Abstract
Background
Autonomic nervous system dysfunction and sympathetic nervous system over-activity play important roles in the development of hypertension associated with chronic kidney disease (CKD). In adults, increased blood pressure variability (BPV) appears to be directly related to sympathetic over-activity with increased risk of end-organ damage and cardiovascular events. Decreased heart rate variability (HRV) has been observed in adults with CKD, and is an independent predictor of mortality.
Methods
The purpose of this study was to evaluate BPV and HRV in pediatric patients enrolled in the Chronic Kidney Disease in Children Study. Ambulatory blood pressure monitoring data were available for analysis of 215 person-visits from 144 children that were not receiving antihypertensive medications.
Results
BPV and HRV were determined by standard deviation and coefficient of variation for heart rate and systolic and diastolic blood pressure for each patient averaged for wake/sleep periods during 24-h monitoring. Uniformly lower values were displayed during sleep versus wake periods: BPV was 20 % lower during sleep (p<0.001) and HRV was 30 % lower during sleep (p<0.001). A significant increase in systolic BPV was observed in hypertensive children compared to children with normal blood pressure (6.9 %, p=0.009). Increased diastolic BPV was detected among hypertensive children during sleep period compared to children with normal blood pressure (11.5 %, p=0.008). There was a significant decrease in HRV in hypertensive compared to normotensive children (−8.2 %, p=0.006).
Conclusions
These findings are similar to those in adult patients and may underscore childhood origin and natural progression of adverse cardiovascular outcomes in adults with CKD.
Keywords: Heart rate variability, Blood pressure variability, Chronic kidney disease, Hypertension, Pediatrics
Introduction
Autonomic nervous system (ANS) dysfunction and sympathetic nervous system (SNS) over-activity play important and distinct roles in the development of hypertension associated with chronic kidney disease (CKD) [1, 2]. In adults, increases in blood pressure variability (BPV) are directly related to SNS over-activity and increased risk of end-organ damage [3–8], as well as a higher rate of subsequent cerebrovascular and cardiovascular events, even after adjustment for mean blood pressure (BP) levels [9–11]. Heart rate variability (HRV) is also a useful, noninvasive tool to assess ANS function and may provide insight into understanding the role of the ANS in the pathogenesis of BP abnormalities in patients with CKD [12–14]. It has been demonstrated that decreases in HRV were observed in patients with hypertension, and was also found to be an independent predictor of mortality after myocardial infarction [13]. In addition, adult patients with CKD have abnormally low HRV, which has been associated with increased risk for progression on to end-stage renal disease (ESRD) and sudden death [15–18]. Therefore, BPV and HRV appear to correlate with the development of cardiovascular complications in a synergistic manner [19]. Lower HRV implies greater mortality [20, 21], and higher BPV appears to correlate with increased risk of cardiovascular disease [8–11].
As a measure of SNS activity, BPV and HRV may be important and practical considerations for the evaluation and management of hypertension. Evidence is accumulating for a role of SNS over-activity in producing renal and cardiac damage, particularly in adult patients with CKD, but this has not been investigated in a diverse pediatric population with CKD. The purpose of the present study was to describe and evaluate BPV and HRV from high-quality 24-h ambulatory blood pressure monitor (ABPM) measurements among pediatric patients enrolled in the Chronic Kidney Disease in Children (CKiD) study.
Methods
Study population and design
The present study is a longitudinal analysis of data obtained from patients enrolled in the CKiD study, an observational prospective cohort study of children with mild-to-moderate CKD, conducted at 48 participating pediatric nephrology centers in North America. The CKiD study protocol has been reviewed and approved by the Institutional Review Boards (IRB) of each participating center. Details of the study design have been previously published [22]. Eligibility criteria for enrollment in the CKiD study included: age 1 to<17 years, Schwartz estimated GFR≥30 to<90 ml/min/1.73 m2 [23], no prior organ transplantation, and signed written informed consent for participation by a parent or guardian. Assent/consent was also obtained from the study participant per local IRB requirements. Children were followed annually after an initial enrollment visit (visit 1); ABPM data were obtained at visit 2 (1 year after initial enrollment) through visit 6 (5 years after initial enrollment). For this analysis, patients were only included if they were not diagnosed with hypertension prior to completing 24 h ABPM, were not receiving any antihypertensive medications, and successfully completed 24-h ABPM as outlined below.
