Abstract
Objective
Many patients with rheumatoid arthritis (RA) benefit from tumor necrosis factor-α blocking treatment (anti-TNF), but about one third do not respond. The objective of this study was to replicate and extend previously found associations between anti-TNF treatment response and genetic variation in the TNF-, NF-κB- and pattern recognition receptor signalling pathways.
Methods
Forty-one single nucleotide polymorphisms (SNPs), including 34 functional, in 28 genes involved in inflammatory pathways were assessed in 538 anti-TNF naive Danish RA patients with clinical data. Multivariable logistic regression analyses were performed to test associations between genotypes and treatment response at 3–6 months using the European League Against Rheumatism (EULAR) response criterion. American College of Rheumatology treatment response (ACR50) and relative change in 28-joint disease activity score (relDAS28) were used as secondary outcomes. Subgroup analyses were stratified according to smoking status, type of anti-TNF drug and IgM-Rheumatoid Factor (IgM-RF) status. False discovery rate (FDR) controlling was used to adjust for multiple testing.
Results
Statistically significant associations with EULAR response were found for two SNPs in NLRP3(rs4612666) (OR (odds ratio) for good/moderate response = 0.64 (95% confidence interval: 0.44–0.95), p = 0.025, q = 0.95) and INFG(rs2430561) (OR = 0.40 (0.21–0.76), p = 0.005, q = 0.18) and among IgM-RF positive patients for TNFRS1A(rs4149570) (0.59 (0.36–0.98), p = 0.040, q = 0.76). Current smokers who carried the NLRP3(rs4612666) variant allele were less likely to benefit from anti-TNF treatment (OR = 0.24 (0.10–0.56), p = 0.001, q = 0.04).
Conclusions
In a population of Danish RA patients, we confirm the NLRP3 gene as associated with EULAR anti-TNF response as previously reported. The NLRP3 variant (T) allele is associated with lower treatment response, in particular among current smokers. Furthermore, we find that a functional polymorphism in the interferon-γ gene is associated with anti-TNF response. All findings should be tested by replication in independent validation cohorts and augmented by assessing cytokine levels and activities of the relevant gene products.
Introduction
Tumour necrosis factor α inhibitors (anti-TNF) have improved the treatment of rheumatoid arthritis (RA); however, the effect varies and approximately one third of patients do not respond [1]. Furthermore, the anti-TNF drugs are expensive and have potentially severe side effects including adverse immunological reactions and increased risk of serious infections [2]. Thus, biomarkers predictive of anti-TNF treatment outcome are likely to improve treatment of patients with RA.
Accordingly, several studies have evaluated biochemical markers, which alone or in combination with clinical parameters are associated with treatment response [3], [4]. Until now, a locus in the PTPRC gene that was first identified in a genome-wide association study (GWAS) [5], [6] is the only confirmed genetic marker that can predict treatment response [7], [8]. Recently, polymorphisms in genes encoding the NLRP3-inflammasome (NLRP3, CARD8) [9], nuclear factor κ B (NF-κB) and Toll-like receptor (TLR) signalling pathways (TLR2, TLR4, MyD88, CHUK) [10] have been associated with European League Against Rheumatism (EULAR) response to anti-TNF treatment.
Anti-TNF treatment blocks TNF from binding to TNF receptor 1 (TNFR1), thereby preventing NF-κB signaling. It seems likely that genetic variants in genes encoding proteins in the inflammatory pathways involved in NF-κB activation are involved in the differential response to anti-TNF drugs.
NF-κB is a central regulator of inflammation and regulates the expression of more than 150 genes (http://www.bu.edu/nf-kb/gene-resources/target-genes/) including TNFA (TNF-α), TNFAIP3 (A20), TLR2, TLR9, CD14, NFKBIA (IκBα), NFKB1 (NF-κB1/p50-RelA), IL1B, IL1RN, IL6, IL10, IL17A and IFNG.
The NLRP3 gene (cryopyrin/NALP3) encodes one of several proteins forming the NLRP3-inflammasome, an intracellular innate immune sensor that also includes the cysteine protease, caspase-1. Upon inflammasome activation by cellular stress and damage [11], caspase-1 controls activation and cellular release of IL-1β and IL-18, both strong pro-inflammatory cytokines.
NF-κB activation [12] as well as increased TLRs [13] and NLRP3 [14] expression can be detected in synovial tissue from RA patients, but several other molecules activating the pathway (TNF-α, IL-1β) [15] or regulated by the pathway, e.g. IL-17 [15], are central in the pathogenesis of RA.
The objective of this study was to replicate and extend the reported associations between anti-TNF treatment response and genes in the TNF-, NF-κB- and pattern recognition receptor signalling pathways (TLRs and NLRP3). We assessed 41 mainly functional polymorphisms based on available knowledge to allow a biological interpretation of associations with treatment response to anti-TNF, thus potentially adding new knowledge on the underlying causes of treatment success and failure. We used the nationwide Danish database with prospectively collected clinical data of patients with RA (the DANBIO registry).
Methods
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Regional Ethics Committee of Central Denmark Region (M-20100153 and S-20120113) and the Danish Data Protection Agency (J. 2010-41-4719). The Regional Ethics Committee of Central Denmark Region gave exemption from informed consent requirements because samples were taken for other reasons and data were not identifiable.
Patients and samples
The DANBIO registry includes data on patients with inflammatory joint diseases, monitored prospectively as part of routine care during treatment with synthetic and biologic disease-modifying anti-rheumatic drugs (DMARDs) [16]. We linked the clinical data from DANBIO with blood samples available from the routine screening for tuberculosis performed prior to treatment with anti-TNF agents.
We included 538 anti-TNF naïve patients with RA in the study. All these patients initiated their first anti-TNF treatment, had clinical variables registered at baseline and follow-up in DANBIO, and had blood samples available.
Biological material (after whole blood analysis for Mycobacterium tuberculosis infection) was collected at Statens Serum Institut from September 2009 and at Aarhus University Hospital from January 2011 and until 1st of July 2012.
