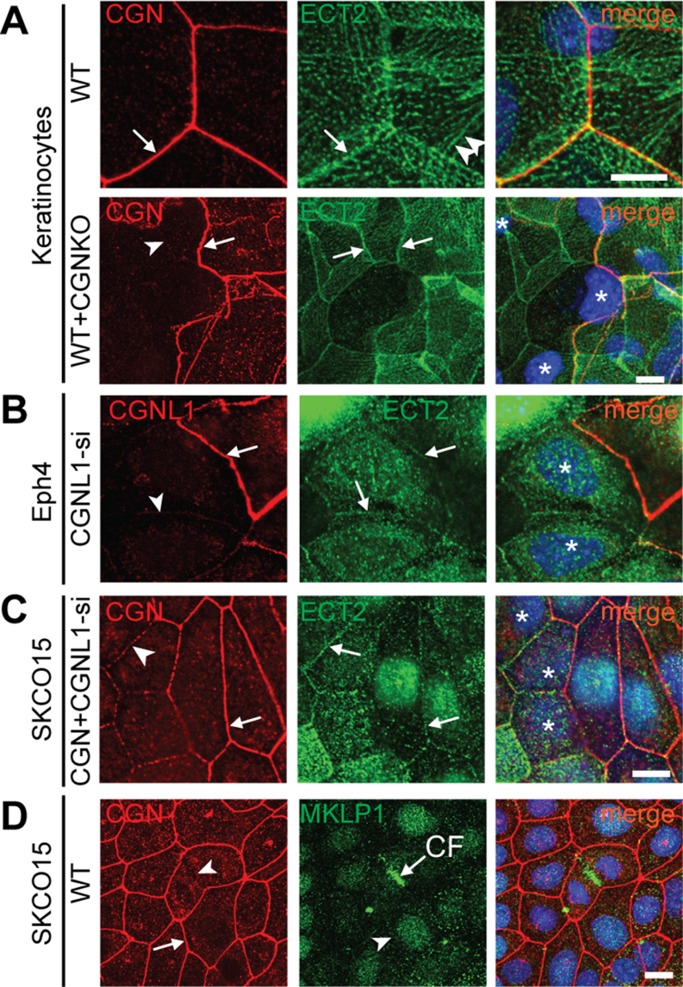
The Rac1 inhibitor MgcRacGAP regulates Rac1 activation and TJ barrier development during junction assembly in epithelial cells. CGN and CGNL1 recruit MgcRacGAP to the TJ and interact with MgcRacGAP.
Abstract
The regulation of Rho-family GTPases is crucial to direct the formation of cell–cell junctions and tissue barriers. Cingulin (CGN) and paracingulin (CGNL1) control RhoA activation in epithelial cells by interacting with RhoA guanidine exchange factors. CGNL1 depletion also inhibits Rac1 activation during junction assembly. Here we show that, unexpectedly, Madin–Darby canine kidney epithelial cells depleted of both CGN and CGNL1 (double-KD cells) display normal Rac1 activation and tight junction (TJ) formation, despite decreased junctional recruitment of the Rac1 activator Tiam1. The expression of the Rac1 inhibitor MgcRacGAP is decreased in double-KD cells, and the barrier development and Rac1 activation phenotypes are rescued by exogenous expression of MgcRacGAP. MgcRacGAP colocalizes with CGN and CGNL1 at TJs and forms a complex and interacts directly in vitro with CGN and CGNL1. Depletion of either CGN or CGNL1 in epithelial cells results in decreased junctional localization of MgcRacGAP but not of ECT2, a centralspindlin-interacting Rho GEF. These results provide new insight into coordination of Rho-family GTPase activities at junctions, since apical accumulation of CGN and CGNL1 at TJs during junction maturation provides a mechanism to spatially restrict down-regulation of Rac1 activation through the recruitment of MgcRacGAP.
INTRODUCTION
The precise spatiotemporal control of the activity of Rho-family GTPases is essential in many cellular processes, including the establishment and maintenance of cell–cell junctions and the formation of epithelial barriers (Nusrat et al., 1995; Braga et al., 1997; Takaishi et al., 1997; Jou et al., 1998; Yamada and Nelson, 2007). Rho-family GTPases exist in active (GTP-bound) and inactive (GDP-bound) states, and the transition between these states depends on a finely tuned antagonism between activating guanine-nucleotide exchange factors (GEFs) and inhibitory GTPase-activating proteins (GAPs; Schmidt and Hall, 2002; Rossman et al., 2005; Tcherkezian and Lamarche-Vane, 2007).
Tight junctions (TJs) form, together with the zonula adhaerens (ZA), a belt-like apical junctional complex (AJC) in epithelial cells, and are uniquely responsible for the barrier function of epithelia through the formation of claudin-based paracellular channels and pores (Furuse and Tsukita, 2006; Anderson and Van Itallie, 2009). The transmembrane proteins of TJ and ZA are clustered at the sites of cell–cell contact by distinct complexes of cytoplasmic adaptor proteins, some of which provide anchoring to the actin and microtubule cytoskeletons (Shin et al., 2006; Meng and Takeichi, 2009). RhoA and Rac1 are the major GTPases of the Rho family, which are implicated in the regulation of TJs and the ZA through the dynamic reorganization and contractility of the actin cytoskeleton (Hall, 2012). However, the molecular mechanisms of their regulation are not completely understood. For example, although GEFs for RhoA and Rac1 have been found to interact with junctional proteins (reviewed in Citi et al., 2011; McCormack et al., 2013), little is known about the function of specific RhoA and Rac1 GAPs in modulating the establishment of the TJ barrier. Furthermore, it is not known whether any Rac-GAP protein interacts with specific components of the cytoplasmic plaque of the TJ. In contrast, GAPs for Cdc42 are required for TJ integrity and interact with junctional proteins (Wells et al., 2006; Elbediwy et al., 2012).
Two key players in the fine-tuning of RhoA activity in epithelial cells are cingulin (CGN), which is specifically localized in the cytoplasmic plaque region of TJs (Citi et al., 1988), and paracingulin (CGNL1; also known as JACOP), which is localized to both the TJ and ZA (Ohnishi et al., 2004; Guillemot and Citi, 2006b). CGN and CGNL1 are structurally related and recruit the Rho GEF GEF-H1 to junctions of Madin–Darby canine kidney (MDCK) cells and p114-RhoGEF to junctions of corneal cells (Citi et al., 2000, 2009, 2012; Aijaz et al., 2005; Guillemot and Citi, 2006a; Guillemot et al., 2008; Terry et al., 2011). CGNL1, unlike CGN, is also required for efficient junctional recruitment of the Rac1 GEF Tiam1 in MDCK cells, and depletion of CGNL1, but not of CGN, results in loss of the waves of increased Rac1 activation observed during junction assembly (Guillemot et al., 2008). Here, by studying the junction assembly phenotype of MDCK cells depleted of both CGN and CGNL1 (double-KD cells), we find that MgcRacGAP (also known as RacGAP1, RacGAP50C, or Cyk-4; Toure et al., 1998; Jantsch-Plunger et al., 2000; Somers and Saint, 2003) plays a role in Rac1-dependent regulation of the dynamic establishment of the TJ barrier and interacts with both CGN and CGNL1.
RESULTS
In double-KD cells, Rac1 activation, junction assembly, and establishment of the TJ barrier are similar to those in wild-type cells, despite decreased junctional recruitment of Tiam1
Stable lines of MDCK epithelial cells depleted of both CGN and CGNL1 were reported previously (Guillemot et al., 2013) and show increased RhoA activation at confluence, consistent with decreased junctional recruitment of GEF-H1, similar to cells depleted of either CGN alone (CGN-KD) or CGNL1 alone (CGNL1-KD; Guillemot and Citi, 2006a; Guillemot et al., 2008). In addition, they show normal expression and localization of proteins of the zonula adhaerens and decreased expression of the transcription factor GATA-4 (Guillemot et al., 2013). Here we investigate the dynamics of junction assembly of double-KD cells, starting from our previous observations that in CGNL1-KD cells Rac1 activation is decreased during junction assembly, junction assembly is delayed, and the peak in transepithelial electrical resistance (TER) observed during the calcium switch is abolished (Guillemot et al., 2008).
