Abstract
Introduction
Multiple studies associate prenatal and perinatal complications with increased risks for autism spectrum disorders (ASDs). The objectives of this study were to utilize a twin study design to 1) Investigate whether shared gestational and perinatal factors increase concordance for ASDs in twins, 2) Determine whether individual neonatal factors are associated with the presence of ASDs in twins, and 3) Explore whether associated factors may influence males and females differently.
Methods
Data from medical records and parent response questionnaires from 194 twin pairs, in which at least one twin had an ASD, were analyzed.
Results
Shared factors including parental age, prenatal use of medications, uterine bleeding, and prematurity did not increase concordance risks for ASDs in twins. Among the individual factors, respiratory distress demonstrated the strongest association with increased risk for ASDs in the group as a whole (OR 2.11, 95% CI 1.27–3.51). Furthermore, respiratory distress (OR 2.29, 95% CI 1.12–4.67) and other markers of hypoxia (OR 1.99, 95% CI 1.04–3.80) were associated with increased risks for ASDs in males, while jaundice was associated with an increased risk for ASDs in females (OR 2.94, 95% CI 1.28–6.74).
Conclusions
Perinatal factors associated with respiratory distress and other markers of hypoxia appear to increase risk for autism in a subgroup of twins. Future studies examining potential gender differences and additional prenatal, perinatal and postnatal environmental factors are required for elucidating the etiology of ASDs and suggesting new methods for treatment and prevention.
Keywords: Autism, Prenatal, Perinatal, Pregnancy complications, Twins, Environment
Introduction
Gestational and perinatal complications are implicated in the risk for autism spectrum disorders (ASDs) in multiple studies (Gardener, Spiegelman, & Buka, 2009; 2011; Haglund & Kallen, 2011; Lampi et al., 2012; Larsson et al., 2005; Maramara, He, & Ming, 2014). ASDs are heterogenous disorders believed to arise from combinations of genetic and environmental influences. A recent study suggests that genetic factors account for only approximately 35–40% of the contributing elements (Hallmayer et al., 2011). The remaining 60–65% is likely due to other factors, such as pre- and perinatal factors, and postnatal environmental factors. Since ASDs are neurodevelopmental disorders, neonatally-observed complications that are markers of events or processes that initiate during the prenatal, perinatal, or neonatal periods may be particularly important to consider. A 2009 meta-analysis investigating prenatal risk factors found advanced parental age at birth, maternal prenatal medication use, bleeding during pregnancy, gestational diabetes, parity, and having a mother born abroad all to be associated with increased risks for ASDs (Gardener et al., 2009). A subsequent meta-analysis investigating perinatal and neonatal risk factors in ASDs implicated the following factors: abnormal/breech presentation, umbilical cord complications, fetal distress, birth injury/trauma, multiple birth, maternal hemorrhage, summer birth, low and very low birth weight, small for gestational age (SGA), congenital malformations, low APGAR scores, meconium aspiration, neonatal anemia, ABO or RH incompatibility, and hyperbilirubinemia. Additionally, C-section came close to reaching significance with a 26% increased rate of autism (p=0.06) (Gardener et al., 2011). More recently, Guinchat et al. (2012) suggested maternal pre-eclampsia, prematurity, post-term birth, induced/precipitous/prolonged labor, head circumference, transfer to special care, and markers of hypoxia (e.g. respiratory distress syndrome, assisted ventilation or a diagnosis of asphyxia) are also associated with autism.
Prenatal and perinatal complications may also help explain the gender differential in ASDs. Autism demonstrates a male: female imbalance at a 4:1 ratio. Several studies indicate male infants typically suffer more neurologic dysfunction in comparison to females following gestations complicated by prematurity or birth trauma associated with asphyxia (Aibar, Puertas, Valverde, Carrillo, & Montoya, 2012; Di Renzo, Rosati, Sarti, Cruciani, & Cutuli, 2007; Stevenson et al., 2000; Vatten & Skjærven, 2004). Male infants are more likely to experience intraventricular hemorrhage (IVH), or mortality from prematurity. Surviving males with IVH have been found to have significantly lower full-scale, verbal, and performance IQs, when compared to matched females with similar degrees of prematurity and IVH (Raz et al., 1995). Among infants at risk for hypoxic-ischemic (HI) injury, males are approximately twice as likely to experience prenatal anoxia, hemorrhage, infection, and cerebral birth trauma as compared to females (Donders & Hoffman, 2002; Gualtieri & Hicks, 1985; Lauterbach, Raz, & Sander, 2001; Raz, Debastos, Newman, & Batton, 2010). Furthermore, animal studies investigating various models of HI injury demonstrate that male animals endure greater losses in brain volume, increased disruption of myelination, and increased behavioral disturbances compared to female animals (Hill, Alexander, McCullough, & Fitch, 2011a; Hill & Fitch, 2012; Hill, Threlkeld, & Fitch, 2011b; Lan et al., 2011; Mayoral, Omar, & Penn, 2009). Although studies directly investigating fetal hypoxia and autism risk with relation to gender are scarce, Burstyn et al. (2011) described increased risk of ASD in males, but not females, with fetal hypoxia based on an estimation-maximization algorithm.
Twin studies are of particular value in studying perinatal factors because the in utero environment is largely shared in twin gestations, allowing us to distinguish the influence of shared prenatal factors and individual neonatal factors on ASD risk. Shared factors are always shared by BOTH twins (e.g. parental age, maternal prenatal use of medications, uterine bleeding, and prematurity), while individual factors may affect only one OR both twins (e.g. low birth weight, nuchal cord complications, and medical complications after birth). Approximately 50% of the genome is shared in dizygotic (DZ) twins, while the genome is shared entirely in monozygotic (MZ) twins. Twin pairs may either be ASD concordant (i.e. both twins have an ASD) or ASD discordant (i.e. only one twin has an ASD).Thus, factors that increase ASD concordance in MZ, as compared to DZ, twin pairs may represent a gene-environment interaction, whereas factors associated with similar rates of concordance in MZ and DZ pairs are likely factors that are relatively independent of genetic influences.
