Introduction
Despite significant advances in treatment, age-related macular degeneration (AMD) remains the leading cause of blindness in the elderly of developed nations [1]. Retinal cells experience functional decline with age as a result of metabolic changes and cumulative oxidative stress [2]. Early AMD is characterized by retinal pigment epithelium (RPE) dysfunction [3]. In the early stages of disease, immunomodulatory proteins and inflammatory cells accumulate in the outer retina, with an associated increase in membrane attack complex (MAC) at the RPE and choroidal interface at Bruch’s Membrane [4,5]. In some cases, accumulation of lipo-glyco-proteinacous deposits in the basal lamina, called drusen, become progressively larger and more abundant and may further cause RPE atrophy [6,7].
Growing evidence suggests a key role of inflammatory pathways in pathogenesis of AMD, triggered either by an aberrant innate immunity or autoimmunity mechanism. Variants of genes in the complement system, such as complement factor B (CFB), factor I (CFI), factor H (CFH), complement component 2 (C2), component 3 (C3) and component 7 (C7), correlate with either increased or decreased susceptibility to AMD [8,9]. In the AMD retina, immune cells such as macrophages, microglia, and giant cells localize to areas surrounding drusen deposits [10–12]. Activated microglia is capable of inducing direct damage to photoreceptors as shown by in vitro studies [12,13]. Proteomic studies revealed that drusen contain a number of inflammatory molecules, including C3, C5, C5b-9 and MAC [9,14]. Additionally, amyloid-beta (Aβ) and advanced glycation end products (AGE) are present [15]. In an earlier microarray study, we found that human RPE cells treated with Aβ produced strong upregulation of interleukin-1-beta (IL-1β) [16]. IL-1β is an important upstream mediator of inflammatory response and is involved in signaling macrophage activation and production of cytokines. IL-1β is capable of inducing reactive oxygen species(ROS) in RPE cells [17]. ROS trigger the release of IL-8, which recruits pro-inflammatory cells such as macrophages [16–18]. Macrophages are present in drusen deposits of AMD eyes and play a key role in promoting neovascular proliferation [19,20]. Recent studies demonstrated the presence of anti-retinal auto-antibodies in early AMD [14]. In these patients, components of drusen such as AGE may be involved in triggering autoimmune responses. In vitro, AGE strongly stimulates chemokine CXC motif ligand 10 and 11 (CXCL-10 and CXCL-11) production in RPE cells (unpublished observation). CXCL-11 recruits activated-Th-lymphocytes and B-cells and may serve as a beacon for B-cell accumulation, activation, and proliferation [21,22]. It is likely that this pathway, along with other similar ones, results in increasing tissue exposure to auto-antibodies. Altogether, our in vitro studies indicate that the components of drusen may be involved in the induction of inflammatory pathways facilitating AMD pathology (unpublished observation) [16]. In particular, IL-1β, CFI, radical S-adenosyl methionine domain containing 2 (RSAD2), and CXCL-10/11 were significantly upregulated. Our data also suggested activation of the JAK/STAT pathway, involved in the interferon response. STAT3, a member of the JAK/STAT pathway, was found to accumulate in the RPE cells of choroidalneovascular membranes of AMD patients [23]. With age, accumulations of drusen may increase the susceptibility of the retina to damage inflicted by these pathways. Here, we sought to verify the upregulation of the aforementioned inflammation-related gene products in the normal human retina, and to test whether changes in expression of these genes are related to the two major risk factors of AMD, age and presence of drusen.
Methods
Eye samples
Human donor eyes were obtained from the Eye Bank of British Columbia, Canada. Methods for securing human tissue were in compliance with the Declaration of Helsinki. The protocol was approved by the Clinical Research Ethics Board (CREB) at the University of British Columbia. All tissue samples included in this study were considered normal and excluded tissues from donors with any of the following: evidence of systemic or local infection; progressive central nervous system disease or systemic disease of unknown etiology; lymphoproliferative or myeloproliferative disorders; intrinsic eye disease or previous ocular surgery. All tissues were fixed within 20 hours of death (median time 14 hours). Eyes were divided into two age groups, a “younger” group (n=17), less or equal to 57 years and an “older” group (n=17), greater or equal to 70 years (Table 1).
Table 1.
Donor List.