In confluent monolayers, Rac1 activation in double-KD cells, as measured by a glutathione S-transferase (GST) pull-down assay, was the same as in wild-type (WT) cells (Figure 1A) and CGNL1-KD cells (Guillemot et al., 2008). Rac1 activity as determined by GST-pull down on cell lysates is a reliable readout of junctional Rac1 activation during junction assembly because although Rac1 is necessary for maintenance of cell–cell contacts and protein traffic at steady state, during junction formation the zones of maximal Rac1 and lamellipodia activity are at the periphery of contacting membranes (Yamada and Nelson, 2007). So we examined Rac1 activation during junction formation in the calcium switch, an established experimental protocol to study the dynamic formation of the TJ barrier (Gonzalez-Mariscal et al., 1985). Surprisingly, Rac1 activation in double-KD cells was indistinguishable from that in WT cells; for example, the peaks of Rac1 activity observed at the early (10–30 min) and late (3 h) time points were not abolished (Figure 1B and Supplemental Figure S1A), in contrast to CGNL1-depleted cells (Guillemot et al., 2008) and similar to WT and CGN(−) cells (Supplemental Figure S1A). Instead, RhoA activity of double-KD cells during the calcium switch was high and remained high at 8 h after the beginning of the switch and in confluent monolayers (Figure 1B and Supplemental Figure S1A; Guillemot et al., 2008), as previously shown for single-KD cells (Guillemot and Citi, 2006a; Supplemental Figure S1A).
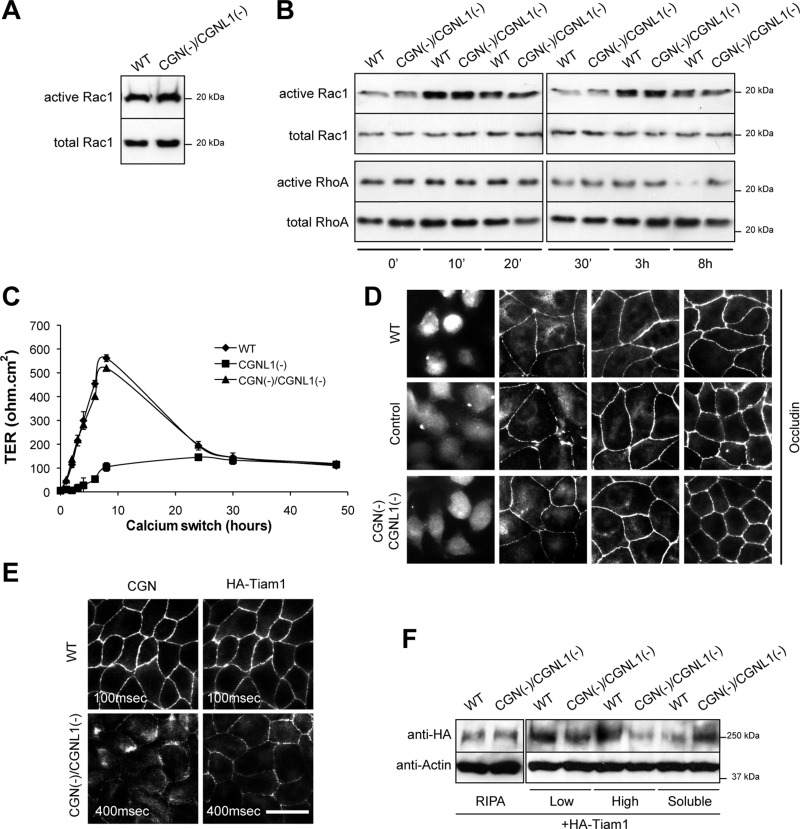
FIGURE 1:
CGN(−)/CGNL1(−) (double-KD) cells show normal Rac1 activation and TJ assembly, despite reduced junctional Tiam1. (A, B) Rac1 activation at steady-state (A) and Rac1 and RhoA activation during the calcium switch (B), as determined by GST pull-down analysis. (C) TER (ohm⋅cm2) of WT, single-KD (CGNL1(−)), and double-KD (CGN(−)/CGNL1(−)) cells during the calcium switch. (D) Occludin immunofluorescence, showing a similar pattern of occludin accumulation at junctions in WT, control, and double-KD cells during the calcium switch (for single-KD CGNL1(−), see Guillemot et al., 2008). (E) Immunofluorescence analysis of exogenous hemagglutinin (HA)-tagged Tiam1 in WT and double-KD cells, showing reduced junctional recruitment of Tiam1 in double-KD cells (exposure time in double-KD cells increased fourfold; see key in each panel). Bar, 10 μm. (F) Immunoblotting with anti-HA (exogenous Tiam1) and anti-actin antibodies of fractionated lysates of WT and double-KD cells (Guillemot et al., 2008). See Supplemental Figure S1 for additional data on single-KD cells.
As an alternative functional assay, we assessed TJ barrier function by examining the pattern of development of the ionic permeability barrier (TER) in double-KD cells. Whereas in CGNL1(–) cells the peak in TER is abolished (Guillemot et al., 2008; Figure 1C), in double-KD cells it is similar to that observed in WT cells (Figure 1C). Distinct stable clones of double-KD cells behaved similarly (Supplemental Figure S1B).
As a third functional assay, we examined the kinetics of accumulation of the transmembrane TJ protein occludin at the junctions of double-KD cells. The behavior of double-KD cells was similar to that of WT cells and cells expressing control short hairpin RNA (shRNA; Figure 1D) and not delayed, as observed in CGNL1(−) cells (Guillemot et al., 2008).
Because in CGNL1(−) cells decreased Rac1 activation and delayed junction assembly correlate with decreased junctional localization of Tiam1 (Guillemot et al., 2008), we asked whether the unexpected phenotype of double-KD cells was due to a rescue in the junctional localization of Tiam1, which could account for the increased Rac1 activation, and normal TJ barrier development. Immunofluorescence analysis showed that in double-KD cells there was decreased junctional localization of Tiam1 compared with WT cells, similar to what observed in single-KD CGNL1(−) cells (Figure 1E; Guillemot et al., 2008). In agreement, immunoblot analysis of soluble and insoluble fractions showed a decrease in the amount of insoluble, junction-associated Tiam1 (Figure 1F). Because this assay indicated that normal Rac1 activation and TJ barrier development in double-KD cells was not due to a rescue in Tiam1 localization, we conclude that Rac1 activation during junction assembly not only depends on Tiam1, but is influenced by additional activators and/or inhibitors of Rac1.
Expression of MgcRacGAP is decreased in double-KD cells, and rescue of normal levels of MgcRacGAP expression results in decreased Rac1 activation and delayed TJ barrier development
We postulated that rescue of Rac1 activation in double-KD cells could be due to either increased expression of a Rac1 GEF or decreased expression of a Rac1 GAP. So we examined the expression of Rac1 GEFs and GAPs, which are associated with junctional proteins such as Asef (Muroya et al., 2007), RICH1 (Wells et al., 2006), MgcRacGAP (Ratheesh et al., 2012), and Vav2 (Noren et al., 2000). By quantitative real-time (RT) PCR, the expression of Asef and RICH1 was not significantly altered in double-KD cells when compared with WT cells (Figure 2A and Supplemental Figure S1C). In contrast, expression of Vav2 and MgcRacGAP was significantly decreased in double-KD cells compared with WT cells (Figure 2A and Supplemental Figure S1C). Because decreased expression of Vav2 (an activator of Rac1) could not explain the increased Rac1 activation in double-KD versus CGNL1(−) cells, we focused on MgcRacGAP, a GAP that is ∼30-fold more active against Rac1 and Cdc42 than RhoA (Toure et al., 1998). Immunoblotting analysis demonstrated that MgcRacGAP expression was decreased at both the mRNA and protein levels in double-KD cells compared with WT cells (Figure 2, B and C) and to either single-KD, CGN(−), or CGNL1(−) cells (Supplemental Figure S1D). Furthermore, analysis of fractionated lysates showed that the decrease in MgcRacGAP levels was observed only in the low-speed insoluble fraction (Figure 2B and Supplemental Figure S1D), suggesting decreased association with the cytoskeleton. The reduced mRNA and protein levels for MgcRacGAP in stable double-KD cells may be due to altered transcriptional regulation (due to reduced GATA-4 levels; Guillemot et al., 2013; our unpublished results) and additional mechanisms that regulate MgcRacGAP mRNA and/or protein stability, which remain to be investigated.