Measurements
Data were obtained from 24-h ABPM performed for the CKiD study as previously described [24]. Ambulatory blood pressure monitoring data were available for analysis of 215 person-visits from 144 children enrolled in the CKiD study over the course of three visits (1, 3, and 5 years after initial enrollment, referred to as CKiD study visit 2, visit 4, and visit 6, respectively). Monitoring was performed for approximately 24 h, with measurements obtained every 20 min through the day and night. In order to qualify as an adequate study, at least 75 % of attempted readings needed to be successful during both wake and sleep periods [24]. A diary was kept during the monitoring period to record the time of sleep, time of waking, and time of any napping. The means for systolic (SBP) and diastolic (DBP) blood pressure, heart rate (HR), HRV, and BPV were determined for overall 24 h, wake, and sleep periods.
Ambulatory hypertension was defined as mean wake or sleep SBP or DBP that was≥pediatric 95th percentile for ambulatory BP limits (adjusted for age, gender, and height) or 25 % of SBP or DBP measurements≥95th percentile for either wake or sleep period [24, 25] from patients’ 24-h ABPM analysis. Normative data for ABPM and the definition of ambulatory hypertension were adapted from American Heart Association recommendations on ABPM for children and adolescents [24, 25].
Glomerular filtration rate (GFR) was measured directly by iohexol plasma disappearance (iGFR) or estimated GFR (eGFR) (when iGFR was unavailable), details of which have been previously published [26, 27]. Additional data used in the current analysis included gender, self-reported race/ethnicity, underlying cause of CKD (glomerular vs. non-glomerular diagnosis), duration of CKD (as measured by years since diagnosis as well as percent of life with CKD), and age at the visit when ABPM was completed.
Blood pressure variability and HRV were determined by the standard deviation (SD) and coefficient of variation (CV) for SBP, DBP, and HR for each patient averaged for wake and sleep periods during the 24-h ABPM assessment. Subjects were stratified by stage of CKD, based on iGFR or eGFR, (divided into three groups: GFR≥60 ml/min/m2, GFR 30–59 ml/min/m2, GFR<30 ml/min/m2) and the presence or absence of ambulatory hypertension.
Statistical analyses
Data with up to three repeated measurements for both wake and sleep periods for each child were analyzed using a linear mixed model, which included a random subject effect to account for up to six repeated measurements of the same subjects at different visits (i.e., measurements for wake and sleep periods at three visits). Each model included four factors of primary interest: activity (wake/sleep), presence of ambulatory hypertension (normotensive/hypertensive), visit (time), and degree of CKD based on iGFR or eGFR (ml/min/1.73 m2, categorized as GFR≥60 ml/min/m2, GFR 30 to 59 ml/min/m2, GFR<30 ml/min/m2) and their interactions; non-significant interactions were removed from the final model. Each analysis adjusted for age, gender, race, and percent of life since CKD diagnosis at visit 2. The outcome variables, BPV and HRV were log transformed in order to stabilize the variance. P<0.05 indicated statistical significance.
Results
Data were analyzed for 215 person-visits from visit 2, 4, and 6 of the CKiD study, comprised of 144 children that were not previously diagnosed with hypertension, and were not receiving any antihypertensive medications. Patient demographics and clinical characteristics are outlined in Table 1. The median age of the person-visits was 12.1 years (interquartile range [IQR] 8.5–15.6), with 66 % being male, and 15 % of African-American race. Underlying cause of CKD was nonglomerular in 93.8 % of children. The proportion of children with ambulatory hypertension was 57 %. The median GFR was 47.9 ml/min/1.73 m2 (IQR 33.7–60.8) with the following distribution: 27 % with GFR≥60 ml/min/1.73 m2, 56 % with GFR 30 to 59 ml/min/1.73 m2, and 17 % with GFR<30 ml/min/1.73 m2. The median percent of life with CKD was 76.9 % (IQR 55.7–99.2).
