Abstract
Mesenchymal stem cells (MSCs) have well-established paracrine effects that are proving to be therapeutically useful. This potential is based on the ability of MSCs to secrete a range of neuroprotective and anti-inflammatory molecules. Previous work in our laboratory has demonstrated that intravenous injection of MSCs, treated with superparamagnetic iron oxide nanoparticle fluidMAG-D resulted in enhanced levels of glial-derived neurotrophic factor, ciliary neurotrophic factor, hepatocyte growth factor and interleukin-10 in the dystrophic rat retina. In this present study we investigated whether the concentration of fluidMAG-D in cell culture media affects the secretion of these four molecules in vitro. In addition, we assessed the effect of fluidMAG-D concentration on retinoschisin secretion from genetically modified MSCs. ELISA-assayed secretion of these molecules was measured using escalating concentrations of fluidMAG-D which resulted in MSC iron loads of 0, 7, 120, or 274 pg iron oxide per cell respectively. Our results demonstrated glial-derived neurotrophic factor and hepatocyte growth factor secretion was significantly decreased but only at the 96 hour’s time-point whereas no statistically significant effect was seen with ciliary neurotrophic factor secretion. Whereas no effect was observed on culture media concentrations of retinoschisin with increasing iron oxide load, a statistically significant increase in cell lysate retinoschisin concentration (p = 0.01) was observed suggesting that increasing fluidMAG-D concentration did increase retinoschisin production but this did not lead to greater secretion. We hypothesize that higher concentrations of iron-oxide nanoparticle fluidMAG-D have an effect on the innate ability of MSCs to secrete therapeutically useful molecules and also on secretion from genetically modified cells. Further work is required to verify these in vitro finding using in vivo model systems.
Index Terms: Blindness, magnetic nanoparticles, retina, stem cells
I. Introduction
Mesenchymal stem cells (MSCs) are multipotent non hematopoietic stromal cells found within many tissues. They are characteristically able to differentiate into osteocytes, chondrocytes, and adipocytes, and have a major role in regenerating mesenchymal lineages in both physiologic and pathologic conditions [1], [2]. Interest in the therapeutic potential of MSCs was originally focused on their tissue regenerative properties but more recently their paracrine effects, in terms of neurotrophic and immunomodulatory abilities have become increasingly recognized. MSCs are known to protect injured neurons, stimulate angiogenesis in stroke, promote wound healing, and inhibit fibrosis through their paracrine effectors. MSCs have been shown to secrete neuroprotective molecules such as: brain-derived neurotrophic factor, ciliary neurotrophic factor (CNTF), insulin-like growth factor 1, nerve growth factor, vascular endothelial growth factor, and basic fibroblast growth factor, as well as immunomodulatory molecules: matrix metalloprotease-9, tumor necrosis factor-alpha, interleukin 10 (IL-10), and hepatocyte growth factor (HGF) [1].
Ex vivo expanded MSCs have, therefore, been investigated as a potential cell based therapy in a wide range of clinical applications with over 100 MSC clinical trials currently listed by the U.S. National Institutes of Health trial database (www.clinicaltrials.gov). The remote delivery of magnetized MSCs, via the systemic circulation, to sites of disease has recently been the focus of work for a number of research groups [3]–[5]. The use of superparamagnetic iron oxide nanoparticles (SPIONs) to magnetize cells is based on a number of advantages: 1) the degree of magnetization possible means that external magnetic fields can be used to target the cells to specific locations; 2) such cells can be visualized by magnetic resonance imaging; 3) in vivo, SPIONs are converted into nontoxic ions; and 4) such biodegradability would avoid long-term toxicity as a consequence of long term storage [6]. Both in vitro [7] and in vivo studies [8], [9] have reported that such particles are nontoxic. However, it has been suggested that SPIONs do have some effect on MSC physiology. For instance it has been shown that SPIONs might also disrupt normal iron-oxide homeostasis. It has also been shown that they can cross the blood-brain-barrier, accumulate in other organs and that cell agglomeration in the presence of an external magnetic field might cause harmful vascular embolization [10].
