Abstract
Age-related macular degeneration (AMD) is the leading cause of permanent, irreversible, central blindness (scotoma in the central visual field that makes reading and writing impossible, stereoscopic vision, recognition of colors and details) in patients over the age of 50 years in European and North America countries, and an important role is attributed to disorders in the regulation of the extracellular matrix (ECM). The main aim of this article is to present the crucial processes that occur on the level of Bruch’s membrane, with special consideration of the metalloproteinase substrates, metalloproteinase, and tissue inhibitor of metalloproteinase (TIMP).
A comprehensive review of the literature was performed through MEDLINE and PubMed searches, covering the years 2005–2012, using the following keywords: AMD, extracellular matrix, metalloproteinases, tissue inhibitors of metalloproteinases, Bruch’s membrane, collagen, elastin.
In the pathogenesis of AMD, a significant role is played by collagen type I and type IV; elastin; fibulin-3, -5, and -6; matrix metalloproteinase (MMP)-2, MMP-9, MMP-14, and MMP-1; and TIMP-3. Other important mechanisms include: ARMS2 and HTR1 proteins, the complement system, the urokinase plasminogen activator system, and pro-renin receptor activation.
Continuous rebuilding of the extracellular matrix occurs in both early and advanced AMD, simultaneously with the dysfunction of retinal pigment epithelium (RPE) cells and endothelial cells. The pathological degradation or accumulation of ECM structural components are caused by impairment or hyperactivity of specific MMPs/TIMPs complexes, and is also endangered by the influence of other mechanisms connected with both genetic and environmental factors.
Keywords: AMD, Extracellular Matrix, Metalloproteinases, Tissue Inhibitors of Metalloproteinases, Collagen, Bruch’s Membrane, Elastin
Bacground
The extracellular matrix (ECM) is composed of stromal substance (stroma) and basement membranes that are stretched along the border between this matrix and the tissues, that is, between the epithelium and the endothelium. The ECM represents a supportive frame for the cells (tissues and organs) and creates the internal environment for nutrient transport, product metabolism, and signal conduction (for proper functioning and protection against outer undesirable factors). The ECM also takes part in regulating the physiological processes of differentiation, proliferation, migration, and cell adhesion [1–4]. The physiological balance between synthesis and disintegration of the ECM components allows for both creation and maintenance of proper tissue architecture. The key role in the continuous rebuilding of connective tissue, both physiological and pathological (processes of growth and reconstruction), is played by endopeptidases, also called metalloproteinases, or matrix metalloproteinases (MMPs), which are able to degrade all of the structural elements of the ECM: basement membranes, cytokines, growth factors, chemokines, and receptors on the cell membranes [1–6]. MMPs are synthesized and secreted as latent enzymes; they become active in the presence of zinc and calcium ions and act on the surface of the cell or in the area around it. MMPs can be divided into several types on the basis of the decomposition of substrates: collagenases, gelatinases, stromelysin, elastase, and membrane metalloproteinase [1–3,5]. The production and secretion of metalloproteinases is controlled genetically [1–3,5]. The activity of MMPs is strictly regulated by tissue-inhibitor metalloproteinases (TIMPs), with the 4-endogenic TIMPs comprising TIMP-1, -2, -3, and -4 [1–3,5,6]. MMP/TIMP complexes are responsible for regulating the cascade of enzymatic reactions occurring in embryogenesis, morphogenesis, cell apoptosis, inflammatory reaction, angiogenesis, and other processes [1–8].
Disorders of regulation of the substrate complex/MMPs/TIMPs (impairment or hyperactivity) leads to general pathological conditions such as rheumatoid arthritis, heart diseases, blood vessel diseases, atherosclerosis, ulcers, multiple sclerosis, metastasis, and autoimmune diseases [1–3,5,7,8], as well as eye diseases such as retinal dystrophy [9–11], age-related macular degeneration (AMD) [12–17], pseudoexfoliation syndrome [18–20], fibrosis of the lenticular capsule after phacoemulsification treatment [21–25], diabetic retinopathy [26,27], proliferative diabetic vitreoretinopathy [16], and epiretinal membrane of proliferative diabetic retinopathy [28].
AMD can be caused by many factors, but advanced age and its related physiological cell apoptosis and tissue involution, together with genetic predisposition, are the strongest risk factors [9,12,14,29–34]. Other important factors include sex [35], environmental influences such as smoking cigarettes [36], heart and vascular disorders, hypertension, dyslipidemia/hypercholesterolemia, diabetes, obesity, improper diet, and sedentary lifestyle [37–42]. The pathological area involved in AMD is represented by photoreceptors, the retinal pigment epithelium (RPE), ECM (Bruch’s membrane [BrM]), and the choriocapillaris [12,17,33]. The pathological features of AMD are triggered by the deregulation of metabolic processes on the level of RPE cells; however, the area of disorders lies within the ECM [12–14,29,43–45].
The most significant symptom of early AMD is the presence of inflammatory deposits, the soft drusen that appear in the area of the BrM, but which are larger than 125 μm and confluent; these in turn represent an important risk factor for further development of AMD [44,46–48]. Most patients with advanced AMD have dry AMD in the form of geographical atrophy of the central retina covering the photoreceptors/RPE cells/BrM/choriocapillaris complex [14]. About 10–15% of all patients with advanced AMD have the wet, or neovascular, form (choroidal neovascularization, CNV) [14], which occurs in 4% of patients who are over 75 years old [34]. As a result of pathological angiogenesis, the vessels from the choroid appear to proliferate – after crossing the ECM border, they penetrate under the retina and their immaturity and the leakiness of their vascular walls causes multiple leaks of serum and lipoproteins to occur, as well as numerous hemorrhages [8,14,49,50]. The overall process finally results in scarring and irreversible loss of central vision.
Molecular Character of Changes in the ECM in AMD
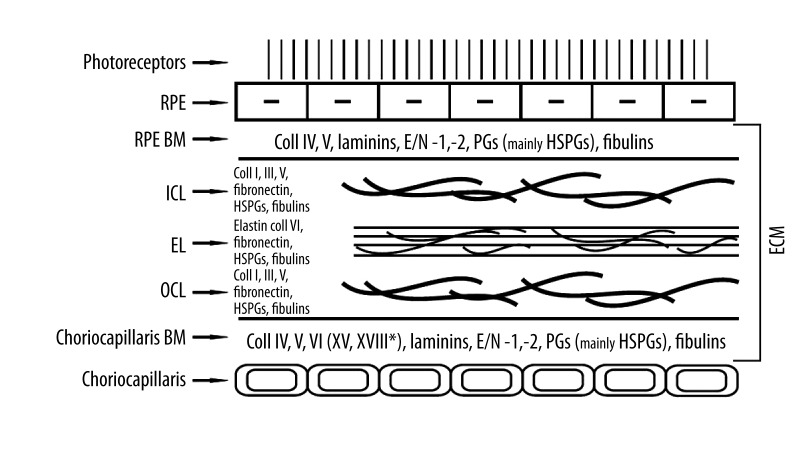
The 2–4 μm-thick BrM [17] is the ECM and acts as a frame for the metabolically active RPE cells, as well as a physical barrier for their passage and that of the endothelial vessels; moreover, it regulates the diffusion of nutrient molecules between the retina and the choroid [17,51–53]. The BrM is composed of 2 basement membranes – 1 for the RPE cells and 1 for the endothelium of the choriocapillaris – which are rich in collagen type IV (non-fibrillar), fibronectin, and laminin. Between the basement membranes are 2 layers (inner and outer) of structural collagen, composed of collagen type I and type III (fibrillar). Both of the collagen layers embrace the middle layer of the BrM, like a sandwich built mainly of elastin [14,15] (Figure 1).
Figure 1.
The composition of regular human extracellular matrix (Bruch’s membrane). E/N – entactin/nidogen, PGs – proteoglycans, HSPGs – heparan sulphate proteoglycans.
RPE cells control the synthesis of all the structural elements of the BrM, mainly the most abundant proteins – collagen type I, collagen type IV, and laminin (which is not a collagen) – as well as the metalloproteinases and their tissue inhibitors [54]. The synthesis of metalloproteinases undergoes complex regulation by growth factors, cytokines, endothelins, interleukins, prostaglandins, and growth hormones [1,55]. There are 2 forms of secreted metalloproteinases: free or anchored in the cell membrane (membrane-type metalloproteinases; MT-MMPs) [1–3,5]. The role of MMPs is not limited to the degradation of the BrM; growth factors are secreted from cell membranes that increase the accessibility of MMPs and strengthen their activity [1–3,5]. Increased activity of MMPs stimulates the secretion of TIMPs, which bind to inactive pro-MMPs or active metalloproteinases. TIMP-1 inhibits the activity of MT-MMPs; TIMP-2 and -3 can bind to all types of MMPs; and TIMP-4 inhibits MMP-1, -2, -3, -7, and -9 [2,5,56] (Table 1).
Table 1.