This study investigated whether certain shared pre- or perinatal factors increase the likelihood of concordance for ASDs among twin pairs in which at least one twin has an ASD. Shared factors that increase risks for ASDs should increase ASD concordance in twins. Based on the extant literature, we hypothesized that amongst the shared factors available for analysis from our study, parental age, uterine bleeding, and prematurity would be associated with increased concordance for ASDs in this cohort of twins. We also explored whether individual perinatal factors were associated with the presence of ASDs in both concordant and discordant twin pairs. Amongst these, we believed low birth weight and markers of hypoxia would be associated with higher risks for ASDs. Finally, we investigated whether certain factors were more likely to be risk factors in males as compared to females, and whether risk factors were significantly greater in MZ relative to DZ pairs.
Methods
This study was prospectively approved by the California Health and Welfare Agency Committee for the Protection of Human Subjects and by the institutional review board at Stanford University. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. Informed consent from parents or legal guardians of participants was obtained after the nature of the procedures had been fully explained.
Participants
Subjects were identified and included in the California Autism Twin Study (CATS), as described in detail in Hallmayer et al. (Hallmayer et al., 2011). In brief, twin pairs were selected for inclusion if at least one child in the pair had a documented ASD diagnosis. Exclusion criteria included known neurogenetic conditions associated with autism (e.g. fragile X syndrome, Down syndrome, tuberous sclerosis and neurofibromatosis) and mental age less than 18 months. ASD diagnoses (or lack thereof) were verified using the Autism Diagnostic Interview – Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994; Rutter, Le Couteur, & Lord, 2003) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000; Lord, Rutter, DiLavore, & Risi, 1999). The presence of an ASD was determined if the individual met criteria for a broad phenotype ASD as described in Hallmayer et al. (Hallmayer et al., 2011) using published criteria for combined ADI-R and ADOS subscores (Risi et al., 2006). Parents completed a medical history questionnaire asking a combination of closed and open-ended questions regarding pregnancy, labor, and complications during and after birth. The analytic sample for which parents completed and returned a medical history questionnaire for both twins ultimately included 388 subjects (194 twin pairs) ranging from ages 4–18 years at time of assessment. Maternal and paternal age, gestational age, and birth weight were obtained from birth records through the California Department of Public Health. Additionally, the following variables were specifically ascertained in the medical history questionnaire completed by parents: maternal prenatal medication use, uterine bleeding, presence of jaundice, breathing problems and oxygen requirement after birth. To address the key question of hypoxic events as a risk factor for ASDs, we also created a variable for “markers of hypoxia”. Gardener et al. (2011) found that several potential risk factors for ASD (ie, growth retardation, fetal distress, nuchal cord, low APGAR score, respiratory distress, resuscitation, meconium aspiration, and cesarean delivery) may be associated with an increased risk of hypoxia (Gardener et al., 2011). Thus, “markers of hypoxia” was coded as present if any of the following were noted by parents throughout the questionnaire in closed or open-ended questions: growth retardation, presence of meconium, fetal distress, unscheduled C-section due to low fetal heart rate and/or distress, umbilical cord complications, respiratory distress or breathing problems, oxygen requirement after birth, resuscitation, or low APGAR scores. Because twin births have high rates of scheduled cesarean, cesarean birth was otherwise not included as a marker of hypoxia. In total, the following variables were included in our analysis: Shared factors: maternal and paternal age at birth in years, maternal prenatal medication use (Y/N), uterine bleeding (Y/N), gestational age in weeks; and individual factors: very low birth weight < 1500 grams, low birth weight < 2500 grams, small for gestational age (SGA), and medical complications after birth including jaundice (Y/N), respiratory distress (Y/N), oxygen requirement after birth (Y/N), and the summary variable “markers of hypoxia”.
Statistics
To investigate the contribution of shared perinatal factors to ASD risk, ASD concordance within the twin pair was defined as the outcome variable. We employed logistic regression to compare ASD concordance in twin pairs exposed and not exposed to each factor. A separate model was run for each shared factor.
To investigate the contribution of individual factors to the risk of ASD, we defined ASD in individual twins as the outcome variable and used chi square analysis first in all twin pairs, and secondly, only in twin pairs discordant for ASD. Although our primary focus was on whether or not an individual risk factor was associated with presence of ASD, and not concordant or discordant status (as was the case for shared factors), we also conducted our analysis separately for discordant twins to examine if the individual risk factors associated with ASD was different in discordant twin pairs only than when all twins were included. Therefore, we employed chi-square analysis first in all twin pairs, and secondly in discordant pairs only to investigate individual factors. We defined rows by the presence or absence of a risk factor and columns as the presence or absence of an ASD. Two-sided significance from Pearson’s chi square tests was reported for all factors with the exception of tests in which at least one cell had a value less than five. In this case, two-sided significance from Fisher’s exact test was employed. We corrected for multiple tests using Bonferroni correction. It is not possible to examine the individual risk factors associated with ASD in concordant pairs only, as both twins have ASD. However, we conducted a separate chi-square analysis of ASD subjects only to examine whether the prevalence of our risk factors differed according to concordant or discordant status.
Individual factors positively associated with ASDs were then further investigated with a second set of chi square analyses stratified by zygosity of twin pair and a third set of chi square analyses with the sample stratified by gender. Pearson’s chi square tests were reported for all factors with the exception of those tests in which at least one cell had less than five individuals. In this case, two-sided significance from Fisher’s exact test was employed. Odds ratios for MZ and DZ twins and for males and females were compared using the Breslow-Day test of homogeneity.