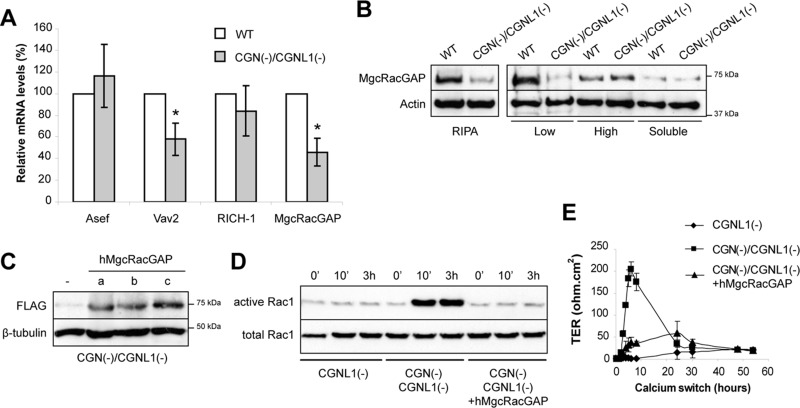
FIGURE 2:
MgcRacGAP levels are reduced in double-KD MDCK cells, and rescue of MgcRacGAP expression inhibits Rac1 activation and the peak in TER during development of the TJ barrier. (A) Histogram showing the relative mRNA levels for Asef, Vav2, Rich-1, and MgcRacGAP in double-KD cells vs. WT cells, taking WT levels as 100, as determined by quantitative RT-PCR. (B) Immunoblotting analysis of either total lysates (RIPA) or fractionated lysates of WT and double-KD cells. Low, pellet after centrifugation at low speed (13,000 × g). High, pellet after high-speed (100,000 × g) centrifugation of the supernatant obtained after low-speed centrifugation. Soluble, Triton-soluble supernatant after centrifugation at 100,000 × g of the low-speed supernatant. (C) Immunoblotting of total (RIPA) lysates from three independent double-KD rescue clones (a–c) stably expressing or not (−) an exogenous human (h) FLAG-tagged MgcRacGAP. (D) Rac1 activation in either single-KD (CGNL1(−)) or double-KD cells expressing (clone a) or not the exogenous MgcRacGAP protein during the calcium switch. Clones b and c are shown in Supplemental Figure S1E. (E) TER profile in the calcium switch for the stable clones described in D. Clones b and c are shown in Supplemental Figure S1F.
Next we tested the hypothesis that the decreased expression of MgcRacGAP plays a mechanistic role in the increased Rac1 activation and normal development of the epithelial paracellular permeability barrier of double-KD cells. We established stable lines expressing exogenous FLAG-tagged MgcRacGAP in the background of double-KD cells (Figure 2C) and asked whether the exogenous MgcRacGAP expression could revert the phenotype of double-KD cells to that of CGNL1(−), single-KD cells. GST pull-down analysis of activated Rac1 showed that when exogenous MgcRacGAP was expressed, the increased Rac1 activation detected at different time points during the calcium switch in double-KD cells was suppressed, thus reverting the phenotype to that of single-KD, CGNL1(−) cells (Figure 2D and Supplemental Figure S1E). Furthermore, Rac1 inactivation induced by exogenous MgcRacGAP expression correlated with a strong reduction in the peak of TER detected at 8 h after the calcium switch in double-KD cells, resulting in a phenotype that was similar to that of single-KD, CGNL1(−) cells (Figure 2E and Supplemental Figure S1F). We also attempted to generate stable lines depleted of MgcRacGAP through shRNA expression in order to test directly the role of MgcRacGAP in junction assembly in WT cells. However, such lines could not be isolated, probably due to the essential role of MgcRacGAP in cytokinesis (Glotzer, 2009).
Cingulin and paracingulin are required for efficient junctional recruitment of MgcRacGAP in different types of epithelial cells
Having established that modulating MgcRacGAP expression levels affects Rac1 activation and the dynamics of establishment of the TJ barrier to ions, we asked whether MgcRacGAP is localized at TJs through interaction with CGN, CGNL1, or both. To do this, we examined the localization of MgcRacGAP in epithelial cells in which CGN, CGNL1, or both were depleted through either shRNA or small interfering RNA (siRNA). In addition, we studied the localization of MgcRacGAP in mixed cultures of primary keratinocytes isolated from WT and CGN-KO mice (Figure 3).
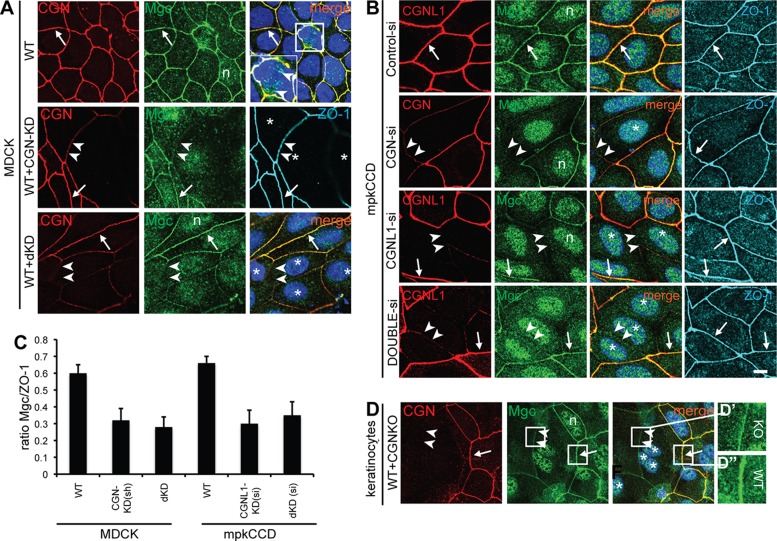
FIGURE 3:
CGN and CGNL1 are required for the efficient recruitment of MgcRacGAP to epithelial junctions. (A, B) Double immunofluorescence of CGN and MgcRacGAP (Mgc) in WT MDCK cells in cocultures of WT and CGN-KD MDCK cells, WT and double-KD MDCK cells (A), or mouse kidney (mpkCCDCl4) cells, after siRNA control, si-CGN, si-CGNL1, and si-double (CGN and CGNL1) treatment. Cells were labeled also with rat anti–ZO-1 to identify junctions. Arrows, junctions labeled by both MgcRacGAP and CGN antibodies. Double arrowheads, junctions with decreased labeling for both CGN and MgcRacGAP and normal labeling for ZO-1. The square area in A and magnified inset shows labeling for MgcRacGAP (arrowheads) in the mitotic spindle. Asterisks, positions of nuclei of KD cells. Single arrowheads, junctions with reduced CGNL1 staining and normal MgcRacGAP staining. n, nuclear labeling for MgcRacGAP. (C) Semiquantitative analysis of junctional labeling intensity for MgcRacGAP (expressed as a ratio of MgcRacGAP to ZO-1 pixel intensity in the same junctional areas) in WT, CGN-KD, dKD junctions of MDCK clonal lines or WT, CGNL1-KD, dKD si-treated mouse kidney cells. (D) Double immunofluorescence of CGN and MgcRacGAP in cocultures of primary keratinocytes derived from either WT or CGN KO mice. Magnified insets in D′ and D′′ show intensity-adjusted images of junctions, to show the lower levels (but not absence) of MgcRacGAP in junctional areas between KO cells (D′) vs. junctions between WT cells (D′′). Bar, 5 μm.
