Abstract
Alcohol intake is one of the few modifiable risk factors for breast cancer. Current alcohol intake has been associated with mammographic density, a strong intermediate marker of breast cancer risk, though few studies have examined the effect of both current and average lifetime alcohol intake. We interviewed 262 participants from a New York birth cohort (born 1959–1963) and obtained mammograms from 163 (71.5% of participants with a mammogram). We collected information on alcohol intake by beverage type separately for each decade of life. We used multivariable linear models to assess the associations between current and average lifetime alcohol intake and mammographic density using a quantitative measure of density from digitized images. Overall, current alcohol intake was more strongly associated with mammographic density than average lifetime alcohol intake; compared with nondrinkers, those with current intake of seven or more servings per week had on average 12.3% (95% CI: 4.3, 20.4) higher density, adjusted for average lifetime alcohol intake, age, and body mass index. We observed a consistent inverse association for red wine intake and mammographic density, suggesting that the positive association between mammographic density and overall alcohol intake was driven by other types of alcoholic beverages. Our findings support an association between current alcohol intake and increased mammographic density independent of the effect of average lifetime alcohol intake. If replicated, our study suggests that reducing current alcohol consumption, particularly beer and white wine intake, may be a means of reducing mammographic density regardless of intake earlier in life.
Keywords: Alcohol, Mammographic density, Breast cancer, Risk factors, Life course
Introduction
Moderate alcohol intake has been consistently associated with increased risk of breast cancer in numerous epidemiologic studies, as well as in animal models (reviewed in [1-4]). Some studies suggest that the association between cumulative lifetime alcohol intake and breast cancer risk may be more relevant to risk than measures of current intake [5, 6]. Mammographic density, defined as the proportion of the breast that is composed of dense tissue (epithelial, stromal tissue, and collagen/connective tissue) compared to the overall breast area [7-9], is one of the strongest intermediate markers of breast cancer risk (reviewed in [9-11]). Studies that have examined the association between alcohol use and mammographic density have primarily focused on current alcohol intake. Some studies support a weak but significant positive association between current alcohol intake and mammographic density in both pre and postmenopausal women [12-14]. Other studies report a non-significant positive trend [15-18] or a null association [19-22]. There is more limited data on the association between timing of alcohol intake and beverage type and mammographic density, and little to no data on the association between beverage type at different life periods and mammographic density. We examined mammographic density and alcohol intake during different life periods (≤20, 20s, 30s, and 40s) by specifically asking about beverage type consumption, frequency, and quantity, during each life period in addition to current intake in participants of The New York Women’s Birth Cohort, an adult follow-up of members of a New York City birth cohort born between 1959 and 1963.
Methods
Study population
This study used data from The New York Women’s Birth Cohort, an adult follow-up study of women enrolled in the New York site of the National Collaborative Perinatal Project (NCPP) [23]. Between 1959 and 1963, 2,138 births at Columbia Presbyterian Hospital were included in the NCPP; including 1,026 female infants of which 841 (82%) were followed until age seven. These 841 girls made up the eligible cohort for adult follow-up. Girls who dropped out of the cohort before age seven were not eligible because of incomplete baseline data and lack of updated address information [24].
Baseline data were collected prospectively by a standardized NCPP protocol and included prenatal (parental age, maternal height and pre-pregnancy weight, weight gain during pregnancy, parity, smoking, race, socioeconomic status [SES], pregnancy conditions) and birth and postnatal data (child weight, height, and head circumference taken by direct measurement at birth, infancy, and childhood until age 7).
Adult follow up and mammogram collection
In 2001, we initiated adult follow-up of NCPP female participants [24]. Of the 841 eligible girls, we were able to obtain paper records with contact information for 779 (93.0%) and successfully traced 375 (44.5%). Of those traced, 262 (70.1%) completed the study questionnaire. The remainder did not participate because of failure to complete the questionnaire (n = 76, 20%), refusal (n = 18, 4.8%), illness (n = 3, 0.8%), and death (n = 16, 4.3%).
The study questionnaire was self-administered and contained questions on adult body size (height at 20, current and weight at 20, 30, 40, current), sociodemographic characteristics (education, occupation, marital status, income, race), adult health and reproductive events (age at menarche, fertility and hormonal medications, and pregnancy history), physical activity, as well as a detailed history of tobacco and alcohol intake.