Table 1.
Characteristics of study population (215 person-visits)
| Characteristic | Median (IQ) or n (%) |
|---|---|
| Visit 2 (1 year after initial enrollment in CKiD) | 104 (48.4) |
| Visit 4 (3 years after initial enrollment in CKiD) | 74 (34.4) |
| Visit 6 (5 years after initial enrollment in CKiD) | 37 (17.2) |
| Number of children with two visits | 43 (30) |
| Number of children with three visits | 14 (10) |
| Age at visit (years) | 12.1 (8.5–15.6) |
| Male gender (of 144 children) | 95 (66) |
| African American race (of 144 children) | 21 (15) |
| Non-glomerular diagnosis (of 144 children) | 135 (93.8 %) |
| Iohexol or estimated GFR | 47.9 (33.7–60.8) |
| Currently hypertensive (by ABPM) | 122 (57) |
| Percent of life with CKD at visit | 76.9 (55.7–99.2) |
| Systolic SD during wake period | 9.70 (8.24–11.26) |
| Systolic SD during sleep period | 7.43 (6.28–9.05) |
| Diastolic SD during wake period | 8.83 (7.91–10.37) |
| Diastolic SD during sleep period | 7.16 (6.22–9.03) |
| HR standard deviation during wake period | 11.64 (9.96–13.84) |
| HR standard deviation during sleep period | 8.65 (6.50–10.42) |
| GFR<30 ml/min/1.73 m2 | 37 (17) |
| GFR 30–59 ml/min/1.73 m2 | 121 (56) |
| GFR≥60 ml/min/1.73 m2 | 57 (27) |
CKiD Chronic Kidney Disease in Children; GFR glomerular filtration rate; ABPM ambulatory blood pressure monitoring; CKD chronic kidney disease; HR heart rate; SD standard deviation
Results of the longitudinal mixed models are outlined in Table 2 and Fig. 1, presenting the SD of systolic and diastolic BPVand HRV. The results from models using CVas outcomes are not presented except where indicated for HRV, since the results were otherwise largely consistent with SD. Variability was consistently lower during the sleep period compared to the wake period. Systolic and diastolic BPV and HRV were significantly lower during the sleep period (−21.6 %, p< 0.001; −22.4 %, p< 0.001; −29.2 %, p< 0.001, respectively).
Table 2.
Results of linear mixed models for three primary outcomes
| Effect | Systolic SD relative % difference (95 % CI) |
Diastolic SD relative % difference (95 % CI) |
Heart rate SD relative % difference (95 % CI) |
|---|---|---|---|
| Sleep vs. wake | −21.6 (−25.4, −17.6) p<0.001 | −22.4 (−27.0, −17.5) p<0.001 | −29.2 (−33.6, −24.5) p<0.001 |
| Hypertensive vs. normal | 6.9 (1.7, 12.4) p=0.009 | −0.8 (−6.2, 4.9) p=0.77 | −2.9 (−8.7, 3.3) p=0.35a |
| Hypertensive vs. normal × sleep vs. wake interaction | n/a | 11.5 (2.9, 20.9) p=0.008 | n/a |
| Visit 4 vs. visit 2 | 2.1 (−3.1,7.5) p=0.44 | −2.9 (−7.4, 1.8) p=0.21 | −2.9 (−8.8, 3.4) p=0.35 |
| Visit 6 vs. visit 2 | 10.4 (3.3, 18.1) p=0.004 | −1.9 (−7.7, 4.3) p=0.53 | 3.6 (−4.5, 12.4) p=0.39 |
| i/e GFR 30–60 vs. >60 ml/min/1.73 m2 | 3.7 (−2.6, 10.3) p=0.25 | 3.5 (−2.4, 9.8) p=0.25 | 6.1 (−1.9, 14.7) p=0.14 |
| i/e GFR <30 vs. >60 ml/min/1.73 m2 | 3.0 (−5.1, 11.8) p=0.48 | 3.6 (−4.1, 12.0) p=0.36 | 6.5 (−3.9, 18.1) p=0.23 |
