Abstract
Background
Magnesium (Mg) alloy is a metal-based biodegradable material that has received increasing attention in the field of clinical surgery, but it is currently seldom used in intestinal anastomosis. This study was conducted to comprehensively assess a ternary magnesium (Mg)-zinc (Zn)-strontium (Sr) alloy’s biological superiorities as a preparation material for intestinal anastomosis ring.
Material/Methods
Mouse L-929 fibroblasts were cultured with Mg-Zn-Sr alloy extract and compared with both positive (0.64% phenol) and negative (original broth culture) controls. The cell morphology of different groups was examined using microscopy, and a cytotoxicity assessment was performed. Fresh anticoagulated human blood was mixed with Mg-Zn-Sr alloy extract and compared with both positive (distilled water) and negative (normal saline) controls. The absorbance of each sample at 570 nm was used to calculate the Mg-Zn-Sr alloy hemolysis ratio in order to test the Mg alloy’s blood compatibility. Bacterial cultures of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus were added to Mg-Zn-Sr alloy block samples and compared with positive (Ceftazidime), negative (316LSS stainless steel), and blank controls. The broth cultures were sampled to compare their bacterial colony counts so as to evaluate the antibacterial properties of the Mg-Zn-Sr alloy. The Mg-Zn-Sr alloy was surface-coated with a layer of poly(lactic-co-glycolic acid) carrying everolimus. The surface morphology and degradability of the coating were examined so as to demonstrate feasibility of coating, which can release the drug evenly.
Results
The experiments proved that Mg-Zn-Sr alloy has good biocompatible, antibacterial, and drug-loaded coating performances, which are lacking in existing intestinal anastomosis devices/materials.
Conclusions
The Mg-Zn-Sr alloy increases biocompatibility, and yields a safer and better therapeutic effect; therefore, it is a novel biomaterial that is feasible for use when preparing biodegradable intestinal anastomosis rings.
MeSH Keywords: Anastomosis, Surgical, Anti-Infective Agents, Drug-Eluting Stents, Magnesium, Materials Testing
Background
In recent years, metal-based biodegradable magnesium (Mg) alloys have received substantial attention in the clinical surgery field. A number of studies have been conducted on the applications of Mg alloys in orthopedic, cardiovascular, and dental surgery. The biodegradability of Mg alloys has been clearly demonstrated. Magnesium metal has a very low standard electrode potential (−2.37V/SCE), and the oxide film that forms on its surface cannot have a protective effect; thus, in the general environment magnesium alloys offer high-performance degradability. In neutral and acidic environments, the corrosion reaction of magnesium is as follows:
If magnesium alloys are implanted into the human body, they will mainly be in contact with the tissues and fluids, so the most important factors resulting in degradation should be hydrolysis (including acid-base interaction and self-catalysis) and enzymatic hydrolysis. Body fluids contain a large number of Cl−; in a Cl−-rich phenomenon, the corrosion of magnesium metal can be further intensified. As a biodegradable implant material, it is suitable for the preparation of short-term or temporary medical devices. However, researchers have found that the corrosion rate of pure magnesium is excessively fast. In order to improve the corrosion resistance, researchers have proposed a variety of methods. The main methods include surface coating and alloying. In 2006, a biodegradable Mg-alloy intravascular stent was first developed by Biotronik in Germany and applied at the St. Blasius Hospital in Belgium. The implanted Mg-alloy stent degraded within 2–3 months, during which the incidences of restenosis and other complications were reduced. In the following year, Erbel [1] reported a clinical trial that involved stent implantation into the coronary arteries of 63 patients using the same brand of biodegradable Mg-alloy intravascular stent, further demonstrating the feasibility of the clinical application of Mg alloys.
Magnesium and magnesium alloys are lightweight metallic materials and have a far higher specific mechanical strength than biodegradable polymer material. The specific modulus of the pure magnesium is 133 GPa/(g/cm3) and that of magnesium alloy even reaches 480 GPa/(g/cm3) [2]. It can completely solve the current clinical problem that biodegradable polymer material has poor mechanical strength and rigidity; therefore, it is more suitable for being used as a biological implant material.
Anastomosis rings are among the sutureless intestinal anastomosis techniques in use worldwide. The principle of an anastomosis ring is to invert and fix both edges of the anastomosis to the intestinal anastomosis ring (IAR) via a purse-string suture. The closed IAR forms a mechanical lock and allows the apposition of intestinal serosa and serosa, further achieving the goal of anastomosis [3]. In 1985, Hardy et al. [3] introduced a biodegradable anastomosis ring onto the market with the trade name Valtrac™. This product, which is made of biodegradable polyglyconate and barium sulfate, has been used globally for nearly 30 years and is recognized by the majority of gastrointestinal surgeons. Compared to other anastomosis techniques such as the manual thread and anastomat, the biodegradable anastomosis ring is applied using a simple procedure, thus saving time and effort. Due to the mechanical locking and sutureless anastomosis techniques, biodegradable anastomosis rings have a strong anti-burst capability [4,5], which can support digestive tract expansion, decrease foreign body response and scar formation brought about by degradation, and reduce the incidence of complications such as intestinal fistula and intestinal stenosis. The polymer materials have poor mechanical properties, excessively rapid degradation rate, and high cost; therefore, there is great need for a new material with which to prepare BAR, which is cheaper, more corrosion-resistant, and possesses better mechanical properties than polymer materials in order to guarantee the security of anastomosis and be affordable. Herein, we propose that an Mg alloy with better mechanical and biological properties than polymer materials (polyglyconate) could be used to produce an IAR with altogether more satisfactory properties.
In this study, we employed an Mg-Zn-Sr alloy for IAR preparation. The 3 metal components of the Mg-Zn-Sr alloy are abundant in nature and are essential elements within the human body. Of these, Sr mostly occurs in bone tissue and promotes osteogenesis. Strontium ranelate plays a role in the treatment of osteoporosis [6,7]. Addition of a small amount of Sr can effectively refine the crystal grain, improve the casting and mechanical properties, and increase the corrosion and heat resistance of Mg alloy [8]. Bornapour [9] investigated the corrosion rate in simulated body fluid (SBF) of a series of Mg–Sr alloys, with Sr in the range of 0.3–2.5%, and found that the Mg-0.5% Sr alloy showed the slowest corrosion rate. The MgZn phase formed in the Mg-Zn alloy has a dual effect of solid solution and ageing strengthening, thus increasing the strength and improving the corrosive properties of Mg [10]. Geng [11] employed the fusion casting method when preparing Mg-Zn-Ca alloys with 4%, 6%, and 8% Zn and found that the Mg-Zn-Ca alloy with 4% Zn exhibited the highest corrosion resistance in Hank’s simulated body fluid (SBF). Therefore, an Mg alloy with 4% Zn and 0.5% Sr was selected for the present study.