For current alcohol intake (defined as average weekly intake within 6 months of questionnaire), we calculated total servings per week based on frequency of consumption and number of servings per time alcohol was consumed. Participants also reported the average number of times they drank per week and number of servings by beverage type (beer, red wine, white wine, hard liquor) for 4 time periods: prior to age 21, 21–29, 30–39, and 40 and greater. For average lifetime alcohol intake, we used the average reported alcohol consumption between the ages of 21 and 39. We calculated grams per day using a standard conversion of 13.2 grams of ethanol per 12-ounce serving of beer, 11.7 grams of ethanol per 4-ounce serving of wine, and 14.1 grams of ethanol per 1.5-ounce serving of hard liquor [25].
Mammographic density assessment
Two hundred twenty-eight (87%) participants reported ever having had a mammogram, and of these 166 (72.8%) provided completed medical release authorization forms. We successfully obtained films for 163 women, but excluded 12 (11 due to poor image quality, 1 taken after breast cancer diagnosis); our analyses are based on a sample of 151. Films were digitized using a Kodak Lumisys Film Digitizer (Kodak LS85). Mammographic density assessments were made using Cumulus, a computer-assisted thresholding program [8] in which the reader outlines the total breast area and dense area, and the software measures their size by identifying the number of pixels within the outlined areas. We calculated absolute breast area and dense area by converting the measure in pixels to cm2. Percent mammographic density was calculated as dense area divided by breast area multiplied by 100. All density assessments were performed blinded to exposure status and date and chronology of films if multiple dates were available.
All films for a participant were read together in random order by date, and 10% of the films were read twice. The Pearson correlations for repeated films were 0.87 for breast area, 0.93 for dense area, and 0.90 for percent mammographic density. All available cranio-caudal (CC) films for a participant were read, and analyses are based on the film taken closest to the date of questionnaire, using the left CC (others have previously reported a very high correlation between left and right side breast density measures in the range of 0.92–0.96 [26]). The study was approved by the Internal Review Board at Columbia Medical Center.
Statistical analyses
We assessed the correlation between servings of alcohol by beverage type and life period using Pearson correlation coefficients for continuous measures of grams per day. We used linear regression models to evaluate the association between mammographic density and current alcohol intake (0, 1–6, and 7 or more drinks per week) and average lifetime alcohol intake (0, ≤4, >4 grams per day). We used multivariable linear regression to simultaneously model average lifetime and current alcohol intake, adjusted for age and body mass index (BMI). In addition, we explored the impact of alcohol consumption by period of intake. For analyses by beverage type, we evaluated each type of alcohol by itself, and then all types per life period simultaneously adjusted for age and BMI. We defined statistical significance as associations having a two-sided P-value less than 0.05.
We assessed confounding of the association between mammographic density and alcohol intake by entering potential confounders individually into age-adjusted models. Confounding was assessed separately for current and average lifetime alcohol intake. Variables were determined to be confounders if their inclusion in the model changed the parameter estimates for the association between alcohol intake and mammographic density by more than 10 percent. Potential confounders included current BMI (kg/m2), physical activity, cigarette smoking, use of oral contraceptives (OC) and hormone replacement therapy (HRT), menopausal status, age at first full term pregnancy and nulliparity, race, first degree family history of breast cancer, breastfeeding, education and annual household income. In addition to the main outcome measure of percent mammographic density, we ran supplemental models with absolute dense tissue area (cm2).
Results
We did not observe significant differences in current and average lifetime alcohol intake between participants who reported never having a mammogram (n = 34), those who had a mammogram and provided authorization for release of mammograms (n = 166), and those who had a mammogram and did not provide authorization for release of mammograms (n = 62) (data not shown). Among participants, 54% reported current drinking; 9% consumed 7 or more servings per week of alcohol, and 45% consumed 1–6 servings/week (Table 1). We assessed the correlation between servings per beverage type by life period of intake and observed the strongest correlations for red wine intake between adolescence and twenties (r = 0.73, P < 0.0001), and hard liquor between adolescence and twenties (r = 0.94, P < 0.0001) and twenties and thirties (r = 0.84, P < 0.0001).
Table 1.