Finally, to determine the ranking and linkage among the variables, we completed a backward stepwise multiple regression analysis using the Wald method. We again performed the analysis first using all twin pairs, and then discordant pairs only. We also performed the analysis in all male-only twin pairs. Subject numbers, however, were too small to complete in female only pairs. Variables included in the stepwise regression included both shared and individual factors. Continuous variables consisted of maternal age, paternal age, gestational age, and birth weight, while maternal prenatal medication use, uterine bleeding, jaundice, respiratory distress, oxygen requirement after birth, and the summary variable “markers of hypoxia” were entered into the model as categorical variables. A standard cut-off of 0.5 and 20 max iterations were specified, as well as an entry point of 0.05 and removal at 0.1.
Results
A total of 388 individuals (194 twin pairs) were included in analysis. This sample consisted of 50 monozygotic male (MZM) pairs (16 ASD discordant, 34 ASD concordant), 8 monozygotic female (MZF) pairs (5 ASD discordant, 3 ASD concordant), 52 dizygotic male (DZM) pairs (42 ASD discordant, 10 ASD concordant), 13 dizygotic female (DZF) pairs (9 ASD discordant, 4 ASD concordant), and 71 dizygotic sex discordant (DZSD) pairs (65 ASD discordant, 6 ASD concordant).
None of the shared factors (maternal age, paternal age, medication, uterine bleeding, or gestational age) were significantly associated with an increased risk for ASD concordance within twin pairs (Table 1). In contrast, respiratory distress, jaundice, having an oxygen requirement after birth, and the presence of a marker for hypoxia were all significantly more common in twin individuals with ASDs than twin individuals without ASDs (Table 2a). Only respiratory distress remained significantly associated with ASD risk after Bonferroni correction. Similarly, when limited to only discordant twin pairs, respiratory distress and having an oxygen requirement after birth were significantly more common in twin individuals with ASDs, and again, only respiratory distress remained significant after Bonferroni correction (Table 2b). Chi square analysis comparing the prevalence of risk factors in ASD concordant twin pairs relative to discordant twin pairs did not reveal any significant differences for any of the individual factors.
Table 1.
Odds of ASD Concordance by Shared Factors in ASD Twin Pairs in the California Autism Twin Study: Logistic Regression Analysis
| Variable | N | B | SE B | Exp(B): Estimated Odds Ratio (95% CI) |
P | Adjusted Bonferroni p (5 tests) |
|---|---|---|---|---|---|---|
| Paternal age, years | 171 | 0.03 | 0.02 | 1.03 (0.99–1.08) | 0.14 | 0.68 |
| Maternal age, years | 175 | 0.01 | 0.03 | 1.01 (0.95–1.07) | 0.77 | 1 |
| Medication during pregnancy | 193 | 0.08 | 0.33 | 1.08 (0.57–2.06) | 0.80 | 1 |
| Uterine bleeding | 191 | 0.97 | 0.53 | 2.65 (0.94–7.45) | 0.07 | 0.33 |
| Gestational age | 192 | 0.03 | 0.05 | 1.03 (0.93–1.14) | 0.59 | 1 |
B = logistic coefficient associated with the intercept, SE B = the standard error around the coefficient B
Table 2.
| (a) Odds of ASD by Perinatal Factors in Twin Individuals in the California Autism Twin Study: Chi Square Analysis, All Twin Pairs | ||||||
|---|---|---|---|---|---|---|
| Individual Factors | Twins with ASDs (N = 251) |
Unaffected co-twins without ASDs (N=137) |
Odds Ratio (95% CI) | p |
Bonferroni adjusted p (7 tests) |
|
| Birth Weight | ||||||
| Very low birth weight) < 1500 g | 16 | 9 | 0.97 (0.42–2.25) | 0.93 | 1 | |
| >1500 g | 234 | 127 | ||||
| Missing | 1 | 1 | ||||
| Low birth weight) <2500 g | 98 | 60 | 0.82 (0.54–1.25) | 0.35 | 1 | |
| >2500 g | 152 | 76 | ||||
| Missing | 1 | 1 | ||||
| Adjusted birth weight | ||||||
| Small for Gestational Age | 11 | 8 | 0.74 (0.29–1.90) | 0.53 | 1 | |
| Appropriate or Large for Gestational Age | 235 | 127 | ||||
| Missing | 5 | 2 | ||||
| Respiratory Distress | ||||||
| Present | 80 | 25 | 2.11 (1.27–3.51) | 0.004 | 0.03 | |
| Absent | 167 | 110 | ||||
| Missing | 4 | 2 | ||||
| Jaundice | ||||||
| Present | 113 | 45 | 1.69 (1.09–2.62) | 0.02 | 0.13 | |
| Absent | 132 | 89 | ||||
| Missing | 6 | 3 | ||||
| Oxygen after Birth | ||||||
| Required | 77 | 25 | 1.96 (1.18–3.27) | 0.009 | 0.06 | |
| Not required | 168 | 107 | ||||
| Missing | 6 | 5 | ||||
| Markers of hypoxia | ||||||
| Present | 97 | 37 | 1.71 (1.08–2.71) | 0.02 | 0.15 | |
| Absent | 147 | 96 | ||||
| Missing | 7 | 4 | ||||
| (b) Odds of ASD by Perinatal Factors in Twin Individuals in the California Autism Twin Study: Chi Square Analysis, Discordant Twin Pairs Only | ||||||||
|---|---|---|---|---|---|---|---|---|
| Individual Factors | Twins with ASDs (N = 137) |
Unaffected co-twins without ASDs (N=137) |
Odds Ratio (95% CI) | p |
Bonferroni adjusted p (7 tests) |
|||
| Birth Weight | ||||||||
| Very low birth weight) < 1500 g | 10 | 9 | 1.12 (0.44–2.85) | 0.81 | 1 | |||