On this occasion, we investigated the feasibility and superiority of the Mg alloy as a material for IAR preparation by examining the biocompatibility, antibacterial properties, and feasibility of drug-loaded coating. This work was a prospective study of the development of the biodegradable Mg-alloy IAR, which provides fundamental data and a feasibility analysis for the further development of this medical device.
Material and Methods
Cytotoxicity assay
To assess the cytotoxicity of the Mg-Zn-Sr alloy, ISO 10993-5 guidelines were used: Medium extraction with a 72-h extraction period and MTT assay to evaluate the possible toxic effects of leachables released from alloy blocks during extraction [12]. Firstly, Mg-Zn-Sr alloy blocks were sterilized with diethylene oxide, then were put into a sterile culture bottle. According to the proportion of alloy surface area and volume of leaching solution 3 cm2/L, RPMI1640 culture liquid was added into the bottle. After incubation at 37°C and 5% CO2 for 72 h, Mg alloy extract was produced. Via recovery and passage, mouse fibroblast cells (L929 cells) in vigorous growth period were made into cell suspension with trypsin digestion, adjusting the cell density of 5×104/ml. Then they were seeded into three 96-well plates, each with 6 holes, and each hole 100 ul. Under the condition of 37°C and 5% CO2, cells were continuously cultured for 24 h until cells attached to the wall. With the original culture medium discarded, L-929 cells were incubated with the Mg-Zn-Sr alloy extract (experimental group), the negative control group (original broth culture), and positive control group (0.64% phenol), each group set in 6 parallel samples. The broth culture was sampled on days 2, 4, and 7 for examination of cell morphology under an inverted phase-contrast microscope. Thereafter, 100 μL of dimethyl sulfoxide (DMSO) was added to each well and the plates were oscillated at room temperature for 10 min, allowing the MTT formazan crystals to dissolve completely. Finally, the OD value of each well was measured with a microplate reader at 570 nm. The relative growth rate (RGR) of L-929 cells was obtained for the preliminary quantitative assessment of Mg-Zn-Sr alloy cytotoxicity, which was calculated as follows:
According to the grading standard, RGR values were converted to 6 grades (i.e., 0–V) (Table 1). The evaluation criteria were as follows: (1) Cytotoxicity grade 0 or 1 indicated that the material was qualified for use; (2) Cytotoxicity grade 2 indicated that comprehensive evaluation of the material in combination with morphological analysis was required; and (3) Cytotoxicity grades 3–4 indicated that the material was not qualified for use [13,14] (Table 1).
Table 1.
The relationship between cell RGR and cytotoxicity grade in Pharmacopoeia in the United States.
| RGR | Cytotoxicity grade |
|---|---|
| ≥100 | 0 |
| ≥80 | 1 |
| ≥50 | 2 |
| ≥30 | 3 |
| ≥0 | 4 |
Hemolysis test
Mg-Zn-Sr alloy blocks were sterilized with diethylene oxide, then the Mg-Zn-Sr alloy blocks were immersed in 10 mL of normal saline in test tubes, with positive and negative control groups prepared with 10 mL of distilled water and 10 mL of normal saline, respectively (6 parallel samples in each group were chosen). All test tubes were incubated in a 37°C water bath for 30 min. To prepare the anticoagulated blood samples, 8 mL of arterial blood was collected from healthy adults (a healthy volunteer from the physical examination center of Xiangya hospital) and immediately mixed with 10 mL of normal saline containing 0.5 mL of 3.8% sodium citrate. Each tube was mixed with 0.2 mL of anticoagulated blood, continuously incubated in a 37°C water bath for 60 min, and then centrifuged at 2500 rpm for 5 min. The supernatant was loaded into 96-well plates and the absorbance of samples was measured on a microplate reader at 550 nm. According to ISO 10993-4: 2002 [15], The mean absorbance of 6 samples in each group is defined as the absorbance of each group, and the average absorbance of the negative control group should be less than 0.03, while that of positive group should be greater than 0.8±0.3. The hemolysis ratio was calculated as follows:
A is the average absorbance of each group. If the hemolysis ratio is less than or equal to 5%, then the material complies with the hemolysis requirements for medical materials.
Antibacterial assessment
Mg-Zn-Sr alloy blocks (10×5×5 mm) and 316LSS stainless steel blocks (10×10×10 mm) were sterilized with diethylene oxide prior to use. The 316LSS stainless steel served as an additive of the negative control group. Another negative control group (blank control group) was prepared with pure bacterial broth culture free of additives. Ceftazidime, a broad-spectrum antibiotic susceptible to Gram-negative and Gram-positive bacteria, served as an additive to the positive control group (0.1-g dosage). The bacterial cultures included Gram-negative Escherichia coli (ATCC25922) and Pseudomonas aeruginosa (ATCC27853), and Gram-positive Staphylococcus aureus (ATCC6538). The 3 bacteria were inoculated into the brain heart infusion extract. After an 18-h incubation period, the broth cultures were prepared as cell suspensions with a cell density of 1.0×105 colony-forming units (CFU)/mL. To each test tube was added 0.5 mL of cell suspension, followed by dilution with 4.5 mL of brain-heart infusion extract (final cell density of 1.0×104 CFU/mL). To the experimental group was added Mg-Zn-Sr alloy block, and to the positive, negative, and blank control groups were added ceftazidime powder (0.1 g), 316LSS stainless steel block, and no additive, respectively. The cultures were incubated at 37°C. Because of cell proliferation, the OD of bacterial broth cultures is bound to change. We chose the OD value at 590 nm (OD590) to determine the bacterial growth. The number of bacterial colonies was assayed at specific time points, with 0.2£ OD590 £0.6. The schedules for bacterial colony counting were as follows: 4.5 h, 6 h, and 7.5 h for E. coli; 6 h, 7 h, and 8 h for S. aureus; and 6 h, 7.5 h, and 9 h for P. aeruginosa. At specific time points, broth cultures (0.1-mL aliquots) were sampled and spread on the corresponding agar plates. The number of bacterial colonies on each plate was counted and converted into CFU/mL. Six samples were taken from each test tube at the same time point and the average number of bacterial colonies was calculated as experimental data. The results are presented as mean ± standard deviation. The difference between the experimental and positive or negative control groups was evaluated by analysis of variance using the SPSS 13.0 statistical software (P<0.05 was considered statistically significant), then using Student-Newman-Keuls method to compare mean value of every 2 groups.