| Donor | Age | Sex | Diagnosis | IL-1β | CFI | STAT3 | CXCL-11 | CXCL-10 | RSAD2 | No Drusen | Drusen <25μm | Drusen ≥25μm | Drusen ≥63μm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 70 yr Old | |||||||||||||
| 1 | 74 | M | sub-arachnoid hemorrhage | X | X | X | X | X | |||||
| 2 | 70 | F | glioblastoma multiform | X | X | X | X | X | X | X | X | ||
| 3 | 71 | F | lung cancer | X | X | X | X | X | X | X | X | ||
| 4 | 70 | M | hepatitis B and colon cancer with metastasis | X | X | X | X | X | X | ||||
| 5 | 74 | M | bowel cancer | X | X | X | X | X | |||||
| 6 | 75 | M | heart disease (cardiac arrest) | X | X | X | X | X | X | X | X | ||
| 7 | 72 | M | pancreatic cancer | X | X | X | X | X | X | X | X | ||
| 8 | 71 | M | colon cancer with metastasis | X | X | X | X | X | X | X | X | ||
| 9 | 74 | M | bone cancer with metastasis | X | X | X | X | X | X | X | X | ||
| 10 | 72 | F | gastroenteritis and chronic renal failure | X | X | X | X | X | X | X | X | ||
| 11 | 72 | M | respiratory arrest | X | X | X | X | X | X | ||||
| 12 | 80 | M | renal failure | X | X | X | X | X | X | X | |||
| 13 | 70 | M | cardiogenic arrest | X | X | X | X | X | X | X | X | ||
| 14 | 72 | F | large left cerebral vascular accident | X | X | X | |||||||
| 15 | 80 | F | not available | X | X | X | X | X | |||||
| 16 | 72 | M | renal cancer | X | X | X | X | X | X | X | |||
| 17 | 73 | M | lung and liver cancer | X | X | X | |||||||
| ≤ 57 yr Old | |||||||||||||
| 18 | 47 | F | lung cancer with mets | X | X | X | X | X | X | X | |||
| 19 | 46 | F | lung cancer | X | X | X | X | X | X | ||||
| 20 | 46 | F | brain tumor | X | X | X | X | X | X | ||||
| 21 | 48 | F | liver cancer, cirrhosis | X | X | X | X | X | X | X | X | ||
| 22 | 56 | F | breast cancer with brain metastasis | X | X | X | X | X | X | X | |||
| 23 | 54 | F | Glioblastoma | X | X | X | X | X | X | X | |||
| 24 | 55 | M | Prostate cancer | X | X | X | X | X | X | X | |||
| 25 | 55 | M | metastatic esophageal cancer | X | X | X | X | X | |||||
| 26 | 42 | F | lung cancer with metastasis | X | X | X | X | X | X | ||||
| 27 | 17 | M | multiple trauma due to car accident | X | X | X | X | X | X | X | |||
| 28 | 57 | M | bladder cancer | X | X | X | X | X | |||||
| 29 | 47 | M | appendix cancer | X | X | X | X | X | X | X | |||
| 30 | 55 | F | lung cancer | X | X | X | |||||||
| 31 | 55 | F | lung cancer | X | X | X | X | X | X | ||||
| 32 | 44 | M | Brain cancer | X | X | X | X | X | X | X | |||
| 33 | 28 | F | ovarian cancer with metastasis | X | X | X | X | X | X | X | |||
| 34 | 35 | F | cercical cancer with metastasis | X | X | X | X | X | X | ||||
Tissue preparation and immunohistochemistry
Eye tissues were prepared as previously described by Seth et al. [4] and embedded in paraffin to obtain 6 μm sections through the pupil and optic nerve axis. Sections that included the macular region were selected for this study.
Antibodies against IL-1β, CFI, RSAD2, phosphorylated signal transducer and activator of transcription 3 (p-STAT3), CXCL-10, and CXCL-11 were used to stain sections from both age groups. Antibody dilution and source are documented in Table 2. Paraffin sections were deparaffinized and rehydrated by standard procedures. Sections underwent antigen retrieval in protease K solution (20 μg/ml, pH 8.0) for 10 minutes at room temperature. Sections were then washed twice in phosphate buffered saline (PBS, pH 7.4), treated with 0.3% H2O2 for 15 minutes, and blocked for 20 minutes with 3% horse or goat serum diluted in 0.3% Triton X (TX)-100-PBS solution to minimize non-specific staining. Tissue sections were allowed to incubate in specific primary antibodies diluted in serum and PBS with 0.3% TX-100 for 24 to 48 hours at 4°C, based on different target antigens. Two methods were employed to obtain negative control sections. In one approach, sections were incubated in the absence of primary antibody. In the second method, sections were treated with an irrelevant IgG isotype at the same concentration and in place of the primary antibody incubation (Table 2). All subsequent steps were identical. Both controls resulted in very low background staining confirming the specificity of the immunohistochemical staining patterns shown here.
Table 2.
Proteins studied and antibodies used.
| Protein/Marker of Inflammation | Results of stimulation studies | Description | Primary Antibody | Irrelevant IgG | Working Concentration | Manufacturer | Detection System |
|---|---|---|---|---|---|---|---|
| IL-1 beta | Aβ | Interleukin-1 beta, produced by activated macrophage, mediates inflammatory response including cellular proliferation, differentiation, and apoptosis | Mouse monoclonal anti-human IL-1 beta | Mouse IgG1 | 1 to 200 | Sigma Aldrich | AEC; Sigma Aldrich |
| CFI | Aβ | Complement factor I is a serine protease that inactivates C3b and C4b | Mouse monoclonal anti-human CFI | Mouse IgG1 | 1 to 250 | Lifespan Biosciences | Vector Blue; Vector Laboratories |
| p-Stat3 | AMD membranes | Stat3 is an important signalling molecule for cytokines/growth factor receptors. Here we stained for activated Stat3 (Phospho-Stat3 (Tyr705) (D3A7)). STAT3 is part of the JAK/STAT pathway. | Rabbit monoclonal anti-mouse phospho-Stat3 | Rabbit IgG | 1 to 100 | Cell Signaling Technology | Vector Blue; Vector Laboratories |
| CXCL-11 | AGE | IFN-inducible T cell α chemoattractant is a member of the C-X-C chemokine family and is expressed in IFN-γ-treated astrocytes, monocytes, keratinocytes, bronchial epithelial cells and neutrophils | Rabbit polyclonal anti-human CXCL-11 | Rabbit IgG | 1 to 100 | Santa Cruz Biotechnology, INC | AEC; Sigma Aldrich |
| CXCL-10 | Early biomarker of AMD | CXCL-10 (IP-10) is a member of the C-X-C chemokine family (pro-inflammatory) | Mouse monoclonal anti-human IP-10 | Mouse IgG1 | 1 to 100 | Santa Cruz Biotechnology, INC | AEC; Sigma Aldrich |
| RSAD2 | Aβ, AGE | RSAD2 is involved in antiviral defence (against Hep C, cytomegalovirus, and HIV-1) | Rabbit polyclonal anti-human RSAD2 | Rabbit IgG | 1 to 100 | Santa Cruz Biotechnology, INC | Vector Blue; Vector Laboratories |
Antibody detects STAT3 when STAT3 is phosphorylated at tyrosine705. Do not cross react with phopho-EGFR or other phosphor-tyrosine STAT proteins.