| >1500 g | 126 | 127 | ||||||
| Missing | 1 | 1 | ||||||
| Low birth weight) <2500 g | 51 | 60 | 0.76 (0.47–1.23) | 0.27 | 1 | |||
| >2500 g | 85 | 76 | ||||||
| Missing | 1 | 1 | ||||||
| Adjusted birth weight | ||||||||
| Small for Gestational Age | 3 | 8 | 0.36 (0.09–1.39) | 0.12 | 0.84 | |||
| Appropriate or Large for Gestational Age | 132 | 127 | ||||||
| Missing | 2 | 2 | ||||||
| Respiratory Distress | ||||||||
| Present | 45 | 25 | 2.20 (1.25–3.86) | 0.005 | 0.04 | |||
| Absent | 90 | 110 | ||||||
| Missing | 2 | 2 | ||||||
| Jaundice | ||||||||
| Present | 59 | 45 | 1.54 (0.94–2.52) | 0.09 | 0.62 | |||
| Absent | 76 | 89 | ||||||
| Missing | 2 | 3 | ||||||
| Oxygen after Birth | ||||||||
| Required | 44 | 25 | 2.12 (1.20–3.73) | 0.009 | 0.06 | |||
| Not required | 89 | 107 | ||||||
| Missing | 4 | 5 | ||||||
| Markers of hypoxia | ||||||||
| Present | 51 | 37 | 1.61 (0.96–2.70) | 0.07 | 0.15 | |||
| Absent | 82 | 96 | ||||||
| Missing | 4 | 4 | ||||||
Markers of hypoxia = Summary variable including growth retardation, presence of meconium, fetal distress, unscheduled C-section due to low fetal heart rate and/or distress, umbilical cord complications, respiratory distress or breathing problems, oxygen requirement after birth, resuscitation, or low APGAR scores
Markers of hypoxia = Summary variable including growth retardation, presence of meconium, fetal distress, unscheduled C-section due to low fetal heart rate and/or distress, umbilical cord complications, respiratory distress or breathing problems, oxygen requirement after birth, resuscitation, or low APGAR scores
We further examined factors found to be significant in twins with ASDs (respiratory distress, jaundice, having an oxygen requirement after birth, and the presence of a marker for hypoxia) in subgroups defined by zygosity and gender in all twin pairs. Increases in odds ratios were similar in MZ and DZ twin pairs, but reached statistical significance only for DZ pairs (Table 3). Furthermore, respiratory distress and markers of hypoxia were significant risk factors for ASD only among males. Although these odds ratios were nearly double in males relative to females, they were not significantly different (Table 3). In contrast, jaundice was a significant risk factor in females, but not males. Again, although the odds ratio was nearly twice as great in females, the increase in odds was not significant (Table 3).
Table 3.
Perinatal Factors in ASD Twin Individuals in the California Autism Twin Study, subgrouped by zygosity and gender, All Twin Pairs
| Chi Square Analysis | Twins with ASDs (N=251) |
Unaffected co-twins without ASDs (N=137) |
Chi Square comparing presence vs. absence of risk factor |
Breslow-Day Test of Homogeneity comparing MZ vs. DZ or Male vs. Female |
||||
|---|---|---|---|---|---|---|---|---|
| Perinatal Factors | Present | Absent | Present | Absent | Odds Ratio (95% CI) | p | P | |
| Respiratory Distress | ||||||||
| MZ | 36 | 56 | 5 | 15 | 1.93 (0.65–5.77) | 0.23 | 0.97 | |
| DZ* | 44 | 111 | 20 | 95 | 1.88 (1.04–3.42) | 0.04 | ||
| Missing | 4 | 2 | ||||||
| Male* | 72 | 140 | 11 | 49 | 2.29 (1.12–4.67) | 0.03 | 0.35 | |
| Female | 8 | 27 | 14 | 61 | 1.29 (0.49–3.44) | 0.62 | ||
| Missing | 4 | 2 | ||||||
| Jaundice | ||||||||
| MZ | 42 | 50 | 9 | 11 | 1.03 (0.39–2.71) | 0.96 | 0.28 | |
| DZ* | 71 | 82 | 36 | 78 | 1.88 (1.13–3.11) | 0.01 | ||
| Missing | 6 | 3 | ||||||
| Male | 92 | 118 | 20 | 40 | 1.56 (0.85–2.85) | 0.15 | 0.23 | |
| Female* | 21 | 14 | 25 | 49 | 2.94 (1.28–6.74) | 0.01 | ||
| Missing | 6 | 3 | ||||||
| Oxygen | ||||||||
| MZ | 34 | 58 | 6 | 14 | 1.37 (0.48–3.89) | 0.56 | 0.59 | |
| DZ* | 43 | 110 | 19 | 93 | 1.91(1.04–3.51) | 0.03 | ||
| Missing | 6 | 5 | ||||||
| Male | 68 | 142 | 13 | 46 | 1.69 (0.86–3.35) | 0.13 | 0.95 | |
| Female | 9 | 26 | 12 | 61 | 1.76 (0.66–4.68) | 0.25 | ||
| Missing | 6 | 5 | ||||||
| Markers of hypoxia | ||||||||
| MZ | 44 | 47 | 6 | 14 | 2.18 (0.77–6.19) | 0.14 | 0.46 | |
| DZ | 53 | 100 | 31 | 82 | 1.40 (0.83–2.38) | 0.21 | ||
| Missing | 7 | 4 | ||||||
| Male* | 86 | 124 | 15 | 43 | 1.99(1.04–3.80) | 0.04 | 0.33 | |
| Female | 11 | 23 | 22 | 53 | 1.15 (0.48–2.76) | 0.75 | ||
| Missing | 7 | 4 | ||||||
p<0.05,
MZ=Monozygotic, DZ=Dizygotic; Markers of hypoxia = Summary variable including growth retardation, presence of meconium, fetal distress, unscheduled C-section due to low fetal heart rate and/or distress, umbilical cord complications, respiratory distress or breathing problems, oxygen requirement after birth, resuscitation, or low APGAR scores
Table 4 illustrates the final models resulting from the stepwise regressions. With all twin pairs included, both respiratory distress and gestational age remained in the model. However, only respiratory distress reached significance. When limited to ASD discordant pairs, only respiratory distress remained in the final model. The order in which variables were removed varied widely depending on the subset analyzed (Table 4).