Preparation of Mg-Zn-Sr alloy surface coating
The Mg-Zn-Sr alloy was polished, sequentially washed with acetone and distilled water in an ultrasonic cleaning device, and dried. Poly(lactic-co-glycolic acid) (PLGA) was dissolved in chloroform (4% by weight), to which everolimus (>98% purity, final concentration: 0.015 g/mL) was added. Mg-Zn-Sr alloy sheets (10×5×3 mm) were immersed in the prepared chloroform solution for 30 min and then pulled out at a constant speed using the Czochralski infiltration method. The alloy sheets were allowed to naturally evaporate for 30 min and were repeatedly coated following the same procedure. Finally, the alloy sheets were dried in an oven to remove the solvent. After repeated folding and bending, the surface morphology of the alloy sheets was examined by scanning electron microscopy (Hitachi S-650, Japan). The coated alloy sheets were transferred into Hank’s simulated body fluid and incubated at 37°C under 5% CO2. Samples were taken every other day, washed with distilled water, and dried to measure the change in the mass according to the following equation: the percentage of mass loss (%) = (W0−W1) / W0 ×100%, where W0 is the sample mass before degradation, and W1 is the sample mass after degradation. There was a total of 5 samples mean values are selected.
Results
Cytotoxicity of the Mg-Zn-Sr alloy
After 2-day incubations, in 3 groups nearly all the cells adhered to the wall as they developed and demonstrated clear shapes, normal nucleoplasm proportions, and uniform cytoplasms. The adherent L-929 cells of the positive control group remained as normal in the aspect of morphology as the negative control group and the experimental group, but the difference in number of cells was apparent; under the microscope at low magnification, the proliferating cells of the positive control group were significantly fewer than that of the other 2 groups.
After 4-day incubations, the cells showed normal morphologies with a few senescent cells present in all groups, but the difference in cellular numbers between the positive control group and the other 2 groups was becoming increasingly evident.
After 7-day incubations, in the positive control group, the small amount of cells under the microscope meant that proliferation and growth rate of cells were much slower than that of the other 2 groups. In the other groups, the cells continued to proliferate and grow, so they were relatively mature.
In this experiment, compared with negative control group and the experimental group, changes in cellular morphology of the positive control group were not obvious, but the slowing of the proliferation rate was significant. We thought that this was mainly due to abnormality of the cellular mitosis mechanism, causing cell cycle stagnation in the G1 phase (Figure 1).
Figure 1.
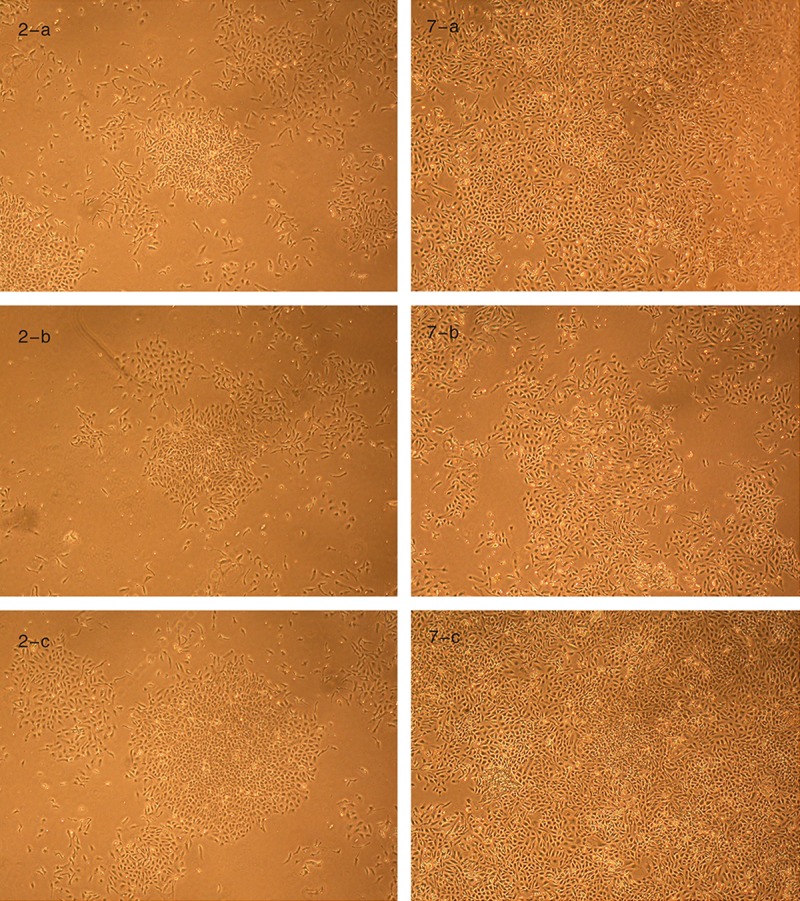
Cell morphology in the 3 groups after 2,7-D incubation. Groups a, b, and c are the experimental group, positive control group, and negative control group, respectively. In the above chart, the cellular numbers of group a and c were more than in the corresponding group b, and the cellular number of group a was almost the same as group c.
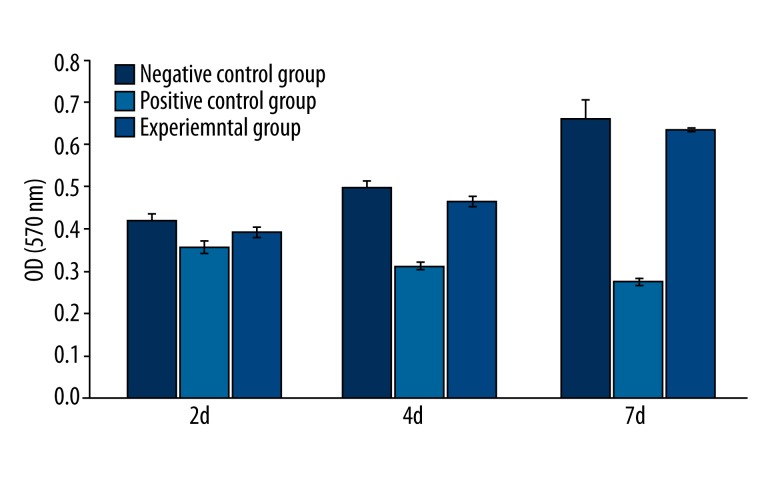
The OD value of the cytotoxicity results of different groups and alloys are shown in Table 2 and 3, Figure 2.