After incubation in the primary antibodies, sections were thoroughly washed and incubated in secondary antibodies for 30 to 60 minutes at room temperature and rinsed twice before proceeding to alkaline phosphatase (Vector Blue, Vector Laboratories) or 3-Amino-9-ethylcarbazole (AEC, Sigma Aldrich) development. For Vector Blue chromogenic reaction, sections were first treated with Avidin-Biotinylated enzyme complex-alkaline phosphatase (ABC-AP) solution for 30 minutes and then developed for 1 hour at room temperature (counterstained with Nuclear Fast Red: Crystal Mount; Biomeda). Sections developed with AEC were pre-incubated for 30 minutes with ABC before incubation with AEC for 5 minutes (counterstained with hematoxylin). Tissue sections from both age groups were developed simultaneously to ensure comparable processing.
Statistical analysis
Statistical analysis was conducted by examination of four sections per eye per each antibody in a masked manner. For each molecule immunostaining intensities were scored semi-quantitatively using 40x objective lens (Eclipse 80i; Nikon, Tokyo, Japan), graded from 0 to 3. A score of 0 indicates no detectable staining above background as determined by comparison with the negative control sections. By systematic scanning through the slides, we identified ones with the strongest staining and classified them as having a score of 3. For staining levels that fall between 0 and 3, a score of 1 was given to samples with the weakest immunolabeling, while a score of 2 represented intermediate immunolabeling. We have also provided a sample range of the semi-quantitative analysis: an example of the strongest immunolabeling is shown in Figure 1, with 1A representing +1, 1B and 1C representing +3. Data were scored in the retina (ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), inner segment (IS), and outer segment (OS)), retinal pigment epithelium (RPE), Bruch’s membrane, choroid, and drusen sites. Sub-retinal layers were scored individually; scores of all retinal layers were summed and averaged to evaluate expression level in the overall retina. Drusen size was determined using RPE cell size as reference (RPE diameter measured parallel to Bruch’s membrane was estimated at 12–14 μm) [24]. Statistical analysis was done using the Mann-Whitney test (GraphPad Software, Inc., La Jolla, CA). A one-tailed test was selected based on the directional hypothesis that accumulation of pro-inflammatory modulators increased with age and presence of drusen. Significance level was set at P ≤ 0.05.
Figure 1.
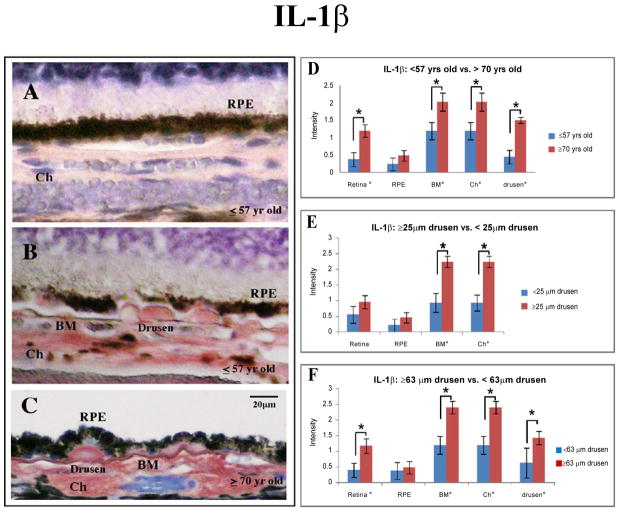
Immunostaining for IL-1β with AEC development system (red) and counterstained with Mayer’s hematoxylin (blue). IL-1β expression levels in human eye tissue, analyzed by age groups and size of drusen. (A) Younger group (≤ 57 yr) with drusen ≤ 25 μm; note faint immunolabeling pattern (semi-quantitative grading of 1). (B) Eye tissue from the younger group (≤ 57 yr) with drusen ≥ 25 μm (arrows). Note immunoreactivity for IL-1β in drusen and through choroid (semi-quantitative grading of 3). RPE cells are mildly immunostained. (C) Strong immunoreactivity in drusen, Bruch’s membrane, and choroid in eyes from older group (≥ 70 yr, semi-quantitative grading of 3) with drusen ≥ 63 μm (arrows). Scale bar 20 μm. (D) Aside from the retinal pigmented epithelial (RPE) cells, tissue from older group (n = 12) are more immunoreactive for IL-1β (retinal layers through to the choroid) than younger group (n = 15) (black asterisk, p ≤ 0.05). (E) Eyes with clinically significant drusen (≥ 25 μm, n = 15) were more immunoreactive for IL-1β in Bruch’s membrane and choroid compared to eyes without clinically significant drusen (n = 12). (F) Eyes with drusen ≥ 63 μm (n = 12) were more immunoreactive for IL-1β in the retinal layers, Bruch’s membrane, choroid, and drusen than eyes with drusen ≤ 63 μm (n = 8).
Categorization of drusen by size
Drusen were grouped into three categories based on their size. Tissues were initially classified to contain “observable drusen” if there are discrete lipo-proteinaceous deposits between the basal lamina of the RPE and Bruch’s membrane with diameters ≥ 4 μm. Next, we classified “clinically significant drusen” to be those with a diameter of ≥ 25 μm [24]. This is based on the fact that, during clinical exams, the limit of resolution through fundoscopy is between 25 to 30 μm. Lastly, due to the physiological significance associated with size we further sub-categorized samples into those with drusen ≥ 63 μm in diameter [24,25]. We randomly sampled 20 sections at 20x magnification from each eye to determine the presence and size of drusen in individual eyes. These categories are shown for each eye on Table 1.
Result
Younger eyes (≥ 57 years) versus older eyes (≥ 70 years)
In order to determine the age-related difference in expression for components of the inflammatory pathways, we compared immunoreactivity in younger eyes (≤ 57 years) with older eyes (≥ 70 years) (Table 3A). Four of the six antigens studied here demonstrated age-related changes. IL-1β immunoreactivity was significantly stronger in the retina, Bruch’s membrane, choroid and drusen deposits in tissue obtained from older group (Figure 1C, D).
Table 3.