Structural elements of the ECM crucial in the development of early and/or advanced age-related macular degeneration: their characteristics, biological function, and digesting matrix metalloproteinases (MMPs).
| Substrate | Localization/Biological Function | Substrate-digesting MMPs | Role in development |
|---|---|---|---|
| Basement membranes (BMs) | |||
| Collagen IV | Main, and most abundant element, of the BM RPE (α3α4α5 molecule – is responsible for fluid/membrane barrier) and the endothelium (α1α1α2 molecule) [57–63]. Stabilizes and integrates BM and takes part in the process of angiogenesis and haemostasis [59,61]. Despite its prevalence and abundance, it is not required for initiating the BM process [62] | −2, −9 [2,8] | SD/CNV [54,59,61] |
| Laminin(s) (12 isoforms) LAMA(α), LAMB(β), LAMC(γ) | “Big” non-collagen element of BM – proteoglycan [64–68]. Initiates and is necessary for the BM process [64,65,69,70] and binds to collagen directly or by means of entactin/nidogen – see below [59,71]. Supports the structural integrity of the BM and adhesion of the RPE and endothelium. Also mediates signal transmission by means of the following cell receptors: integrins, proteoglycans, and glycoproteins (e.g., dystroglycan) – see below [59] | −2, −9, −7, −19, MT1-MMP [2,5] | |
| Entactin/Nidogen 2 isoforms: (E/N-1, -2) | “Small” components of BM – proteoglycans [68]. Necessary for membrane formation and binding of laminin to collagen and fibulins – see below [59,72–74]. Responsible for adhesion by mediation of integrins or other receptors [59,75] | −3, −13 [2,5] | |
| Proteoglycans (PGs) | Chondroitin/dermatan sulphate and mainly heparan sulphate. With proteins of PGs, glycosaminoglycans create strong hydrophilic and water-absorbing gel-like structures, in which collagen fibres are drowned [76]. Support the histo-architecture, regulate adhesion and differentiation of cells and the activity of growth factors, and play a key role in selective ultrafiltration of the ECM [47,58,77–80] | −3, −10, −11 [2,5] | |
| ICL, EL, OCL | |||
| Collagen I | Main element of the ICL and OCL [59,81]. Renovation of fibres, adhesion of cells, interactions in the ECM [82–84] | −1, −2, −8, −9, −13, −18, MT-MMPs: −14, −15, −16, −24 [2,5,8] | SD/CNV [54,8,85,86] |
| Elastin | Main structural element of the EL. Influences the elasticity and endurance of the ECM [87–89] | −2, −9, −12 [2,8] | CNV [87,88] |
| Fibronectin | Proteoglycan. Shapes and degrades collagen [84] and facilitates adhesion of the RPE and the endothelium by binding integrins to collagen (see below) [58,76]. Also necessary for the formation of choroid vessels [85] | −2, −9, −7 [2,5,8] | |
| Fibulins (mainly-3, -5, -6) | “Small” components of the BM, ICL, EL, and OCL. Form, stabilize, and anchor elastic fibres to the BM by binding elastin and laminin to integrins [9,68,90–92] FBLN-3 inhibits the CNV by limiting secretion of MMP-2, -3, and -9 [92] |
SD/*GA, *CNV [9,92–96] | |
| Integrins 2 isoforms: (ITG-A, -B) | Next to dystroglycan, they are the main surface receptors of the RPE and endothelium for collagen, laminin, and fibronectin [97–102]. Polygonal network of laminin appears as a result of the binding of neighbouring cells, anchored to integrins or dystroglycans. The process is steered by cellular actin [103] | ||
BM – basement membrane; ICL – internal collagenous layer; EL – elastic layer; OCL – outer collagenous layer.
Familial.
The elastic layer of the BrM plays a key role in the pathogenesis of advanced neovascular AMD [87,88]. With increasing age, the number of basophilous fibers increases, which in turn leads to calcification and synchronously to the loss of elasticity of the BrM [14]; the developing destruction of the elastic fibres releases proteins, which are strong pro-angiogenic factors [89,104]. Regardless of an individual’s age, the elastic layer is 3 to 6 times thinner and 5 times more porous in the area of the macula than it is on the rim of the retina [87]. The thinner and more porous elastic layer causes the BrM to become a weaker frame for the RPE cells and may trigger CNV development in this part of the retina [87,88]. Some researchers [87,105,106] suggest that the defects in the BrM are essential factors – whereas others [107] maintain that they are not – for the CNV to enter the area of the ECM of the macula. Elastin has strong structural and functional connections with fibulins [92–95] and enters into a reaction with the age-related maculopathy susceptibility 2 (ARMS2) protein (see below) [9]. Elastin is degraded by (metallo)elastase MMP-12, and gelatinase A (MMP-2) and B (MMP-9) [1,2,5,108,109]. Smoking cigarettes is thought to be a strong stimulator for the destruction of elastic fibers; this key modifiable environmental risk factor for the development of AMD also triggers auto-immunological processes against elastin [110,111].
Metalloproteinases of the ECM and Their Tissue Inhibitors are Important for the Pathogenesis of Both Dry and Wet AMD
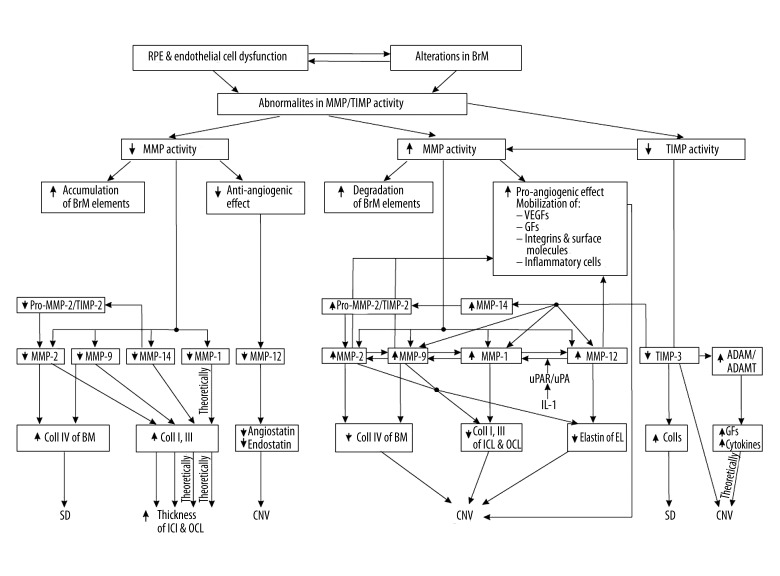
In AMD, RPE cell dysfunction results in disorders in the activity of MMPs/TIMPs and their substrates. MMP-2 and MMP-9, being regulated by TIMP-2 and TIMP-1, respectively [2,5,8], play a key role in the pathogenesis of AMD [43,45,112,113] and in both early dry AMD [13,43,45] and advanced wet (neovascular) AMD [4]. MMP-2 and MMP-9 can digest the main structural elements of the ECM: collagen IV, collagen V, fibronectin, and laminin [1,2,5]. Loss of MMP-2 activity leads mainly to an increase in collagen IV, as well as to an accumulation of deposits under the RPE layer [114]. MMP-2 is the most abundant enzyme synthesized by RPE cells [43,45,113]. The disordered activity of MMP-2 is the main cause of early AMD development [13,43,45]. MMP-2 is secreted as an inactive pro-enzyme that is activated by MMP-14 near the pro-MMP-2/TIMP-2/MMP-14 complex [2,35,115]. The activation process takes place exclusively when the TIMP-2 level is low in relation to that of MMP-14 in this 3-element complex [2,115]. MMP-14 indirectly influences collagen by activating MMP-2, but also directly influences it by causing an increase in collagen I and III [35].
MMP-1 and TIMP-3 also have important roles in the development of AMD. MMP-1 degrades collagen I, II, III [1,2,5] and the decrease in its activity favors the development of soft drusen. In AMD-prone eyes (family history, influence of crucial environmental risk factors), the activation of MMP-1 by lysosomal enzymes in ageing and dysfunctional REP cells, together with the simultaneous effect of the MMP-9 and urokinase plasminogen activator receptor/urokinase plasminogen activator (uPAR/uPA) system (activated by interleukin-1), leads to the development of advanced neovascular AMD [15,116,117]. TIMP-3 strongly binds to glycosaminoglycans in basement membranes [44] and has a role in cell apoptosis [10,118,119]. RPE cells, when exposed to blue light (relative AMD risk factor), diminish TIMP-3 production, which consequently causes an increase in the amounts of various collagens and in the development of early dry AMD [15,120]; on the other hand, TIMP-3 demonstrates a protective influence, since it inhibits the development of neovascularization [121] (Figure 2).
Figure 2.
Matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP) significant in the pathogenesis of early and late AMD. IL-1 – interleukin-1.