Candidate gene analyses
We chose to focus on genes involved in the NF-κB, TNF-α and pattern recognition receptor signalling pathways (see full list of polymorphisms in Table S1). Candidate polymorphisms in genes in these pathways were identified by searching for “polymorphism AND Gene name AND (reporter gene OR luciferase OR ELISA OR RT-PCR OR flow cytometry OR EMSA)” in PubMed in August 2011 [17], [18]. After an extensive literature search approximately 100 polymorphisms were found and 41 were subsequently chosen primarily based on evidence of biological effect and secondly based on association with autoimmune disease. We screened for linkage disequilibrium (LD) in SNAP, a web-based database based on the International HapMap Project [19]. The selected polymorphisms had expected minor allele frequencies (MAF) ranging from 5% to 48%.
The polymorphisms were genotyped by PCR-based KASP genotyping assay by KBioscience (KBioscience, Hoddesdon, United Kingdom - www.lgcgenomics.com) on extracted DNA (Maxwell 16 LEV Blood DNA Kit, Promega, Madison, Wisconsin, USA) as described by Bank et al. [20]. Genotyping of TNF (TNF-α) rs1799724 and rs1800630 failed due to their close proximity to each other, causing bias in genotype failure. All other chosen assays had a call rate exceeding 97%.
Outcome measures, data handling, and statistical methods
Patient data from the DANBIO database included pre-treatment and follow-up data on tender and swollen joint counts (28 joints), C-reactive protein (CRP, mg/l) and patient global score on a 100 mm VAS-scale, which were combined to calculate the disease activity score (DAS28) [21]. Furthermore, data on baseline DMARD treatment (yes/no), type of anti-TNF drug, IgM rheumatoid factor (IgM-RF) status (ICD-10: seropositive RA DM5.9/seronegative RA DM6.0+DM6.9), sex, age, smoking status (never/previous/current) and functional status assessed by the health assessment questionnaire (HAQ, range: 0–3, with 3 being completely disabled), were also drawn from DANBIO. Titers on IgM-RF and anti-citrullinated protein antibodies (ACPA) were not available.
The baseline (pre-treatment) visit was defined as a visit 0–30 days before start of anti-TNF treatment and follow-up as a visit within 60–180 days after treatment onset (at the contact closest to 120 days if more than one visit was registered).
Primary outcome was EULAR response criteria (good, moderate or none) at follow-up [21].
Univariate statistical analysis was used to assess the association between EULAR good/moderate response and genotypes under a dominant model (chi-square tests). Multivariable logistic regression analyses were then performed to investigate association between EULAR good or moderate response and genotype with the variant alleles grouped together (dominant model) and, furthermore, the individual genotypes with the homozygote wildtype as the reference genotype.
The final model included the following baseline covariates: sex, age, HAQ, CRP, baseline DMARD and IgM-RF status. Smoking was not included in the final analyses as data were missing for 15% of patients. However, in patients with available data, we assessed gene-smoking interaction for all polymorphisms by stratifying patients into current-, previous- and never smokers. We also stratified patients into anti-TNF subgroups to check drug specific associations. The remaining variables were also checked for interaction with the polymorphisms using Wald’s test and likelihood-ratio test.
Secondary outcomes were the relative change in DAS28 (relDAS28 = (baseline DAS28-follow-upDAS28)/baseline DAS28) and the American College of Rheumatology outcome measure, ACR50 response. Furthermore, we used ACR70 response in case of statistically significant association for ACR50 response and gene variants. Multivariate linear regression analysis was performed for relDAS28.
All statistical analyses were performed in Stata version 12 (StataCorp, Texas, USA). The Genetic Power Calculator was used for power analysis of discrete traits [22].
At the 5% significance level and MAF of 0.1, 0.25 and 0.4 we had >80% power to detect effect sizes of 1.5, 1.5 and 1.5, respectively, assuming a dominant genetic model. For a recessive model the corresponding effect sizes were 2.9, 1.8 and 1.6, respectively.
We performed correction for multiple testing using False Discovery Rate (FDR) classical one-stage method set at 0.05 (q-value) [23].
Results
Study population
Clinical and demographic baseline characteristics of the 538 patients are presented in Table 1. The patients were treated with the following anti-TNF drugs: infliximab (31.2%), etanercept (30.9%), adalimumab (24.9%), golimumab (9.1%) or certolizumab (3.9%). Overall, EULAR responses (good, moderate, and none) were achieved for 42.9%, 27.5% and 29.6%, respectively.
Table 1. Baseline clinical and demographic characteristics.
| All RA patients | Seropositive RA patients | Seronegative RA patients | |
| No. | 538 (−) | 407 (75.7%) | 131 (24.3%) |
| Female | 407 (75.7%) | 303 (74.4%) | 105 (80.2%) |
| Age/years (SD) | |||
| at treatment start | 55.0 (13.0) | 55.7 (12.8) | 53.0 (13.6) |
| Smoking status | |||
| Current | 142 (31.8%) | 110 (32.7%) | 32 (29.1%) |
| Previous | 151 (33.9%) | 120 (35.7%) | 31 (28.2%) |
| Never | 153 (34.3%) | 106 (31.6%) | 47 (42.7%) |
| Missing data | 92 (−) | 71 (−) | 21 (−) |
| DMARD | 453 (84.2%) | 343 (84.3%) | 110 (84.0%) |
| VAS Patient global score (0–100)/Mean (SD) | 62.6 (22.6) | 60.8 (22.9) | 68.5 (20.7) |
| VAS Physician global score (0–100)/Mean (SD) | 38.4 (20.7) | 38.0 (20.2) | 39.8 (22.3) |
| VAS pain score (0–100)/Mean (SD) | 58.0 (22.8) | 55.9 (23.2) | 64.4 (20.2) |
| TJC 0-28/Mean (SD) | 9.5 (7.3) | 9.0 (7.0) | 11.1 (8.1) |
| SJC 0-28/Mean (SD) | 5.4 (4.6) | 5.6 (4.5) | 4.8 (4.8) |
| HAQ score/Mean (SD) | 1.2 (0.7) | 1.2 (0.7) | 1.3 (0.7) |
| CRP/mg/mL (SD) | 19.7 (25.5) | 20.5 (27.0) | 17.2 (20.3) |
| DAS28/mean (SD) | 4.9 (1.2) | 4.8 (1.2) | 5.0 (1.1) |
| Anti-TNF drug | |||
| Infliximab (%) | 168 (31.2%) | 122 (30.0%) | 46 (35.1%) |
| Etanercept (%) | 166 (30.8%) | 124 (30.5%) | 42 (32.1%) |
| Adalimumab (%) | 134 (24.9%) | 105 (25.8%) | 29 (22.1%) |
| Golimumab (%) | 49 (9.1%) | 38 (9.3%) | 11 (8.4%) |
| Certolizumab (%) | 21 (3.9%) | 18 (4.4%) | 3 (2.3%) |
| EULAR response | |||
| Good (%) | 231 (42.9%) | 178 (43.7%) | 53 (40.5%) |
| Moderate (%) | 148 (27.5%) | 108 (26.5%) | 40 (30.5%) |
| None (%) | 159 (29.6%) | 121 (29.7%) | 38 (29.0%) |
| ACR50 response (%) | 170 (31.6%) | 131 (32.2%) | 39 (29.8%) |
| RelDAS28 response (SD) | 0.28 (0.32) | 0.28 (0.34) | 0.285 (0.27) |
SD: standard deviation, DMARD: disease modifying anti-rheumatic drugs, VAS: visual analogue scale, TJC: tender joint count, SJC: swollen joint count, HAQ: health assessment score, CRP: C-reactive protein, DAS28: disease activity score (28-joints), EULAR: European League Against Rheumatism, ACR50: American College of Rheumatology, 50% improvement, RelDAS28: relative change in DAS28.