Semiquantitative analysis of IL-1β, CFI, RSAD2, STAT3, and CXCL-11immunoreactivity.
| A | B | C | D | ||
|---|---|---|---|---|---|
| ≥70 yrs old vs ≤57 yrs Old | ≥25 μm drusen vs <25 μm drusen | ≤57 yrs old ≥4 μm drusen vs <4 μm drusen | ≥63 μm drusen vs <63 μm drusen | ||
| IL-1β | Retina |
 * * |
- | - |
 * * |
| RPE | - | - | - | - | |
| BM |
 * * |
 * * |
- |
 * * |
|
| Ch |
 * * |
 * * |
- |
 * * |
|
| Drusen |
 * * |
NA | NA |
 - - |
|
| RSAD2 | Retina | - | - | *

|
- |
| RPE | - | - | - | - | |
| BM | - |
 * * |
- | - | |
| Ch | - | * | - | - | |
| Drusen | - | NA | NA | - | |
| p-STAT3 | Retina |
 * (GCL/INL/ONL) * (GCL/INL/ONL) |
 *(GCL/INL/ONL) *(GCL/INL/ONL) |
 * * |
 *(GCL/INL) *(GCL/INL) |
| RPE | - | - | - | - | |
| BM | - | - | - | *

|
|
| Ch | - | - | - | - | |
| Drusen | - | NA | NA | - | |
| CXCL-10 | Retina | * (GCL)

|
 * * |
- | - |
| RPE | - | - | - | - | |
| BM | - | - | - | - | |
| Ch | - | - |
 * * |
- | |
| Drusen | - | - | - | - | |
| CXCL-11 | Retina | * (OPL)

|
- |
 * * |
 * * |
| RPE | - | - |
 * * |
- | |
| BM | *

|
- |
 * * |
- | |
| Ch | - | - |
 * * |
- | |
| Drusen | - | NA | NA | - | |
| CFI | Retina | - | - | - | - |
| RPE | - | - | - | - | |
| BM | - | - | - | - | |
| Ch | - | - | - | - | |
| Drusen | - | NA | NA | - | |
Mann-Whitney test (one-tail unequal variance) with significance set at p≤ 0.05 (black asterisk). Arrowhead denotes population with stronger immunolabeling.
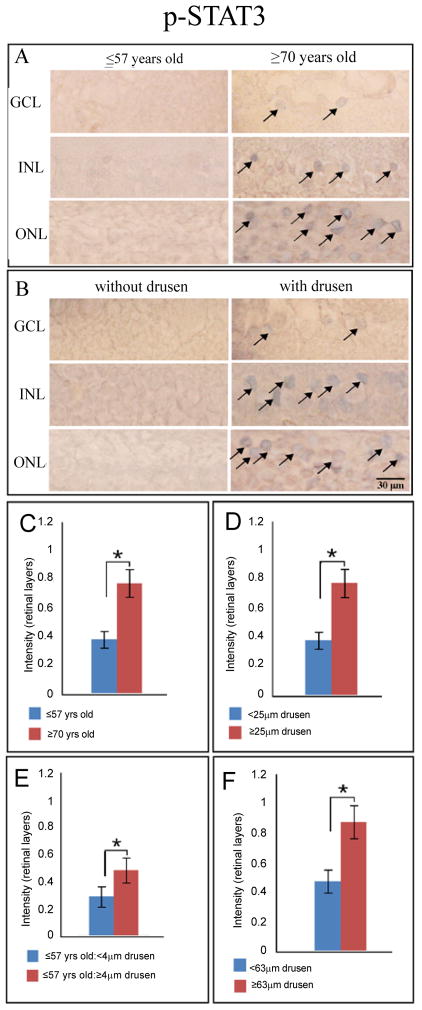
Immunoreactivity for p-STAT3, the activated form of STAT3, was significantly stronger in the older group for the combined retinal layers (Figure 2A, C). Specifically, this observation was found to be statistically significant in the GCL, INL, and ONL. For CXCL-11, the overall retinal layers immunostained significantly stronger in the combined retinal layers, particularly OPL of younger eyes. In addition, younger eyes also showed significantly stronger CXCL-11 expression in Bruch’s membrane. Similar to CXCL-11, CXCL-10 immunoreactivity was significantly stronger in the combined retinal layers of younger eyes, specifically in the GCL. There were no discernable differences in immunoreactivity between the two age groups for CFI and RSAD2.
Figure 2.
p-STAT3 levels in human eye tissue visualized with Vector Blue (blue) and counterstained with neutral red (pink). (A) Cells (arrows) in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer are found to be immunoreactive (vector blue) for phosphorylated (activated) STAT3 in the older group (≥ 70 yr). (B) Similarly, immunolabeling profile is seen in eyes with drusen ≥ 25 μm regardless of age. Scale bar 30 μm. p-STAT3 accumulation significantly differs (p ≤ 0.05, black asterisk) in the retinal layers between the analyzed groups. STAT3 activation is greater in older eyes (C, ≥ 70 yr, n = 14), in eyes with clinically significant drusen (D, ≥ 25 μm, n = 14) and particularly in eyes with large drusen (F, ≥ 63 μm, n = 10). In the younger group (E, ≤ 57 yr, n = 14), the presence of drusen ≥ 4 μm (n = 7) is associated with increased STAT3 activation compared to eyes without drusen (n = 7).
Eyes with drusen: ≤25μm versus ≥25 μm (independent of age)
Next, we investigated whether the presence of clinically significant drusen (≥ 25 μm) was correlated with changes in expression levels of components of the inflammatory pathway (Table 3B). Antibodies against IL-1β localized strongly to Bruch’s membrane and choroid of eyes with drusen ≥ 25 μm. We did not observe differences in accumulation of IL-1β in the retinal layers or the RPE between the two analyzed groups (Figure 1B, C, E).
p-STAT3 stained more robustly in the combined retinal layers of drusen-containing eyes in particular, significantly higher activation of STAT3 was found in the ganglion cell layer, inner and outer nuclear layers of eyes with clinically significant drusen (Figure 2B, D). CXCL-10 demonstrated similar staining patterns to p-STAT3 in retina.