| African American vs. other races | −3.0 (−10.3, 4.8) p=0.44 | −2.3 (−9.3, 5.3) p=0.55 | −6.7 (−15.5, 3.1) p=0.17 |
| Female vs. male gender | −9.8 (−14.9, −4.4) p<0.001 | −5.6 (−10.7, −0.3) p=0.041 | 4.3 (−3.1, 12.3) p=0.26 |
| Age at visit 2 | 1.3 (0.6, 2.0) p<0.001 | 0.2 (−0.4, 0.9) p=0.49 | 0.3 (−0.6, 1.1) p=0.53 |
| % Life with CKD at visit 2 | 0.5 (−9.6, 11.7) p=0.92 | 9.7 (−0.9, 21.4) p=0.074 | 21.8 (6.4, 39.3) p=0.004 |
Bolded values represent statistically significant results/values
CI confidence interval; SD standard deviation; GFR glomerular filtration rate; CKD chronic kidney disease
Heart rate coefficient of variation relative % difference (95 % CI): −8.2 (−13.7, −2.4), p=0.006
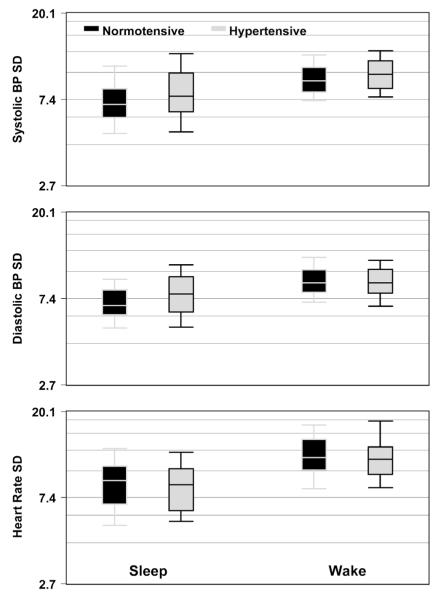
Fig. 1.
Comparison of blood pressure (BP) standard deviation (SD) and heart rate SD by sleep-wake periods and hypertensive condition
A significant increase in systolic BPV was observed in hypertensive children compared to children with normal blood pressures on ABPM (6.9 % higher, p=0.009). Although no significant difference in diastolic BPV was detected overall, when we evaluated differences within the wake/sleep periods, significantly increased diastolic BP variability was detected among hypertensive children during the sleep period compared to children with normal BP (Table 2). For diastolic BPV, hypertensive children during the sleep period were 11.5 % higher than children with normal BP (p=0.008). Figure 1 illustrates these interactions with box plots for hypertensive and non-hypertensive children during the wake and sleep periods. The middle panel plot demonstrates that during the sleep state, the hypertensive children demonstrated more diastolic BPV compared to the non-hypertensive children; whereas during the wake period, there is virtually no difference between the two groups (p=0.77). There was also a significant decrease in HRVamong the hypertensive compared to normotensive children (footnote Table 2, CV: −8.2 %, p=0.006).
These models also show that systolic BPV increased over the 4 years of follow-up. Specifically, systolic BPV was 10.4 % higher at visit 6 relative to visit 2 (p=0.004), and was 2 % higher at visit 4 compared to visit 2 (p=0.44). There were no significant differences noted for diastolic BPV or HRV over time.
For each year increase in baseline age, there was a 1.3 % increase in systolic BPV (p<0.001). A significant difference was detected based on duration of CKD—with each increase percent of life with CKD, there was a significant increase in HRV (21.8 %, p=0.004). No significant differences in BPV were detected based on duration of CKD. In addition, no significant differences in HRVor BPV were detected between races and among the different GFR groups/degree of CKD.
Females exhibited significantly less systolic and diastolic BPV compared to males (−9.8 %, p<0.001 and −5.6 %, p=0.041, respectively). No significant difference in HRV between genders was detected.