Descriptive statistics and current and lifetime alcohol intake among New York Women’s Birth Cohort participants
| N | % | Mean | (SD) | |
|---|---|---|---|---|
| Age at mammogram | 151 | 42.38 | (2.11) | |
| Body mass index (kg/m2) | 145 | 27.60 | (6.50) | |
| Race | ||||
| Non-hispanic white | 39 | 25.8 | ||
| Non-hispanic black | 52 | 34.4 | ||
| Hispanic | 60 | 39.8 | ||
| Menopausal status | ||||
| Premenopausal | 137 | 90.7 | ||
| Postmenopausal | 14 | 9.3 |
| Servings per week |
||||
|---|---|---|---|---|
| Alcohol | N | % | Mean | (SD) |
| Current alcohol use | ||||
| 0 Drinks/week | 69 | 46.0 | ||
| 1–6 Drinks/week | 67 | 45.0 | ||
| >7 Drinks/week | 14 | 9.0 | ||
| Lifetime alcohol | ||||
| Nondrinker | 44 | 30.0 | ||
| Ever | 107 | 70.0 | 5.89 | (9.09) |
| Prior to age 21 | ||||
| Nondrinker | 73 | 48.3 | ||
| Ever drinker | 78 | 51.7 | 5.85 | (9.96) |
| Beer | 54 | 69.2 | 4.14 | (6.93) |
| Red wine | 18 | 23.1 | 1.11 | (1.68) |
| White wine | 22 | 28.2 | 1.41 | (2.05) |
| Liquor | 51 | 65.4 | 3.56 | (7.95) |
| Twenties (age 21–29) | ||||
| Nondrinker | 52 | 34.4 | ||
| Ever drinker | 99 | 65.6 | 5.80 | (10.50) |
| Beer | 58 | 58.6 | 4.72 | (9.13) |
| Red wine | 35 | 35.4 | 1.54 | (2.89) |
| White wine | 45 | 45.5 | 0.85 | (0.66) |
| Liquor | 74 | 74.7 | 2.81 | (6.71) |
| Thirties (age 30–39) | ||||
| Nondrinker | 54 | 35.8 | ||
| Ever drinker | 97 | 64.2 | 5.39 | (8.41) |
| Beer | 55 | 56.7 | 3.16 | (4.67) |
| Red wine | 47 | 48.5 | 2.68 | (4.75) |
| White wine | 55 | 56.7 | 1.33 | (1.61) |
| Liquor | 68 | 70.1 | 2.21 | (4.10) |
| Forties (age 40 and above) | ||||
| Nondrinker | 64 | 46.4 | ||
| Ever drinker | 74 | 53.6 | 4.37 | (6.42) |
| Beer | 49 | 66.2 | 1.42 | (2.62) |
| Red wine | 37 | 50.0 | 2.40 | (3.99) |
| White wine | 51 | 68.9 | 1.55 | (3.34) |
| Liquor | 60 | 81.1 | 0.74 | (1.47) |
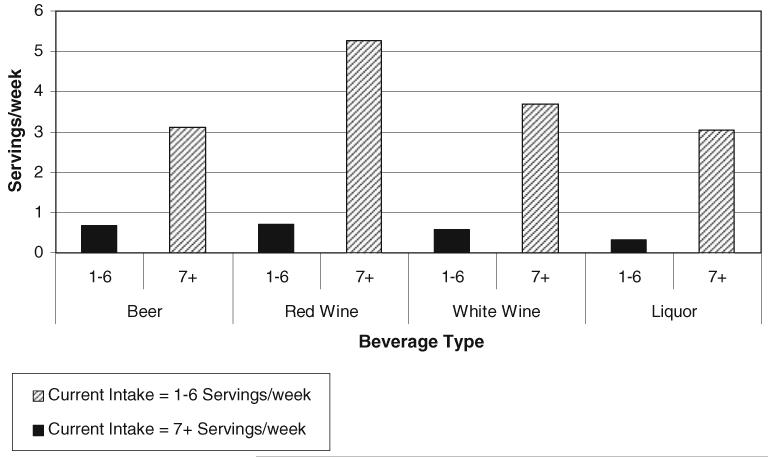
We observed differences in beverage type between lighter current drinkers (1–6 servings per week) and heavier drinkers (seven or more servings per week). Figure 1 presents the mean servings per week of each beverage type in the 40s among current lighter drinkers (1–6 servings per week) and heavier drinkers (seven or more servings per week). The most common beverage types among heavier drinkers included red wine (mean = 5.3 servings per week) and white wine (mean = 3.7 servings per week) compared to other beverage types. Lighter current drinkers drank significantly fewer servings of alcohol overall, and were more likely to drink red wine (mean = 0.71 servings per week) and beer (mean = 0.68 servings per week) compared to other beverage types.
Fig. 1.