In confluent WT MDCK cells, MgcRacGAP staining at junctions was continuous and largely colocalized with CGN (arrows in Figure 3, A, B, and D, and Supplemental Figure S2). However, in dividing cells, only MgcRacGAP and not CGN labeling was detected in the mitotic spindle (inset in Figure 3A, WT). In addition, unlike CGN and CGNL1, MgcRacGAP was also detected in the nucleus (marked “n” in Figure 3 and Supplemental Figure S2). In mixed cultures of WT MDCK cells, together with either CGN-KD or double-KD stable clonal cells (WT+dKD), MgcRacGAP labeling at junctions was reduced, albeit not abolished, in junctions between KD cells compared with junctions between WT cells (double arrowheads in Figure 3A). A similar reduction of junctional MgcRacGAP labeling was obtained by depleting CGN in MDCK cells, using siRNA instead of shRNA (Supplemental Figure S2A).
To examine the effect of CGNL1 depletion on the junctional localization of MgcRacGAP, we used mouse kidney cells (mpkCCDCl4) because the mouse anti-CGNL1 antibody required for double immunofluorescence with rabbit anti-MgcRacGAP does not recognize canine CGNL1. In mouse kidney cells, depletion of CGN, CGNL1, or both by siRNA resulted in reduced but not absent junctional staining for MgcRacGAP (Figure 3B). Semiquantitative analysis of MgcRacGAP staining at junctions, expressed as a ratio to ZO-1 staining in the same junctional area, showed a similar decrease, by ∼50%, in both single- and double-KD canine and mouse kidney cells (Figure 3C).
As a third cell model system, we examined differentiated keratinocytes isolated from WT and CGN-KO mice and grown in mixed cultures (Figure 3D). Both CGN and MgcRacGAP showed a continuous, linear (zonular), and mostly overlapping labeling of junctions in WT keratinocytes (arrow in Figure 3D). In contrast, in junctions between CGN-KO keratinocytes, MgcRacGAP labeling was decreased but not absent (arrowheads in Figure 3D and magnified insets in Figure 3, D′ and D′′), indicating that even in the absence of any junctional CGN, a fraction of MgcRacGAP remains localized at junctions.
To extend our analysis to additional epithelial cell types, we also examined the effect of either CGN or CGNL1 depletion in human intestinal colon carcinoma cells (SKCO-15) and mouse mammary epithelial cells (Eph4). In both of these cell types (and also in MCF-7 cells; unpublished data) depletion of CGN, either alone or in combination with CGNL1 resulted in decreased junctional staining for MgcRacGAP (Supplemental Figure S2, B and C). In contrast, depletion of CGNL1 alone resulted in decreased junctional labeling for MgcRacGAP only in Eph4 (Supplemental Figure S2C) but not in SKCO-15 cells (single arrowhead in Supplemental Figure S2B, CGNL1-si) or human breast cancer (MCF-7) cells (unpublished data).
Taken together, these observations demonstrate that CGN, in all the epithelial cell types we tested, and CGNL1, in all the cell types we tested with the exception of SKCO-15 and MCF-7 cells, are required for the recruitment of MgcRacGAP to the AJC.
Cingulin and paracingulin interact with MgcRacGAP
To explore whether the requirement of CGN and CGNL1 for the junctional recruitment of MgcRacGAP depends on an interaction of MgcRacGAP with these proteins, we carried out immunoprecipitation and GST pull-down experiments. Immunoblot analysis of either CGN or CGNL1 immunoprecipitates showed that endogenous MgcRacGAP is present in a complex with CGN and CGNL1 in epithelial cells (Figure 4A). Furthermore, specific coimmunoprecipitation was also detected between tagged, exogenously expressed MgcRacGAP, CGN, and CGNL1 in MDCK cells (Supplemental Figure S3).
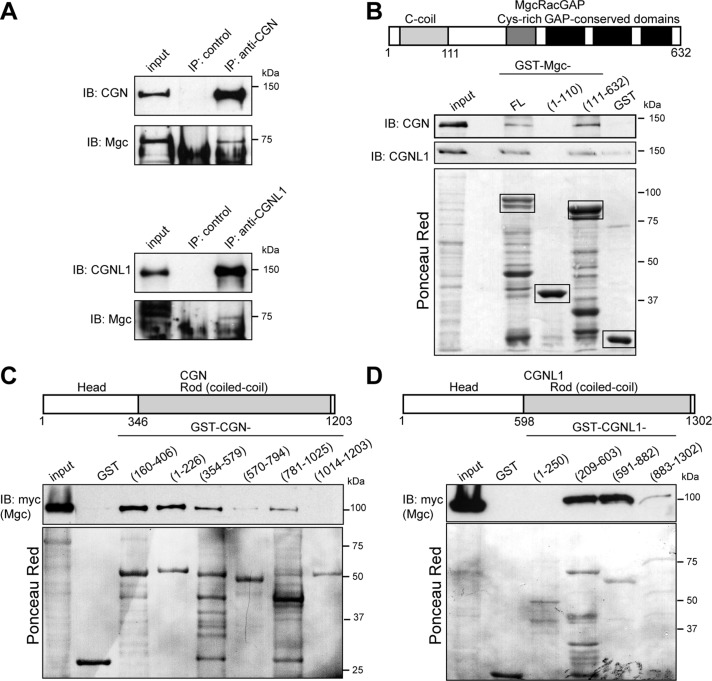
FIGURE 4:
CGN and CGNL1 form a complex and interact directly with McRacGAP. (A) Immunoblotting of CGN (top, from MDCK cell lysates) or CGNL1 (bottom, from Eph4 cell lysates) immunoprecipitates, using antibodies against CGN/MgcRacGAP or CGNL1/MgcRacGAP, respectively. Input: 1/40 of volume used for immunoprecipitation. MgcRacGAP shows different mobility and forms in MDCK vs. Eph4 cells. (B) Domain organization of MgcRacGAP and immunoblotting analysis of CGN in GST pull downs of full-length CGN interacting with either full-length (FL) or truncated constructs (1–110, 111–632) of MgcRacGAP fused to GST. (C, D) Domain organization of CGN (C) and CGNL1 (D) and immunoblotting analysis of myc-tagged MgcRacGAP in GST pull downs of the indicated fragments of either CGN or CGNL1 fused to GST. Images of Ponceau red–labeled membranes below immunoblots show protein loadings for GST fusion proteins.
Next we asked whether MgcRacGAP can interact directly with CGN and CGNL1, by incubating recombinant, bacterially expressed MgcRacGAP constructs with full-length CGN or CGNL1, the latter expressed in baculovirus-infected insect cells. Both CGN and CGNL1 were detected by immunoblotting when either full-length MgcRacGAP or an N-terminally truncated construct of MgcRacGAP (residues 111–632) were used as bait, but not when a fragment containing the coiled-coil domain of MgcRacGAP (1–111) was used (Figure 4B). Thus the region of MgcRacGAP that interacts with CGN and/or CGNL1 is distinct from the region (N-terminal 120 residues) that interacts with ECT2 and MKLP1 (Mishima et al., 2002; Somers and Saint, 2003) and partially overlaps with the sites of interaction with anillin (D'Avino et al., 2008; Gregory et al., 2008) and PRC1 (Ban et al., 2004).
To map the regions of CGN and CGNL1 that interact with MgcRacGAP, we incubated bacterially expressed constructs of either CGN or CGNL1 with myc-tagged MgcRacGAP. In the case of CGN, the strongest binding was observed with fragments of the CGN globular head domain (residues 160–406, 1–226), although fragments of the coiled-coil rod (residues 354–579, 781–1025) also interacted with MgcRacGAP (Figure 4C). In the case of CGNL1, the strongest interaction was observed with the C-terminal two-thirds of the globular head domain (residues 209–603) and the N-terminal one-third of the coiled-coil rod domain (residues 591–882; Figure 4D).
In summary, CGN and CGNL1 form a complex and interact directly with MgcRacGAP.