Discussion
In this study, examining BPV and HRV in a pediatric cohort with mild-to-moderate CKD, subjects exhibited circadian patterns characterized by elevated BPV and HRV during the wake period, and lower values during the sleep period. These findings are consistent with results described in adults [6, 28–30]. In adults, BP and HR follow a highly reproducible circadian pattern characterized by elevated values while awake/active, and lower values with rest/sleep [28–30]. It has also been demonstrated that there is an increased incidence of acute myocardial infarction (MI), ischemic stroke, and sudden death during the early morning period [6, 7, 31–33]. In addition, beta-blockers, which can diminish these physiologic processes, have been shown to decrease BPVand prevent the morning peak of onset of acute MI [34]. Differences involving BPV were also detected based on age and gender: older children had increased systolic BPV compared to younger children, and girls exhibited less systolic and diastolic BPV than boys. A significant difference was also detected when comparing children across visits/over time–in comparing visit 6 with visit 2, there was a significant increase in systolic BPV. This increase in systolic BPVover time may reflect the differences that were detected with increased age. These observed differences likely reflect the well-identified age and gender disparities in the overall prevalence of hypertension and cardiovascular disease [31–39].
Increased SNS activity acts as a mechanism for both initiating and sustaining BP elevation, and is associated with increased cardiovascular risks, as has been demonstrated in adult patients with hypertension, CKD, obstructive sleep apnea, obesity and heart failure [2–5, 40–42]. Sympathetic over-activity, in addition to sustaining BP elevation, has also been associated with increased left ventricular hypertrophy, ventricular arrhythmias, sudden death, hyperlipidemia, insulin resistance, and increased risk of end-organ damage [8, 31–33, 41–43]. Epidemiologic studies have demonstrated that systolic and diastolic BPV are independently associated with the incidence of cardiovascular morbidity and mortality and this effect is greater in hypertensive compared to normotensive patients [32, 33]. In addition, end-organ damage accompanying hypertension is more pronounced among those with increased BPV [8, 31–33, 43]. This evidence further suggests that an individual’s prognosis may depend not only on overall mean BP values, but additionally on degree of BPV.
Chen et al. [35] demonstrated that even after adjusting for mean childhood BP levels, adult hypertension was associated with increased BPV in terms of SD of serial BP measurements obtained during childhood. Additional studies have also demonstrated that greater BPV either over 24 h or over a prolonged period is associated with hypertension in adulthood [36, 37].
In our study, hypertensive patients were found to have increased systolic and diastolic BPV in comparison with normotensive patients. In particular, hypertensive patients had increased systolic variability during both awake and sleep periods, and significantly elevated diastolic BPV during the sleep period. These findings are consistent with previous reports of an association between hypertension, elevated BPV, and increased SNS activity in adults [44–47]. These studies have demonstrated that the degree of BPV bears an independent relationship with end-organ damage associated with hypertension [44–49].
In addition, our study also demonstrated decreased HRV in hypertensive children, compared with normotensive children. In adults, HRV has been proposed as an indicator of cardiovascular health-lower HRV has been associated with a higher risk of cardiovascular disease and death [50]. A decline in HRV has been noted to occur early in the development of hypertension and decreases in ANS function appear to precede the development of clinical hypertension [50].
Although there was no significant difference in HRV and BPV based on degree of CKD, several studies have indicated the presence of SNS over-activity in CKD and its relationship with arterial hypertension [3, 51–53]. In addition, lower HRV has also been associated with a higher risk of progression to ESRD [14–16, 18]. This underscores the importance of HRV as a relatively under recognized predictor of adverse cardiovascular and renal outcomes in patients with CKD.