Primary outcome EULAR response
Statistically significant associations with EULAR response were found for polymorphisms in NLRP3 (rs4612666), IFNG (rs2430561) and TNFRSF1A (rs4149570) (Table 2, Table S2).
Table 2. Genotypes of associated polymorphisms and adjusted odds ratios for associations between gene variants and EULAR anti-TNF treatment response.
| EULAR GOOD/MODERATE | EULAR GOOD | |||||||||||||||
| GENE(SNP) | GENOTYPE | FREQ. | NONE | MODERATE | GOOD | ADJ.OR | 95% CI | P-VALUE | ADJ. OR | 95% CI | P-VALUE | |||||
| All RA | ||||||||||||||||
| IFNG | TT | 137 | 34 | 37 | 66 | Ref. | Ref. | |||||||||
| rs2430561 | TA | 263 | 74 | 71 | 118 | 0.81 | (0.50–1.31) | 0.395 | 0.75 | (0.45–1.27) | 0.285 | |||||
| AA | 114 | 40 | 38 | 36 | 0.59 | (0.34–1.02) | 0.059 | 0.40 | (0.21–0.76) | 0.005** | ||||||
| TA or AA | 377 | 114 | 109 | 154 | 0.73 | (0.47–1.15) | 0.177 | 0.63 | (0.38–1.03) | 0.067 | ||||||
| NLRP3 | CC | 275 | 69 | 84 | 122 | Ref. | Ref. | |||||||||
| rs4612666 | CT | 210 | 73 | 54 | 83 | 0.62 | (0.42–0.92) | 0.018* | 0.62 | (0.40–0.97) | 0.037* | |||||
| TT | 31 | 9 | 8 | 14 | 0.85 | (0.37–1.96) | 0.707 | 0.89 | (0.36–2.24) | 0.808 | ||||||
| CT or TT | 241 | 82 | 62 | 97 | 0.64 | 0.44–0.95) | 0.025* | 0.65 | (0.43–1.00) | 0.050* | ||||||
| Seropositive RA | ||||||||||||||||
| IFNG | TT | 106 | 26 | 30 | 50 | Ref. | Ref. | |||||||||
| rs2430561 | TA | 205 | 56 | 55 | 94 | 0.85 | (0.49–1.46) | 0.547 | 0.83 | (0.43–1.51) | 0.544 | |||||
| AA | 78 | 30 | 21 | 27 | 0.51 | (0.26–0.96) | 0.038* | 0.42 | (0.20–0.87) | 0.020* | ||||||
| TA or AA | 283 | 86 | 76 | 121 | 0.73 | (0.43–1.22) | 0.229 | 0.69 | (0.39–1.21) | 0.196 | ||||||
| NLRP3 | CC | 212 | 51 | 62 | 99 | Ref. | Ref. | |||||||||
| rs4612666 | CT | 156 | 55 | 39 | 62 | 0.58 | (0.37–0.92) | 0.020* | 0.58 | (0.35–0.96) | 0.035* | |||||
| TT | 25 | 9 | 5 | 11 | 0.59 | (0.24–1.43) | 0.241 | 0.64 | (0.24–1.71) | 0.375 | ||||||
| CT or TT | 181 | 64 | 44 | 73 | 0.58 | (0.37–0.90) | 0.016* | 0.59 | (0.36–0.96) | 0.032* | ||||||
| TNFRSF1A | GG | 137 | 33 | 40 | 64 | Ref. | Ref. | |||||||||
| rs4149570 | GT | 196 | 68 | 47 | 81 | 0.59 | (0.36–0.98) | 0.040* | 0.63 | (0.37–1.09) | 0.102 | |||||
| TT | 56 | 15 | 18 | 23 | 0.89 | (0.43–1.85) | 0.760 | 0.82 | (0.37–1.82) | 0.619 | ||||||
| GT or TT | 252 | 83 | 65 | 104 | 0.65 | (0.40–1.04) | 0.074 | 0.67 | (0.39–1.13) | 0.130 | ||||||
Logistic regression, adjusted (Adj.) for sex, age, HAQ, CRP, DMARD at baseline, IgM RF status (seropositive/seronegative). CI: confidence interval, Freq.: Frequency, OR: odds ratio, EULAR: European League Against Rheumatism, P-value: *<0.05, **<0.01.