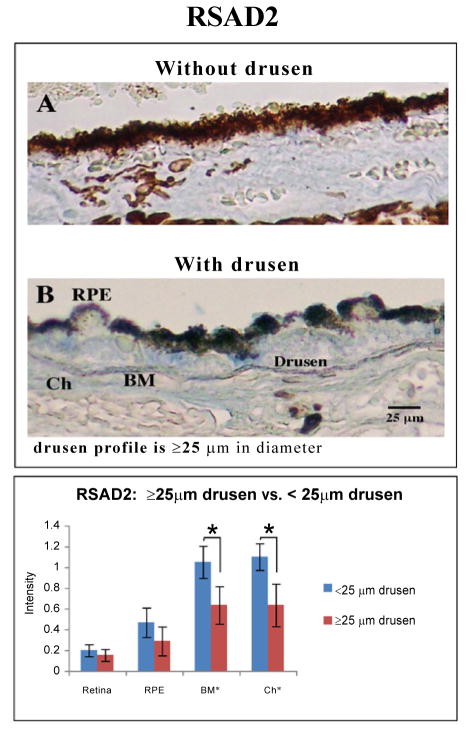
RSAD2, similar to IL-1β was most immunoreactive in Bruch’s membrane and through the choroid (Figure 3B, C). In contrast, CFI and CXCL-11 levels did not differ with the presence of clinically significant drusen, in this age independent analysis.
Figure 3.
Immunostaining for RSAD2 with Vector Blue (blue) and counterstained with neutral red (pink). (B) Representative sections from eyes with drusen ≥ 25 μm shown that areas around drusen (≥ 25 μm), in RPE and Bruch’s membrane are more immunoreactive for RSAD2 than (A) the background reactivity seen in tissues with small (≤ 25 μm) or no drusen. Scale bar 25 μm. (C) Eyes with clinically significant drusen (≥ 25 μm, n = 15) are found to have stronger immunostaining for RSAD2 particularly in the Bruch’s membrane and choroid (p ≤ 0.05, black asterisk) than those without clinically significant drusen (n=13).
Younger eyes (≤ 57 years): ≤ 4 μm drusen versus ≥ 4 μm drusen (controlled for age)
Individual components of drusen (Aβ, AGE) have been found to upregulate pro-inflammatory factors in cultured RPE cells (unpublished observation) [16]. Thus, it is important to evaluate for any correlation between presence of drusen and changes in the expression and/or accumulation of inflammatory factors in the eye. Although drusen ≤ 25 μm are not considered clinically significant, the pro-inflammatory Aβ is present in these deposits [26]. Thus, we compared candidate gene expression levels in younger eyes with (n=10) or without (n=7) drusen (≥ 4 μm).
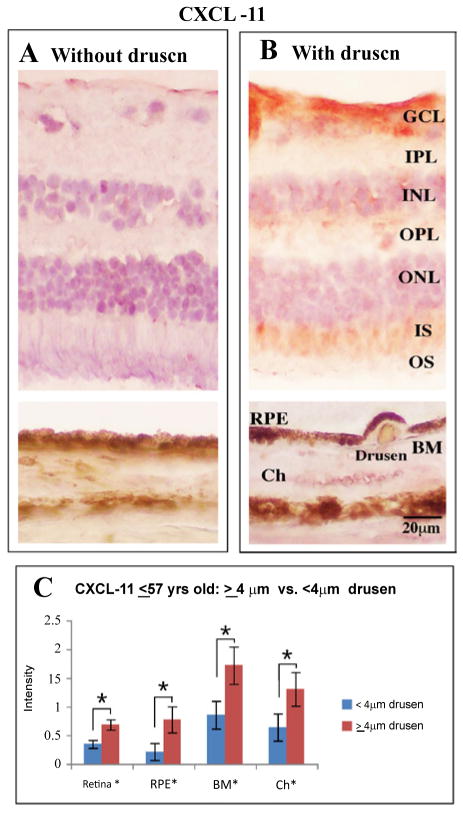
Expression of CXCL-11was observed to be stronger in the drusen group for the combined retinal layers, RPE, Bruch’s membrane, and choroid (Table 3C, Figure 4). Specifically, in the retina, this observation was found to be statistically significant for the ganglion cell layer and outer segments. p-STAT3 was also strong in the retinal layers in young eyes with drusen, while the immunoreactivity against CXCL-10 was strong in the choroid layer of young eyes with drusen (Figure 2, 5). We found no significant difference in IL-1β and CFI immunostaining between the retinas of younger eyes with or without drusen.
Figure 4.
Immunolabeling for CXCL-11 with Vector Blue (blue) and counterstained with neutral red (pink). Representative sections from younger eyes (≥ 57 yr, n = 16) are shown. Samples were analyzed for the presence of drusen (≥ 4 μm, n = 9). Within the younger age-group, eyes with drusen (B) have greater accumulation of CXCL-11 throughout the retina and choroid. Scale bar 20μm. (C) Within tissue of younger eyes (≤ 57 yr, n = 16), those with any observable drusen (≥ 4 μm, n = 9) were found to stain stronger (p ≤ 0.05, black asterisk) for chemokine, CXCL-11 from the retina through to the choroid.
Figure 5.
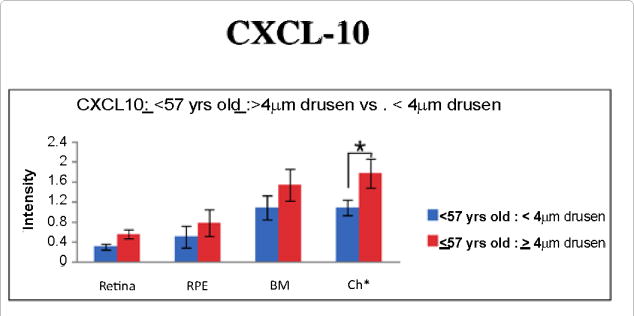
In the younger age group (≤ 57 yr, n = 15), immunoreactivity against CXCL-10 is significantly greater in the choroid of eyes with drusen (≥ 4 μm, p ≤ 0.05, n = 9).