There are several limitations to our study. Data from three CKiD study visits provided up to four years of longitudinal data. However, this time frame may be too short to detect significant differences in HRVand BPV due to CKD progression. The number of children with data at all three visits is also limited. However, we are confident that the data is of high quality given the strict requirements for a successful ABPM [24], particularly given the challenges of 24-h measurement among children and adolescents. Additionally, by restricting data to subjects who were not receiving antihypertensive medications, a large confounder was removed, thus ensuring a homogenous study sample, at least with respect to treatment. An additional limitation of this study is that BPVand HRV are indirect measurements of SNS activity and ANS function from sympathetic effector responses. Indeed, these variables are non-specific measures of SNS activity rather than direct measurement of SNS activity through the use of sympathetic nerve recordings/muscle sympathetic nerve activity. Implementation of such procedures was well beyond the scope of the CKiD study. However, the estimation of SNS activity from indirect measures by 24-h ABPM may serve as a foundation for future, more precise assessment.
Our findings of decreased HRV and increased BPV in hypertensive children with CKD are similar to that encountered in adult patients and may underscore the childhood origin and natural progression of SNS over-activity and adverse cardiovascular outcomes in adults. The SNS contributes to the overall control of circulation and should be regarded as an important therapeutic target in order to lower arterial BP in hypertensive patients. Optimal antihypertensive treatment should therefore focus on the reduction of overall average BP levels and degree of variability with improved regulation of SNS over-activity—using BPV and HRV as a marker or indicator of activity. Further studies are needed to assess whether recently developed antihypertensive medications induce not only a reduction in BP but also a decrease in variability and SNS activity.
Perspectives
Overall, this is a unique study in that it is the first examination of BPV and HRV within a large multi-center, prospective cohort of children with CKD in whom precise measurement of BP by 24-h ABPM and standardized data collection methods across all participating centers, including GFR were achieved. An important finding from this study of pediatric CKD is that children with uncontrolled hypertension have higher systolic and diastolic BPV and lower HRV compared to children without hypertension. Given the known association between increased BPV and decreased HRV as a marker of SNS over-activity and ANS dysfunction, with resultant increased risk for cardiovascular disease and CKD progression in adults, incorporation of treatment strategies designed to reduce SNS over-activity should be an important consideration in the management of pediatric CKD.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia and the University of Pennsylvania (Susan Furth, MD, Ph.D.), a Central Biochemistry Laboratory at the University of Rochester (George Schwartz, MD), and a data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.). The CKiD prospective cohort study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, UO1-DK-082194, UO1-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
Footnotes
Disclosures None.
Contributor Information
Gina-Marie Barletta, Pediatric Nephrology, Dialysis and Transplantation, Phoenix Children’s Hospital, 1919 E Thomas Road, Phoenix, AZ 85016, USA.
Joseph Flynn, Seattle Children’s Hospital, Seattle, WA, USA.
Mark Mitsnefes, Cincinnati Children’s Hospital, Cincinnati, OH, USA.
Joshua Samuels, University of Texas, Houston, TX, USA.
Lisa Aronson Friedman, John’s Hopkins, Baltimore, MD, USA.
Derek Ng, John’s Hopkins, Baltimore, MD, USA.
Christopher Cox, John’s Hopkins, Baltimore, MD, USA.
Timothy Poffenbarger, University of Texas, Houston, TX, USA.
Bradley Warady, Children’s Mercy Hospital, Kansas City, MO, USA.