Histological and Clinical Aspects of Changes in the ECM and Other Mechanisms (Apart from MMP/TIMP Complexes) That Regulate the ECM in Both Early and Advanced AMD
As a result of RPE cell ageing and disorders in their functioning, the structure of the ECM changes [82]. The amount of collagen I [122], III, IV, and V increases [123], as does the amount of non-soluble collagen (responsible for facilitating the accumulation of metabolic waste and lipoproteins, growth factors, and cytokines [123]), and the network of collagen fibers in both collagen layers of the BrM becomes irregular [124]. An increase in glycosaminoglycans is partially responsible for the changes in the metabolism of collagen [78]. The elastic layer of the BrM loses its flexibility and becomes porous and fragile [125]. In addition, the amount of collagen increases, mainly in the internal collagenous layer [78,122,123]; basal laminar deposits (BLamD) [126–128] and drusen [126,128] are formed; and fats accumulate [129–131]. All of these factors cause a minimum triple thickness of the BrM [78,126,128], which worsens filtration to and from the retina [78,132]. The situation is further worsened by the decrease in blood flow caused by the diminishing vascular lumen and the amount of choriocapillaries [133–136] (Figure 3).
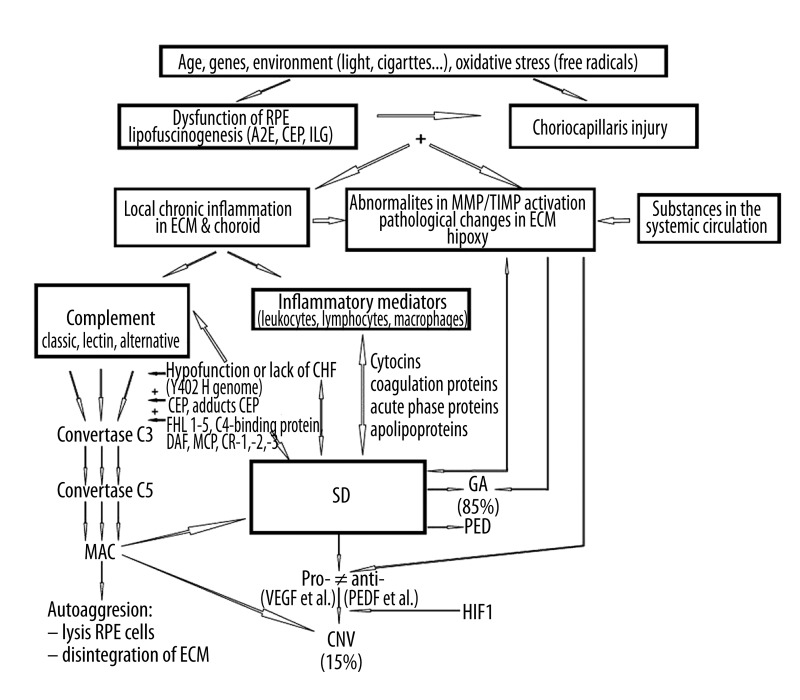
Figure 3.
Pathological changes in the retinal pigment epithelium, extracellular matrix, and choroid in age-related macular degeneration. PED - pigment epithelial detachment. * Programmed cell death – apoptosis; ** cell death caused by separation from its BMs – anoikis [14].
The symptoms of RPE and ECM change disorders in early AMD are crucial for the pathogenesis, invisible clinically, of early and advanced BLamD and basal linear deposits (BLinD) [125,137–139]. BLamD are stored and accumulated between the cytoplasmic and the basement membrane of RPE cells [125,137,140], whereas BLinD are stored and accumulated in the ECM between the RPE basement membrane and the internal collagen layer of the BrM [125,137,138]. BLamD comprise membrane proteins and fibrous long-spacing collagen, with cyclic repeatability of the collagen polymer every 100 to 120 nm [125,137], which react together with antibodies against elements of the basement membrane such as collagen type IV, proteoglycans, and laminin [125,137]. The early BLamD does not cause separation of the RPE cells from the internal collagen layer of the BrM, but accumulated, advanced, and thick BLamD can cause the separation of the RPE from the ECM. This separation, together with the decrease in permeability of the BrM (which becomes thicker and hydrophobic), simultaneous with the worsened blood supply caused by the choroid’s capillary vessels, worsens the metabolism of the RPE cells, affecting the ability of these cells to supply the photoreceptors [141–144]. Both BLamD and BlinD are an index of the degree of degeneration of photoreceptors and RPE cells, which correlates positively with a decrease in visual acuity and clinically visible pigment changes in the macula [125,139,140]. The deposits in question also influence the development of advanced forms of AMD [139]. The accumulated basal deposits may trigger disorders caused by changes in the ECM and deepen the process of lipofuscinogenesis, an accumulation of lipofuscin that takes place in the RPE; ie, age pigment, which develops from fully or partially digested outer segments of photoreceptors as a result of the gradual failure of liposomal enzymes in the RPE [145–147]. In the basal deposits, present in patients with neovascular AMD, 2 new forms of collagen aggregates, from untypical collagen polymerization, have been reported. The first form appears as parallel stripe-shaped structures, singly and/or in clusters, and is stretched from the internal surface of BLamD to the ECM; this form is morphologically similar to the collagen of the short-chained type. The second form appears as aggregates, similar to long-fiber collagen, and is periodically separated with characteristic dense strands [148]. According to the authors, the leakage of various factors from the neovascularization that trespasses into the area of the BrM and from the basal deposit may both induce physico-chemical changes in the ECM, which in turn may trigger untypical polymerization of the collagen molecules into aggregates similar to the stripes and long fibers of collagen [148]. Deposition of these new structures increases the BLamD thickness to a high degree and simultaneously worsens the nutrient and oxygen supply of the RPE/photoreceptors, which in turn maintains neovascularization [148].
The basal deposits, with diameters of over 25–30 μm, turn into clinically visible soft drusen, which are conducive to serous separation of the RPE from the ECM [9,139,149–151]. They are a risk factor for developing advanced forms of AMD during the next 5 to 10 years [152,153]; i.e., the geographical atrophy or neovascular form of AMD [139,154–157]. Eyes without drusen and with only the pigment changes (resulting from the elimination from the ECM of the RPE cells, which are loaded with lipofuscin and have lost their microvilli and ability to adhere to the ECM) [141–144,158], show a slower AMD process and have a lower risk of developing the advanced form of AMD during the next 5 to 10 years [152,153]. Drusogenesis takes place and lasts in the ECM for many years. It is a complex and multifactorial process, as evident from the composition of drusen: phospholipids, glycolipids, saturated and unsaturated fatty acids, cholesterol, approximately 130 various kinds of proteins, carbohydrates, fibronectin, ubiquitin, vitronectin, integrins, apolipoproteins, and immunoglobulins. Drusen also contains components of the complement complex, including, among others, the membrane attack complex (MAC), adducts of the carboxyethylpyrrole (CEP) protein (see below), MMPs, TIMPs, and others [14,114,149,159–162]. Drusogenesis is a side effect of both dysfunction and degeneration of RPE cells; however, it may also indicate a chronic para-inflammatory (which in some aspects is similar to inflammation, but not classic inflammation) and immunological condition in the ECM [163–169]. The phagocytes (leukocytes, lymphocytes, and macrophages) and complement system attempt to clear the ECM of the accumulated metabolic waste of the RPE [170–172].
The classic and alternative pathways of the complement system (which does not require the presence of a typical pathogen), activated by factors that are dependent on and independent from lipofuscinogenesis (an oxidized albuminous element of lipofuscin, bis-retinoid pyrimidine A2E, is one of the strongest cytotoxic stimulants of the alternative pathway, and is only exceeded by CEP and isolevuglandin), lead to the creation of the MAC and the destruction (lysis) of the aggressor’s cells after the spontaneous hydrolysis of the C3 factor (classic pathway) or the C3b factor (alternative pathway) [170,172–177]. The complement system is an integral element of the inborn (not specific) immunological response, and it supports phagocytosis and controls the inflammatory reaction process [170,172]. However, in conditions of insufficient control in the AMD, the complement system starts to attack both the drusen material and its own RPE cells, especially in patients with the Y402H gene polymorphism, which causes hypofunction or lack of the protective complement factor H, which in turn slows down the cascade of reactions connected with an increase in MAC, especially within the range of the alternative pathway. This leads to deep disintegration of the ECM [178–181]. Apart from complement factor H, other serum (plasmatic) suppressors of the activity of the complement system are important, including the family of factor H-like proteins 1–5, C4-binding proteins, and surface protein such as membrane decay-accelerating factor CD55, membrane cofactor protein CD46, and receptors of the complement system CR1, CR2, and CR3 [177,182]. In the mobilization of the immune system and the auto-aggressive response regarding RPE cells and the ECM, a pivotal role is also played by the CEP protein and its adducts, which are generated in blood as a result of CEP conjugation with circulating proteins such as plasma albumin [149,183]. CEP is a derivative of the free-radical oxidation of phospholipid docosahexaenoic acid (DHA), which is the main representative of polyunsaturated fatty acids, which are abundant in plasmatic membranes of photoreceptors digested by the RPE cells [183,184]. DHA is especially prone to the oxidation process, which is also favored by relatively high oxygen partial pressure in the central part of the retina [184–186]. Oxidized DHA-CEP products and CEP adducts are present in the lipofuscin of RPE cells, the drusen of the ECM, and systemic circulation [149,183]. They are strongly immunogenic, and therefore constitute a target for the immune system and stimulate the production of anti-CEP antibodies and anti-CEP auto-antibodies (because the adducts are endogenic compounds with antigen features) [149,183]. CEP adducts caused the development of human AMD (drusen, geographical RPE atrophy, neovascularization) in immunized animals [187, 188]. They also stimulate angiogenesis independently from vascular endothelial growth factor (VEGF; see below) [189]. In the neovascular type of AMD, the ECM is affected by the influence of the pro-angiogenic forces (generated by the RPE) on the choroid and therefore becomes the area of neovascularization invasion. Hypoxia, but also hyperactivity of the complement system, together with the inflammatory process, leads to disturbances in the pro/anti-angiogenic balance. The increased RPE expression of the angiogenesis-stimulating factors (mainly VEGF-A, -B, -C, and -D and platelet-derived growth factors PDGF-A and B) over the anti-angiogenic factors (mainly pigment epithelium-derived factor) causes the expansion of the pathological vessels from the choroid to the area of the BrM [49,50,107,190]. Hypoxia-inducible factor-1 is a key substance in activating the transcription of the pro-angiogenic factors, including VEGF [107,190]. RPE is the main source of pigment epithelium-derived factor, and its decreased expression derives from the degeneration of the RPE cells [190] (Figure 4).