Mean current alcohol intake (servings/week) by beverage type drank in 40s, New York Women’s Birth Cohort
Table 2 presents the results of linear regression analysis of the relationship between current and average lifetime alcohol intake and mammographic density. Current alcohol intake of seven or more servings per week had a significant, positive association with mammographic density, even after adjustment for age and BMI. The association remained after further adjustment for average lifetime alcohol intake; those who drank seven or more servings per week of alcohol had on average 12.3% (95% Confidence Interval (CI) = 4.3–20.4) higher mammographic density and 16.3 cm2 (95% CI = 5.6–27.1) higher dense area compared to nondrinkers.
Table 2.
Current and lifetime alcohol intake and mammographic density, New York Women’s Birth Cohort
| Percent density Age-adjusted |
Percent density Age and BMI adjusted |
Absolute dense area (cm2) Age and BMI adjusted |
||||
|---|---|---|---|---|---|---|
| βa (95% CI) |
βb (95% CI) n = 145 |
βa (95% CI) |
βb (95% CI) n = 145 |
βa (95% CI) |
βb (95% CI) n = 145 |
|
| Current alcohol intake (drinks per week) | n = 150 | n = 145 | n = 145 | |||
| 1–6 Drinks:0 Drinks | 0.82 (−3.71, 5.34) | −0.83 (−5.67, 4.01) | 0.75 (−3.61,5.10) | −0.09 (−4.79, 4.60) | 2.71 (−3.11, 8.54) | 2.21 (−4.06, 8.49) |
| 7+ Drinks:0 Drinks | 11.92 (4.19, 19.64) | 11.97 (3.79, 20.16) | 11.36 (3.75,18.97) | 12.32 (4.28, 20.36) | 14.16 (3.98, 24.34) | 16.33 (5.58, 27.07) |
| Average lifetime alcohol intake (grams per day) | n = 151 | n = 146 | n = 146 | |||
| ≤4 Grams/day:0 Grams/day | 5.99 (0.46, 11.52) | 6.25 (0.45, 12.05) | 3.75 (−1.68, 9.18) | 3.88 (−1.82, 9.58) | 4.63 (−2.63, 11.88) | 3.56 (−4.06, 11.18) |
| >4 Grams/day:0 Grams/day | 3.84 (−1.57, 9.26) | 1.80 (−3.95, 7.55) | 1.62 (−3.74, 6.99) | −0.40 (−6.07, 5.27) | 0.62 (−6.56, 7.79) | −2.67 (−10.24, 4.91) |
Current and lifetime alcohol modeled individually
Current and lifetime alcohol modeled simultaneously
For average lifetime alcohol consumption, low to moderate consumers (≤4 grams per day) had on average 6.0% higher density (95% CI = 0.5, 11.5) than nondrinkers, adjusted for age. The association remained significant after adjusting for current intake. However, after adjusting for BMI, the association between mammographic density and moderate average lifetime alcohol was attenuated and not statistically significant (β ≤ 4 grams per day = 3.8, 95% CI = −1.7, 9.2 relative to nondrinkers). There was no association with higher levels of average lifetime alcohol consumption (β >4 grams per day = 1.6, 95% CI = −3.7, 7.0 relative to nondrinkers, adjusted for age and BMI). We observed a similar, but not statistically significant, pattern for linear regression when the outcome was absolute dense area.
We investigated the association between alcohol intake in different periods of life (prior to age 21, 21–29, 30–39, and 40 and greater) and mammographic density. Alcohol intake prior to age 21 (any versus none) had a borderline significant inverse association with mammographic density, adjusted for age and BMI (β = −4.2, 95% CI = −8.5, 0.1). Overall alcohol intake from age 21 to 29 was not associated with mammographic density. Overall intake from age 30 to 39 was significantly positively associated with mammographic density although after adjusting for BMI the association was not significant (data not shown).