Susan Furth, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
References
- 1.Flynn JT, Woroniecki RP. Pathophysiology of hypertension. In: Avner ED, Harmon WE, Niaudet P, editors. Pediatric nephrology. 5th ed Lippincott, Williams & Wilkins; Philadelphia, PA: 2004. pp. 1153–1177. [Google Scholar]
- 2.Phillips JK. Pathogenesis of hypertension in renal failure: role of the sympathetic nervous system and renal afferents. Clin Exp Pharmacol Physiol. 2005;32:415–418. doi: 10.1111/j.1440-1681.2005.04204.x. [DOI] [PubMed] [Google Scholar]
- 3.Converse RL, Jacobsen NJ, Toto RD, Jost CMT, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 4.Koomans HA, Blankestinjn PJ, Joles JA. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J Am Soc Nephrol. 2004;15:524–537. doi: 10.1097/01.asn.0000113320.57127.b9. [DOI] [PubMed] [Google Scholar]
- 5.Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension. 2004;43:699–706. doi: 10.1161/01.HYP.0000121881.77212.b1. [DOI] [PubMed] [Google Scholar]
- 6.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 7.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Di Rienzo M, Parati G, Grassi G. Sympathetic activity, blood pressure variability and end-organ damage in hypertension. J Hum Hypertens. 1997;11:S3–S8. [PubMed] [Google Scholar]
- 9.Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens. 2009;22:842–847. doi: 10.1038/ajh.2009.103. [DOI] [PubMed] [Google Scholar]
- 10.Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Schwartz JE, Shimada K, Kario K. Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens. 2009;22:46–51. doi: 10.1038/ajh.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH, Syst-Eur investigators Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Thomson BJ, McAreavey D, Neilson JM, Winney RJ, Ewing DJ. Heart rate variability and cardiac arrhythmias in patients with chronic renal failure. Clin Auton Res. 1991;1:131–133. doi: 10.1007/BF01826209. [DOI] [PubMed] [Google Scholar]
- 13.Ewing DJ. Heart rate variability: an important new risk factor in patients following myocardial infarction. Clin Cardiol. 1991;14:683–685. doi: 10.1002/clc.4960140811. [DOI] [PubMed] [Google Scholar]
- 14.Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, Finkelstein F, Hinderliter A, Pop-Busui R, Rajagopalan S, Saran R. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant. 2012;27:700–709. doi: 10.1093/ndt/gfr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg AA, Mars RL, Goldman DS, Percy RF. Effect of end-stage renal disease on decreased heart rate variability. Am J Cardiol. 1998;82(1156–1158):A10. doi: 10.1016/s0002-9149(98)00580-3. [DOI] [PubMed] [Google Scholar]
- 16.Di Leo R, Vita G, Messina C, Savica V. Autonomic function in elderly uremics studied by spectral analysis of heart rate. Kidney Int. 2005;67:1521–1525. doi: 10.1111/j.1523-1755.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty CM, Burr RL. Comparison of heart rate variability in survivors and non-survivors of sudden cardiac arrest. Am J Cardiol. 1992;70:441–448. doi: 10.1016/0002-9149(92)91187-9. [DOI] [PubMed] [Google Scholar]
- 18.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–325. doi: 10.1093/ndt/18.2.318. [DOI] [PubMed] [Google Scholar]
- 19.Albaladejo P, Copie X, Boutouyrie P, Laloux B, Déclère AD, Smulyan H, Bénétos A. Heart rate, arterial stiffness, and wave reflections in paced patients. Hypertension. 2001;38:949–952. doi: 10.1161/hy1001.096210. [DOI] [PubMed] [Google Scholar]
- 20.Singh RB, Cornélissen G, Weydahl A, Schwartzkopff O, Katinas G, Otsuka K, Watanabe Y, Yano S, Mori H, Ichimaru Y, Mitsutake G, Pella D, Fanghong L, Zhao Z, Rao RS, Gvozdjakova A, Halberg F. Circadian heart rate and blood pressure variability considered for research and patient care. Int J Cardiol. 2003;87:9–30. doi: 10.1016/s0167-5273(02)00308-x. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez-Villegas JF, Lam-Espinosa E, Ramirez-Moreno DF, Calvo-Echeverry PC, Agredo-Rodriguez W. Heart rate variability dynamics for the prognosis of cardiovascular risk. PLoS One. 2011;6:e17060. doi: 10.1371/journal.pone.0017060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 24.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60:43–50. doi: 10.1161/HYPERTENSIONAHA.111.189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S, American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment–A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young and the Council for High Blood Pressure Research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancia G, Parati G, Castiglioni P, Tordi R, Tortorici E, Glavina F, Di Rienzo M. Daily life blood pressure changes are steeper in hypertensive than in normotensive subjects. Hypertension. 2003;42:277–282. doi: 10.1161/01.HYP.0000084632.33942.5F. [DOI] [PubMed] [Google Scholar]