Table 2.
The OD values of each group in cytotoxicity assay.
| Mean value | Standard deviation | |
|---|---|---|
| Negative control group | ||
| 2 day | 0.4183 | 0.0190 |
| 4 day | 0.4959 | 0.0145 |
| 7 day | 0.6601 | 0.0472 |
| Positive control group | ||
| 2 day | 0.3562 | 0.0161 |
| 4 day | 0.3123 | 0.0098 |
| 7 day | 0.2749 | 0.0092 |
| Experimental group | ||
| 2 day | 0.3911 | 0.0129 |
| 4 day | 0.4642 | 0.0129 |
| 7 day | 0.6334 | 0.0045 |
Table 3.
The result of the Mg-Zn-Sr alloy’s cytotoxicity.
| Time | Group | Relative growth rate (RGR) | Cytotoxicity grade |
|---|---|---|---|
| 2-day | Negative control group | 100% | 0 |
| Experimental group | 93% | 1 | |
| Positive control group | 85% | 1 | |
| 4-day | Negative control group | 100% | 0 |
| Experimental group | 94% | 1 | |
| Positive control group | 63% | 2 | |
| 7-day | Negative control group | 100% | 0 |
| Experimental group | 96% | 1 | |
| Positive control group | 42% | 3 |
Cells (L-929) were cultured in contact with Mg-Zn-Sr alloy extract (experimental group) and compared with the positive control group (0.64% phenol) and negative control group (original broth culture). Cytotoxicity assessment of the Mg-Zn-Sr alloy was then determined via an MTT assay. RGR (%) = (optical density [OD] value of the experimental group/OD value of the negative control group) ×100%.
Figure 2.
The OD values of each group. The OD values for the positive control group are obviously lower than that of both the experimental group and negative control group.
During incubation, the RGR of the L-929 cells in contact with the Mg-Zn-Sr alloy was 93% on day 2, 94% on day 4, and 96% on day 7. Accordingly, the cytotoxicity of the alloy was rated as grade 1. The MTT assay results showed that the ternary Mg-Zn-Sr alloy was a qualified material, as it exhibited no cytotoxic effects and complied with the basic requirements for biomaterials.
Hemolysis ratio of the Mg-Zn-Sr alloy
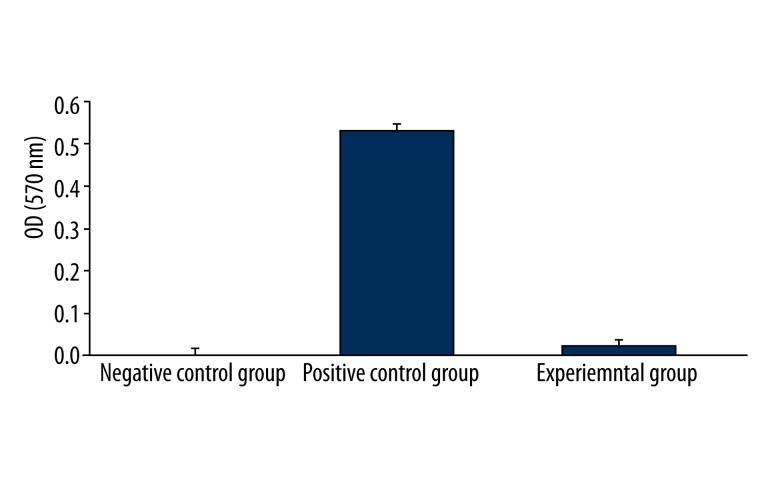
The hemolysis ratio of the ternary Mg-Zn-Sr alloy was 2.45%, complying with the requirements for biomedical materials (<5%). This result indicated that the Mg-Zn-Sr alloy has no hemolytic effects and meets the requirements for clinical application (Table 4, Figure 3).
Table 4.
OD values of each group at 570 nm in hemolysis test.
| Group | Mean value | Standard deviation |
|---|---|---|
| Negative control group | 0.0020 | 0.0002 |
| Positive control group | 0.5359 | 0.0141 |
| Experimental group | 0.0151 | 0.0005 |
Figure 3.
OD values of each group at 570 nm. Fresh anticoagulated human blood was allowed to make contact with the Mg-Zn-Sr alloy extract (experimental group) and compared with the positive control group (0.64% phenol) and negative control group (normal saline). Six parallel samples were prepared for each group, and the absorbance of each sample was measured at 570 nm with an ultraviolet-visible spectrophotometer.
Antibacterial action of the Mg-Zn-Sr alloy
After the Mg-Zn-Sr alloy was added, evident bubbling occurred in the test tubes. The alloy surface lost the original metallic luster and exhibited a relatively obscure color. This observation demonstrated that the Mg-Zn-Sr alloy was subject to corrosion in the test tubes, leading to the formation of hydroxide – Mg (OH)2 and hydrogen (H2). Along with the degradation of the Mg-Zn-Sr alloy, the concentration of Mg (OH)2 in the solution increased, significantly increasing the pH of the solution. For most bacteria, the optimum growth pH condition is neutral or weakly alkaline (i.e., pH 7.2–7.6) [16]. When the pH exceeds the tolerance range of the organism, antibacterial action will be achieved (Figure 4).
Figure 4.

The 4 Escherichia coli culture groups (left to right: experimental group, blank control group with no additive, negative control group with 316L stainless steel, and positive control group with antibiotics). The magnesium alloy of the experimental group precipitated a corrosion reaction in the culture liquid, a large number of bubbles emerged on the surface of the liquid, and there are also obvious signs of corrosion on the surface of the magnesium alloy block, but no such phenomenon occurred in the control group.
