and, as the skin may have stretched during the pairing, remove any gross excess, then suture or staple the skin closed for each animal. All postoperative care is as for the parabiosis surgery, and animals should be housed one individual per cage, at least until the skin heals.
References
- Andresen RH, Hass GM, Madden DA, Monroe CW. Postparabiotic tissue reactions of rabbits to musculofascial cross-transplants. J Exp Med. 1957;105:85–92. doi: 10.1084/jem.105.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Obituary - Paul Bert. Boston Med Sur J. 1886;115:485. [Google Scholar]
- Bert P. Expériences et Considérations Sur la Greffe Animale. J Anatomie Physiologie. 1864;1:69–87. [Google Scholar]
- Binhammer RR, Schneider M, Finerty JC. Time as a factor in postirradiation protection by parabiosis. Am J Physiol. 1953;175:440–442. doi: 10.1152/ajplegacy.1953.175.3.440. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bunster E, Meyer RK. An improved method of parabiosis. Anat Rec. 1933;57:339–343. [Google Scholar]
- Butenko GM. Active mechanisms of dysfunction in the process of aging. Vestn Akad Med Nauk SSSR. 1990;2:0–23. [PubMed] [Google Scholar]
- Butenko GM, Gubrii IB. Primary immune response in parabionts of different ages. Biull Eksp Biol Med. 1980;89:435–437. [PubMed] [Google Scholar]
- Butenko GM, Gubrii IB. Mechanism of immune response inhibition during parabiosis of animals of different ages. Biull Eksp Biol Med. 1981;92:318–319. [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel A. Concerning Visceral Organisms. J Exp Med. 1913;18:155–161. doi: 10.1084/jem.18.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll HW, Kimeldorf DJ. Protection through parabiosis against the lethal effects of exposure to large doses of x-rays. Science. 1967;156:954–955. doi: 10.1126/science.156.3777.954. [DOI] [PubMed] [Google Scholar]
- Carroll HW, Kimeldorf DJ. Mechanisms of protection against gastrointestinal and hematopoietic radiation lethality by parabiosis. Radiat Res. 1969;39:770–776. [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Conboy IM. Parabiosis in aging research and regenerative medicine. In: Parekkadan B, Yarmush M, editors. Methods in Bioengineering: Stem Cell Bioengineering. Norwood, MA: Artech House; 2009. pp. 125–142. [Google Scholar]
- Finerty J. Parabiosis in physiological studies. Physiol Rev. 1952;32:277–302. doi: 10.1152/physrev.1952.32.3.277. [DOI] [PubMed] [Google Scholar]
- Finerty JC, Panos TC. Parabiosis intoxication. Proc Soc Exp Biol Med. 1951;76:833–835. doi: 10.3181/00379727-76-18647. [DOI] [PubMed] [Google Scholar]
- Finerty JC, Binhammer R, Schneider M. Protection of irradiated rats by parabiosis. Tex Rep Biol Med. 1952;10:496–500. [PubMed] [Google Scholar]
- Frol’kis VV, Paramonova GI, Shaposhnikov VM. Effect of heterochronic parabiosis on liver microsomal enzyme oxidation. Dokl Akad Nauk. 1996;347:828–830. [PubMed] [Google Scholar]
- Gubrii IB, Reznikov AG, Demchenko VN, Butenko GM. Cessation of ovarian cycles in a young mouse while in parabiosis with an older one. Biull Eksp Biol Med. 1987;103:205–208. [PubMed] [Google Scholar]
- Hall CE, Hall O, Nevis AH. Prolongation of survival by parabiosis in strain 129 dystrophic mice. Am J Physiol. 1959;196:110–112. doi: 10.1152/ajplegacy.1958.196.1.110. [DOI] [PubMed] [Google Scholar]
- Hilgard HR, Cornelius EA, Dalmasso AP, Martinez C, Good RA. Immune mechanisms in parabiosis intoxication. J Exp Med. 1964;119:567–579. doi: 10.1084/jem.119.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruza Z. Increase of cholesterol turnover of old rats connected by parabiosis with young rats. Exp Gerontol. 1971;6:103–107. doi: 10.1016/0531-5565(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans NY Acad Sci. 1972;34:582–587. doi: 10.1111/j.2164-0947.1972.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Lunsford WR, McCay CM, Lupien PJ, Pope FE, Sperling G. Parabiosis as a method for studying factors which affect aging in rats. Gerontologia. 1963;7:1–8. doi: 10.1159/000211170. [DOI] [PubMed] [Google Scholar]
- McCay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7–17. doi: 10.1159/000210677. [DOI] [PubMed] [Google Scholar]
- Nisbet NW. Parabiosis in immunobiology. Transplant Rev. 1973;15:123–161. doi: 10.1111/j.1600-065x.1973.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Pope F, Lunsford W, McCay CM. Experimental prolongation of the life span. J Chronic Dis. 1956;4:153–158. doi: 10.1016/0021-9681(56)90015-7. [DOI] [PubMed] [Google Scholar]
- Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbruch R, Heyde M. Ueber Parabiose kunstlich vereinigter Warmbliiter. Munch Med Wchnschr. 1908;55:153–156. [Google Scholar]
- Schmidt G. Stand und Ziele der Parabioseforschung, auf Grund eigener Untersuchungen. Deutsche Ztschr F Chir. 1922;171:141–156. [Google Scholar]
- Sidorenko AV, Gubrii IB, Andrianova LF, Macsijuk TV, Butenko GM. Functional rearrangement of lymphohemopoietic system in heterochronically parabiosed mice. Mech Ageing Dev. 1986;36:41–56. doi: 10.1016/0047-6374(86)90137-5. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Chute RN, Farrington EM. Protection of the hematopoietic system by parabiosis. Lab Invest. 1960;9:191–198. [PubMed] [Google Scholar]