Figure 4.
Schematic presentation of the mechanisms causing early and advanced forms of age-related macular degeneration. A2E – bis-retinoid pyrimidine, CEP – carboxyethylpyrrole protein, ILG – isolevuglandin, CFH – complement factor H, FHL – family of factor H-like proteins, DAF – membrane decay-accelerating factor, MCP – membrane cofactor protein, CR – complement receptor, MAC – membrane attack complex, GA – geographical atrophy, PED – pigment epithelium detachment, VEGF – vascular endothelial growth factor, PEDF – pigment epithelium-derived factor, HIF-1 – hypoxia-inducible factor-1.
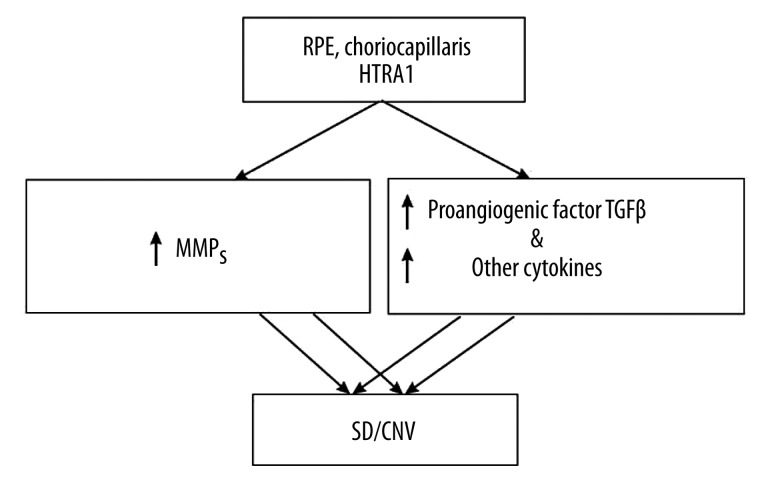
In the degradation of the ECM proteins (as well as in the case of cell growth and survival processes), a crucial role is played by the serine protease HTRA1 (high temperature requirement factor A 1), also called the heat shock protein, which is secreted in stress-related conditions. Expressions of HTRA1 are present in RPE cells, endothelium cells of the choroid, drusen, and subretinal neovascular membranes. HTRA1 is related to early and advanced neovascular AMD and causes an increase in the secretion of metalloproteinases and/or binding the pro-angiogenic factor transforming growth factor-β [191–193] (Figure 5).
Figure 5.
The relation of high-temperature requirement factor A 1 (HTRA 1) to early and advanced age-related macular degeneration. TGFβ – transforming growth factor β.
Proteins coded by the genes connected with the development of maculopathy (genetically conditioned) include MMP-9 [112]; TIMP-3 [194]; fibulins -3, -5, and -6 [93,95,96,195]; and elastin [17,88], as well as ARMS2 protein [9,196]. The secretory cytosolic (but not the mitochondrial) ARMS2 protein [196] regulates the ECM [9]. It is present in the retina and the RPE; however, it mainly accumulates in the intercapillary spaces of the choroid, where it interacts with elements of the basement membranes, including collagen type IVα2 (COL4A2) and fibulin-1 and -6 (FBLN-1, -6), as well as with ECM proteins, collagen type Iα1 (COL1A1) and fibronectin1 (FN1) [9,90,91]. Homozygous patients, having no ability to synthesize the ARMS2 protein [198], show a higher susceptibility to the development of drusen and AMD [9]. ARMS2 shows functional interaction with the protein network of the ECM; many of these proteins are present in the development of maculopathy [90,91,195,198,199]. The importance of ARMS2 in the pathomechanism of AMD underlines, as pointed out by researchers [9], the direct relationship of ARMS2 to fibulin-6, mutations of which lead to damage in the elastic fibers of the BrM (Figure 6).
Figure 6.
Protective influence of the age-related maculopathy susceptibility 2 protein (ARMS 2) on the structural and functional elements of the extracellular matrix. FBN – fibulins, FN – fibronectin.
The uPAR/uPA system influences the development of advanced neovascular AMD [15]. Both RPE cells and the choriocapillaris epithelium secrete uPAR and inactive uPA [116]. Activated uPA causes the conversion of inactive plasminogen into plasmin (i.e., the protease), which degrades laminin, fibronectin, and other proteins of the ECM [200]. Moreover, uPA also reduces cell adhesion and decreases cellular mobility [98]. uPA, stimulated by, among others, interleukin-1 and other cytokines, synergizes with MMP-9 and elastase in collagen VI and elastin degradation, which in turn enables CNV and the RPE cells to enter the area of the ECM [15]. uPA activity is blocked by 2 endogenous inhibitors (plasminogen activator inhibitors PAI-1 and PAI-2) [200] (Figure 7).
Figure 7.
The relationship between the urokinase plasminogen activator receptor/urokinase plasminogen activator system and the development of choroid neovascularization/age-related macular degeneration. IL-1 – interleukin-1, LAMs – laminins, FN1 – fibronectin1.
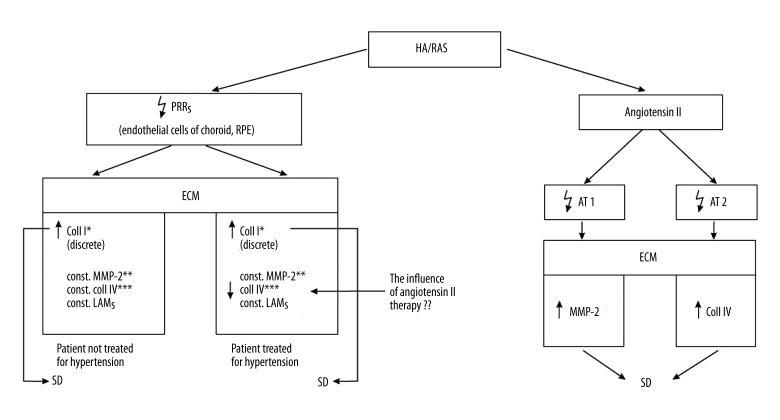
Activation of the pro-renin receptor (PRR), the pivotal element of the renin-angiotensin system, causes an increase in collagen I accumulation, with the level of collagen IV and laminin unchanged, in an animal model [54]. Activation of the PRR receptors does not influence the MMP-2/MMP-14/TIMP-2 complex and therefore does not cause an increase in the levels of collagen IV and laminin, which are the main cause of the development of drusen [54]. As was hypothetically assumed by the authors, activation of PPR may influence the development of early AMD because the increase in collagen I leads to the growth of deposits and to the thickening of the BrM, which together represent early signs of AMD [54]. The increase of PPR expression and collagen I, with unchanged levels of collagen IV and laminin, were also observed in the retinas of patients with hypertension who were untreated during their lifetimes [54]. On the other hand, in the retinas of patients with AMD who were treated for hypertension, an increase in PRR expression and in the amount of collagen I, but a decrease in collagen IV and an unchanged level of laminin, have been demonstrated [54]. Changes in the amounts of collagen I and IV and an unchanged level of laminin are probably induced by drugs that block angiotensin II receptors [54]. In the situation in which AMD occurs simultaneously with hypertension, the crucial pathological regulator of the ECM is angiotensin II. Tests on animals have shown that angiotensin II stimulates the RPE cells to a higher expression of mRNA for the production of the AT1 receptors of angiotensin, the activation of which leads to a greater release of the active form of MMP-2 by the RPE [41]. MMP-2 plays a key role in the early development of AMD as a result of the enhancement of the deposits stored under the RPE layer [41,54]. It has also been shown that in the pathway in which the AT2 receptors of angiotensin are activated, angiotensin II stimulates the RPE cells to collect collagen IV [41]. In the case of early AMD occurring together with hypertension, drug-induced blocking of angiotensin II receptors may be of therapeutic importance and at the same time protect against AMD degeneration [41] (Figure 8).
Figure 8.