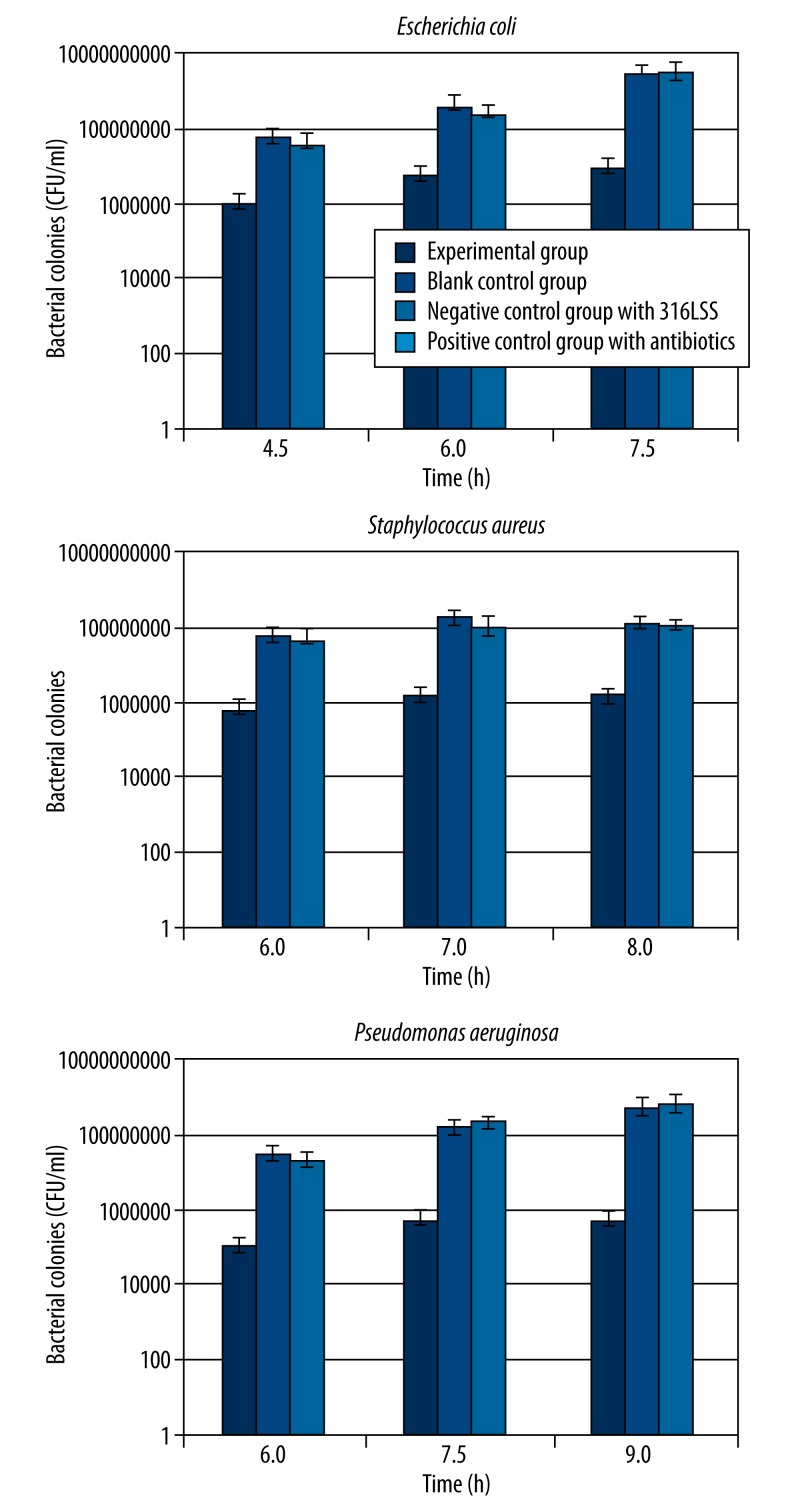
When the bacterial colonies in the experimental group, the negative group, and the positive group were compared, we found that the overall mean indexes of groups were statistically significant (P<0.05), meaning that the added materials affected bacterial proliferation differently. Then we applied Student-Newman-Keuls method to compare mean value of every 2 groups, the results showed that bacterial colonies in the experimental group relative to the negative group was statistically significant, reflecting the clear antibacterial advantage. However, there was a significant difference between the experimental and positive control group as well, indicating that the antibacterial effect of the Mg-Zn-Sr alloy did not approach that of 0.1 g of ceftazidime (Table 5, Figure 5).
Table 5.
The result of antibacterial experiment.
| Time (h) | Experimental group bacterial colonies (CFU/ml( | Blank control group bacterial colonies (CFU/ml) | Negative control group with 316LSS bacterial colonies (CFU/ml) | Positive control group with antibiotics bacterial colonies (CFU/ml) |
|---|---|---|---|---|
| Escherichia coli | ||||
| 4.5 | (8.6±1.2)×105 | (5.2±1.3)×107 | (3.5±0.7)×107 | 0 |
| 6.0 | (4.9±1.5)×106 | (3.5±0.7)×108 | (2.0±0.2)×108 | 0 |
| 7.5 | (7.6±1.4)×106 | (2.4+1.1)×109 | (2.7±0.9)×109 | 0 |
| Staphylococcus aureus | ||||
| 6.0 | (6.0±1.2)×105 | (6.1±1.5)×107 | (4.6±1.3)×107 | 0 |
| 7.0 | (1.4±0.3)×106 | (1.5±0.5)×108 | (1.0±0.4)×108 | 0 |
| 8.0 | (1.7±0.6)×106 | (1.3±0.3)×108 | (1.1±0.4)×108 | 0 |
| Pseudomonas aeruginosa | ||||
| 6.0 | (1.7±0.7)×105 | (2.9±1.0)×107 | (2.1±1.2)×107 | 0 |
| 7.5 | (3.9±0.9)×105 | (1.7±0.6)×108 | (2.5±0.5)×108 | 0 |
| 9.0 | (4.2±1.3)×105 | (6.9±1.5)×108 | (8.6±1.2)×108 | 0 |
Figure 5.
The number of bacterial colonies on each plate. Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus with control, Mg-Zn-Sr alloy, 316LSS and ceftazidime treatment groups. Control, no additive; Mg-Zn-Sr, Mg-Zn-Sr alloy turnings; 316LSS, 316L stainless steel; ceftazidime, antibiotic. Data are presented as median CFU ml/L. Columns of the same group were not significantly different (P>0.05) at the given time point.