Cingulin and paracingulin are not required for junctional recruitment of the Rho GEF ECT2
MgcRacGAP interacts with and is required for the junctional recruitment of ECT2, a centralspindlin-associated Rho GEF, at cadherin-based junctions of human breast cancer cells (MCF-7; Ratheesh et al., 2012). Because depletion of either CGN or CGNL1 reduced the junctional localization of MgcRacGAP, we asked whether ECT2 is also affected under these conditions.
The localization of ECT2 was investigated in different types of epithelial cells: canine and mouse kidney cells, mouse primary keratinocytes, human intestinal cells, and mouse mammary epithelial cells (Figure 5 and Supplemental Figure S4). In canine and mouse kidney cells, no junctional labeling for ECT2 could be detected (unpublished data). In keratinocytes, ECT2 labeling was detected at junctions, but it was not zonular like that of MgcRacGAP, and it only partially colocalized with CGN (arrows in Figure 5A, WT, and Supplemental Figure S4B), suggesting that it is associated with the ZA rather than the TJ. In addition, prominent ECT2 labeling was detected along lateral regions of cell–cell contact, clustered along aligned cable-like structures, some of which reached the zonular junctional region, where they apparently connected with continuing cables on the adjoining cells (double arrowheads in Figure 5A, WT, and Supplemental Figure S4A). These observations indicate that ECT2 is distributed along lateral spot-like adherens junctions associated with actomyosin bundles, which terminate at the zonula adhaerens and may be part of a transcellular network across neighboring cells that provides continuity across contractile units (Ebrahim and Kachar, 2013). Of importance, no decrease in ECT2 labeling was observed in CGN-KO keratinocytes (arrowhead in Figure 5A, WT+CGNKO) compared with junctions between WT keratinocytes (arrow in Figure 5A, WT+CGNKO). In SKCO-15 cells (Supplemental Figure S4) and Eph4 cells (unpublished data), labeling for ECT2 was highly heterogeneous in intensity and distribution in different cells, mostly detectable in the cytoplasm and nucleus in a pattern that suggested cell cycle–dependent expression and localization (Liot et al., 2011; Matthews et al., 2012) and was more rarely detectable at junctions (Supplemental Figure S4). We found no correlation between intensity and distribution of ECT2 labeling and depletion of either CGN or CGNL1 in Eph4 or SKCO-15 cells (Figure 5, B and C, and Supplemental Figure S4B). In human mammary carcinoma cells (MCF-7), labeling for both ECT2 and MgcRacGAP was junctional, and CGN depletion decreased junctional MgcRacGAP, whereas ECT2 was not affected by depletion of either CGN or CGNL1 (unpublished data). These observations indicate that ECT2 distribution is highly variable depending on cell type, the decreased levels of junctional MgcRacGAP in CGN/CGNL1-depleted cells do not correlate with reduced junctional localization of ECT2, and neither CGN nor CGNL1 is required for junctional recruitment of ECT2. This is consistent with the observation that depletion of CGN, CGNL1, or both has no detectable effect on the organization of cadherin-based junctions (Guillemot et al., 2008, 2013; Pulimeno et al., 2011).
FIGURE 5:
Depletion of CGN and CGNL1 does not affect the junctional recruitment of the Rho GEF ECT2. (A) Double immunofluorescence analysis (CGN, red; ECT2, green; 4′,6-diamidino-2-phenylindole [DAPI], blue; in merge images) of WT keratinocytes (top) or mixed cultures of WT and CGN-KO keratinocytes (bottom). Additional images are shown in Supplemental Figure S4A. Note the presence of ECT2 (arrow) in cells lacking CGN (arrowhead). (B, C) Double immunofluorescence analysis (CGN, red; ECT2, green; DAPI, blue; in merge images) of Eph4 cells treated with siRNA against CGNL1 or SKCO-15 cells treated with siRNA against both CGN and CGNL1 (C), showing both WT cells, containing junctional CGNL1/CGN (arrow) and CGNL1(CGN)-depleted cells (arrowhead). Note the presence of ECT2 (arrow) in cells lacking CGNL1/CGN. (D) Double immunofluorescence analysis (CGN, red; MKLP1, green) of confluent SKCO-15 cells, showing that MKLP1 is not detected at junctions (arrow), but only at the cleavage furrow (CF) of dividing cells. Asterisks, positions of nuclei of siRNA-CGN–depleted/KO cells. Single arrowheads, sites with reduced CGN/CGNL1 labeling (KD or KO) but detectable ECT2 labeling. Arrows, junctions between WT cells showing both CGN/CGNL1 and ECT2 labeling. Bar, 5 μm (B–D), 2 μm (A).
Finally, we examined the localization of the kinesin MKLP1 (KIF23), the second subunit of the centralspindlin complex, in SKCO-15 cells, and detected strong labeling only at the cleavage furrow of dividing cells but no junctional labeling in interphase cells (Figure 4D), suggesting that the centralspindlin complex dissociates upon completion of mitosis, and MKLP1 does not associate with junctions.
DISCUSSION
We provide evidence for a new molecular mechanism regulating the activity of Rac1 at epithelial junctions, based on observations on the phenotype of stable MDCK lines depleted of both CGN and CGNL1. Our data indicate that a component of the centralspindlin complex, MgcRacGAP, is involved in regulation of Rac1 activation and dynamics of TJ barrier formation during epithelial junction assembly, and is recruited to apical zonular junctions by CGN and CGNL1. We also provide evidence that the localization of MgcRacGAP in interphase epithelial cells is distinct from that of the second component of the centralspindlin complex, MKLP1, and of ECT2.
MgcRacGAP is a Rac-specific GAP (Toure et al., 1998) fundamental for mammalian viability, since its deficiency in mouse embryos leads to preimplantation lethality (Van de Putte et al., 2001). As a component of the centralspindlin complex, MgcRacGAP plays a critical role during cytokinesis, by regulating assembly of the central spindle, in part by affecting RhoA through the Rho GEF ECT2 (Mishima et al., 2002; Yuce et al., 2005; White and Glotzer, 2012) but mainly by inhibiting Rac1 activity (D'Avino et al., 2004; D'Avino and Glover, 2009; Canman et al., 2008; Bastos et al., 2012). What role, if any, MgcRacGAP plays in Rac1 regulation at junctions of interphase cells has not been determined. A study reported that the centralspindlin complex is localized at the ZA of breast cancer (MCF-7) cells by anchoring with the N-terminus of α-catenin to support ECT2-controlled Rho signaling at junctions (Ratheesh et al., 2012). Here we show that in interphase epithelial cells, MgcRacGAP colocalizes with CGN, a specific marker of TJ, whereas ECT2 is distributed heterogeneously at the ZA, along lateral contacts, and in the cytoplasm/nucleus in different cell types. In addition, whereas depletion of either CGN or CGNL1 results in decreased MgcRacGAP accumulation at junctions, we did not observe any decrease in the junctional localization of ECT2, in apparent disagreement with the idea that MgcRacGAP recruits ECT2 to junctions (Ratheesh et al., 2012), and confirming the lack of effect of CGN/CGNL1 depletion on ZA organization (Guillemot et al., 2004, 2008, 2012, 2013; Guillemot and Citi, 2006a; Pulimeno et al., 2011). However, because even in double-KD kidney epithelial cells we observed residual junctional staining for MgcRacGAP, this discrepancy can be explained by the presence of two pools of MgcRacGAP, one at the TJ, which is recruited by CGN and CGNL1, and one at the ZA, recruited by the α-catenin/E-cadherin complex (Ratheesh et al., 2012; Priya et al., 2013). The proportion of MgcRacGAP that associates with TJ versus ZA, and hence its regulation, may be different in cancer cell lines versus spontaneously immortalized cell lines and may depend on the composition and degree of “maturation” of these junctions. Further studies should characterize the interactions of MgcRacGAP with components of the ZA, and its regulation at the apical junctional complex. On the other hand, our observations on different types of CGN/CGNL1-depleted cells, and the finding that MgcRacGAP forms a complex and interacts directly with CGN and CGNL1 strongly support the idea that CGN and CGNL1 play a major role in recruiting MgcRacGAP to the TJ.