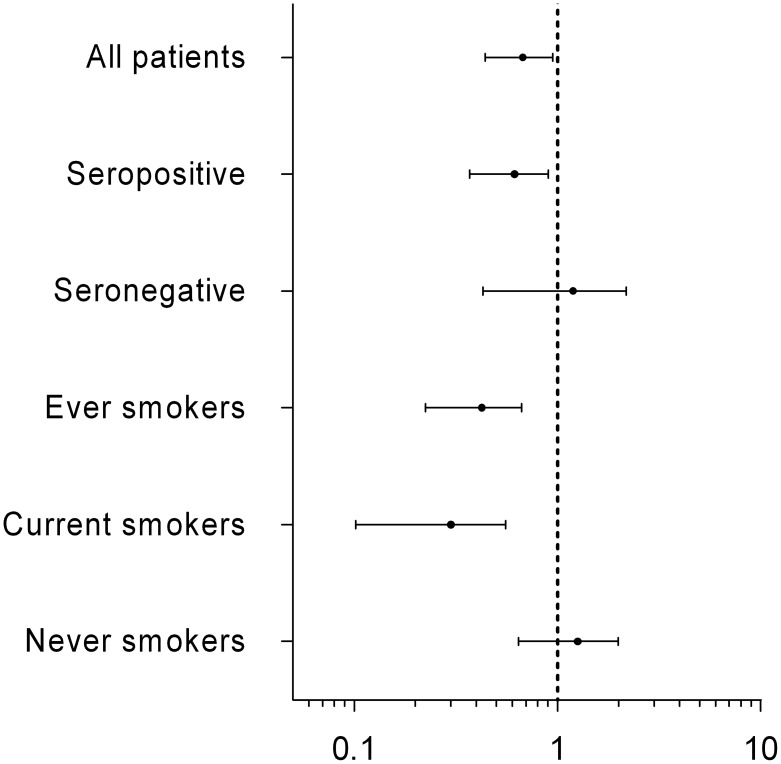
NLRP3 rs4612666 variant allele carriers had a significantly lower chance of achieving EULAR good/moderate response (OR = 0.64, p = 0.025, q = 0.95) and EULAR good response (OR = 0.65, p = 0.050, q = 0.97). Stratification according to both smoking status and to IgM RF status revealed even lower OR among seropositive RA patients and current smokers (Table 2, Figure 1).
Figure 1. Odds ratio (OR) for association between NLRP3 (rs4612666) variant allele and EULAR good/moderate response (log scale, 95% confidence interval).
Patients stratified on diagnose (seropositive-/seronegative RA) or smoking status. Smoking as independent predictor of EULAR good/moderate response: OR = 1.018, p = 0.941.
For IFNG rs2430561, the homozygous variant genotype was significantly associated with a lower chance of EULAR good response (OR = 0.40, p = 0.005, q = 0.18), and–among seropositive RA patients–with a lower chance of both EULAR good/moderate response (OR = 0.51, p = 0.038, q = 0.83) and EULAR good response (OR = 0.42, p = 0.020, q = 0.70).
TNFRSF1A rs4149570 was associated with EULAR good/moderate response (OR = 0.59, p = 0.040, q = 0.760) in the seropositive subgroup analysis, exclusively.
Secondary outcomes relDAS28 and ACR50
Of the three polymorphisms associated with EULAR response, NLRP3 rs4612666 (seropositive RA: p = 0.024, q = 0.65) and TNFRSF1A rs4149570 (seropositive RA: p = 0.034, q = 0.65) were also significantly associated with the relDAS28 (Table 3, Table S3a–b).
Table 3. Adjusted odds ratio (OR) for associations between gene variants and ACR50 and relDAS28 response to anti-TNF treatment.
| ACR50 response | relDAS28 | ||||||||||
| GENE (SNP) | GENOTYPE | Freq. | No | Yes | Adj. OR | 95% CI | P-VALUE | Adj. Coeff. | 95% CI | P-VALUE | |
| ALL RA | |||||||||||
| IL1B | GG | 255 | 181 | 74 | |||||||
| rs1143623 | GC | 223 | 149 | 74 | 1.22 | (0.82–1.80) | 0.330 | 0.02 | (−0.04–0.08) | 0.489 | |
| CC | 37 | 20 | 17 | 2.14 | (1.05–4.35) | 0.037* | 0.02 | (−0.09–0.13) | 0.693 | ||
| GC/CC | 260 | 169 | 91 | 1.32 | (0.91–1.93) | 0.145 | 0.02 | (−0.04–0.08) | 0.466 | ||
| TLR4 | GG | 253 | 183 | 70 | |||||||
| rs5030728 | GA | 215 | 134 | 81 | 1.58 | (1.06–2.35) | 0.023* | 0.01 | (−0.05–0.07) | 0.786 | |
| AA | 46 | 32 | 14 | 1.21 | (0.61–2.42) | 0.584 | 0.01 | (−0.09–0.11) | 0.794 | ||
| GA/AA | 261 | 166 | 95 | 1.51 | (1.03–2.21) | 0.033* | 0.01 | (−0.05–0.06) | 0.748 | ||
| NLRP3 | CC | 275 | 183 | 92 | |||||||
| rs4612666 | CT | 210 | 147 | 63 | 0.86 | (0.58–1.27) | 0.434 | −0.06 | (−0.12–0.00) | 0.049* | |
| TT | 31 | 21 | 10 | 0.97 | (0.44–2.17) | 0.947 | −0.04 | (−0.16–0.08) | 0.524 | ||
| CT/TT | 241 | 168 | 73 | 0.87 | (0.60–1.27) | 0.467 | −0.06 | (−0.11–0.00) | 0.050* | ||
| SEROPOSITIVE RA | |||||||||||
| IL17A | GG | 176 | 127 | 49 | |||||||
| rs2275913 | GA | 172 | 106 | 66 | 1.73 | (1.09–2.75) | 0.021* | 0.01 | (−0.06–0.08) | 0.752 | |
| AA | 44 | 30 | 14 | 1.30 | (0.63–2.69) | 0.471 | 0.05 | (−0.06–0.16) | 0.363 | ||
| GA/AA | 216 | 136 | 80 | 1.63 | (1.05–2.54) | 0.030* | 0.02 | (−0.05–0.09) | 0.562 | ||
| NLRP3 | CC | 212 | 137 | 75 | |||||||
| rs4612666 | CT | 156 | 109 | 47 | 0.80 | (0.51–1.25) | 0.330 | −0.08 | (−0.15–0.01) | 0.024* | |
| TT | 25 | 17 | 8 | 0.90 | (0.37–2.19) | 0.813 | −0.08 | (−0.22–0.06) | 0.256 | ||
| CT/TT | 181 | 126 | 55 | 0.81 | (0.53–1.25) | 0.345 | −0.08 | (−0.15–0.01) | 0.018* | ||
| TLR4 | TT | 147 | 90 | 57 | |||||||
| rs12377632 | TC | 189 | 133 | 56 | 0.64 | (0.40–1.02) | 0.061 | −0.03 | (−0.10–0.04) | 0.400 | |
| CC | 50 | 36 | 14 | 0.59 | (0.29–1.21) | 0.152 | −0.02 | (−0.13–0.09) | 0.708 | ||
| TC/CC | 239 | 169 | 70 | 0.63 | (0.41–0.98) | 0.042* | −0.03 | (−0.10–0.04) | 0.412 | ||
| TNFRSF1A | GG | 137 | 87 | 50 | |||||||
| rs4149570 | GT | 196 | 137 | 59 | 0.77 | (0.48–1.23) | 0.268 | −0.08 | (−0.15–0.01) | 0.034* | |
| TT | 56 | 38 | 18 | 0.81 | (0.41–1.60) | 0.544 | −0.04 | (−0.14–0.07) | 0.506 | ||
| GT/TT | 252 | 175 | 77 | 0.78 | (0.50–1.21) | 0.267 | −0.07 | (−0.14–0.00) | 0.051 | ||
Adj. OR: adjusted odds ratio for ACR50 and coefficient (Coeff.) for relative change in DAS28 (relDAS28). Adjusted for gender, age, HAQ-, DMARD at baseline, CRP, IgM RF status (seropositive/seronegative). Freq.: frequency.