Table 4.
Backwards Wald Stepwise Regressions: Final Models
| N | Variable | B | SE B | Wald | df | p | Exp(B): Estimated Odds Ratio (95% CI) |
Order of variables removed | |
|---|---|---|---|---|---|---|---|---|---|
| All Twins | 318 (Missing 70) | Gestational Age | 0.08 | 0.48 | 2.83 | 1 | 0.09 | 1.08 (0.99–1.19) | 1. Markers of hypoxia 2. Uterine bleeding 3. Maternal age 4. Medication during pregnancy 5. Oxygen requirement after birth 6. Birth weight 7. Paternal age 8. Jaundice |
| Respiratory Distress | 1.03 | 0.33 | 9.51 | 1 | 0.002 | 2.79 (1.45–5.37) | |||
| ASD Discordant Twins | 224 (Missing 50) | Respiratory Distress | 0.76 | 0.32 | 5.83 | 1 | 0.02 | 2.13 (1.15–3.96) | 1. Maternal age 2. Paternal age 3. Medication during pregnancy 4. Uterine bleeding 5. Gestational age 6. Markers of hypoxia 7. Oxygen requirement after birth 8. Jaundice 9. Birth weight. |
| All Male Twins | 233 (Missing 42) | Gestational Age | 0.11 | 0.06 | 3.02 | 1 | 0.08 | 1.12 (0.99–1.27) | 1. Markers of hypoxia 2. Oxygen requirement after birth 3. Paternal Age 4. Uterine bleeding 5. Jaundice 6. Birth weight 7. Maternal age 8. Medication during pregnancy |
| Respiratory Distress | 1.25 | 0.47 | 6.96 | 1 | 0.008 | 3.49 (1.38–8.82) | |||
| Male Discordant Twins | 151 (Missing 30) | Respiratory Distress | 0.88 | 0.42 | 4.34 | 1 | 0.04 | 2.41 (1.05–5.54) | 1. Uterine bleeding 2. Oxygen requirement after brith 3. Markers of hypoxia 4. Birth weight 5. Jaundice 6. Paternal age 7. Maternal age 8. Gestational age 9. Medication during pregnancy |
B = logistic coefficient associated with the intercept, SE B = the standard error around the coefficient B
Finally, among the subset of MZ ASD discordant pairs, if only one twin experienced a risk factor associated with a marker of hypoxia, it was always the twin with an ASD (Table 5). However, many of the ASD discordant pairs included twin pairs where either both twins or neither twin possessed the hypoxia associated risk factor (Table 5).
Table 5.
Description of Parent-reported Risk Factors in MZ ASD Discordant Pairs in the California Autism Twin Study
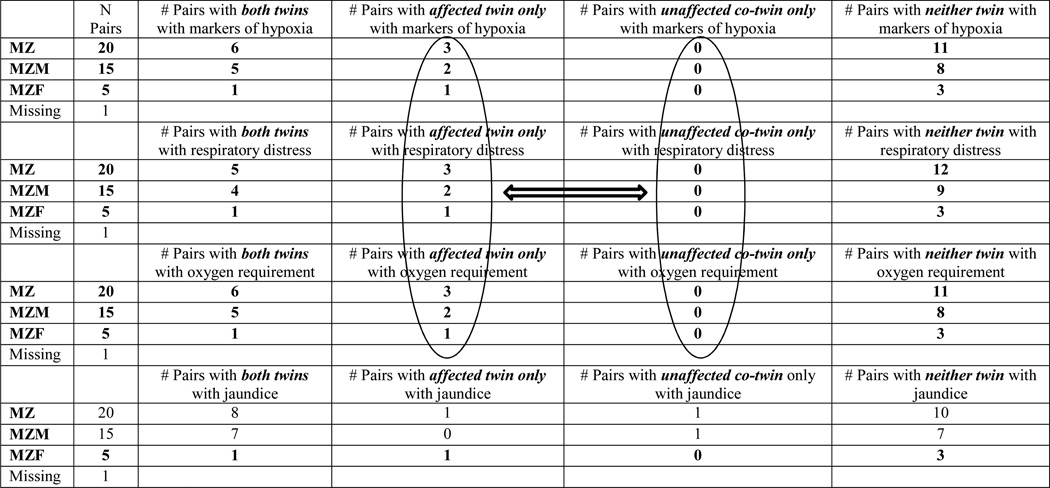 |
MZ=Monozygotic, MZM = Monozygotic Male, MZF = Monozygotic Female; Markers of hypoxia = Summary variable including growth retardation, presence of meconium, fetal distress, unscheduled C-section due to low fetal heart rate and/or distress, umbilical cord complications, respiratory distress or breathing problems, oxygen requirement after birth, resuscitation, or low APGAR scores
Discussion
Our findings are consistent with previous studies suggesting that specific prenatal and perinatal events associated with markers of hypoxia may increase the risk for ASDs in some children. Parent-reported respiratory distress, having an oxygen requirement at birth, and the general category “markers of hypoxia” were associated with increased risks of ASDs. However, concordance risks associated with these factors were not significantly increased in MZ, relative to DZ, twin pairs, suggesting these events are largely independent of autosomal genetic risk factors. In the MZ ASD discordant pairs in which only one twin possessed a given risk factor (respiratory distress, markers of hypoxia, and oxygen requirement after birth), it was always present in the twin with ASD. However, 6 of the 20 MZ pairs in which both twin individuals experienced the risk factor were discordant for ASD, suggesting that these particular markers of hypoxia are not associated with increased risk for ASD in all individuals.