Concerning the function of MgcRacGAP in interphase cells, we show that in double-KD MDCK cells, where MgcRacGAP levels are decreased, RhoA activation during the calcium switch is indistinguishable from WT and single-KD (CGNL1(−)) MDCK cells (Guillemot et al., 2008; present study). In contrast, rescuing MgcRacGAP levels dramatically affects Rac1 signaling during junction assembly, supporting the notion that MgcRacGAP acts primarily on Rac1 and not RhoA signaling (D'Avino and Glover, 2009). The correlation between Rac1 activation and the pattern of TER development in the double-KD cell lines strengthens the idea that Rac1 activation regulates the development of the paracellular barrier to ions. The barrier to ions depends on claudins, which polymerize on a scaffold of cytoplasmic TJ proteins, which are linked directly or indirectly to the actin cytoskeleton (Umeda et al., 2006; Fanning and Anderson, 2009; Citi et al., 2012). Although the precise molecular mechanism that generates the peak in TER observed during junction assembly in MDCK cells is not known, it can be speculated that it is due to a transient, cytoskeleton-dependent “tightening” of the claudin-based barrier, the differential kinetics of assembly of “tight” (barrier) versus “leaky” (pore) claudin isoforms (Kolosov et al., 2014), or a combination of these mechanisms. Because TER development is also modulated by Tiam1, a Rac1 GEF that interacts with Par3 and CGNL1 (Chen and Macara, 2005; Mertens et al., 2005; Guillemot et al., 2008); by RICH1, a Cdc42/Rac1 GAP that interacts with angiomotin (Wells et al., 2006); and by β2-syntrophin, which modulates the localization of Tiam1 and Rac1 along the apicobasal axis (Mack et al., 2012), we conclude that Rac1 is a key regulator of TER development, and we hypothesize that this regulation occurs primarily by controlling cytoskeletal organization and cytoskeletal anchoring of claudins. Of importance, the ability of MgcRacGAP to modulate TJ barrier development must depend on its spatially restricted activity at the cytoplasmic, cytoskeleton-linked “plaque” of the TJ, and our data indicate that this is through its interaction with CGN and CGNL1. Therefore, accumulation of CGN and CGNL1 at TJs during junction maturation provides a mechanism to spatially restrict down-regulation of Rac1 activation at TJs, through recruitment of MgcRacGAP.
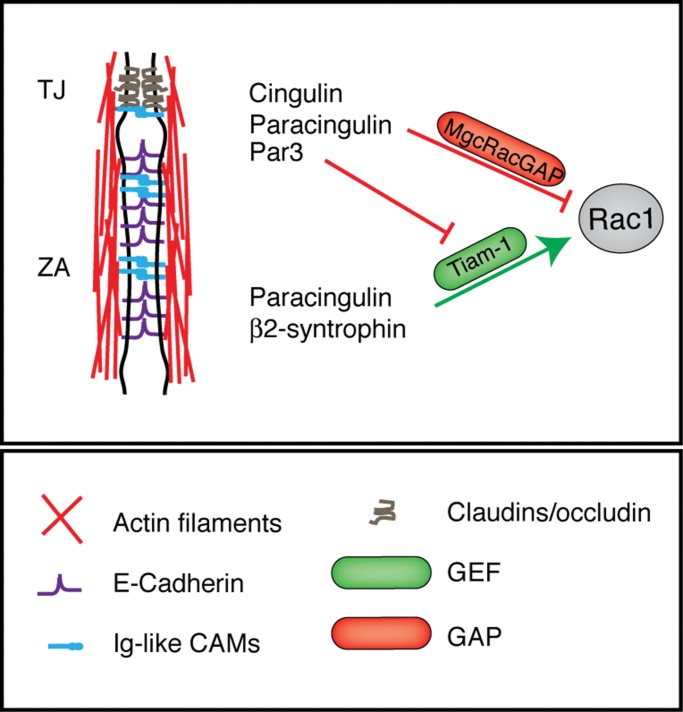
Taken together, the results presented here and in previous studies (Aijaz et al., 2005; Guillemot and Citi, 2006a; Guillemot et al., 2008) suggest a working model in which CGN and CGNL1 have multiple functions in fine-tuning RhoA and Rac1 activation in MDCK cells (Figure 6). In confluent cells, they both inhibit RhoA activation by sequestering GEF-H1, whereas during junction assembly, CGNL1 promotes Rac1 activation by supporting junctional recruitment of Tiam1, whereas both CGN and CGNL1 inhibit Rac1 activation at TJ through recruitment of MgcRacGAP. So we propose that the dynamics of junction assembly and TER development result at least in part from a balance between Tiam1-mediated Rac1 activation and MgcRacGAP-mediated Rac1 inhibition. Changes in the levels of expression of either Tiam1 or MgcRacGAP or their interacting partners at junctions, and additional regulatory mechanisms, may affect such equilibrium, resulting in altered spatiotemporal dynamics of junction assembly. To further test this model, it will be necessary to explore in more detail the phenotype of CGN and CGNL1 KO model systems, and the regulation of the molecular interactions between different GEFs, GAPs, and cytoplasmic junctional proteins.
FIGURE 6:
The control of Rac1 signaling at the apical junctional complex. Simplified scheme showing the AJC and the putative molecular interactions of GEFs and GAPs with CGN, CGNL1, and other junctional proteins. Green lines indicate activation; red lines indicate inhibition. A key for the graphical objects is shown at the bottom.
In summary, our data reveal a new mechanism of fine-tuning of junction assembly and development of the TJ barrier, by which MgcRacGAP controls Rac1 activation at apical junctions, where it is recruited through its interaction with CGN and CGNL1. Our results also support the idea that the spatiotemporal control of Rho GTPases is coordinated by the cell context–dependent makeup of GTPase activators and inhibitors and interacting junctional proteins.
MATERIALS AND METHODS
Antibodies and plasmids
Commercial antibodies were purchased against hemagglutinin (Covance, Meyrin, Switzerland), FLAG (M2; Sigma-Aldrich, Buchs, Switzerland), MgcRacGAP (rabbit sc-98617; Santa Cruz), ECT2 (07-1364; Millipore), MKLP1 (sc-867; Santa Cruz), and CGNL1 (mouse sc-162681; Santa Cruz, Muttenz, Switzerland). The mouse anti-CGNL1 antibody did not cross-react with canine CGNL1 and therefore could not be used to immunolocalize CGNL1 in double labeling of MDCK cells with rabbit anti-MgcRacGAP or for immunoprecipitations of CGNL1 from MDCK cells. The rat anti-ZO-1 monoclonal antibody (R40-76) was a kind gift of D. Goodenough (Harvard University, Cambridge, MA). Antibodies against MgcRacGAP (immunoblotting) and a plasmid (pMXs-neo) encoding FLAG-tagged human MgcRacGAP were kind gifts from T. Kitamura (Institute of Medical Science, University of Tokyo, Tokyo, Japan; Hirose et al., 2001) and A. Touré (Institut Cochin, Paris, France; Toure et al., 1998). Secondary antibodies for immunofluorescence (Jackson ImmunoResearch Europe, Newmarket, UK) were Alexa 488 anti-rabbit, Cy3 anti-mouse, and Cy5 anti-rat. Constructs for bacterial expression of MgcRacGAP GST fusion proteins (full-length and truncated) were produced by PCR amplification and subcloning into the pGEX4T1 vector. MgcRacGAP was subcloned into pcDNA3.1myc-His (EcoRI-NotI) for establishing stable MDCK cell lines. All new constructs were verified by sequencing. Polyclonal antiserum against CGN (Cardellini et al., 1996), other constructs, and antibodies were as previously described (Guillemot et al., 2004, 2008; Guillemot and Citi, 2006a; Paschoud and Citi, 2008).