In our recent in vivo study, we showed that intravenous, fluidMAG-D-labeled MSCs can be magnetically targeted to a specific locus in the dystrophic rodent retina [3]. Specifically to assess toxicity, we undertook a MTT assay (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay) using bone-marrow derived MSCs in the presence of three different concentrations (0.05, 0.25 and 0.5 mg/mL) of SPION fluidMAG-D. We observed at least 95% cell survival at each of these concentrations. ELISA measurements from rat retinal tissue showed that this magnetic cell therapy resulted in enhanced tissue concentrations of neuroprotectant molecules: glial cell derived neurotrophic factor (GDNF) and CNTF; and anti-inflammatory factors: IL-10 and HGF. In this present study we proposed to determine whether exposing MSCs to fluidMAG-D influenced the cells ability to secrete these four molecules. In addition we proposed to determine whether fluidMAG-D had any effect on MSCs genetically altered to release a novel therapeutic molecule. For this latter work we elected to use MSCs genetically modified to secrete retinoschisin, an extracellular matrix molecule deficient in x-linked retinoschisis, the commonest cause of blindness in young children [11].
II. Experiment
A. MSC Harvest and Culture
MSCs were isolated from inguinal adipose tissue based on our previously described methods (where MSCs were isolated based on their phenotypic characteristics of adherence to plastic surfaces and ability to differentiate into adipocytes, chondrocytes or osteocytes) [3]. Postnatal day 21 Sprauge-Dawley rat tissue was used for work on innate MSC secretion (of GNTF, CNTF, IL-10 and HGF). Postnatal day 20 C57BL/6 mouse tissue was used for work on MSCs genetically modified to secrete retinoschisin. Briefly, fresh tissue was harvested and distributed at 5 g per well of a 6-well dish. The tissue was macerated with surgical scissors in 3 mL of 1 mg/mL Collagenase 1 (Sigma) in DPBS (Life Technologies) and incubated at 37 °C for 1 h. Collagenase-dissociated tissue was filtered through a sterile 40 μm cell strainer into a 50 mL tube and centrifuged at 1000 g for 10 min. The supernatant was removed and the cell pellet was resuspended in MesenCult MSC Basal Medium (Stemcell Technologies) supplemented with 15% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin (Life Technologies). The cells were plated in a 6-well dish and allowed to adhere for 48 h in a 37 °C incubator with 5% CO2. Following the initial incubation, medium was replaced and upon reaching confluency the cells were trypsinized and transferred to a T-75 cell culture flask.
B. MSC Transfection
For stable transfections, the human retinoschisin 1 (RS1) cDNA (gift from Dr. R. Molday) was cloned into pIRES2-DsRed2 (Clontech) under the control of the CMV promoter. This plasmid carries the neomycin-resistance gene for the selection of transfected cells with the antibiotic G418. Mouse MSCs were electroporated using the Gene Pulser Xcell system (BioRad). Briefly, MSCs from an early passage (3–5) were trypsinized, counted and collected by centrifugation. One million cells were re-suspended in 500 μL electroporation buffer, transferred into a 0.4 cm electroporation cuvette (BioRad) and mixed thoroughly with 100 μL plasmid DNA (1μg/μL). A square wave pulse of 220 V for 25 ms was applied to the cells, which were then quickly plated in a 6-well dish in 2 ml MSC medium and incubated in a 37 °C incubator. Twenty four hours later, the medium was replaced with selection medium 200 μg/ml G418 (Life Technologies) in MSC medium and selection continued for two weeks. Expression of RS1 was confirmed by visualization of the reporter gene DsRed2, using a Zeiss LSM 510 META confocal laser scanning system attached to a Zeiss Axiovert 200 M inverted microscope.
C. Incubation With Magnetic Nanoparticles
Rat MSCs and RS1-expressing mouse MSCs were collected from confluent plates and cultured overnight at a density of 10,000 cells per well of 24-well plates. FluidMag-D particles, superparamagnetic iron oxide nanparticles coated with starch of average size 2000 nm (ChemiCell GmbH, Germany), were diluted in MSC medium at concentrations of 0.05 mg/mL, 0.25 mg/mL and 0.5 mg/mL and 350 μl from each dilution was distributed to designated wells. These concentrations were chosen to correspond with previous studies using 0.05 mg/mL [3] and 0.5 mg/mL [4]. Cells incubated without fluidMag-D served as control. The secretion of GDNF, CNTF, IL-10, HGF and RS1 was examined at 24 and 96 h after the addition of fluidMag-D. Experiments were done in duplicates. To determine the iron load carried by MSCs treated with different fluidMag-D concentrations, 50 mL aliquots containing 250,000 cells from all four groups were suspended in distilled water and underwent inductively coupled plasma atomic emission spectroscopy (Exova, Surrey, Canada). These experiments were done in triplicate.