This result was possibly related to the small amount of the Mg-Zn-Sr alloy used in the reaction system. We will consider testing the relationship between the quantity and the antibacterial effect of the Mg-Zn-Sr alloy in similar reaction systems. As the present study aimed to clarify the antibacterial properties of the Mg-Zn-Sr alloy – that is, a qualitative rather than quantitative analysis of the alloy’s antibacterial action – the goal of the experimental design was indeed achieved.
Characteristics of drug-loaded coating on the Mg-Zn-Sr alloy
The coated Mg-Zn-Sr alloy had a smooth surface on which the polymer was evenly distributed without blocking. No peeling or curling was observed after the alloy sheets were bent and folded. SEM (scanning electron microscopy) showed a lack of cracks, peeling, curling, or alternate blocking on the surface of the coated Mg-Zn-Sr alloy. These observations suggest that the bonding force of PLGA and the Mg-Zn-Sr alloy meets the application requirements for biomaterial coatings. After immersion in simulated body fluid, the PLGA coating retained its smooth and dense appearance without any defects such as holes or damage (Figure 6).
Figure 6.

The coated Mg-Zn-Sr alloy’s Surface morphology by SEM. White points in the figure are some of the non-conductive dust and other substances on the surface.
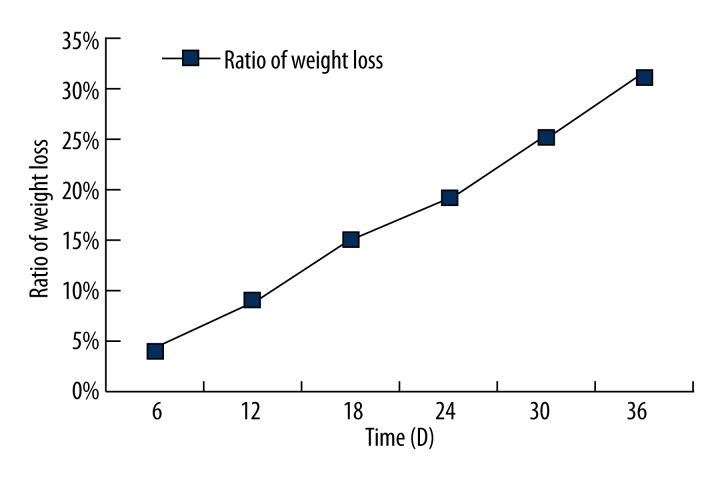
During the simulated body fluid immersion test, the surface coating showed no bubbling or peeling while degrading uniformly and slowly. Overall, PLGA degraded in a linear pattern, suggesting that the degradation rate was uniform and slow. This also demonstrated that the coating was subject to planar degradation rather than massive degradation, which ensured slow release of the drug and maintained its concentration and action time (Figure 7).
Figure 7.
PLGA coating’s weight loss-time coordinates.
Discussion
Due to the excellent biological and mechanical properties of Mg and its alloys, substantial efforts have been made to develop Mg alloy medical devices for cardiovascular and orthopedic surgeries. However, no reports have been published regarding Mg alloy-related medical devices for intestinal anastomosis.
Biocompatibility is the first issue that is encountered in biomaterials research, as it is the key link as to whether a biomaterial can be used in clinical research. In the present study, we conducted a preliminary assessment of the biocompatibility of a ternary Mg-Zn-Sr alloy via a cytotoxicity assay and hemolysis test. The MTT assay is a method for testing cell survival and growth. The principle is that succinate dehydrogenase in the mitochondria of living cells can deoxidize exogenous MTT into water-insoluble violet crystals (Formazan) and then form deposits in the cells, but dead cells do not have such a function. Dimethylsulfoxide (DMSO) can dissolve formazan in living cells, and the enzyme-linked immunosorbent detector measures its OD value at 570 nm wavelength, which can indirectly reflect the number of living cells. Therefore, the MTT assay is used to detect the relative number of cells and the relative vitality [17]. As DMSO causes damage to cells, MTT assay cannot be used to dynamically detect a single cell’s viability, rather, only at 1 point of time. Because the magnesium alloy block is impossible to degrade over a short time period, the experimental time needed is a little longer than for other tests; in the meantime, we also absorb experiences from other cytotoxicity tests of Mg-Ca and Mg-Zn alloys [18,19]. Therefore, we selected the 2nd, 4th, and 7th day’s broth culture as samples. The hemolysis test is the most commonly performed blood evaluation experiment, and since all devices that may be in contact with blood are required for the hemolysis performance evaluation, it has created a widely accepted qualified indicator. The hemolysis test is measured by levels of erythrocyte lysis and hemoglobin release caused by instruments, materials, or extraction liquid in vitro. Because of high sensitivity, simple operation, and good reproducibility, it has long been considered one of the most meaningful screening tests [20]. Our results from the MTT assay and hemolysis test indicate that the Mg-Zn-Sr alloy has the potential for use as an implantation material and complies with the index for the safety performance of biomaterials.
Bacterial infections are a burden for the majority of patients, which greatly increase the time and cost of hospitalization, and risk of mortality. This issue is particularly evident in gastrointestinal surgery, as a variety of bacteria already exists in the intestinal tract, which, together with the superinfection of patients caused by immune decline, foreign bacteria invasion, and postoperative antibiotic use, increase the chance of intestinal anastomosis infection. Because it can cause inflammatory exudation, edema, delayed healing, and necrosis of anastomotic stoma, bacterial infection is considered to be one of the most important causes of anastomotic leakage. Clinical anastomotic leakage is associated with an increased mortality rate, ranging from 10% to 15%, and increased overall or local recurrence in patients who have undergone resection for rectal cancer [21]. The use of numerous antibiotics cannot effectively control the infection, which raises a series of issues relating to superinfection and drug resistance. Studies have been conducted to identify absorbable antimicrobial biomaterials with broad-spectrum antibacterial properties that subvert bacterial defense mechanisms while causing no toxic effects on their users. In the present study, the antibacterial experiment results demonstrated that Mg and Mg alloys represent such biomaterials, which exhibit broad-spectrum antibacterial properties that differ from those of traditional antibiotics. This ensures that the antibacterial action of the Mg alloy cannot be easily resisted via defense mechanisms of traditional microbes, such as loss of enzyme activity, anti-bacterial target alterations, and antibiotic activation failure. Zn also has antibacterial effect, and zinc oxide is listed as “generally recognized as safe (GRAS)” by the U.S. Food and Drug Administration and is used in antibacterial sprays [22]. The inhibitory effect of the Mg alloy is undoubtedly a highly favorable factor for intestinal anastomotic healing. If the material used for preparing anastomotic devices has inherent anti-bacterial properties, it will not only obviate the need for the use of antibiotics but also reduce the incidence of relevant complications such as intestinal infections and intestinal fistulas. These advantages are characteristics of the Mg alloy, which are lacking in anastomotic devices currently in use.