The influence of arterial hypertension (HA) and activated renin-angiotensin system (RAS) on the development of early age-related macular degeneration. PRRs – pro-renin receptors, LAMs – laminins, AT 1,2 – angiotensin receptors 1,2. * Aggravation effect on AMD by favoring accumulation of coll I, but not by stimulating activity of MMP-2; ** when degrading activity of MMP-2 stays unchanged, the synthesis of coll I increases; *** ↑ activation of PRRs do not stimulate the pro-MMP-2/TIMP-2/MMP-14 complex and therefore coll IV and LAMs, the main causes of SD, remain stable.
Conclusions
In both early and advanced AMD, the ECM is the area of dynamic changes connected with the activity of its specific regulators – metalloproteinases and their tissue inhibitors. However, it is also under the positive and negative influence of other regulatory systems. The importance of the ECM can be seen by the fact that transplants of RPE cells exclusively, without the BrM, do not bring about the required results because, apart from the immunological problems connected with transplant rejection, there also occurs the problem of the ground matrix; the cells are unable to adhere to the age-changed and insufficient BrM and/or are iatrogenically damaged during the surgical removal of the CNV [110]. The ECM has a crucial influence on both the survival and functioning of the transplanted RPE cells [99,110]. We are not able to treat the atrophic form of AMD, and the therapies applied in neovascular AMD (ie, treating neovascularization by means of photodynamic therapy, or blocking its stimulants by drugs injected into the vitreous camera of the eye (pan anti-VEGF Lucentis® or off ophthalmological label pan anti-VEGF Avastin®) may only slow down AMD advancement [201–204]. Better insight into the pathological mechanisms acting in the area of the ECM may lead to the development of new and improved strategies for AMD treatment.
Abbreviations
- AMD
age-related macular degeneration
- ARMS2
age-related maculopathy susceptibility 2
- BLamD
basal laminar deposits
- BLinD
basal linear deposits
- BM
basic membrane
- BrM
Bruch’s membrane
- CEP
carboxyethylpyrrole
- CNV
choroidal neovascularization
- DHA
docosahexaenoic acid
- ECM
extracellular matrix
- GA
geographic atrophy
- HTRA1
high temperature requirement factor A-1
- MAC
membrane attack complex
- MMP
matrix metalloproteinase
- MT-MMP
membrane-type metalloproteinase
- PRR
pro-renin receptor
- RPE
retinal pigment epithelium
- SD
soft drusen
- TIMP
tissue inhibitor of metalloproteinases
- uPAR/uPA
urokinase plasminogen activator receptor/urokinase plasminogen activator
- VEGF
vascular endothelial growth factor
Footnotes
Source of support: Self-financing unit
References
- 1.Chang C, Werb Z. The many faces of metalloproteinases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Nagase H, Woessner JF. Matrix Metalloproteinases. J Biol Chem. 1999;274(31):21491–94. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 4.Steen B, Sejersen S, Berglin L, et al. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–200. [PubMed] [Google Scholar]
- 5.Visse R, Nagase H. Matrix Metaloproteinases and tissue inhibitors of metaloproteinases. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 6.Wright JK, Cawston TE, Hazleman BL. Transforming growth factor beta stimulates the production of the tissue inhibitor of metalloproteinases (TIMP) by human synovial and skin fibroblasts. Biochim Biophys Acta. 1991;1094:207–10. doi: 10.1016/0167-4889(91)90010-u. [DOI] [PubMed] [Google Scholar]
- 7.Liotta L, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–36. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen M, Arkell J, Jackson CJ. Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960–70. doi: 10.1016/s1357-2725(01)00007-3. [DOI] [PubMed] [Google Scholar]
- 9.Kortvely E, Hauck SM, Duetach G, et al. ARMS2 is a constituent of the extracellular matrix providing a link between familial and sporadic age-related macular degenerations. Invest Ophtalmol Vis Sci. 2010;51(1):79–89. doi: 10.1167/iovs.09-3850. [DOI] [PubMed] [Google Scholar]
- 10.Jones SE, Jomary C, Neal MJ. Expression of TIMP3 mRNA is elevated in retinas affected by simplex retinitis pigmentosa. Feder Euro Bioch Soc Letters. 1994;352:171–74. doi: 10.1016/0014-5793(94)00951-1. [DOI] [PubMed] [Google Scholar]
- 11.Yeow KM, Kishnani NS, Hutton M, et al. Sorsby’s fundus dystrophy tissue inhibitor of metalloproteinases- 3 (TIMP-3) mutants have unimpaired matrix metalloproteinaseinhibitory activities, but affect cell adhesion to the extracellular matrix. Matrix Biol. 2002;21:75–88. doi: 10.1016/s0945-053x(01)00180-9. [DOI] [PubMed] [Google Scholar]
- 12.De Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 13.Marin-Castaño ME, Striker GE, Alcazar O, et al. Repetitive nonlethal oxidant injury to retinal pigment epithelium decreased extracellular matrix turnover in vitro and induced sub-RPE deposits in vivo. Invest Ophthalmol Vis Sci. 2006;47:4098–112. doi: 10.1167/iovs.05-1230. [DOI] [PubMed] [Google Scholar]
- 14.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 15.Elner SG, Elner VM, Kindzelskii AL, et al. Human RPE cell lysis of extracellular matrix: functional urokinase plasminogen activator receptor (uPAR), collagenase and elastase. Exp Eye Res. 2003;76:585–95. doi: 10.1016/s0014-4835(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 16.Sebag J, Hageman GS. Interfaces. Eur J Ophthalmol. 2000;10(1):1–3. doi: 10.1177/112067210001000101. [DOI] [PubMed] [Google Scholar]
- 17.Sivaprasad S, Bailey TA, Chong VNH. Bruch’s membrane and the vascular intima: is there a common basis for age-related changes and disease? Clin Exp Ophthalmol. 2005;33:518–23. doi: 10.1111/j.1442-9071.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 18.Hammer TH, Schlotzer-Schrehardt U, Naumann G. Unilateral or asymetric pseudoexfoliation syndrome? An ultrastructural study. Arch Ophthalmol. 2001;119(7):1023–31. doi: 10.1001/archopht.119.7.1023. [DOI] [PubMed] [Google Scholar]
- 19.Ritch R, Schlotzer-Schrehardt U. Exfoliation Syndrom. Survey of Ophthalmol. 2001;45(4):265–316. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 20.Schlotzer-Schrehardt U, von der Mark K, Sakai LY, Naumann GO. Increased extracallular deposition of fibrillin-containing fibrils in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 1997;38:970–84. [PubMed] [Google Scholar]
- 21.Ishibashi T, Araki H, Sugai S, et al. Detection of proteoglycans in human posterior capsule opacification. Ophthalmic Res. 1995;27:208–13. doi: 10.1159/000267707. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi T, Hatae T, Inomata H. Collagen types in human posterior capsule opacification. J Cataract Refract Surg. 1994;20:643–46. doi: 10.1016/s0886-3350(13)80655-4. [DOI] [PubMed] [Google Scholar]
- 23.Nishi O, Nishi K, Fujiwara T, et al. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. Br J Ophthalmol. 1996;80:63–68. doi: 10.1136/bjo.80.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saika S, Kawashima Y, Miyamoto T, et al. Immunolocalization of prolyl 4 hydroxylase subunits, a-smooth muscle actin, and extracellular matrix components in human lens capsules with lens implants. Exp Eye Res. 1998a;66:283–94. doi: 10.1006/exer.1997.0434. [DOI] [PubMed] [Google Scholar]
- 25.Saika S, Kawashima Y, Miyamoto T, et al. Immunohistochemical identification of proteoglycan types in fibrotic human capsules with intraocular lens implants. Jpn J Ophthalmol. 1998b;42:368–72. doi: 10.1016/s0021-5155(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 26.Chronopoulos A, Trudeau K, Roy S, et al. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res. 2011;36(8):747–53. doi: 10.3109/02713683.2011.585735. [DOI] [PubMed] [Google Scholar]
- 27.Comer GM, Ciulla TA. Pharmacotherapy for diabetic retinopathy. Curr Opin Ophthalmol. 2004;15(6):508–18. doi: 10.1097/01.icu.0000143685.60479.3b. [DOI] [PubMed] [Google Scholar]
- 28.Hosoda Y, Okada M, Matsumura M, et al. Epiretinal membrane of proliferative diabetic retinopathy: an immunohistochemical study. Ophthalmic Res. 1993;25(5):289–94. doi: 10.1159/000267327. [DOI] [PubMed] [Google Scholar]
- 29.Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med. 2000;342:483–92. doi: 10.1056/NEJM200002173420707. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 31.Evans JR. Risk factors for age-related macular degeneration. Prog Retin Eye Res. 2001;20:227–53. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 32.McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol. 2001;119:1455–62. doi: 10.1001/archopht.119.10.1455. [DOI] [PubMed] [Google Scholar]
- 33.McConnell V, Silvestri G. Age-related macular degeneration. Ulster Med J. 2005;74:82–92. [PMC free article] [PubMed] [Google Scholar]
- 34.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 35.Elliot SJ, Catanuto P, Espinosa-Heidmann DG, et al. Estrogen receptor b protects against in vivo injury in RPE cells. Exp Eye Res. 2010;90:10–16. doi: 10.1016/j.exer.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alcazar O, Hawkridge AM, Collier TS, et al. Proteomics Characterization of cell membrane blebs in human retinal pigment epithelium cells. Mol Cell Proteomics. 2009;8(10):2201–11. doi: 10.1074/mcp.M900203-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman E. The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2000;130:658–63. doi: 10.1016/s0002-9394(00)00643-7. [DOI] [PubMed] [Google Scholar]
- 38.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol. 2000;118:351–58. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 39.Jonas JB, Hayreh SS, Martus P. Influence of arterial hypertension and diet-induced atherosclerosis on macular drusen. Graefes Arch Clin Exp Ophthalmol. 2003;241(2):125–34. doi: 10.1007/s00417-002-0615-3. [DOI] [PubMed] [Google Scholar]