Eyes with drusen: ≤ 63 μm versus ≥ 63 μm (independent of age)
Next, we examined whether any sub-populations exist within eyes carrying drusen. This analysis was motivated by the observation that 95.5% to 98.8% of the human population has one or more drusen [27–30]. While the most frequently observed drusen are ≤ 63μm and considered relatively benign, the prevalence of drusen ≥63 μm increases with age and is considered a risk factor for age related maculopathy [24]. Thus, it is important to discern between these two sizes of drusen. The drusen diameters found in this study range from 4 μm to 126 μm. We observed that drusen ≥ 63 μm are found mostly outside of the macular area at peripheral location, while drusen ≤ 63 μm are distributed more evenly amongst foveal, parafoveal and peripheral regions (data not shown). Using the morphological classification provided by Rudulf et al. [31], drusen ≥ 63 μm identified in our study were predominantly hard drusen.
Of the 25 eyes found with drusen deposits, 12 eyes had drusen deposits that were ≥ 63 μm. In this group of eyes (≥ 63 μm), expression of IL-1β was found to be significantly stronger in the combined retinal layers, Bruch’s membrane, choroid, and drusen. Immunoreactivity to CXCL-11 was significantly stronger in the combined retinal layers of eyes with drusen ≥ 63 μm, although significant differences were not observed in individual neuronal layers of the retina (Table 3D). Interestingly, p-STAT3 immunoreactivity was stronger in Bruch’s membrane in eyes with drusen ≤ 63 μm. However, expression of p-STAT3 in combined retinal layers was stronger in eyes with drusen ≥ 63μm. In particular, the ganglion cell and the inner nuclear layers show greater p-STAT3 expression in eyes with drusen ≥ 63 μm (Table 3D). No differences in CFI, RSAD2, and CXCL-10 expression levels were observed between the two groups (Table 3D).
Discussion
Here, we examined the expression levels of multiple candidate biomarkers of AMD by immunohistochemistry in human postmortem eye tissues and correlated the results with age and presence of drusen, two known risk factors for AMD development and progression. Most of the targets we tested were positively correlated with age and/or presence of drusen. This would be expected if drusen indeed induces inflammation via Aβ and/or AGEs [16].
IL-1β
IL-1β is strongly implicated in the pathogenesis of chronic inflammatory diseases [32]. First, aberrant auto-upregulation of IL-1β leads to excessive inflammation. Second, it promotes angiogenesis through upregulation of VEGF, the target of choice for slowing the exudative or wet form of AMD [33]. In this study, eyes from the older group demonstrated significantly stronger immunoreactivity for IL-1β than those from the younger group. Since the tissue samples were from individuals who had no known or diagnosed eye diseases, this result suggests that older eyes are more prone to an inflamed state. This is also consistent with studies identifying age as one of the biggest risk factors of AMD [1].
Additional examination of IL-1β accumulation showed that regardless of age, eyes with clinically significant drusen have significantly stronger immunereactivity localized to Bruch’s membrane and choroid layers (Figure 1). IL-1β is secreted by a number of cell types into local environment and systemic circulation. Serum levels of IL-1β do not change significantly with aging [34]. Also, in vitro stimulation of RPE cells with Aβ resulted in increased IL-1β expression [16,35]. While we cannot rule out an abnormal retinal accumulation of IL-1β derived from systemic circulation, at least part of the IL-1β accumulation with aging and drusen may be explained by increased IL-1β secretion by RPE cells. Furthermore, a sub-analysis of all eyes with drusen indicated that staining for IL-1β was significantly stronger in eyes with large drusen (≥ 63 μm), which we found to be enriched in the peripheral. Peripheral drusen, while producing little structural disruption, may have indirect effects on the cells in the macular region [36]. Interestingly, peripheral drusen ≥ 63 μm is strongly associated with CFHY402H polymorphism [37]. Taken together, these results suggest that, while expression and accumulation of IL-1β in eye tissue is correlated with the presence of drusen deposits, it is drusen ≥ 63 μm that are associated with increased levels of IL-1β. It is important to note that advanced age can potentially confound our conclusions in this analysis because the prevalence of drusen ≥ 63 μm is correlated with increasing age. Thus, further studies designed to control for this confounding factor are warranted.
p-STAT3
STAT3 is an oncogene and can be activated by IFNs; it induces transcriptional responses that block apoptosis and promote cell survival during inflammation [38,39]. In tumour cell lines, STAT3 can induce VEGF production either directly via activation of VEGF transcription or indirectly viahypoxia-inducible factor 1α [40]. We observed increased immunoreactivity for p-STAT3 with increasing age and with presence of drusen. The increase in pSTAT3 level is particularly prominent in retina with drusen ≥ 63 μm in diameter (Figure 2). In an age-controlled analysis, we continued to see STAT3 activation to be associated with presence of drusen ≥ 4 μm. These associations raise the possibility that components of drusen promote the activation of STAT3, which acts to protect the retina from apoptotic damages, and to promote exudative AMD through VEGF upregulation.
RSAD2
Increased RSAD2 expression has been reported in age-related diseases such as atherosclerosis, the pathology of which is associated with deposition of drusen components such as Aβ and AGE [26,41]. In atherosclerosis, local inflammation could cause increased RSAD2 expression, leading to abnormal lipid accumulation [42]. Lipid accumulation in Bruch’s membrane is associated with upregulation of VEGF in the choriocapillaris of mice [43]. Previously, we found that stimulation of RPE cells with either Aβ or AGE increased RSAD2 expression [16] (unpublished observation). Here, we reported increased RSAD2 immunoreactivity in eyes with drusen, but not in aged eyes. These data indicate that drusen component such as Aβ/AGE might upregulate RSAD2 expression through induction of local inflammation. The consequent lipid accumulation may further lead to VEGF upregulation in the choriocapillaris, a result that is implicated in neovascular AMD.