Taking into account the high binding capacity of the Mg alloy and high polymers, as well as the inter-miscibility of drugs and high polymers, we prepared the Mg-alloy sheets with a drug-loaded coating and performed a series of experiments to confirm that the coating and the magnesium alloy have a good binding capacity, and the coating performs well in terms of slow degradation, which ensured slow release of the drug and maintained its concentration and action time. Everolimus is an analogue of rapamycin, which was used as an immunosuppressive agent in transplantation surgery in early studies. Later, when employed in the present study, it was gradually recognized that everolimus has an anticancer effect. More recently, everolimus has successfully passed stage III clinical trials and was approved for use in treatment of pancreatic neuroendocrine tumors [23]. Anticancer drugs such as everolimus and other drugs that promote the growth of local tissues or topical antibiotics all can be loaded into the coating. Previously, 5-Fu and other drugs were applied to the abdominal cavity after radical resection of cancer tissue to achieve the anticancer effect of early chemotherapy postoperatively. However, this approach has a short duration of action and limited anticancer effect. At present, fluorouracil implants are commonly used as slow-release carriers in the clinical setting for colorectal cancer [24]. However, these materials are non-biodegradable and exist as foreign bodies in vivo, and thus can induce complications such as intestinal obstruction. The IAR with a drug-loaded coating proposed in the present study is an ideal combination of drug, coating material, and device material. A Mg-alloy IAR such as this plays a role in anastomosis and supports the intestine, while the loaded drug creates a treatment effect. The loaded drug will be evenly distributed and uniformly released while slowly degrading in the intestinal tract without leaving residual foreign bodies.
In view of the many excellent biological properties of magnesium alloy, we attempted to prepare an IAR using a Mg-Zn-Sr alloy. In contrast to currently used anastomotic instruments, it has a few advantages: (1) magnesium is widely available, so its price is lower than polymer materials, The Mg alloy (as cast condition) costs less than 10 dollars/kg, while the polylactic acid (raw material) costs 8000 dollars/kg. (2) As an essential element, magnesium is completely harmless to humans. (3) Magnesium alloy has better mechanical properties than polymer materials. (4) Because the use of Mg alloys enables the control of the degradation rate by adjusting surface modification, as well as alloy composition and proportion [25,26], it is possible to prepare an IAR with an Mg alloy at different degradation rates. For instance, the large intestine generally heals at a slower rate than the small intestine. Thus, one can prepare IARs suitable for different degradation periods by regulating the component proportion of the alloy, thus further achieving personalized design and improving the security of the IARs. (5) Magnesium metal has an antibacterial property not possessed by intestinal anastomotic devices currently used. (6) The feasibility of the drug-loaded coating is one of the advantages of intestinal anastomosis ring made of magnesium alloy. It plays a role in anastomosis and also achieves the therapeutic effect.
In this study, we referred to the indices of the physical and mechanical properties of the Mg alloy, as well as the design concept for IAR products from Tyco (USA). Regarding the production process, we employed the laser sintering technique to prepare the IAR model with nylon polymers and then performed intestinal anastomosis in animals, as shown in Figure 8.
Figure 8.
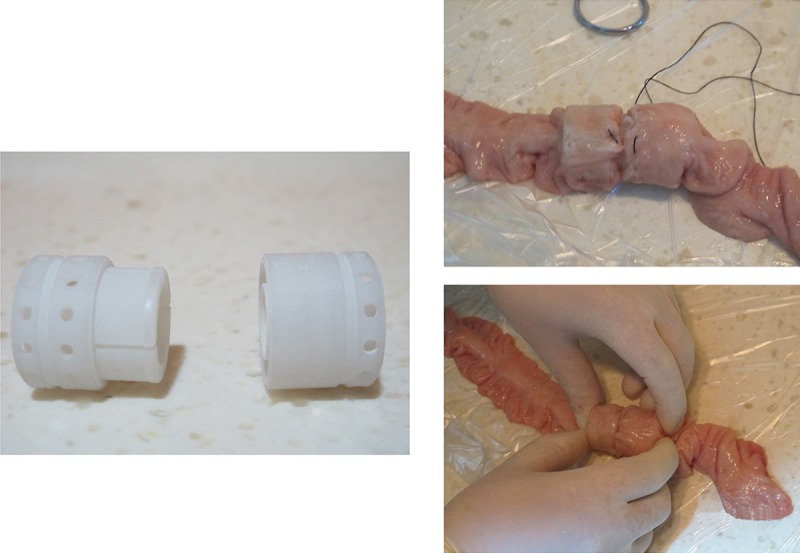
Model of the intestinal anastomosis rings and the course of anastomotic operation with anastomosis ring model.
Our next step is to prepare an IAR with the Mg-Zn-Sr alloy using the laser sintering technique and to perform in vivo animal experiments for comprehensive assessment of anastomotic strength, in vivo biocompatibility, and other potential issues that may occur during material degradation. We will further compare the properties of the Mg-alloy IAR with those of silk suture, anastomat, and an anastomosis ring of polymer materials to verify the superiority of the Mg-alloy IAR.
Conclusions
The focus of this study was to confirm the good biocompatibility, and antibacterial and drug-loaded coating properties of the Mg-Zn-Sr alloy. Good mechanical properties and controllable degradation rate have already been proven. These high-performing qualities are not offered by materials used in intestinal anastomotic instruments currently in use, and it is reported that the BAR has no obvious advantage in complications, especially anastomotic leakage, as compared with silk suture and stapler [4,5,27]. Biodegradable Mg-Zn-Sr IAR is a product of the combination of state-of-art medical concepts and the advantages of materials science. It may bring about unprecedented innovation for the development and application of gastrointestinal surgery. However, a number of issues remain, necessitating further research.