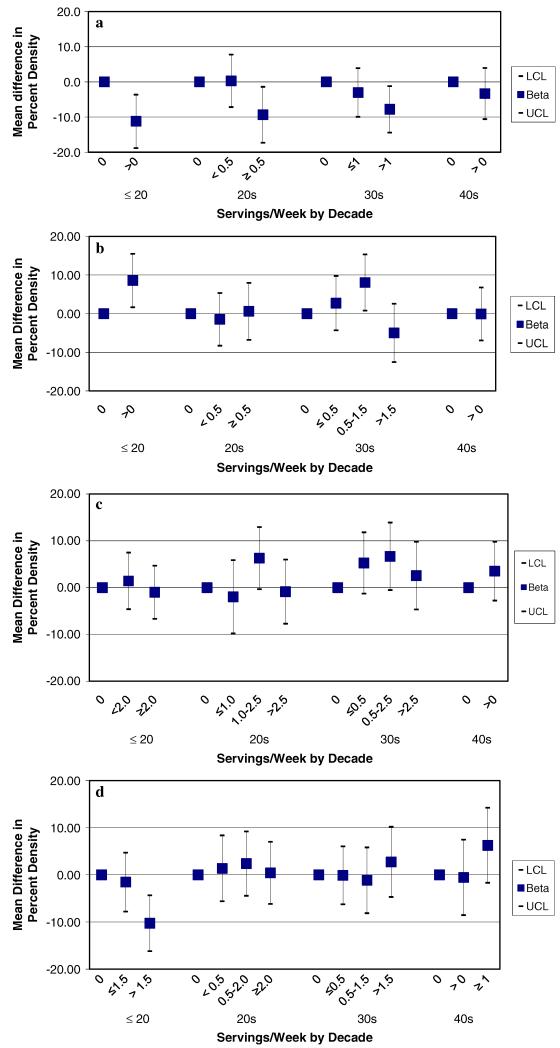
We assessed the association between alcohol consumption in different life periods and mammographic density according to type of beverage consumed (Fig. 2, Panel a–d). We observed a consistent statistically significant inverse association for red wine consumption prior to age 21, 21–29 and 30–39 (Fig. 2, Panel a). White wine consumption prior to age 21 and in the thirties was associated with increased mammographic density, although we did not observe a dose response for intake in the thirties (Fig. 2, Panel b). Beer intake in the twenties and thirties was associated with elevated mammographic density; however the association was not statistically significant and we did not observe the same pattern for intake prior to age 21 and in the forties (Fig. 2, Panel c). Hard liquor prior to age 21 had a statistically significant inverse association with mammographic density; however it was not significantly associated with mammographic density in other life periods. We did observe elevated mammographic density for hard liquor intake in the forties, although this was not statistically significant (Fig. 2, Panel d).
Fig. 2.
a Red Wine Intake in Adolescence, 20s, 30s, 40s and Mammographic Density, New York Women’s Birth Cohort. *Adjusted for age, BMI, and intake of other alcoholic beverage types (Beer, White Wine, Hard Liquor). b White Wine Intake in Adolescence, 20s, 30s, 40s and Mammographic Density, New York Women’s Birth Cohort. *Adjusted for age, BMI, and intake of other alcoholic beverage types (Beer, Red Wine, Hard Liquor). c Beer Intake in Adolescence, 20s, 30s, 40s and Mammographic Density, New York Women’s Birth Cohort. *Adjusted for age, BMI, and intake of other alcoholic beverage types (Red Wine, White Wine, Hard Liquor). d Hard Liquor Intake in Adolescence, 20s, 30s, 40s and Mammographic Density, New York Women’s Birth Cohort. *Adjusted for age, BMI, and intake of other alcoholic beverage types (Beer, Red Wine, White Wine)
Supplemental models using absolute density as the outcome resulted in similar inferences. When we stratified our linear models by race, current alcohol intake of seven or more servings per day was positively associated with mammographic density in all racial groups, but was only statistically significant among white and black participants (data not shown).
Discussion
We observed a positive association between current alcohol intake (seven or more servings per week) and percent and absolute mammographic density; the association remained after adjusting for BMI and additionally for average lifetime alcohol intake. Average lifetime alcohol intake was not associated with mammographic density after adjusting for current alcohol intake, BMI and age. Overall, the positive association we observed for current intake is consistent with much of the literature on alcohol and mammographic density [12-18], although the association was not statistically significant in four of these studies [15-18], and an additional four studies reported a null association [19-22]. The majority of the studies on alcohol intake and mammographic density (excluding [18], [15]) assessed current intake and did not account for patterns of earlier exposure.
We assessed the association between patterns of alcohol consumption and mammographic density by examining intake by decade of life. Alcohol consumption prior to age 21 was inversely associated with mammographic density in our sample. This conflicts with results from Vachon et al. [18], who observed a nonsignificant higher density in women who were moderate/heavy drinkers in adolescence compared to low/nondrinkers. Their study had a low proportion of adolescent drinkers (10.8% compared to 51.7% in our sample). We found that for other life periods, alcohol intake was generally positively associated with mammographic density; however most associations were not significant after adjusting for BMI. This finding was consistent with results from Herrinton et al. [15], who reported that moderate drinkers, with intake of 14–51 grams per week, were more likely to have higher density compared to infrequent and nondrinkers, regardless of the timing of intake (age 21–30, 30–49, and 50). Heavier drinkers also had a higher prevalence of higher mammographic density prior to age 50.