- 40.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy. Ophthalmology. 2003;110:1273–80. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 41.Praddaude F, Cousins SW, Pêcher C, Marin-Castano ME. Angiotensin II-induced hypertension regulates AT1 receptor subtypes and extracellular matrix turnover in mouse retinal pigment epithelium. Exp Eye Res. 2009;89:109–18. doi: 10.1016/j.exer.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–43. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- 43.Deryugina EI, Bourdon MA, Reisfeld RA, Strongin A. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res. 1989;58:3743–50. [PubMed] [Google Scholar]
- 44.George SJ. Tissue inhibitors of metalloproteinases and metalloproteinases in atherosclerosis. Curr Opin Lipidol. 1998;9:413–23. doi: 10.1097/00041433-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Marin-Castaño ME, Csaky KG, Cousins SW. Nonlethal oxidant injury to human retinal pigment epithelium cells causes cell membrane blebbing but decreased MMP-2 activity. Invest Ophthalmol Vis Sci. 2005;46:3331–40. doi: 10.1167/iovs.04-1224. [DOI] [PubMed] [Google Scholar]
- 46.Pauleikhoff D, Barondes MJ, Minassian D, et al. Drusen as risk factors in age-related macular disease. Am J Ophthalmol. 1990;109:38–43. doi: 10.1016/s0002-9394(14)75576-x. [DOI] [PubMed] [Google Scholar]
- 47.Sheridan CM, Hiscott P, Grierson I. The role of glycoproteins and integrins in human RPE-induced collagen matrix contraction. Exp Eye Res. 2000;71:S119. [Google Scholar]
- 48.Vinding T. Occurrence of drusen, pigmentary changes and exudative changes in the macula with reference to age-related macular degeneration An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh) 1990;68:410–14. doi: 10.1111/j.1755-3768.1990.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 49.Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization. Basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52:3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Witmer AN, Vrensen GF, van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 51.Agtmael TV, Bruckner-Tuderman L. Basement membranes and human disease. Cell Tissue Res. 2010;339:167–88. doi: 10.1007/s00441-009-0866-y. [DOI] [PubMed] [Google Scholar]
- 52.Bai X, Dilworth DJ, Weng Yi-C, et al. Developmental distribution of collagen IV isoforms and relevance to ocular diseases. Matrix Biology. 2009;28:194–201. doi: 10.1016/j.matbio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schittny JC, Yurchenco PD. Basement membranes: molecular organization and function in development and disease. Curr Opin Cell Biol. 1989;1(5):983–88. doi: 10.1016/0955-0674(89)90069-0. [DOI] [PubMed] [Google Scholar]
- 54.Alcazar O, Cousins SW, Striker GE, Marin-Castano ME. (Pro)renin receptor is expressed in human retinal pigment epithelium and participates in extracellular matrix remodeling. Exp Eye Res. 2009;89:638–47. doi: 10.1016/j.exer.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Nagasse H. Activation mechanism of matrix metalloproteinases. Biol Chem. 1997;378:151–60. [PubMed] [Google Scholar]
- 56.Ellerbroek SM, Stack SM. Membrane associated matrix metalloproteinases in metastasis. Bioessays. 1999;21:940–49. doi: 10.1002/(SICI)1521-1878(199911)21:11<940::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Miyamura N, Ninomiya Y, Handa JT. Distribution of the collagen IVisoforms in human Bruch’s membrane. Br J Ophthalmol. 2003;87(2):212–15. doi: 10.1136/bjo.87.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das A, Frank RN, Zhang NL, Turczyn TJ. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch’s membrane. Arch Ophthalmol. 1990;108(3):421–29. doi: 10.1001/archopht.1990.01070050119045. [DOI] [PubMed] [Google Scholar]
- 59.Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48(10):1291–306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 60.Gould DB, Marchant JK, Savinova OV, et al. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum Mol Genet. 2007;16(7):798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- 61.Favor J, Gloeckner CJ, Janik D, et al. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175:725–36. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parkin JD, San Antonio JD, Pedchenko V, et al. Mapping structural landmarks, ligand binding sites, and missense mutations to the Collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutation. 2011;32(2):127–43. doi: 10.1002/humu.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 65.Ekblom M, Falk M, Salmivirta K, et al. Laminin isoforms and epithelial development. Ann NY Acad Sci. 1998;23(857):194–211. doi: 10.1111/j.1749-6632.1998.tb10117.x. [DOI] [PubMed] [Google Scholar]
- 66.Marshall GE, Konstas AG, Reid GG, et al. Type IV collagen and laminin in Bruch’s membrane and basal linear deposit in the human macula. Br J Ophthalmol. 1992;76:607–14. doi: 10.1136/bjo.76.10.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–14. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volonghi I, Pezzini A, del Zotto E, et al. Role of COL4A1 in basement-membrane integrity and cerebral small-vessel disease. The COL4A1 Stroke Syndrome. Curr Med Chem. 2010;17(13):1317–24. doi: 10.2174/092986710790936293. [DOI] [PubMed] [Google Scholar]
- 69.Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–56. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- 70.Smyth N, Vatansever HS, Murray P, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–60. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKee KK, Harrison D, Capizzi S, Yurchenco PD. Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem. 2007;282:21437–47. doi: 10.1074/jbc.M702963200. [DOI] [PubMed] [Google Scholar]
- 72.Bader BL, Smyth N, Nedbal S, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–56. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura N, Toyoshima T, Kojima T, Shimane M. Entactin-2: a new member of basement membrane proteins with high homology to entactin/ nidogen. Exp Cell Res. 1998;241:36–45. doi: 10.1006/excr.1998.4016. [DOI] [PubMed] [Google Scholar]
- 74.Kohfeldt E, Sasaki T, Gohring W, Timpl R. Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol. 1998;282:99–109. doi: 10.1006/jmbi.1998.2004. [DOI] [PubMed] [Google Scholar]
- 75.Chakravarti S, Tam MF, Chung AE. The basement membrane glycoprotein entactin promotes cell attachment and binds calcium ions. J Biol Chem. 1990;265:10597–603. [PubMed] [Google Scholar]
- 76.Gumbiner B. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 77.Gohring W, Sasaki T, Heldin CH, Timpl R. Mapping of thebinding of platelet derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur J Biochem. 1998;255:60–66. doi: 10.1046/j.1432-1327.1998.2550060.x. [DOI] [PubMed] [Google Scholar]
- 78.Hewitt AT, Nakazawa K, Newsome DA. Analysis of newly synthesized Bruch’s membrane proteoglycans. Invest Ophthalmol Vis Sci. 1989;30(3):478–86. [PubMed] [Google Scholar]
- 79.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 80.Miettinen A, Stow JL, Mentone S, Farquhar MG. Antibodies to basement membrane heparan sulfate proteoglycans bind to thelaminae rarae of the glomerular basement membrane (GBM) and induce subepithelial GBM thickening. J Exp Med. 1986;163:1064–84. doi: 10.1084/jem.163.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guymer R, Luthert P, Bird A. Changes in Bruch’s membrane and related structures with age. Prog Retin Eye Res. 1999;18:59–90. doi: 10.1016/s1350-9462(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 82.Gautieri A, Uzel S, Vesentini S, et al. Molecular and mesoscale mechanisms of osteogenesis imperfecta disease in collagen fibrils. Biophysical J. 2009;97:857–65. doi: 10.1016/j.bpj.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28:209–21. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sweeney SM, Orgel JP, Fertala A, et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J Biol Chem. 2008;283:21187–97. doi: 10.1074/jbc.M709319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imai H, Honda S, Kondo N, et al. The upregulation of angiogenic gene expression in cultured retinal pigment epithelial cells grown on type I collagen. Curr Eye Res. 2007;32:903–10. doi: 10.1080/02713680701604749. [DOI] [PubMed] [Google Scholar]
- 86.Roberts JM, Forrester JV. Factors affecting the migration and growth of endothelial cells from icrovessels of bovine retina. Exp Eye Res. 1990;50:165–72. doi: 10.1016/0014-4835(90)90227-l. [DOI] [PubMed] [Google Scholar]
- 87.Chong NHV, Keonin J, Luthert PJ, et al. Decreased Thickness and Integrity of the Macular Elastic Layer of Bruch’s Membrane Correspond to the Distribution of Lesions Associated with Age-Related Macular Degeneration. Am J Pathol. 2005;166(1):240–51. doi: 10.1016/S0002-9440(10)62248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kondo N, Honda S, Ishibashi K, et al. Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:1101–5. doi: 10.1167/iovs.07-1145. [DOI] [PubMed] [Google Scholar]
- 89.Nackman G, Karkowski F, Halpern V, et al. Elastin degradation products induce adventitial angiogenesis in the Anidjar/Dobrin rat aneurysm model. Surgery. 1997;122:39–44. doi: 10.1016/s0039-6060(97)90262-2. [DOI] [PubMed] [Google Scholar]
- 90.Fisher SA, Rivera A, Fritsche LG, et al. Case-control genetic association study of fibulin-6 (FBLN6 or HMCN1) variants in age-related macular degeneration (AMD) Hum Mutat. 2007;28:406–13. doi: 10.1002/humu.20464. [DOI] [PubMed] [Google Scholar]
- 91.Schultz DW, Klein ML, Humpert AJ, et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 2003;12:3315–23. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Marmorstein LY. Focus on molecules: Fibulin-3 (EFEMP1) Exp Eye Res. 2010;90:374–75. doi: 10.1016/j.exer.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu L, Garland D, Yang Z, et al. The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Hum Mol Genet. 2007;16:3411–22. doi: 10.1093/hmg/ddm198. [DOI] [PubMed] [Google Scholar]