Cell culture, transfection, and other techniques
MDCK, SKCO-15 (human intestinal colon carcinoma; a kind gift from A. Nusrat, Emory University, Atlanta, GA; Ivanov et al., 2010), and Eph4 cells (mouse mammary epithelial cells; a kind gift of E. Reichmann, University of Zurich, Zurich, Switzerland; Maschler et al., 2005) were cultured in DMEM containing 10% fetal bovine serum (FBS), nonessential amino acids, and appropriate antibiotics for selection (Guillemot et al., 2013). Mouse kidney collecting duct cells (mpkCCDCl4; a kind gift of E. Feraille, University of Geneva, Geneva, Switzerland; Gonin et al., 2001) were cultured as described previously (Pulimeno et al., 2010). Stable clones of MDCK cells expressing shRNAs to deplete CGN, CGNL1, or both CGN and CGNL1 (double-KD) or rescue MgcRacGAP constructs were obtained as described (Guillemot and Citi, 2006a; Guillemot et al., 2008, 2013). For siRNA-mediated depletion, the following target sequences were used (sense): 1) CGN: GTGACCAGGAGGTGGAACA (human and mouse), AGCTCAGAAGGCTTCCAGA (canine); 2) CGNL1: AGTGGCTGAACTTCAGAGA (human), GACTTAAAGAGCCGGATTA (mouse). Transfections were carried out using Lipofectamine2000 (DNA) and RNAiMax (siRNA), and cells were analyzed by immunofluorescence 48–72 h after transfection. Calcium switch, measurement of TER, Rac1 and RhoA activation assays, immunoblotting, and immunofluorescence were as described (Citi et al., 2001; Guillemot and Citi, 2006a; Guillemot et al., 2008; Paschoud and Citi, 2008).
GST pull downs, immunoprecipitation, and cell fractionation
For GST pull downs, full-length CGN and CGNL1 were from baculovirus-infected insect cells and MgcRacGAP from HEK293 cells transfected with exogenous, myc-tagged MgcRacGAP (Citi et al., 2001; Guillemot et al., 2008). Coimmunoprecipitation experiments were carried out as described (Guillemot et al., 2008), except that lysates were sonicated, and antibodies (2 μg) were incubated with Dynabeads Protein G (ReF. 1004D; Life Technologies Europe, Zug, Switzerland; 20 μl, prewashed three times with phosphate-buffered saline containing 5% skimmed milk) for 1 h at 4°C before incubation with cell lysates (overnight at 4°C) and washing with coimmunoprecipitation buffer. MDCK lysates were used for CGN-MgcRacGAP immunoprecipitations and Eph4 cell lysates for CGNL1-MgcRacGAP immunoprecipitation because the mouse anti-CGNL1 antibody does not cross-react with canine CGNL1. The anti-MgcRacGAP antibody did not work for immunoprecipitation. For fractionation, cells were lysed in cytoskeleton stabilizing buffer (CSK; 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 6.8, 250 mM sucrose, 150 mM KCl, 1 mM ethylene glycol tetraacetic acid, 3 mM MgCl2, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail). The CSK lysate was centrifuged for 15 min at 13,000 × g, and the pellet was resuspended, washed with CSK buffer, centrifuged again, and taken as the “low-speed” cytoskeleton fraction. The soluble fraction was centrifuged for 120 min at 100,000 × g, and the pellet was washed and taken as the “high-speed” Triton X-100 insoluble cytoskeleton fraction. The supernatant from high-speed centrifugation was taken as the “soluble” fraction. Equivalent protein loadings from each fraction were analyzed by SDS–PAGE and immunoblotting.
Primary keratinocyte cultures
Keratinocytes were isolated from WT and CGN KO (Guillemot et al., 2012) newborn mice, following a protocol approved by the Ethics Committee of the University of Geneva and the Cantonal Veterinary Office (Authorization 1027/3853/53). Skins were dissected and incubated with dispase II (04942078001; Roche), 2.5 U/ml (3.1 mg/ml) at 37°C for 2 h, in low-calcium medium (DMEM/F12 3:1, pH 7.2, supplemented with 15% calcium-free FBS, 100 U/ml penicillin/streptomycin, 0.1 nM choleratoxin, 6 × 10−4 mg/ml hydrocortisone, 5 × 10−3 mg/ml insulin, 5 × 10−3 mg/ml transferrin, and 0.02 nM 3′,5-triiodo-l-thyronine). The epidermis was then separated from the dermis, minced into pieces, and incubated with trypsin 0.25%-1× EDTA (253000062; Invitrogen) for 15 min at 37°C. The cell suspension was filtered through a 70-μm cell strainer and centrifuged at 1500 rpm for 5 min. Cells were seeded at a density of 0.25 × 106 cells/cm2 and incubated at 37°C in the presence of 5% CO2. Immunofluorescence analysis of keratinocytes was performed after calcium-induced induction of differentiation, which promotes junction formation: CaCl2 (0.25 M stock) was added to a final concentration of 1.2 mM, and keratinocytes were fixed (cold methanol) for immunofluorescence labeling at different times after calcium addition.
Quantitative RT-PCR
mRNA levels for GEFs and GAPs were analyzed by SYBR green–based RT-PCR (Guillemot and Citi, 2006a; Guillemot et al., 2008, 2013), using the following primers: Asef (ARHGEF4) forward, 5′-GCGGACCAACGTCATCAAC-3′; reverse, 5′-CCCGCAGGTGCTTGATGTA-3′; Vav2 forward, 5′-AAGAACTCAATGACCCCCCTGA-3′; reverse, 5′-AGGTTGATGAAAATGGCTGCC-3′; RICH1 forward, 5′-TGGCAGCGGCTACCTCTG-3′; reverse, 5′-TCGGCGTGCTGAATGATG-3′; MgcRacGAP forward, 5′-TGAATTTGCGGAATCTGTTTGA-3′; reverse, 5′-TGGAGTTCATTTCCTTCACTGAGA-3′.
Confocal microscopy
Cells were observed using Zeiss conventional (Axiovert TV100) and confocal (LSM700) microscopes. For quantification of MgcRacGAP labeling in CGN-KD and double-KD cells, cells were triple labeled with mouse anti-CGN/CGNL1, rabbit anti-MgcRacGAP, and rat anti–ZO-1. ZO-1 was used as an internal reference for junction/plane of focus to exclude the possibility that reduced junctional labeling for MgcRacGAP could be due to shift of plane of focus. For quantifications using ImageJ (National Institutes of Health, Bethesda, MD), for each marker the cytoplasmic area (devoid of junctions) was taken as background and subtracted from the junctional labeling. The mean pixel intensity of MgcRacGAP junctional labeling was measured in junctional segments or representative images in WT, single-KD, and double-KD cells (n = 5) and ratioed to ZO-1 labeling in the corresponding junctions (Pulimeno et al., 2011).
Statistical analysis
All experiments were carried out at least in triplicate, and histogram values show means ± SD. Values were considered statistically significant (*) when p < 0.05 between experiments (Student's t tests). For immunoblots and immunofluorescence data, one representative example is shown.
Supplementary Material
Acknowledgments
This study was funded by the Swiss National Science Foundation (Grants 31003A_116763, 31003A_135730/1, and 31003A_152899/1), the Swiss Cancer League (KFS-2813-08-2011), and the Canton and Republic of Geneva. We thank the colleagues cited in the text for kind gifts of reagents.
Abbreviations used:
- AJC
apical junctional complex
- Asef
APC-stimulated guanine nucleotide exchange factor
- CGN
cingulin
- CGNL1
paracingulin
- ECT2
epithelial cell transforming sequence 2 oncogene
- GAP
GTPase-activating protein
- GEF
guanidine exchange factor
- HA
hemagglutinin
- KD
knockdown
- KO
knockout
- MDCK
Madin–Darby canine kidney
- MgcRacGAP
male germ cell Rac GTPase-activating protein
- MKLP1
mitotic kinesin-like protein
- RICH1
RhoGAP interacting with CIP4 homologues protein 1
- TER
transepithelial electrical resistance
- Tiam1
T-cell lymphoma invasion and metastasis 1
- TJ
tight junction
- WT
wild type
- ZA
zonula adhaerens
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-11-0680) on May 7, 2014.