- 29.White WB. Ambulatory blood pressure monitoring in clinical practice. N Engl J Med. 2005;348:2377–2378. doi: 10.1056/NEJMp030057. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z-Y, Zhao ZY, Wang YQ, Yan ZH, Cui J, Li YY. Quantitative study of circadian variation of ambulatory blood pressure in Chinese healthy, hypertensive, and diabetes subjects. Clin Exp Hypertens. 2005;3:187–194. [PubMed] [Google Scholar]
- 31.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983–988. doi: 10.1161/01.hyp.32.6.983. [DOI] [PubMed] [Google Scholar]
- 32.Psaty BM, Furberg CD, Kuller LH, Cushman M, Savage PJ, Levine D, O’Leary DH, Bryan RN, Anderson M, Lumley T. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality. Arch Intern Med. 2001;161:1183–1192. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 33.Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A, on behalf of the ELSA investigators Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2001;19:1981–1989. doi: 10.1097/00004872-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone MT, Mittleman M, Tofler G, Muller JE. The path-ophysiology of the onset of morning cardiovascular events. Am J Hypertens. 1996;9:22S–28S. doi: 10.1016/0895-7061(95)00403-3. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Srinivasan SR, Yao L, Li S, Dasmahapatra P, Fernandez C, Xu J, Berenson GS. Low birth weight is associated with higher blood pressure variability from childhood to young adulthood: the Bogalusa Heart Study. Am J Epidemiol. 2012;176(Suppl 7):S99–S105. doi: 10.1093/aje/kws298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parati G, Mancia G. Blood pressure variability as a risk factor. Blood Press Monit. 2001;6:341–347. doi: 10.1097/00126097-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. doi: 10.1161/01.res.53.1.96. [DOI] [PubMed] [Google Scholar]
- 38.Burt VL, Whelton P, Ej R, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 39.Rosner B, Prineas R, Daniels SR, Loggie J. Blood pressure differences between blacks and whites in relation to body size among US children and adolescents. Am J Epidemiol. 2000;151:1007–1019. doi: 10.1093/oxfordjournals.aje.a010129. [DOI] [PubMed] [Google Scholar]
- 40.Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009;84:822–830. doi: 10.4065/84.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol. 2000;35:S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- 42.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 43.Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900. doi: 10.1161/01.hyp.36.5.894. [DOI] [PubMed] [Google Scholar]
- 44.Parati G, Di Rienzo M, Ulian L, Santucciu C, Girard A, Elghozi JL, Mancia G. Clinical relevance of blood pressure variability. J Hypertens. 1998;16:S25–S33. [PubMed] [Google Scholar]
- 45.Mancia G, Parati G, Di Rienzo M, Zanchetti A. Zanchetti A, Mancia G, editors. Blood pressure variability. Handbook of hypertension. Pathophysiol Hypertens. 1997;17:117–169. [Google Scholar]
- 46.Mancia G, Di Rienzo M, Parati G. Ambulatory blood pressure monitoring use in hypertension research and clinical practice. Hypertension. 1993;21:510–522. doi: 10.1161/01.hyp.21.4.510. [DOI] [PubMed] [Google Scholar]
- 47.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Palatini P, Penzo M, Racioppa A, Zugno E, Guzzardi G, Anaclerio M, Pessina AC. Clinical relevance of night-time blood pressure and of daytime blood pressure variability. Arch Intern Med. 1992;152:1855–1860. [PubMed] [Google Scholar]
- 50.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 51.Cottone S, Panepinto N, Vadalà A, Zagarrigo C, Galione P, Volpe V, Cerasola G. Sympathetic overactivity and 24-hour blood pressure pattern in hypertensives with chronic renal failure. Ren Fail. 1995;17:751–758. doi: 10.3109/08860229509037643. [DOI] [PubMed] [Google Scholar]
- 52.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn P. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–1576. doi: 10.1111/j.1523-1755.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 53.Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol. 2007;2:491–500. doi: 10.2215/CJN.02360706. [DOI] [PubMed] [Google Scholar]