- 94.Marmorstein LY, Munier FL, Arsenijevic Y, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:13067–72. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stone EM, Braun TA, Russell SR, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–53. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 96.Stone EM, Lotery AJ, Munier FL, et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- 97.Anderson DH, Guerin CJ, Matsumoto B, Pfeffer BA. Identification and localization of a beta1 receptor from the integrin family in mammalian retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1990;31:81–93. [PubMed] [Google Scholar]
- 98.Ghosh S, Brown R, Jones JC, et al. Urinary-type plasminogen activator (uPA) expression and uPA receptor localization are regulated by alpha 3 beta 1 integrin in oral keratinocytes. J Biol Chem. 2000;275:23869–76. doi: 10.1074/jbc.M000935200. [DOI] [PubMed] [Google Scholar]
- 99.Sheridan CM, Williams R, Grierson I. Basement membranes and artificial substratesin cell transplantation. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:68–75. doi: 10.1007/s00417-003-0800-z. [DOI] [PubMed] [Google Scholar]
- 100.Timpl R, Brown JC. Aassembly of basement membranes. Bio Essays. 1996;18:123–32. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 101.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36:1405–10. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 102.Wu C. Roles of integrins in fibronectin matrix assembly. Histol Histopathol. 1997;12:233–40. [PubMed] [Google Scholar]
- 103.Schwarzbauer J. Basement membrane: Putting up the barriers. Current Biology. 1999;9:R242–44. doi: 10.1016/s0960-9822(99)80153-5. [DOI] [PubMed] [Google Scholar]
- 104.Tummalapalli C, Tyagi S. Responses of vascular smooth muscle cell to extracellular matrix degradation. J Cell Biochem. 1999;75:515–27. [PubMed] [Google Scholar]
- 105.Kwak N, Okamoto N, Wood J, Campochiaro P. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–64. [PubMed] [Google Scholar]
- 106.Schwesinger C, Yee C, Rohan R. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol. 2001;158:1161–72. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spilsbury K, Garrett K, Shen W, et al. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–44. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banda MJ, Clark EJ, Werb Z. Selective proteolysis of immunoglobulins by mouse macrophage elastase. J Exp Med. 1983;157:1184–96. doi: 10.1084/jem.157.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Banda JJ, Clark EJ, Werb Z. Limited proteolysis by macrophage elastase inactivates human a-1-proteinase inhibitor. J Exp Med. 1980;152:1563–70. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Espinosa-Heidmann DG, Suner IJ, Catanuto P, et al. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47(2):729–38. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- 111.Lee SH, Goswami S, Grudo A, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–69. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 112.Chau KY, Sivaprasad S, Patel N, et al. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye. 2007;21:1511–15. doi: 10.1038/sj.eye.6702722. [DOI] [PubMed] [Google Scholar]
- 113.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation role in cell invasion across collagen barrier. J Biol Chem. 2001;276:16248–56. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- 114.Leu ST, Batni S, Radeke MJ, et al. Drusen are cold spots for proteolysis: expression of matrix metalloproteinases and their tissue inhibitor proteins in age-related macular degeneration. Exp Eye Res. 2002;74:141–54. doi: 10.1006/exer.2001.1112. [DOI] [PubMed] [Google Scholar]
- 115.Alcazar O. MMP-14 and TIMP-2 overexpression protects against hydroquinone-induced oxidant injury in RPE: implications for extracellular matrix turnover. Invest Ophthalmol Vis Sci. 2007;48:5662–70. doi: 10.1167/iovs.07-0392. [DOI] [PubMed] [Google Scholar]
- 116.Alexander JP, Bradley JMB, Gabourel JD, Acott TS. Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990;31:2520–28. [PubMed] [Google Scholar]
- 117.Dhaliwal BS, Steinbrecher UP. Scavenger receptors and oxidized low density lipoproteins. Clin Chim Acta. 1999;286:191–205. doi: 10.1016/s0009-8981(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 118.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–87. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stohr H, Roomp K, Felbor U, Weber BHF. Genomic organization of the human Tissue Inhibitor of Metalloproteinases-3 (TIMP3) Genome Res. 1995;5:483–87. doi: 10.1101/gr.5.5.483. [DOI] [PubMed] [Google Scholar]
- 120.Kamei M, Hollyfield JG. TIMP-3 in Bruch’s membrane: changes during aging and in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:2367–75. [PubMed] [Google Scholar]
- 121.Moses MA, Langer R. A metalloproteinase inhibitor as an inhibitor of neovascularization. J Cell Biochem. 1991;47:230–35. doi: 10.1002/jcb.240470308. [DOI] [PubMed] [Google Scholar]
- 122.Newsome DA, Hewitt AT, Huh W, et al. Detection of specific extracellular matrix molecules in drusen, Bruch’s membrane, and ciliary body. Am J Ophthalmol. 1987;104:373–81. doi: 10.1016/0002-9394(87)90227-3. [DOI] [PubMed] [Google Scholar]
- 123.Karwatowski WS, Jeffries TE, Duance VC, et al. Preparation of Bruch’s membrane and analysis of the age-related changes in the structural collagens. Br J Ophthalmol. 1995;79:944–52. doi: 10.1136/bjo.79.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamamoto T, Yamashita H. Scanning electron microscopic observation of Bruch’s membrane with the osmium tetroxide treatment. Br J Ophthalmol. 1989;73:162–67. doi: 10.1136/bjo.73.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with agerelated macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44(Suppl 1):S10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 126.Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27. [PubMed] [Google Scholar]
- 127.van der Schaft TL, Mooy CM, de Bruijn WC, et al. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232:40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- 128.van der Schaft TL, Mooy CM, de Bruijn WC, et al. Histologic features of the early stages of age-related macular degeneration: A statistical analysis. Ophthalmology. 1992;99:278–86. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- 129.Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch’s membrane: a histochemical and morphologic study. Ophthalmology. 1990b;97:171–78. [PubMed] [Google Scholar]
- 130.Sheraidah G, Steinmetz R, Maguire J, et al. Correlation between lipids extracted from Bruch’s membrane and age. Ophthalmology. 1993;100:47–51. doi: 10.1016/s0161-6420(13)31712-6. [DOI] [PubMed] [Google Scholar]
- 131.Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D. Characterization of peroxidized lipids in Bruch’s membrane. Retina. 1999;19:141–47. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 132.Booji JC, Baas AC, Beisekeeva J, et al. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 133.Chen SJ, Cheng CY, Lee AF, et al. Pulsatile ocular blood flow in asymmetric exudative age-related macular degeneration. Br J Ophthalmol. 2001;85:1411–15. doi: 10.1136/bjo.85.12.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McLeod DS, Taomoto M, Otsuji T, et al. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986–93. [PubMed] [Google Scholar]
- 135.McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–811. [PubMed] [Google Scholar]
- 136.Nasir M, Sugino I, Zarbin MA. Decreased choriocapillaris perfusion following surgical excision of choroidal neovascular membranes in age-related macular degeneration. Br J Ophthalmol. 1997;81:481–89. doi: 10.1136/bjo.81.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005;80:761–75. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 138.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–39. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 139.Sarks SH, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthal Vis Sci. 2007;48:968–77. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- 140.Green WR, Enger C. Age-related macular degeneration histopathologic studies. Ophthalmology. 1993;100:1519–35. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 141.Hillenkamp J, Hussain AA, Jackson TL, et al. The influence of path length and matrix components on ageing characteristics of transport between the choroid and the outer retina. Invest Ophthalmol Vis Sci. 2004;45:1493–98. doi: 10.1167/iovs.03-0765. [DOI] [PubMed] [Google Scholar]
- 142.Hussain AA, Rowe L, Marshall J. Age-related alterations in the diffusional transport of amino acids across the human Bruch’s – choroid complex. J Opt Soc Am A Opt Image Sci Vis. 2002;19(1):166–72. doi: 10.1364/josaa.19.000166. [DOI] [PubMed] [Google Scholar]
- 143.Moore DJ, Clover GM. The effect of age on the macromolecular permeability of human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:2970–75. [PubMed] [Google Scholar]
- 144.Starita C, Hussain AA, Pagliarini S, Marshall J. Hydrodynamics of ageing Bruch’s membrane: implications for macular disease. Exp Eye Res. 1996;62:565–72. doi: 10.1006/exer.1996.0066. [DOI] [PubMed] [Google Scholar]
- 145.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 146.Ng KP, Gugiu B, Renganathan K, et al. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7(7):1397–405. doi: 10.1074/mcp.M700525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Warburton S, Southwick K, Hardman RM, et al. Examining the proteins of functional retinal lipofuscin using proteomic analysis as a guide for understanding its origin. Mol Vis. 2005;15(11):1122–34. [PubMed] [Google Scholar]