The authors declare no commercial affiliation or conflict of interest.
REFERENCES
- Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a002584. a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban R, Irino Y, Fukami K, Tanaka H. Human mitotic spindle-associated protein PRC1 inhibits MgcRacGAP activity toward Cdc42 during the metaphase. J Biol Chem. 2004;279:16394–16402. doi: 10.1074/jbc.M313257200. [DOI] [PubMed] [Google Scholar]
- Bastos RN, Penate X, Bates M, Hammond D, Barr FA. CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J Cell Biol. 2012;198:865–880. doi: 10.1083/jcb.201204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, Oegema K. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–1546. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardellini P, Davanzo G, Citi S. Tight junctions in early amphibian development: detection of junctional cingulin from the 2-cell stage and its localization at the boundary of distinct membrane domains in dividing blastomeres in low calcium. Dev Dyn. 1996;207:104–113. doi: 10.1002/(SICI)1097-0177(199609)207:1<104::AID-AJA10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Citi S, D'Atri F, Cordenonsi M, Cardellini P. Tight junction protein expression in early Xenopus development and protein interaction studies. In: Fleming TP, editor. In: Cell–Cell Interactions. Vol. 256. Oxford, UK: IRL Press; 2001. pp. 153–176. [Google Scholar]
- Citi S, D'Atri F, Parry DAD. Human and Xenopus cingulin share a modular organization of the coiled-coil rod domain: predictions for intra- and intermolecular assembly. J Struct Biol. 2000;131:135–145. doi: 10.1006/jsbi.2000.4284. [DOI] [PubMed] [Google Scholar]
- Citi S, Paschoud S, Pulimeno P, Timolati F, De Robertis F, Jond L, Guillemot L. The tight junction protein cingulin regulates gene expression and RhoA signalling. Ann NY Acad Sci. 2009;1165:88–98. doi: 10.1111/j.1749-6632.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- Citi S, Pulimeno P, Paschoud S. Cingulin, paracingulin and PLEKHA7: signalling and cytoskeletal adaptors at the apical junctional complex. Ann NY Acad Sci. 2012;1257:125–132. doi: 10.1111/j.1749-6632.2012.06506.x. [DOI] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Citi S, Spadaro D, Schneider Y, Stutz J, Pulimeno P. Regulation of small GTPases at epithelial cell-cell junctions. Mol Membr Biol. 2011;28:427–444. doi: 10.3109/09687688.2011.603101. [DOI] [PubMed] [Google Scholar]
- D'Avino PP, Glover DM. Cytokinesis: mind the GAP. Nat Cell Biol. 2009;11:112–114. doi: 10.1038/ncb0209-112. [DOI] [PubMed] [Google Scholar]
- D'Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol. 2004;166:61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avino PP, Takeda T, Capalbo L, Zhang W, Lilley KS, Laue ED, Glover DM. Interaction between anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J Cell Sci. 2008;121:1151–1158. doi: 10.1242/jcs.026716. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Kachar B. Myosin transcellular networks regulate epithelial apical geometry. Cell cycle. 2013;12:2931–2932. doi: 10.4161/cc.26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A, Zihni C, Terry SJ, Clark P, Matter K, Balda MS. Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J Cell Biol. 2012;198:677–693. doi: 10.1083/jcb.201202094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Glotzer M. Cytokinesis: GAP gap. Curr Biol. 2009;19:R162–165. doi: 10.1016/j.cub.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonin S, Deschenes G, Roger F, Bens M, Martin PY, Carpentier JL, Vandewalle A, Doucet A, Feraille E. Cyclic AMP increases cell surface expression of functional Na,K-ATPase units in mammalian cortical collecting duct principal cells. Mol Biol Cell. 2001;12:255–264. doi: 10.1091/mbc.12.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK) J Membr Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol. 2008;18:25–29. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell. 2006a;17:3569–3577. doi: 10.1091/mbc.E06-02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot L, Citi S. Cingulin, a cytoskeleton-associated protein of the tight junction. In: Gonzalez-Mariscal L, editor. In: Tight Junctions. New York: Landes Bioscience-Springer Science; 2006b. pp. 54–63. [Google Scholar]
- Guillemot L, Hammar E, Kaister C, Ritz J, Caille D, Jond L, Bauer C, Meda P, Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J Cell Sci. 2004;117:5245–5256. doi: 10.1242/jcs.01399. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell. 2008;19:4442–4453. doi: 10.1091/mbc.E08-06-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J Cell Sci. 2012;125:5005–5014. doi: 10.1242/jcs.101261. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Spadaro D, Citi S. The junctional proteins cingulin and paracingulin modulate the expression of tight junction protein genes through GATA-4. PLoS One. 2013;8:e55873. doi: 10.1371/journal.pone.0055873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821–5828. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol. 2010;176:134–145. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, Glotzer M. CYK-4: A Rho family GTPase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosov D, Chasiotis H, Kelly SP. Tight junction protein gene expression patterns and changes in transcript abundance during development of model fish gill epithelia. J Exp Biol. 2014;217:1667–1681. doi: 10.1242/jeb.098731. (Pt 10) [DOI] [PubMed] [Google Scholar]
- Liot C, Seguin L, Siret A, Crouin C, Schmidt S, Bertoglio J. APC(cdh1) mediates degradation of the oncogenic Rho-GEF Ect2 after mitosis. PLoS One. 2011;6:e23676. doi: 10.1371/journal.pone.0023676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack NA, Porter AP, Whalley HJ, Schwarz JP, Jones RC, Khaja AS, Bjartell A, Anderson KI, Malliri A. beta2-Syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol. 2012;14:1169–1180. doi: 10.1038/ncb2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- Matthews HK, Delabre U, Rohn JL, Guck J, Kunda P, Baum B. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell. 2012;23:371–383. doi: 10.1016/j.devcel.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J, Welsh NJ, Braga VM. Cycling around cell-cell adhesion with Rho GTPase regulators. J Cell Sci. 2013;126:379–391. doi: 10.1242/jcs.097923. [DOI] [PubMed] [Google Scholar]
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harbor Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- Muroya K, Kawasaki Y, Hayashi T, Ohwada S, Akiyama T. PH domain-mediated membrane targeting of Asef. Biochem Biophys Res Commun. 2007;355:85–88. doi: 10.1016/j.bbrc.2007.01.131. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carmes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J Biol Chem. 2004;279:46014–46022. doi: 10.1074/jbc.M402616200. [DOI] [PubMed] [Google Scholar]
- Paschoud S, Citi S. Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression. Mol Membr Biol. 2008;25:1–13. doi: 10.1080/09687680701474009. [DOI] [PubMed] [Google Scholar]
- Priya R, Yap AS, Gomez GA. E-cadherin supports steady-state Rho signaling at the epithelial zonula adherens. Differentiation. 2013;86:133–140. doi: 10.1016/j.diff.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Pulimeno P, Bauer C, Stutz J, Citi S. PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO-1 and E-cadherin. PLoS One. 2010;5:e12207. doi: 10.1371/journal.pone.0012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulimeno P, Paschoud S, Citi S. A role for ZO-1 and PLEKHA7 in recruiting paracingulin to tight and adherens junctions of epithelial cells. J Biol Chem. 2011;286:16743–16750. doi: 10.1074/jbc.M111.230862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–828. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and mvhogenesis. Nat Cell Biol. 2011;13:159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, Reibel L, Gacon G. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Van de Putte T, Zwijsen A, Lonnoy O, Rybin V, Cozijnsen M, Francis A, Baekelandt V, Kozak CA, Zerial M, Huylebroeck D. Mice with a homozygous gene trap vector insertion in mgcRacGAP die during pre-implantation development. Mech Dev. 2001;102:33–44. doi: 10.1016/s0925-4773(01)00279-9. [DOI] [PubMed] [Google Scholar]
- Wells CD, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- White EA, Glotzer M. Centralspindlin: at the heart of cytokinesis. Cytoskeleton (Hoboken) 2012;69:882–892. doi: 10.1002/cm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.