Additionally, significant associations with ACR50 outcome (Table 3, Table S3a-c) were found for variant allele carriers of TLR4 rs5030728 (OR = 1.51, p = 0.033, q = 0.82) and for carriers of the homozygote variant genotype of IL1B rs1143623 (OR = 2.14, p = 0.037, q = 0.90) among all RA patients.
Among seropositive RA patients, the variant alleles of TLR4 rs12377632 (OR = 0.63, p = 0.042, q = 0.61) and IL17A rs2275913 (OR = 1.63, p = 0.030, q = 0.61) were significantly associated with ACR50 response.
Statistically significant associations were found between ACR70 response (13.9% of patients) and IL1B (rs1143623, rs1143627), and IL17A (rs2275913) but not IL1B (rs4848306) and TLR4 (rs12377632, rs1554973, rs5030728) (data not shown).
Smoking and anti-TNF drug stratified analyses
Subgroup analyses stratifying for smoking status and type of anti-TNF drug was performed (Table S4+S5).
Among current smokers, the variant alleles of NLRP3 rs4612666 (OR = 0.24, p = 0.001, q = 0.04) and IL4R rs1805010 (OR = 2.69, p = 0.028, q = 0.56) were significantly associated with EULAR good/moderate response.
In patients treated with infliximab three polymorphisms (IL1B (rs4848306) (OR = 2.84, p = 0.004, q = 0.16), LY96 (rs11465996) (OR = 2.21, p = 0.023, q = 0.45), NLRP3 (rs4612666) (OR = 0.50, p = 0.041, 0.53)) were associated with EULAR good/moderate response. For etanercept-treated patients, the variant allele of TNFRSF1A rs4149570 (OR = 0.40, p = 0.025, q = 0.87) was associated with EULAR good/moderate response. No drug-specific association with any polymorphism was found for adalimumab-treated patients. However, in the seropositive subgroup significant associations with polymorphisms were found for infliximab (TNF), etanercept (LY96, NFKB1, NLRP3, TNFRSF1A) and adalimumab (IL23R, CD14, TLR5) treated patients.
No associations were found for the combined group of anti-TNF drugs based on monoclonal antibodies (all except etanercept).
Summarized effect of polymorphisms
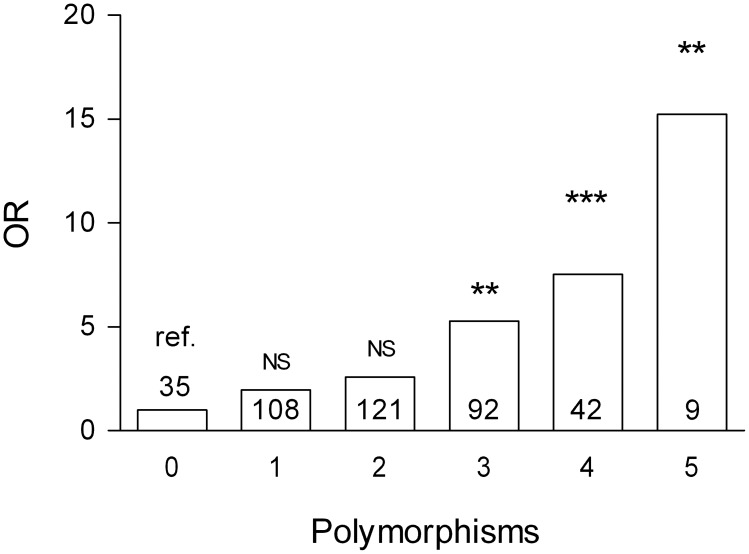
For each seropositive RA patient we counted the alleles (homozygote wildtype or carrier of variant) that were significantly associated with EULAR non-response and calculated a weighted aggregated genetic risk score. The risk of EULAR non response increased per associated polymorphism, and patients with five polymorphisms associated had about 15 times higher risk of EULAR non response than did patients with no associated alleles (Figure 2).
Figure 2. Aggregate genetic risk score.
Weighted odds ratio (OR) for seropositive RA patients’ risk of EULAR non-response according to the number of associated polymorphisms, relative to patients with zero associated polymorphisms. Number of patients in bars. NS: not significant. P-value: **<0.01, ***<0.001.
Discussion
We assessed the associations between 41 inflammatory pathway-related polymorphisms and the response to treatment with anti-TNF in 538 anti-TNF naive RA patients in a genetically homogeneous and clinically well-characterised and closely monitored cohort of RA patients [7]. The polymorphisms were chosen in genes encoding proteins in the TNF-, NFκB- and pattern recognition receptor signalling pathways (TLRs and NLRP3). Most of the chosen polymorphisms (34/41) were functional, i.e., affecting gene expression or protein function and, thus, allowing biological interpretation of the results.