- 148.Reale E, Groos S, Eckardt U, et al. New components of ‘basal laminar deposits’ in age-related macular degeneration. Cells Tissues Organs. 2009;190:170–81. doi: 10.1159/000187632. [DOI] [PubMed] [Google Scholar]
- 149.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome anlysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99(23):14682–87. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83(3):358–68. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Umeda S, Suzuki MT, Okamoto H, et al. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different of macular degeneration in cynomolgus monkey (Macaca fascicularis) FASEB J. 2005;19(12):1683–85. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- 152.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No 18. Arch Ophthalmol. 2005;123:1570–74. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang JJ, Foran S, Smith W, Mitchell P. Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation: The Blue Mountains Eye Study Cohort. Arch Ophthalmol. 2003;121:658–63. doi: 10.1001/archopht.121.5.658. [DOI] [PubMed] [Google Scholar]
- 154.Bressler NM, Munoz B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities Waterman study. Arch Ophthalmol. 1995;113:301–8. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- 155.Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108:1442–47. doi: 10.1001/archopht.1990.01070120090035. [DOI] [PubMed] [Google Scholar]
- 156.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 157.Sarraf D, Gin T, Yu F, et al. Long-term drusen study. Retina. 1999;19:513–19. doi: 10.1097/00006982-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 158.Sarks SH, Sarks JP. Age-related maculopathy: nonneovascular age-related macular degeneration and the evolution of geographic atrophy. In: Ryan S, editor. Medical Retina. 3rd ed. St Louis: Mosby; 2001. pp. 1064–99. [Google Scholar]
- 159.Hageman GS, Mullins RF, Clark WG, et al. Drusen share molecular constituents common to atherosclerotic, elastotic and amyloid deposits. Invest Ophthalmol Vis Sci. 1995;36:432. [Google Scholar]
- 160.Mullins RF, Russel SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis and dense deposit disease. FASEB J. 2000;14(7):835–46. [PubMed] [Google Scholar]
- 161.Mullins RF, Johnson LV, Anderson DH, Hageman GS. Characterization of drusen-associated glycoconjugates. Ophthalmology. 1997;104(2):288–94. doi: 10.1016/s0161-6420(97)30322-4. [DOI] [PubMed] [Google Scholar]
- 162.Pauleikhoff D, Sheraidah G, Marshall J, et al. Biochemical and histochemical analysis of age related lipid deposits in Bruch’s membrane. Ophthalmology. 1994;91:730–34. [PubMed] [Google Scholar]
- 163.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 164.Augustin AJ, Kirchhof J. Inflammation and pathogenesis of age-related macular degeneration. Expert Opin Ther Targets. 2009;13(6):641–51. doi: 10.1517/14728220902942322. [DOI] [PubMed] [Google Scholar]
- 165.Donoso LA, Kim D, Frost A, et al. The role of inflammation in the pathogenesis age-related macular degeneration. Surv Ophthalmol. 2006;51(2):137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hageman GS, Luther PJ, Chong VNH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 167.Johnson LV, Ozaki S, Staples MK, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–49. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 168.Kijlstra A, La Heij E, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13(1):3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 169.Xu H, Chen M, Forrester JV. Para-inflammation in aging retina. Prog Retin Eye Res. 2009;28(5):348–68. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 170.Despriet DD, van Duijn CM, Oostra BA, et al. Complement component C3 and risk of age-related macular degeneration. Ophthalmology. 2009;116(3):474–80. doi: 10.1016/j.ophtha.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 171.Grossniklaus HE, Jing JX, Wallace TM, et al. Macrophages and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;21(8):119–26. [PubMed] [Google Scholar]
- 172.Zipfel PF, Heinen S, Józsi M, Skerka C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol. 2006;43:97–106. doi: 10.1016/j.molimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 173.Gasgue P, Dean YD, McGreal EP, et al. Complement components of innate immune system in health and disease in the CNS. Immunopharmacology. 2000;49:171–86. doi: 10.1016/s0162-3109(00)80302-1. [DOI] [PubMed] [Google Scholar]
- 174.Rodriguez de Cordoba SR, Esparza-Gordillo J, Goicoechea de Jorge E, et al. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–67. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 175.Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophthalmol Vis Sci. 2009;50(3):1392–99. doi: 10.1167/iovs.08-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2006;103(44):16182–87. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zipfel PF. Complement and immune defense: from innate immunity to human diseases. Immunol Lett. 2009;22(126):1–7. doi: 10.1016/j.imlet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 178.Edwards A, Ritter R, Abel K, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–24. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 179.Hageman GS, Anderson D, Johnson L, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposesindividuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haines J, Hauser M, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 181.Klein R, Zeiss C, Chew E, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–89. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29(8):380–87. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 183.Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278(43):42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 184.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 185.San Giovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acid in health and disease of the retina. Prog Retin Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 186.Stilwell W, Wassal SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126(1):1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 187.Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxydative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–98. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Renganathan K, Ebrahem Q, Vasanji A, et al. Carboxyethylpropyrrole adducts, age-related macular degeneration and neovascularization. Adv Exp Med Biol. 2008;613:261–67. doi: 10.1007/978-0-387-74904-4_30. [DOI] [PubMed] [Google Scholar]
- 189.Ebrachem Q, Renganathan K, Sears J, et al. Carboxyethylpropyrrole oxidative protein modifications stimulate neovascularization: Implifications for age-related macular degeneration. Proc Natl Acad Sci USA. 2006;103(36):13480–84. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bhutto IA, McLeod DS, Hasegawa T, et al. Pigment epithelium derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroids and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.De Luca A, De Falco M, Severino A, et al. Distribution of the serine protease HtrA1 in normal human tissues. J Histochem Cytochem. 2003;51:1279–84. doi: 10.1177/002215540305101004. [DOI] [PubMed] [Google Scholar]
- 192.De Wan A, Liu M, Harman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–92. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 193.Yang Z, Camp NC, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–93. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 194.Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1): implications for macular degenerations. J Biol Chem. 2004;279:30469–73. doi: 10.1074/jbc.M403026200. [DOI] [PubMed] [Google Scholar]
- 195.Klein ML, Schultz DW, Edwards A, et al. Age-related macular degeneration: clinical features in a large family and linkage to chromosome 1q. Arch Ophthalmol. 1998;116:1082–88. doi: 10.1001/archopht.116.8.1082. [DOI] [PubMed] [Google Scholar]
- 196.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–36. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 197.Wang G, Spencer KM, Court BL, et al. Age-related macular degeneration associated ARMS2 is not a mitochondrial but cytosolic protein. Invest Ophthalmol Vis Sci. 2009;50(7):3084–90. doi: 10.1167/iovs.08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fritsche LG, Loenhardt T, Janssen A, et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40(7):892–96. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- 199.Iyengar SK, Song D, Klein BE, et al. Dissection of genomewide-scan data in extended families reveals a major locus and oligogenic susceptibility for age-related macular degeneration. Am J Hum Genet. 2004;74:20–39. doi: 10.1086/380912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Andreasen PA, Kjoller L, Christensen L, Duffy MF. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 201.Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology. 2009;116(10 Suppl):15–23. doi: 10.1016/j.ophtha.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 202.Cruess AF, Zlateva G, Pleil AM, Wirostko B. Photodynamic therapy with verteporfin in age-related macular degeneration: asystematic review of efficacy, safety, treatment modifications and pharmacoeconomic properties. Acta Ophthalmol. 2009;87(2):118–32. doi: 10.1111/j.1755-3768.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 203.Ruiz-Moreno JM, Montero JA. Combined treatment of exudative age-related macular degeneration with photodynamic therapy and intravitreal triamcinolone. Clin Ophthalmol. 2008;2(1):71–75. doi: 10.2147/opth.s1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shah GK, Sang DN, Hughes MS. Verteporfin combination regimens in the treatment of neovascular age-related macular degeneration. Retina. 2009;29(2):133–48. doi: 10.1097/IAE.0b013e3181960a28. [DOI] [PubMed] [Google Scholar]