CXCL-10 and CXCL-11
RSAD2 is an antiviral protein, activated by IFN α, β, or γ [44]. Upregulation of RSAD2 suggests a role for interferon in AMD pathogenesis. We further examined this system by looking at the downstream targets of IFN-γ: CXCL-10 and CXCL-11 [21]. Both ligands are agonist of CXC receptor 3 (CXCR3). They promote chemotaxis of T-cells, B-cells, and natural killer cells [21]. Through CXCR3-mediated mechanism, CXCL-10 and 11 inhibit angiogenesis and inhibit endothelial cell proliferation (49, 50 of serum paper). Serum concentration of CXCL-10 increased dramatically with age and with the development of AMD [45,46]. Mo et al. [46] confirmed the accumulation of CXCL-10 in human eye tissue with AMD and proposed that CXCL-10 may function as a biomarker for AMD. Although we found the opposite in terms of age related changes in CXCL-10 concentration, this difference may be accounted for by the different methods used: serum versus histology. However, CXCL-10 accumulation was determined to be greater in eyes with clinically significant drusen. This trend continues in the younger age group.
Not much is known about the effects of aging on CXCL-11 levels. CXCL-11 immunohistochemical level is higher in younger eyes and no difference in staining intensity exists between those with and without clinically significant drusen (Table 3). However, CXCL-11 levels were higher in those eyes with drusen ≥ 63 μm. Moreover, CXCL-11 level was higher in tissue of the younger group with drusen (≥ 4 μm). These findings suggest that the relationship between drusen and CXCL-11 is complex. With normal aging, the macrophage population shifts from the pro-inflammatory M1 to the anti-inflammatory M2, which are associated with diminishing CXCL-11 expression [47]. Cytokines such as CXCL-11 and IL-1β are markers for M1 macrophage phenotype. CXCL-11 is also upregulated in AMD [47]. Our data suggest that CXCL-11 is an early precursor of disease progression, upregulated in younger eyes with drusen. This may warrant further investigation to determine its future role as an early indicator of AMD disease progression.
Summary
With age and drusen accumulation, the environment of the eye tends to shift more towards a pro-inflammatory state (Figure 6) through either IL-1β and/or possibly IFN pathways. Little change is induced in the complement inhibitor, CFI, in association with drusen, suggesting a failure to activate protective mechanisms against complement activation. Alternatively, the lack of change in CFI accumulation level could indicate that that the pro-inflammatory actions of drusen may not yet be enough to trigger inhibition of the complement cascade in healthy eye tissue. Here, we identified three inflammatory molecules in the postmortem human eye, p-STAT3, CXCL-10, and CXCL-11, to be strongly correlated with the presence of drusen within a population controlled for age (≤57 years old, Figure 6). This pattern of accumulation suggests that these molecules are closely involved with the presence of drusen rather than with aging, and may represent a predisposition toward AMD pathogenesis. A recent microarray study on mouse RPE cells reported expression changes in over 315 genes associated with advanced age [48]. Many pathways they identified were also found in our prior microarray study (unpublished observation) [16]. Similarly, Chen et al. [49] found that the mouse neuroretina displayed age-related upregulation of the complement cascade and activation of retinal microglia [49]. Curiously, neither study found expression changes in any of the gene products examined in this study. It is important to note that drusen-like deposits have not been documented in wild-type-mouse eyes [50]. Thus, one interpretation for this discrepancy may be that the genes identified in our microarray studies, and specifically the gene products studied here reflect a drusen-specific response rather than a general response to aging. Our studies now set the stage for future experiments testing the function of each of these potential mediators in the pathogenesis of AMD. One approach might involve assessing the response of each mediator following Aβ and/or AGE injection into young wild-type mice to determine degree of mimicry to AMD pathology.
Figure 6.
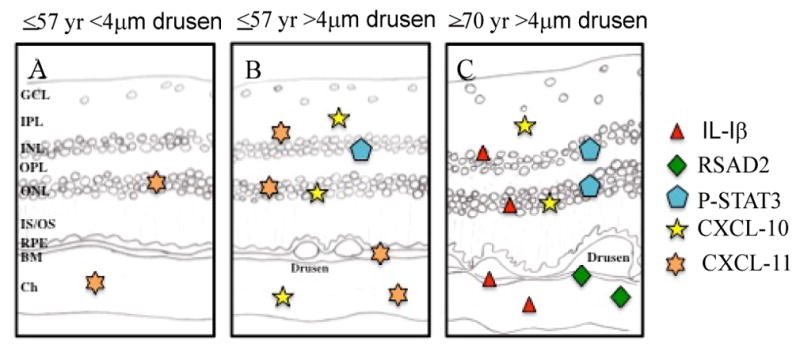
Summary diagram depicting the distribution of IL-1β, RSAD2, p-STAT3, and CXCL-10/11 immunoreactivity in post-mortem eye tissue with respect to aging and present of drusen. Icons denote the relative immunostaining of each molecule in a given retinallocation.
Acknowledgments
We thank Jonathan Tang, Jonathan Coleman, and Dr. Ian Clark for discussion and editing of the manuscript. This work was funded by Canadian Institutes of Health Research (CIHR-MOP-97806) to J.A.M.
Grant Support
Supported by Canadian Institutes of Health Research (CIHR) Grant# MOP-97806 (to JAM).