We analysed treatment response using both EULAR response criteria, relative change in DAS28, and ACR50/ACR70. This enables comparison with other studies.
We were able to confirm the association between the NLRP3 gene and EULAR anti-TNF response recently reported in a well-powered study on UK RA patients [9]. However, in the UK study the specific NLRP3 polymorphism (rs4612666) was not reported as associated with EULAR response but three other polymorphisms in NLRP3 were. Although these three polymorphisms are poor markers for rs4612666 (r2 for linkage with rs4612666: rs4925659, r2 = 0.005; rs10925026, r2 = 0.09; rs4925648, r2 = 0.22), there is relatively strong linkage disequilibrium between rs4612666 and rs10925026 (D’ = 0.81) (Figure S1). Thus, two different polymorphisms in an area of the NLRP3 gene with low recombination have now been associated EULAR good and moderate response vs no response in two different cohorts treated with anti-TNF. This is a strong indicator of a true association of the gene with treatment response. As indicated, there are contradicting results for rs4612666 between our and the UK study [9]. This may be due to a type 1 error in our study. On the other hand it can also reflect differences in the population of the two cohorts, e.g. the proportion of the specific anti-TNF drugs used for treatment is different in the two studies. Also, geographical/ethnic variation may play a role. Further validation is needed to clear this question.
The data also showed that a polymorphism in IFNG–and in subgroups, polymorphisms IL1B, LY96, TLR2 and TNFRSF1A–were significantly associated with EULAR response to anti-TNF treatment. However, due to the low number of observations in subgroups and the fact that they, to our knowledge, have not earlier been linked to a differentiated anti-TNF response, these results should be considered preliminary.
When analysing the secondary outcome (ACR50), polymorphisms in IL1B, IL17 and TLR4 were associated with response to anti-TNF treatment. Another study [10] also found a polymorphism in TLR4 associated with anti-TNF response, though this study used the EULAR response criterion.
The importance of the NLRP3-inflammasome in RA pathogenesis is illustrated by the findings of increased NLRP3 mRNA in synovial tissue from RA patients compared to subjects with osteoarthritis [14]. Moreover, another polymorphism in NLRP3 has been found to be associated with delayed apoptosis of neutrophils suggesting that NLRP3 could influence the resolution of inflammation through a dysregulated innate immune response [24].
Hitomi et al. found the rs4612666 variant allele to cause lower mRNA expression by decreasing the transcriptional enhancer activity (in intron 7) of the NLRP3 gene in a cell expression study [25].
It is premature to give a solid biological interpretation on the association found for NLRP3 (rs4612666) as the association of this specific polymorphism has not been replicated. However, since the association found in this polymorphism remained statistically significant after correction for multiple testing (among smoking variant allele carriers) it is tempting to hypothesize that this SNP is functionally relevant for the outcome of anti-TNF treatment in RA patients.
Smoking produce exogenous reactive oxygen species (ROS) [26] and ROS has been found to increase expression and activation of the NLRP3-inflammasome [27]. This may explain why the largest ORs were found in the subgroup of current smokers in whom increased activation of a differentially expressed NLRP3-inflammasome should have the strongest biological effects (carriers of the NLRP3 variant (T) allele were less likely to benefit from anti-TNF treatment). Taken together, our results indicate a potential impact of smoking on anti-TNF response and suggest that the non-responders may have lower expression of NLRP3 (Table 4).
Table 4. Polymorphisms’ effect on anti−/pro-inflammatory signal and chance of good anti-TNF response (EULAR good/moderate or ACR50).
| GENE/PROTEIN (SNP) | ProteinInflammatoryeffect | Variant allele Expectedeffect on geneexpression | Variant alleleAssociation withAnti-TNF response | Subgroup |
| NLRP3/NLRP3 | Pro- | ↓[25] | ↓* | All |
| rs4612666 C>T | Smokers | |||
| Seropositive | ||||
| Infliximab | ||||
| TNFRS1A/TNFR1 | Pro- | ↑[32] | ↓* | Seropositive |
| rs4149570 G>T | Etanercept | |||
| IL17A/IL-17 | Pro- | ↑[33] | ↑# | Seropositive |
| rs2275913 G>A | ||||
| TLR4/TLR4 | Pro- | Unknown | ↑# | All |
| rs5030728 G>A | ||||
| IL1B/IL-1β | Pro- | ↑[34] | ↑# | All |
| rs1143623 G>C | Seropositive | |||
| IL1B/IL-1β | Pro- | ↓[34] | ↑* | Infliximab |
| rs4848306 | ||||
| LY96/MD-2 | Pro- | ↑[35] | ↑* | Infliximab |
| rs11465996 C>G | ||||
| INFG/Interferon-γ | Pro−/Anti- | ↓[36] | ↓* | All |
| rs2430561 T>A | Seropositive |
EULAR (*): European League Against Rheumatism ACR50 (#): American College of Rheumatology response criterion (50% improvement).
This study was not designed to detect effect sizes below 1.5 and had low power to perform subgroup analyses. In the seropositive subgroup there was >80% power to detect effect sizes above 1.7 for polymorphisms with MAFs above 0.25, whereas power in the seronegative subgroup was too low for relevant analyses. Though also limited by low power we found it relevant to analyse associations for individual drugs and monoclonal-based anti-TNFs because of the small differences and similarities which exist between them.
We have chosen to present significant result at the 5% significance level. Though most of these results become insignificant after correction for multiple testing, we also present FDR corrected q-value. We cannot exclude that choosing this level of statistical significance may result in type 1 errors but obviously may too conservative testing result in type 2 errors [28]. Due to the exploratory nature of the study we found it relevant to present even weak genetic associations, which may subsequently be replicated in other studies. Other factors than significance level should be considered when interpreting these results [29]. The prior probability of finding associations in functional polymorphisms is higher since earlier studies have found these to result in an altered expression of their corresponding genes. These genes were chosen based on their expected biological effects in the mechanism of action of the anti-TNF treatment.
Also the presence or absence of systematic bias is important for the risk of type 1 error. In this study, the selection of patients was based on the availability of sufficient clinical data to calculate EULAR responses and this could theoretically create a selection bias. Overall, however, the response rates found in this study are comparable to on the response rates in the complete DANBIO cohort [30] and other comparable studies [10], [31]. Genotyping artefacts are theoretically possible and were identified for two polymorphisms but re-genotyping of 94 randomly selected samples in another cohort yielded >99% identical genotypes.