None of the shared factors (maternal age, paternal age, maternal prenatal use of medications, bleeding, or prematurity) were found to be significant risk factors for increased concordance of ASDs in twins. Previous studies have linked increased maternal and paternal age to increased risk for ASDs (Hultman, Sandin, Levine, Lichtenstein, & Reichenberg, 2011; Parner et al., 2012; Sandin et al., 2012) and to increased ASD concordance in same sex twin pairs (Liu, Zerubavel, & Bearman, 2010). Theories regarding the association between parental age and increased risk for ASDs include the potential for more genetic mutations in the gametes of older fathers and mothers, as well as a less favorable in utero environment in older mothers with more obstetrical complications (Kolevzon, Gross, & Reichenberg, 2007; Salem Yaniv et al., 2011; Sandin et al., 2012). Additionally, men and women with a genetic predisposition for ASDs may be more likely to delay childbearing until later years (Grether, Anderson, Croen, Smith, & Windham, 2009). However, the effect sizes in several of these studies have not been large. Thus, although shared factors including maternal and paternal age did not increase concordance in our cohort of twins, it may be that we were not sufficiently powered to detect such effect sizes.
The observation that odds ratios were approximately twice as great in males for respiratory distress, markers of hypoxia, and oxygen requirement as compared to females, but twice as great in females for jaundice suggests that future studies, with larger samples of females, should examine whether perinatal factors differentially impact males and females. Because statistical techniques for comparing ORs such as the Breslow-Day Test of Homogeneity are designed for large samples, this statistical comparison is likely underpowered given our sample size. Thus, neither a true difference NOR lack of a difference in risk based on gender can be concluded from our data. Given that unconjugated bilirubin (UCB) is a substance that can cross the blood-brain barrier and cause neurotoxicity, it is possible that females may physiologically respond differently to elevated levels of UCB compared to males. However, as noted by Newman and Croen, one must be careful making such inferences, as hyperbilirubinemia is a relatively common finding in newborns and may be merely a marker for an underlying, causative factor such as acidosis, sepsis, and hypoalbuminemia (Amin, Smith, & Wang, 2011; Newman & Croen, 2011). Although we hypothesized that fetal hypoxia may specifically be a risk factor for males relative to females, results of this study are difficult to interpret due to a relatively small number of females, congruent with the large gender discrepancy in ASDs. The conjecture that males are at greater risk from hypoxic events is supported by studies showing increased risks of neurotoxicity following HI injury. Theories regarding why males are more at risk include the idea that testosterone has been shown to be neurotoxic in HI injury (Hill et al., 2011b; Nishino et al., 1998), while progesterone and its metabolite allopregnanolone have been shown to be neuroprotective (Knight, Davidson, Young, & Gibson, 2012; Reddy & Rogawski, 2002; Sayeed, Guo, Hoffman, & Stein, 2006; Schumacher et al., 2007; Stein, 2008; Xiao, Wei, Yan, Wang, & Lu, 2008). Furthermore, the mechanisms through which these neurosteroids are either neurotoxic or neuroprotective may be through modulation of excitatory and inhibitory signaling, consistent with the theory that autism is caused by an imbalance in excitation and inhibition in the brain during development (Rubenstein & Merzenich, 2003).
In the stepwise regression models, respiratory distress was the factor most strongly associated with ASD. Perhaps the most straightforward explanation for the mechanism underlying the association of respiratory distress with ASD, is that respiratory distress may lead directly to cerebral hypoxia and neuronal damage. However, indirect mechanisms may also exist. For example, birth insults with perinatal hypoxia have been shown in animal models to lead to long-term changes in dopaminergic function (Boksa & El-Khodor, 2003), and dopamine has long been hypothesized to be a key neurochemical involved in the development of autism spectrum disorders (Previc, 2007). Finally, it is also possible that there may be a cerebral mechanism that independently underlies both the development of respiratory distress, as well as the development of an autism spectrum disorder. Longitudinal investigations are required to address this issue.
Limitations of this study include the fact that the twin study design may account, in part, for prematurity and birth weight not meeting significance as a risk factor. Compared to singleton births, twin births are much more likely to be premature with lower birth weights. Fifty-four percent of twin pairs in this study were born at less than 37 weeks, and 8% were born less than 32 weeks. Birth weight correlated strongly with gestational age (r=0.748, partial correlation controlling for gender). While the findings regarding both prematurity and low birth weights in ASDs are variable, several studies have identified links between these factors and increased risks for ASDs (Gardener et al., 2011; Kolevzon et al., 2007; Lampi et al., 2012; Pinto-Martin et al., 2011). Other study limitations include the limited sample size for specific subgroup comparison, as in the case of the gender comparison. Additionally, information on shared and individual perinatal factors was derived from parent report in response to a standardized questionnaire as opposed to documentation in medical records. Thus, data is limited by parents’ knowledge and recollection of events. Due to the large age range, some twins were already teenagers at the time parents completed the questionnaire, and recollection bias of the parents may be especially pronounced for those with older children. Furthermore, the questionnaire only assessed for qualitative events such as jaundice or oxygen requirement after birth, as opposed to quantitative measures such as bilirubin levels, oxygen concentrations and durations, and medication doses and length of use. The use of perinatal medications in this study, which included infertility medications, estrogen/progesterone, beta-2 agonists, tocolytics, and antidepressants, amongst others, may have been too broad to attain significant medication effects. Exploratory analysis for each of the afore-mentioned categories of medications did not reveal any of them to be significant risk factors. However, the number of women reporting use of each of these was relatively small ranging from 4 for antidepressants to 44 for infertility treatments. Twin pairs included in this study consisted of twins pairs in which at least one of the twins met criteria for an ASD. Although funding for the present study did not allow for collection of typically developing twin pairs, future studies will ideally also include a control twin cohort in which neither twin within a pair meets criteria for an ASD.