Acknowledgement
The authors thank Li Le and Nie jingyuan of Changsha City administration of quality and technology supervision for providing help for conducting antibacterial experiments. All authors have read and approved the final manuscript.
Footnotes
Conflict of interest
All authors declare that there is no conflict of interests or ethical issues.
Source of support: This project has been funded by the Professor’s Research Foundation of Central South University (Grant number: 20130030040001)
References
- 1.Erbel R, Di Mario C, Bartunek J, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369(9576):1869–75. doi: 10.1016/S0140-6736(07)60853-8. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Gao J, Wang Y. Evaluation of cytotoxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid [J] Surface and Coatings Technology. 2004;185(1):92–98. [Google Scholar]
- 3.Hardy TG, Jr, Pace WG, Maney JW, et al. A biofragmentable ring for sutureless bowel anastomosis. An experimental study. Dis Colon Rectum. 1985;28(7):484–90. doi: 10.1007/BF02554090. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs T, Koves I, Orosz Z, et al. Healing of esophageal anastomoses performed with the biofragmentable anastomosis ring versus the end-to-end anastomosis stapler: comparative experimental study in dogs. World J Surg. 2003;27(4):465–72. doi: 10.1007/s00268-002-6723-8. [DOI] [PubMed] [Google Scholar]
- 5.Ghitulescu GA, Morin N, Jetty P, Belliveau P. Revisiting the biofragmentable anastomotic ring: is it safe in colonic surgery? Can J Surg. 2003;46(2):92–98. [PMC free article] [PubMed] [Google Scholar]
- 6.Saidak Z, Marie PJ. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136(2):216–26. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Weitzmann MN. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-kappaB activation. Mol Cell Biochem. 2012;359(1–2):399–407. doi: 10.1007/s11010-011-1034-8. [DOI] [PubMed] [Google Scholar]
- 8.Pan F, Yang MB. Research status on microstructure and mechanical properties of magnesium alloys containing strontium. The Chinese Journal of Nonferrous Metals. 2011;21(10):2382–93. [Google Scholar]
- 9.Bornapour M, Muja N, Shum-Tim D, Cerruti M, Pekguleryuz M. Biocompatibility and biodegradability of Mg-Sr alloys: the formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013;9(2):5319–30. doi: 10.1016/j.actbio.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Song G. Control of biodegradation of biocompatable magnesium alloys. Corrosion Science. 2007;49(4):1696–701. [Google Scholar]
- 11.Geng LY, Song YQ, Zhang YH. Effect of Zinc Content on Corrosion Behavior of Biological Ternary Alloy Mg-Zn-Ca in Hank’s Simulated Body Fluid. Journal of Materials Protection. 2012;45(8):28–30. [Google Scholar]
- 12.Guo XX, He W, Zhang XJ, Hu XM. Cytotoxicity of cationic liposomes coated by N-trimethyl chitosan and their in vivo tumor angiogenesis targeting containing doxorubicin. J Applied Polym Sci. 2013;128(1):21–27. [Google Scholar]
- 13.ISO 10993-5: 1999 Biological evaluation of medical devices-Part 5: Tests for cytotoxicity: in vitro methods
- 14.Wang P, Zheng C, Wen J, et al. Cytotoxicity of a new biomedical titanium alloy Ti-25Nb-10Ta-1Zr-0.2Fe. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(12):1279–83. doi: 10.3969/j.issn.1672-7347.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 15.ISO10993-4: 2002 Biological evaluation of medical devices-Part4: Selection of tests for interactions with blood
- 16.Lu DY. Medical microbiology. 4th ed. Beijing: People’s medical publishing house; 1996. [Google Scholar]
- 17.Yang X, Xi T. [Progress in the studies on the evaluation of biocompatibility of biomaterials]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2001;18(1):123–28. [PubMed] [Google Scholar]
- 18.Li ZJ, Zhang K, Lou SQ, Zheng YF. A Cellular Study on Cytotoxicity of Magnesium – calcium Alloy. Chinese Journal of Bone and Joint Injury. 2007;22(9):740–42. [Google Scholar]
- 19.Zhang Y, Tao H-R, He Y-H, et al. Cytotoxicity and hemolytic properties of biodegradable Mg-Zn alloy. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008;12(41):8162–66. [Google Scholar]
- 20.You SH. Hemocompatibility evaluation and tests for medical devices-Explanation for GB/T16886.4-2003/ISO10993-4: 2002. China Medical Device Information. 2006;12(12):49–54. [Google Scholar]
- 21.Fouda E, El Nakeeb A, Magdy A, et al. Early detection of anastomotic leakage after elective low anterior resection. J Gastrointest Surg. 2011;15(1):137–44. doi: 10.1007/s11605-010-1364-y. [DOI] [PubMed] [Google Scholar]
- 22.Snega S, Ravichandran K, Begum NJ, Thirumurugan K. Enhancement in the electrical and antibacterial properties of sprayed ZnO films by simultaneous doping of Mg and F. Journal of Materials Science: Materials in Electronics. 2013;24(1):135–41. [Google Scholar]
- 23.Oberstein PE, Remotti H, Saif MW, Libutti SK. Pancreatic neuroendocrine tumors: entering a new era. JOP. 2012;13(2):169–73. [PubMed] [Google Scholar]
- 24.Huang Y, Nie Y, Zhang M, et al. [Fluorouracil implants for colorectal cancer: a systematic review and meta-analysis]. Chinese Journal of Gastrointestinal Surgery. 2012;15(4):377–81. [PubMed] [Google Scholar]
- 25.Wang J, Tang J, Zhang P, et al. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J Biomed Mater Res B Appl Biomater. 2012;100(6):1691–701. doi: 10.1002/jbm.b.32707. [DOI] [PubMed] [Google Scholar]
- 26.Rosalbino F, De Negri S, Scavino G, Saccone A. Microstructure and in vitro degradation performance of Mg-Zn-Mn alloys for biomedical application. J Biomed Mater Res A. 2013;101(3):704–11. doi: 10.1002/jbm.a.34368. [DOI] [PubMed] [Google Scholar]
- 27.Ryan S, Seim H, III, Macphail C, et al. Comparison of biofragmentable anastomosis ring and sutured anastomoses for subtotal colectomy in cats with idiopathic megacolon. Vet Surg. 2006;35(8):740–48. doi: 10.1111/j.1532-950X.2006.00218.x. [DOI] [PubMed] [Google Scholar]