A strength of the study is that the clinical data were collected independently and prospectively as part of routine care.
In conclusion, we confirm the NLRP3 gene as associated with anti-TNF treatment response based on EULAR criteria in a Danish cohort of RA patients [9]. The NLRP3 variant (T) allele is associated with a poorer treatment response, in particular among current smokers. Furthermore, we find the homozygous variant genotype of an IFNG polymorphism associated with a poorer anti-TNF response.
Results indicating association with other polymorphisms among subgroups in this cohort should be interpreted with care and all findings tested by replication in independent validation cohorts.
Supporting Information
Linkage Disequilibrium Plot for polymorphisms spanning the NLRP3 -gene. D’ values are shown. Darker grey indicates higher r2.
(PNG)
Chosen polymorphisms and corresponding gene. Associated effect of polymorphism.
(DOCX)
Adjusted odds ratios for associations between gene variants and EULAR anti-TNF treatment response. (a. All RA patients, b. Seropositive RA patients).
(DOCX)
Adjusted odds ratio (OR)/coefficient for associations between gene variants and ACR50 and relDAS28 response to anti-TNF treatment. (a. All RA patients, b. Seropositive RA patients).
(DOCX)
Smoking strata: Odds ratio for minor allele carriers achieving EULAR good/moderate response.
(DOCX)
Anti-tumour necrosis factor (TNF) subgroup odds ratio for EULAR good/moderate response. (a. All RA patients, b. Seropositive RA patients).
(DOCX)
Acknowledgments
We thank Hans Jürgen Hoffmann, Department of Respiratory Diseases, Aarhus University Hospital, Aarhus, Denmark; and Vibeke Østergaard Thomsen, International Reference Laboratory of Mycobacteriology, Statens Serum Institut for collecting samples; Andreas Nicolai Andersen, Statens Serum Institut, for statistics support; Elvira Chapka, Statens Serum Institut for laboratory support; and Niels Steen Krogh, Zitelab Aps, Copenhagen, Denmark for database management. We also thank Department of Medicine, Viborg Regional Hospital, Denmark and OPEN (Odense Patient data Explorative Network), Odense University Hospital, Denmark for supporting this work.
Funding Statement
This study was supported by the Danish Rheumatism Association (www.gigtforeningen.dk), Region of Southern Denmark’s PhD Fund (www.regionsyddanmark.dk) and the Department of Rheumatology, Frederiksberg Hospital (www.frederiksberghospital.dk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, et al. (2010) Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 62: 22–32 10.1002/art.27227 [doi]. [DOI] [PubMed] [Google Scholar]
- 2. Rosenblum H, Amital H (2011) Anti-TNF therapy: safety aspects of taking the risk. Autoimmun Rev 10: 563–568 10.1016/j.autrev.2011.04.010 [doi]. [DOI] [PubMed] [Google Scholar]
- 3. Emery P, Dorner T (2011) Optimising treatment in rheumatoid arthritis: a review of potential biological markers of response. Ann Rheum Dis 70: 2063–2070 10.1136/ard.2010.148015 [doi]. [DOI] [PubMed] [Google Scholar]
- 4. Toonen EJ, Gilissen C, Franke B, Kievit W, Eijsbouts AM, et al. (2012) Validation study of existing gene expression signatures for anti-TNF treatment in patients with rheumatoid arthritis. PLoS One 7: e33199 10.1371/journal.pone.0033199 [doi];PONE-D-11-21951 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui J, Saevarsdottir S, Thomson B, Padyukov L, van der Helm-van Mil AH, et al. (2010) Rheumatoid arthritis risk allele PTPRC is also associated with response to anti-tumor necrosis factor alpha therapy. Arthritis Rheum 62: 1849–1861 10.1002/art.27457 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plant D, Prajapati R, Hyrich KL, Morgan AW, Wilson AG, et al. (2012) Replication of association of the PTPRC gene with response to anti-tumor necrosis factor therapy in a large UK cohort. Arthritis Rheum 64: 665–670 10.1002/art.33381 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krintel SB, Palermo G, Johansen JS, Germer S, Essioux L, et al. (2012) Investigation of single nucleotide polymorphisms and biological pathways associated with response to TNFalpha inhibitors in patients with rheumatoid arthritis. Pharmacogenet Genomics 22: 577–589 10.1097/FPC.0b013e3283544043 [doi]. [DOI] [PubMed] [Google Scholar]
- 8. Umicevic MM, Cui J, Vermeulen SH, Stahl EA, Toonen EJ, et al. (2012) Genome-wide association analysis of anti-TNF drug response in patients with rheumatoid arthritis. Ann Rheum Dis 0: 1–7 annrheumdis-2012-202405 [pii];10.1136/annrheumdis-2012-202405 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, et al. (2013) Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis 0: 1–9 annrheumdis-2013-203276 [pii];10.1136/annrheumdis-2013-203276 [doi]. [DOI] [PubMed] [Google Scholar]
- 10. Potter C, Cordell HJ, Barton A, Daly AK, Hyrich KL, et al. (2010) Association between anti-tumour necrosis factor treatment response and genetic variants within the TLR and NF{kappa}B signalling pathways. Ann Rheum Dis 69: 1315–1320 ard.2009.117309 [pii];10.1136/ard.2009.117309 [doi]. [DOI] [PubMed] [Google Scholar]
- 11. Strowig T, Henao-Mejia J, Elinav E, Flavell R (2012) Inflammasomes in health and disease. Nature 481: 278–286 10.1038/nature10759 [doi]. [DOI] [PubMed] [Google Scholar]
- 12. van LG, Beyaert R (2011) Negative regulation of NF-kappaB and its involvement in rheumatoid arthritis. Arthritis Res Ther 13: 221 ar3324 [pii];10.1186/ar3324 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goh FG, Midwood KS (2012) Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford) 51: 7–23 ker257 [pii];10.1093/rheumatology/ker257 [doi]. [DOI] [PubMed] [Google Scholar]
- 14. Rosengren S, Hoffman HM, Bugbee W, Boyle DL (2005) Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis 64: 708–714 ard.2004.025577 [pii];10.1136/ard.2004.025577 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klareskog L, Catrina AI, Paget S (2009) Rheumatoid arthritis. Lancet 373: 659–672 S0140-6736(09)60008-8 [pii];10.1016/S0140-6736(09)60008-8 [doi]. [DOI] [PubMed] [Google Scholar]