Finally, this study does not establish causality, and confounding factors must be considered. In fact, one of the difficulties in studying perinatal risk factors is that many of the factors are not independent of each other. For example, respiratory distress, oxygen requirement after birth, and the summary variable “markers of hypoxia” are all highly correlated with each other, as are gestational age and birth weight, as well as and maternal and paternal age. Thus, the Bonferroni correction may be an overly conservative correction for multiple tests. Although less conservative techniques such as the Benjamini-Hochberg method were considered, we ultimately chose to utilize the Bonferroni correction in order to minimize Type I error. Nonetheless, even with the more stringent Bonferroni correction, respiratory distress still came out as a positive risk factor. Importantly, one must also consider whether there may be an underlying neurologic or other preceding insult leading to fetuses experiencing distress and thus hypoxia during labor and delivery. Poor response to labor may represent an underlying etiology. Thus, future longitudinal studies should also attempt to identify possible underlying etiologies that may occur earlier during gestation.
In conclusion, we found perinatal events associated with possible hypoxia such as breathing difficulty/respiratory distress, oxygen requirement, and other markers of hypoxia to be associated with increased risk for autism. However, given respiratory distress and markers of hypoxia similarly increased risks of ASDs in both MZ and DZ twin pairs, our findings suggest these risk factors may not be dependent on strong autosomal gene-environment interactions. Given that many of the ASD discordant pairs, including MZ pairs, included twin pairs where either both twins or neither twin possessed a risk factor, our findings also suggest that these factors may be predictors of the development of ASDs in some, but not all individuals with autism. Thus, future studies should consider additional potential risk factors to further our understanding of the etiology of ASDs and to suggest new methods for treatment and prevention.
Acknowledgements
We would like to thank the families who participated in this study for their time and dedication. We are also grateful to the National Institute of Mental Health (Grant R01MH067005) and Autism Speaks for funding the California Autism Twin Study, and for support provided through the Faculty Scholar Fund in the Stanford Autism Center at Lucile Packard Children’s Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Wendy Froehlich-Santino
Developed the hypotheses, designed the approach and analytic plan, conducted the literature search, conducted the statistical analyses, drafted the manuscript and oversaw all aspects of the development of this manuscript.
Amalia Londono Tobon
Contributed to the design of the approach and analytic plan, reviewed of data files, coded and compiled data, instrumental in analysis and drafting of the manuscript.
Sue Cleveland
Contributed to the data collection, diagnostic assessment, literature search, review of data files, and drafting of the manuscript.
Andrea Torres
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Jennifer Phillips
Contributed to the data collection, developed the protocol for the diagnostic assessments, review of data files, and drafting of the manuscript.
Brianne Cohen
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Tiffany Torigoe
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Janet Miller
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Angie Fedele
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Jack Collins
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Karen Smith
Contributed to the data collection, literature search, review of data files, and drafting of the manuscript.
Linda Lotspeich
Contributed to the data collection, development of the protocol, diagnostic criteria, review of data files, and drafting of the manuscript.
Lisa A. Croen
Contributed to the data collection, hypothesis development, development of the protocol, literature search, analytic approach, review of data files, and drafting of the manuscript.
Sally Ozonoff
Contributed to the data collection, hypothesis development, development of the protocol, literature search, analytic approach, review of data files, and drafting of the manuscript.
Clara Lajonchere
Contributed to the data collection, hypothesis development, development of the protocol, literature search, analytic approach, review of data files, and drafting of the manuscript.
Judith K. Grether
Contributed to the data collection, hypothesis development, development of the protocol, literature search, analytic approach, review of data files, and drafting of the manuscript.
Ruth O’Hara
Contributed to the hypothesis development, literature search, analytic approach, and drafting of the manuscript.