We found some differences in the effect of alcohol on mammographic density by beverage type. We observed significantly lower mammographic density for hard liquor intake prior to age 21, but not for other life periods, while we observed elevated density for beer intake in the twenties and thirties, white wine intake in the thirties, and hard liquor intake in the forties. Vachon et al. [17] observed a significant positive association between current white wine intake and density among postmenopausal women, but did not observe significant patterns for beer or liquor consumption. It is possible that some of our results are due to chance given the multiple comparisons; these observations should be investigated in larger populations.
We observed a consistent inverse association between red wine intake at multiple life periods (<21, 21-29, 30–39) and mammographic density. The association remained after adjusting for current intake, BMI, and concurrent intake of other beverage types. Vachon et al. [17] also reported a significant inverse association between current red wine intake and mammographic density, but the association was restricted to postmenopausal women. In contrast, Masala et al. [13] observed a positive association between wine intake and density, although they do not report the type of wine. Studies are mixed in regard to the association between red wine intake and breast cancer risk. While some studies support a positive association between exposure to any type of alcohol and breast cancer [2], there is evidence that red wine may be protective against breast cancer because it contains the polyphenol resveratrol, which may prevent cellular proliferation and have chemopreventive properties [27]. It is plausible that this would be reflected in reduced mammographic density. We were unable to evaluate the effect of consumption of only red wine because fewer than 5% of our sample drank only red wine in any life period, and none drank only red wine overall.
We observed a stronger association between current alcohol consumption and mammographic density compared to average lifetime consumption. One plausible explanation for the stronger association is measurement error because, as with other epidemiologic studies, we relied on self-report of alcohol intake. However, we expect that any measurement error would be non-differential with respect to the outcome, as there is no reason to suspect that women report differently based on their mammographic density. Such non-differential misclassification may be stronger for past lifetime measures of alcohol than for current measures, which may explain in part the lack of an observed association between average lifetime alcohol intake and density. In addition, density was read by a trained reader blinded to exposure, and had a high reliability. Therefore, any measurement error of outcome would also be nondifferential with respect to exposure, and the true association between current alcohol and mammographic density may thus be even stronger.
Further, it is unlikely that the positive association we observed can be explained by residual confounding, as residual confounders would also have to mimic the patterns we observed. We found that only BMI and age were confounders of this association in our data, and the association remained after adjustment for these variables.
Alcohol intake may impact breast cancer risk through mammographic density as well as other pathways. The lack of an association between lifetime alcohol intake and mammographic density should not be taken to indicate that cumulative lifetime exposure to alcohol does not affect breast cancer risk. Alcohol may play a role in multiple stages of carcinogenesis and may act as both an initiator and promoter; potential mechanisms include acetaldehyde, a product of alcohol metabolism, which may be carcinogenic, and the potential for alcohol to increase cellular susceptibility to genetic damage and mutation and enhance carcinogenicity of other substances, affect nutrient levels, influence hormone levels and activate enzymes, and impact cellular proliferation [1, 2, 28, 29]. Some studies [2, 30] suggest that alcohol intake may impact mammographic density by altering insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 3 (IGF-BP3) levels, which are associated with both alcohol intake [30] and mammographic density [31]. Further, there is evidence that several factors may modify susceptibility to alcohol intake in the population, including folate [2, 32-34] and other nutrients, genetic factors (e.g. alcohol metabolizing genotype) [2, 35], BMI [2], HRT use [2, 16], and menopausal status [17].
Our study provides further support for an association between current alcohol intake and mammographic density, an important intermediate marker of breast cancer risk. In the 2005 NHANES study, 63.3% of US women age 25–44 reported current drinking [36]. Given the potential cardiovascular benefits of moderate alcohol consumption, in addition to the potential cancer risk, current recommendations are for women to limit alcohol consumption to one drink per day [1, 37]. Revision of these guidelines to specify beverage type may be needed.
These results suggest that modifying alcohol intake may represent a plausible way to change density. Kerlikowske et al. reported that changing a BI-RADS (Breast Imaging Reporting and Data System) category in density is associated with a change in breast cancer risk [38]. To date, only HRT and Tamoxifen have demonstrated an effect on changing density in prospective studies [39], and only HRT has been shown to change density by a BI-RAD category [40].