- 16. Hetland ML (2011) DANBIO–powerful research database and electronic patient record. Rheumatology (Oxford) 50: 69–77 keq309 [pii];10.1093/rheumatology/keq309 [doi]. [DOI] [PubMed] [Google Scholar]
- 17.Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, et al.. (2014) Associations between functional polymorphisms in the NFkappaB signaling pathway and response to anti-TNF treatment in Danish patients with inflammatory bowel disease. Pharmacogenomics J. tpj201419 [pii];10.1038/tpj.2014.19 [doi]. [DOI] [PubMed]
- 18.Bank S, Andersen PS, Nexo BA, Sode J, Vogel U, et al.. (2014) Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS ONE (Accepted for publication). [DOI] [PMC free article] [PubMed]
- 19. Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, et al. (2008) SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24: 2938–2939 btn564 [pii];10.1093/bioinformatics/btn564 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bank S, Nexo BA, Andersen V, Vogel U, Andersen PS (2013) High-Quality and -Quantity DNA Extraction from Frozen Archival Blood Clots for Genotyping of Single-Nucleotide Polymorphisms. Genet Test Mol Biomarkers 17: 501–503 10.1089/gtmb.2012.0429 [doi]. [DOI] [PubMed] [Google Scholar]
- 21. Fransen J, van Riel PL (2005) The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 23: S93–S99. [PubMed] [Google Scholar]
- 22. Purcell S, Cherny SS, Sham PC (2003) Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19: 149–150. [DOI] [PubMed] [Google Scholar]
- 23. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 57: 289–300. [Google Scholar]
- 24. Blomgran R, Patcha BV, Verma D, Bergstrom I, Soderkvist P, et al. (2012) Common genetic variations in the NALP3 inflammasome are associated with delayed apoptosis of human neutrophils. PLoS One 7: e31326 10.1371/journal.pone.0031326 [doi];PONE-D-10-02541 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hitomi Y, Ebisawa M, Tomikawa M, Imai T, Komata T, et al. (2009) Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol 124: 779–785. [DOI] [PubMed] [Google Scholar]
- 26. Valavanidis A, Vlachogianni T, Fiotakis K (2009) Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 6: 445–462 10.3390/ijerph6020445 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simard JC, Cesaro A, Chapeton-Montes J, Tardif M, Antoine F, et al. (2013) S100A8 and S100A9 Induce Cytokine Expression and Regulate the NLRP3 Inflammasome via ROS-Dependent Activation of NF-κB. PLoS ONE 8: e72138 doi:10.1371/journal.pone.0072138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316: 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plenge RM, Bridges SL, Huizinga TWJ, Criswell LA, Gregersen PK (2011) Recommendations for publication of genetic association studies in Arthritis & Rheumatism. Arthritis & Rheumatism 63: 2839–2847. [DOI] [PubMed] [Google Scholar]
- 30. Hetland ML, Lindegaard HM, Hansen A, Podenphant J, Unkerskov J, et al. (2008) Do changes in prescription practice in patients with rheumatoid arthritis treated with biological agents affect treatment response and adherence to therapy? Results from the nationwide Danish DANBIO Registry. Ann Rheum Dis 67: 1023–1026 ard.2007.087262 [pii];10.1136/ard.2007.087262 [doi]. [DOI] [PubMed] [Google Scholar]
- 31. Coenen MJ, Enevold C, Barrera P, Schijvenaars MM, Toonen EJ, et al. (2010) Genetic variants in toll-like receptors are not associated with rheumatoid arthritis susceptibility or anti-tumour necrosis factor treatment outcome. PLoS One 5: e14326 10.1371/journal.pone.0014326 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang GB, Li CR, Yang J, Wen PQ, Jia SL (2011) A regulatory polymorphism in promoter region of TNFR1 gene is associated with Kawasaki disease in Chinese individuals. Hum Immunol 72: 451–457 S0198-8859(11)00032-2 [pii];10.1016/j.humimm.2011.02.004 [doi]. [DOI] [PubMed] [Google Scholar]
- 33. Espinoza JL, Takami A, Nakata K, Onizuka M, Kawase T, et al. (2011) A genetic variant in the IL-17 promoter is functionally associated with acute graft-versus-host disease after unrelated bone marrow transplantation. PLoS One 6: e26229 10.1371/journal.pone.0026229 [doi];PONE-D-11-15752 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H, Wilkins LM, Aziz N, Cannings C, Wyllie DH, et al. (2006) Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet 15: 519–529 ddi469 [pii];10.1093/hmg/ddi469 [doi]. [DOI] [PubMed] [Google Scholar]
- 35. Gu W, Shan YA, Zhou J, Jiang DP, Zhang L, et al. (2007) Functional significance of gene polymorphisms in the promoter of myeloid differentiation-2. Ann Surg 246: 151–158 10.1097/01.sla.0000262788.67171.3f [doi];00000658-200707000-00023 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV (2000) A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: Absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Human Immunology 61: 863–866 10.1016/S0198-8859(00)00167-1 [doi]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linkage Disequilibrium Plot for polymorphisms spanning the NLRP3 -gene. D’ values are shown. Darker grey indicates higher r2.
(PNG)
Chosen polymorphisms and corresponding gene. Associated effect of polymorphism.
(DOCX)
Adjusted odds ratios for associations between gene variants and EULAR anti-TNF treatment response. (a. All RA patients, b. Seropositive RA patients).
(DOCX)
Adjusted odds ratio (OR)/coefficient for associations between gene variants and ACR50 and relDAS28 response to anti-TNF treatment. (a. All RA patients, b. Seropositive RA patients).
(DOCX)
Smoking strata: Odds ratio for minor allele carriers achieving EULAR good/moderate response.
(DOCX)
Anti-tumour necrosis factor (TNF) subgroup odds ratio for EULAR good/moderate response. (a. All RA patients, b. Seropositive RA patients).
(DOCX)