References
- 1.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-Associated Deposits in the Human Retina. Invest Ophthalmol Vis Sci. 2008;49:743–750. doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The Role of Inflammation in the Pathogenesis of Age-related Macular Degeneration. Surv Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauleikhoff D, Barondes MJ, Minassian D, Chisholm IH, Bird AC. Drusen as risk factors in age-related macular disease. Am J Ophthalmol. 1990;109:38–43. doi: 10.1016/s0002-9394(14)75576-x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman HR, Chan C-C, Ferris FL, III, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein RJ, Zeiss C, Chew EY, Tsai J-Y, Sackler RS, et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel M, Chan C-C. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dastgheib K, Green WR. Granulomatous Reaction to Bruch’s Membrane in Age-Related Macular Degeneration. Arch Ophthalmol. 1994;112:813–818. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- 11.Penfold PL, Wong JG, Gyory J, Billson FA. Effects of triamcinolone acetonide on microglial morphology and quantitative expression of MHC-II in exudative age-related macular degeneration. Clin Experiment Ophthalmol. 2001;29:188–192. doi: 10.1046/j.1442-9071.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 12.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roque RS, Rosales AA, Jingjing L, Agarwal N, Al-Ubaidi MR. Retina-derived microglial cells induce photoreceptor cell death in vitro. Brain Res. 1999;836:110–119. doi: 10.1016/s0006-8993(99)01625-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye. 2011;25:127–139. doi: 10.1038/eye.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurji K, Cui J, Lin T, Harriman D, Prasad S, et al. Microarray Analysis Identifies Changes in Inflammatory Gene Expression in Response to Amyloid-Beta Stimulation of Cultured Human Retinal Pigment Epithelial Cells. Invest Ophthalmol Vis Sci. 2009;51:1151–1163. doi: 10.1167/iovs.09-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D, Elner SG, Bian Z-M, Till GO, Petty HR, et al. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang YS, Jeong M, Park JS, Kim MH, Lee DB, et al. Interleukin-1[beta] stimulates IL-8 expression through MAP kinase and ROS signaling in human gastric carcinoma cells. Oncogene. 2004;23:6603–6611. doi: 10.1038/sj.onc.1207867. [DOI] [PubMed] [Google Scholar]
- 19.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, et al. Macrophage Depletion Diminishes Lesion Size and Severity in Experimental Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 20.Jager MJ, Klaver CCW. Macrophages feel their age in macular degeneration. J Clin Invest. 2007;117:3182–3184. doi: 10.1172/JCI34070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petkovic V, Moghini C, Paoletti S, Uguccioni M, Gerber B. I-TAC/CXCL11 is a natural antagonist for CCR5. J Leukoc Biol. 2004;76:701–708. doi: 10.1189/jlb.1103570. [DOI] [PubMed] [Google Scholar]
- 22.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasler-Kan E, Wunderlich K, Hildebrand P, Flammer J, Meyer P. Activated STAT 3 in choroidal neovascular membranes of patients with age-related macular degeneration. Ophthalmologica. 2005;219:214–221. doi: 10.1159/000085730. [DOI] [PubMed] [Google Scholar]
- 24.Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83:358–368. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 26.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 27.Bressler NM, Bressler SB, West SK, Fine SL, Taylor HR. The Grading and Prevalence of Macular Degeneration in Chesapeake Bay Watermen. Arch Ophthalmol. 1989;107:847–852. doi: 10.1001/archopht.1989.01070010869032. [DOI] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 29.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–210. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–1460. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 31.Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, et al. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinarello CA. IL-1: Discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 33.Jung Y, Liu W, Reinmuth N, Ahmad S, Fan F, et al. Vascular endothelial growth factor is upregulated by interleukin-1β in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 34.Di Iorio A, Ferrucci L, Sparvieri E, Cherubini A, Volpato S, et al. Serum IL-1beta levels in health and disease: a population-based study. ‘The InCHIANTI study’. Cytokine. 2003;22:198–205. doi: 10.1016/s1043-4666(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Ohno-Matsui K, Yoshida T, Shimada N, Ichinose S, et al. Amyloid-beta up-regulates complement factor B in retinal pigment epithelial cells through cytokines released from recruited macrophages/microglia: Another mechanism of complement activation in age-related macular degeneration. J Cell Physiol. 2009;220:119–128. doi: 10.1002/jcp.21742. [DOI] [PubMed] [Google Scholar]
- 36.Al-Hussaini H, Schneiders M, Lundh P, Jeffery G. Drusen are associated with local and distant disruptions to human retinal pigment epithelium cells. Exp Eye Res. 2009;88:610–612. doi: 10.1016/j.exer.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Munch IC, Ek J, Kessel L, Sander B, Almind GJ, et al. Small, Hard Macular Drusen and Peripheral Drusen: Associations with AMD Genotypes in the Inter99 Eye Study. Invest Ophthalmol Vis Sci. 2010;51:2317–2321. doi: 10.1167/iovs.09-4482. [DOI] [PubMed] [Google Scholar]
- 38.Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 41.Peppa M, Uribarri J, Vlassara H. The role of advanced glycation end products in the development of atherosclerosis. Curr Diab Rep. 2004;4:31–36. doi: 10.1007/s11892-004-0008-6. [DOI] [PubMed] [Google Scholar]
- 42.Olofsson PS, Jatta K, Wagsater D, Gredmark S, Hedin U, et al. The antiviral cytomegalovirus inducible gene 5/viperin is expressed in atherosclerosis and regulated by proinflammatory agents. Arterioscler Thromb Vasc Biol. 2005;25:e113–116. doi: 10.1161/01.ATV.0000170130.85334.38. [DOI] [PubMed] [Google Scholar]
- 43.Rudolf M, Schloetzer-Schrehardt U, Michels S, Aherrahou Z, Doehring LC, et al. Lipid Accumulation in Bruch’s Membrane Is Associated With an Increased Expression of Vascular Endothelial Growth Factor (VEGF) in the Choriocapillaries. Invest Ophthalmol Vis Sci. 2005;46:1213. [Google Scholar]
- 44.Chin K-C, Cresswell P. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc Natl Acad Sci U S A. 2001;98:15125–15130. doi: 10.1073/pnas.011593298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196:298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Mo FM, Proia AD, Johnson WH, Cyr D, Lashkari K. Interferon gamma-inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4226–4236. doi: 10.1167/iovs.09-3910. [DOI] [PubMed] [Google Scholar]
- 47.Cao X, Shen D, Patel MM, Tuo J, Johnson TM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61:528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS One. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in Retinal Aging: A Gene Expression Study. Invest Ophthalmol Vis Sci. 2010;61:528–535. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- 50.Luhmann UF, Robbie S, Munro PM, Barker SE, Duran Y, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009;50:5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]