We observed a strong association between heavier current alcohol intake and mammographic density, with a significant 12.3% higher mean density in heavier drinkers compared to nondrinkers. The association between alcohol and density may be driven by specific beverage types, as heavier drinkers were more likely to drink white wine. However, given the inverse association between red wine and density (adjusted for other beverage types), and that heavy drinkers also drank a substantial amount of red wine (5.3 servings per week), it is possible that changing the type of alcohol consumed rather than the total amount could affect density. These results need to be replicated in larger prospective studies so that we can better understand whether altering drinking patterns, and in particular beverage type, may reduce mammographic density and ultimately breast cancer risk.
Acknowledgments
We would like to acknowledge the following individuals for their contributions to this study: Drs. Ezra Susser and Victor Grann, and Tara Kalra. Funded by grants nos. DAMD170210357 and K07CA90685 from the Department of Defense Breast Cancer Research Program and the National Cancer Institute, respectively.
Contributor Information
Julie D. Flom, Department of Epidemiology, Columbia University, Mailman School of Public Health, 722 West 168th Street, Room 731, New York, NY 10032, USA
Jennifer S. Ferris, Department of Epidemiology, Columbia University, Mailman School of Public Health, 722 West 168th Street, Room 731, New York, NY 10032, USA
Parisa Tehranifar, Department of Epidemiology, Columbia University, Mailman School of Public Health, 722 West 168th Street, Room 731, New York, NY 10032, USA.
Mary Beth Terry, Department of Epidemiology, Columbia University, Mailman School of Public Health, 722 West 168th Street, Room 731, New York, NY 10032, USA.
References
- 1.World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 2.Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286(17):2143–2151. doi: 10.1001/jama.286.17.2143. doi:10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 3.Hamajima N, et al. Alcohol, tobacco and breast cancer-collaborative reanalysis of individual data from 53 epidemiological studies, including 58, 515 women with breast cancer and 95, 067 women without the disease. Br J Cancer. 2002;87(11):1234–1245. doi: 10.1038/sj.bjc.6600596. doi:10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiatt RA. Alcohol consumption and breast cancer. Med Oncol Tumor Pharmacother. 1990;7(2-3):143–151. doi: 10.1007/BF02988542. [DOI] [PubMed] [Google Scholar]
- 5.Terry MB, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16(3):230–240. doi: 10.1016/j.annepidem.2005.06.048. doi:10.1016/j.annepidem. 2005.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Longnecker MP, et al. Risk of breast cancer in relation to lifetime alcohol consumption. J Natl Cancer Inst. 1995;87(12):923–929. doi: 10.1093/jnci/87.12.923. doi:10.1093/jnci/87.12.923. [DOI] [PubMed] [Google Scholar]
- 7.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10(1):201. doi: 10.1186/bcr1831. doi:10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byng JW, et al. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629–1638. doi: 10.1088/0031-9155/39/10/008. doi:10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 9.Boyd NF, et al. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7(12):1133–1144. [PubMed] [Google Scholar]
- 10.McCormack VA, sSilva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. doi:10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 11.Boyd NF, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. doi:10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, et al. Plasma lipids, lipoproteins, and mammographic densities. Cancer Epidemiol Biomarkers Prev. 1995;4(7):727–733. [PubMed] [Google Scholar]
- 13.Masala G, et al. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. Int J Cancer. 2006;118(7):1782–1789. doi: 10.1002/ijc.21558. doi:10.1002/ijc. 21558. [DOI] [PubMed] [Google Scholar]
- 14.Vachon CM, et al. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/a:1008926607428. doi:10.1023/A:1008926607428. [DOI] [PubMed] [Google Scholar]
- 15.Herrinton LJ, et al. Do alcohol intake and mammographic densities interact in regard to the risk of breast cancer? Cancer. 1993;71(10):3029–3035. doi: 10.1002/1097-0142(19930515)71:10<3029::aid-cncr2820711024>3.0.co;2-k. doi:10.1002/1097-0142(19930515)71:10<3029:: AID-CNCR2820711024>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Maskarinec G, et al. Alcohol consumption and mammographic density in a multiethnic population. Int J Cancer. 2006;118(10):2579–2583. doi: 10.1002/ijc.21705. doi:10.1002/ijc.21705. [DOI] [PubMed] [Google Scholar]