Joachim Hallmayer
Obtained funding for this study, developed the protocol, diagnostic criteria, analytic approach, and contributed to the hypothesis development, analysis of the data, literature search and drafting of the manuscript.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Aibar L, Puertas A, Valverde M, Carrillo MP, Montoya F. Fetal sex and perinatal outcomes. Journal of Perinatal Medicine. 2012;40:271–276. doi: 10.1515/jpm-2011-0137. [DOI] [PubMed] [Google Scholar]
- Amin SB, Smith T, Wang H. Is neonatal jaundice associated with Autism Spectrum Disorders: a systematic review. Journal of Autism and Developmental Disorders. 2011;41:1455–1463. doi: 10.1007/s10803-010-1169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P, El-Khodor BF. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neuroscience & Biobehavioral Reviews. 2003;27:91–101. doi: 10.1016/s0149-7634(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Wang X, Yasui Y, Sithole F, Zwaigenbaum L. Autism spectrum disorders and fetal hypoxia in a population-based cohort: accounting for missing exposures via Estimation-Maximization algorithm. BMC Medical Research Methodology. 2011;11:2. doi: 10.1186/1471-2288-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gender Medicine. 2007;4:19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16:491–499. doi: 10.1037//0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. British Journal of Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether JK, Anderson MC, Croen LA, Smith D, Windham GC. Risk of autism and increasing maternal and paternal age in a large north American population. Am J Epidemiol. 2009;170:1118–1126. doi: 10.1093/aje/kwp247. [DOI] [PubMed] [Google Scholar]
- Gualtieri T, Hicks R. An immunoreactive theory of selective male affliction. Behavioral and Brain Sciences. 1985;8:427–441. [Google Scholar]
- Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstetricia Gynecologica Scandinavica. 2012;91:287–300. doi: 10.1111/j.1600-0412.2011.01325.x. [DOI] [PubMed] [Google Scholar]
- Haglund NG, Kallen KB. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism. 2011;15:163–183. doi: 10.1177/1362361309353614. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Archives of General Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Alexander ML, McCullough LD, Fitch RH. Inhibition of X-linked inhibitor of apoptosis with embelin differentially affects male versus female behavioral outcome following neonatal hypoxia-ischemia in rats. Developmental Neuroscience. 2011a;33:494–504. doi: 10.1159/000331651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurology Research International. 2012;2012:867531. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. International Journal of Developmental Neuroscience. 2011b;29:381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Molecular Psychiatry. 2011;16:1203–1212. doi: 10.1038/mp.2010.121. [DOI] [PubMed] [Google Scholar]
- Knight SR, Davidson C, Young AM, Gibson CL. Allopregnanolone protects against dopamine-induced striatal damage after in vitro ischaemia via interaction at GABA A receptors. Journal of Neuroendocrinology. 2012;24:1135–1143. doi: 10.1111/j.1365-2826.2012.02319.x. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Archives of Pediatrics & Adolescent Medicine. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, Gissler M, Brown AS, Sourander A. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. Journal of Pediatrics. 2012;161:830–836. doi: 10.1016/j.jpeds.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan WC, Priestley M, Mayoral SR, Tian L, Shamloo M, Penn AA. Sex-specific cognitive deficits and regional brain volume loss in mice exposed to chronic, sublethal hypoxia. Pediatric Research. 2011;70:15–20. doi: 10.1203/PDR.0b013e31821b98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, Schendel D, Thorsen P, Mortensen PB. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161:916–925. doi: 10.1093/aje/kwi123. discussion 926-918. [DOI] [PubMed] [Google Scholar]
- Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15:411–420. [PubMed] [Google Scholar]
- Liu K, Zerubavel N, Bearman P. Social demographic change and autism. Demography. 2010;47:327–343. doi: 10.1353/dem.0.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule – WPS. WPS edn. Los Angeles: 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maramara LA, He W, Ming X. Pre- and Perinatal Risk Factors for Autism Spectrum Disorder in a New Jersey Cohort. Journal of Child Neurology. 2014 doi: 10.1177/0883073813512899. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Mayoral SR, Omar G, Penn AA. Sex differences in a hypoxia model of preterm brain damage. Pediatric Research. 2009;66:248–253. doi: 10.1203/PDR.0b013e3181b1bc34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TB, Croen LA. Jaundice-autism link unconvincing. Pediatrics. 2011;127:e858–e859. doi: 10.1542/peds.2010-3769A. [DOI] [PubMed] [Google Scholar]
- Nishino H, Nakajima K, Kumazaki M, Fukuda A, Muramatsu K, Deshpande SB, Inubushi T, Morikawa S, Borlongan CV, Sanberg PR. Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neuroscience Research. 1998;30:303–312. doi: 10.1016/s0168-0102(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Parner ET, Baron-Cohen S, Lauritsen MB, Jorgensen M, Schieve LA, Yeargin-Allsopp M, Obel C. Parental age and autism spectrum disorders. Annals of Epidemiology. 2012;22:143–150. doi: 10.1016/j.annepidem.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Martin JA, Levy SE, Feldman JF, Lorenz JM, Paneth N, Whitaker AH. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics. 2011;128:883–891. doi: 10.1542/peds.2010-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previc FH. Prenatal influences on brain dopamine and their relevance to the rising incidence of autism. Medical Hypotheses. 2007;68:46–60. doi: 10.1016/j.mehy.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Raz S, Debastos AK, Newman JB, Batton D. Extreme prematurity and neuropsychological outcome in the preschool years. J Int Neuropsychol Soc. 2010;16:169–179. doi: 10.1017/S1355617709991147. [DOI] [PubMed] [Google Scholar]
- Raz S, Lauterbach MD, Hopkins TL, Glogowski BK, Porter CL, Riggs WW, Sander CJ. A female advantage in cognitive recovery from early cerebral insult. Developmental Psychology. 1995;31:958–966. [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH, Jr, Leventhal BL, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism diagnostic interview-revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Salem Yaniv S, Levy A, Wiznitzer A, Holcberg G, Mazor M, Sheiner E. A significant linear association exists between advanced maternal age and adverse perinatal outcome. Archives of Gynecology and Obstetrics. 2011;283:755–759. doi: 10.1007/s00404-010-1459-4. [DOI] [PubMed] [Google Scholar]
- Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:477–486. e471. doi: 10.1016/j.jaac.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Annals of Emergency Medicine. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocrine Reviews. 2007;28:387–439. doi: 10.1210/er.2006-0050. [DOI] [PubMed] [Google Scholar]
- Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Research Reviews. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile LA. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2000;83:F182–F185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatten LJ, Skjærven R. Offspring sex and pregnancy outcome by length of gestation. Early Human Development. 2004;76:47–54. doi: 10.1016/j.earlhumdev.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Critical Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]