- 17.Vachon CM, et al. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9(2):151–160. [PubMed] [Google Scholar]
- 18.Vachon CM, et al. Alcohol intake in adolescence and mammographic density. Int J Cancer. 2005;117(5):837–841. doi: 10.1002/ijc.21227. doi:10.1002/ijc.21227. [DOI] [PubMed] [Google Scholar]
- 19.Gapstur SM, et al. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Bio-markers Prev. 2003;12(10):1074–1080. [PubMed] [Google Scholar]
- 20.Maskarinec G, et al. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat. 2007;104(1):47–56. doi: 10.1007/s10549-006-9387-5. doi:10.1007/s10549-006-9387-5. [DOI] [PubMed] [Google Scholar]
- 21.Sala E, et al. High-risk mammographic parenchymal patterns and anthropometric measures: a case-control study. Br J Cancer. 1999;81(7):1257–1261. doi: 10.1038/sj.bjc.6690838. doi:10.1038/sj.bjc.6690838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brisson J, et al. Diet, mammographic features of breast tissue, and breast cancer risk. Am J Epidemiol. 1989;130(1):14–24. doi: 10.1093/oxfordjournals.aje.a115305. [DOI] [PubMed] [Google Scholar]
- 23.Broman SH. Praeger, New York: 1984. The collaborative perinatal project: an overview. In: Mednick SA, Harway M, Finello KM (eds) Hanbook of longitudinal research; pp. 166–179. [Google Scholar]
- 24.Terry MB, Wei Y, Esserman D. Maternal, birth, and early life influences on adult body size in women. Am J Epidemiol. 2007;166(1):5–13. doi: 10.1093/aje/kwm094. doi:10.1093/aje/kwm094. [DOI] [PubMed] [Google Scholar]
- 25.Harvey EB, et al. Alcohol consumption and breast cancer. J Natl Cancer Inst. 1987;78(4):657–661. [PubMed] [Google Scholar]
- 26.Byng JW, et al. Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev. 1996;5(5):319–327. doi: 10.1097/00008469-199610000-00003. doi:10.1097/00008469-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Jang M, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. doi:10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 28.Longnecker MP. Alcohol consumption and risk of cancer in humans: an overview. Alcohol. 1995;12(2):87–96. doi: 10.1016/0741-8329(94)00088-3. doi:10.1016/0741-8329(94)00088-3. [DOI] [PubMed] [Google Scholar]
- 29.Blot WJ. Alcohol and cancer. Cancer Res. 1992;52(7 (Suppl)):2119s–2123s. [PubMed] [Google Scholar]
- 30.Yu H, Berkel J. Do insulin-like growth factors mediate the effect of alcohol on breast cancer risk? Med Hypotheses. 1999;52(6):491–496. doi: 10.1054/mehy.1998.0828. doi:10.1054/mehy.1998.0828. [DOI] [PubMed] [Google Scholar]
- 31.Byrne C, et al. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3 and Mammographic density. Cancer Res. 2000;60:3744–3748. [PubMed] [Google Scholar]
- 32.Baglietto L, et al. Does dietary folate intake modify effect of alcohol consumption on breast cancer risk? Prospective cohort study. BMJ. 2005;331(7520):807. doi: 10.1136/bmj.38551.446470.06. doi:10.1136/bmj.38551.446470.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negri E, La Vecchia C, Franceschi S. Re: dietary folate consumption and breast cancer risk. J Natl Cancer Inst. 2000;92(15):1270–1271. doi: 10.1093/jnci/92.15.1270-a. doi:10.1093/jnci/92.15.1270-a. [DOI] [PubMed] [Google Scholar]
- 34.Rohan TE, et al. Dietary folate consumption and breast cancer risk. J Natl Cancer Inst. 2000;92(3):266–269. doi: 10.1093/jnci/92.3.266. doi:10.1093/jnci/92.3.266. [DOI] [PubMed] [Google Scholar]
- 35.Terry MB, et al. ADH3 genotype, alcohol intake and breast cancer risk. Carcinogenesis. 2006;27(4):840–847. doi: 10.1093/carcin/bgi285. doi:10.1093/carcin/bgi285. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics Health, United States . With Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2007. [PubMed] [Google Scholar]
- 37.US Department of Health and Human Services and US Department of Agriculture . Dietary guidelines for Americans. 6th edn. US Government Printing Office; Washington DC: 2005. [Google Scholar]
- 38.Kerlikowske K, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99(5):386–395. doi: 10.1093/jnci/djk066. doi:10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 39.Cuzick J, et al. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 40.Rutter CM, et al. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285:171–176. doi: 10.1001/jama.285.2.171. doi:10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]