D. Cell Lysis
Cells were lysed by the addition of 250 μL lysis buffer (10 mM Tris-HCl pH 7.4, 2 mM EDTA, 150 mM NaCl, 0.875% Brij-96, 0.125% NP-40, 1 μg/ml Aprotinin, 1 μg/ml Leupeptin and 174 μg/ml PMSF) to each well of a 24 well plate. Lysates were collected in 0.5 mL tubes and were subjected to 3 freeze-thaw cycles. After a brief centrifugation for 1 min at 10,000 g, the supernatant was collected and used in ELISA.
E. Enzyme-Linked Immunosorbent Assay (ELISA)
Following 24 or 96 h incubation with the respective concentrations of fluidMAG-D, 100 L of cell lysate or culture medium were used to determine the concentration of the following molecules using sandwich ELISA kits. ELISA kits for GDNF (Emax ImmunoAssay System, Promega; Madison, WI), CNTF (RayBiotech; Norcross, GA), IL-10 (Abnova; Taipei City, Taiwan), HGF (B Bridge; Cupertino, CA), and Retinoschisin (Uscn Life Sciences Inc., Wuhan, China) were each used according to the manufacturer’s instructions. Visualization of the chromogenic substrates was measured at 450 nm using a POLARstar Omega plate reader (BMG LABTECH, GmbH, Germany). For innate protein secretion assays (GDNF, CNTF, IL10 and HGF) three technical repeat assays were undertaken from each of two biological replicate samples (n = 6). Data was analyzed as mean values ± standard error of the mean. For retinoschisin assays, two separate biological assays were undertaken at each data-point.
F. Statistical Analysis
In order to determine whether a significant positive or negative effect of fluidMAG-D was present in the results of each set of the ELISA assays, we fit the data to a linear curve using standard simple regression analysis (Sigma Plot versus 10 Systat software, San Jose, CA, U.S.A.), and determined values for the coefficient of determination (R2) and for the F statistic and corresponding P value in order to test the hypothesis that the regression coefficient (i.e. slope) ≠ 0.
III. Results and Discussion
A. Innate MSC Secretion of Neuroprotective Molecules
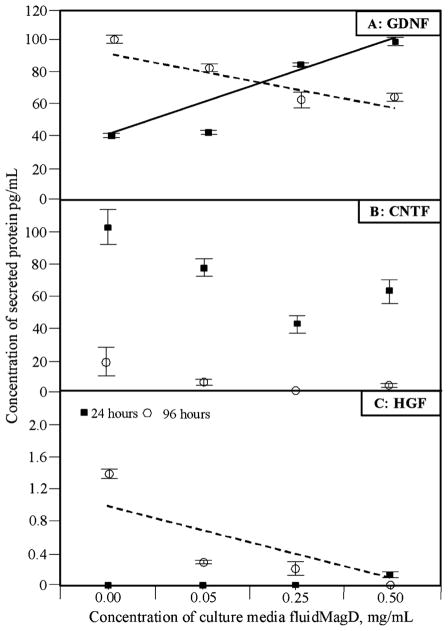
After 24 h of incubation in the presence of iron-oxide nanoparticles, regression analysis suggested that GDNF concentration in culture media significantly increased with increasing fluidMAG-D dose (R2 = 0.75; p < 0.0001). However, this had reversed by 96 h incubation (R2 = 0.82; p < 0.0001) (Fig. 1(a)). This contrasted with CNTF concentration in culture media which suggested a trend of reduced concentration with increasing fluidMAG-D dose at both time-points (Fig. 1(b)). However, these changes were not statistically significant suggesting no effect on CNTF secretion (at 24 hour timepoint R2 = 0.04; p = 0.35 and at 96 h timepoint R2 = 0.04; p = 0.33). These conflicting results between GDNF and CNTF at different time-points suggest that different effects are being triggered by exposure to fluidMAG-D.
Fig. 1.
ELISA assay results from culture media of rat MSCs. Untreated cells were compared with cells cultured with either: 0.05 mg/mL; 0.25 mg/mL; or 0.5 mg/mL fluidMAG-D. Measurements were undertaken after 24 h and 96 h. Measurements obtained for—(A): GDNF (glial-derived neurotrophic factor); (B): CNTF (ciliary neurotrophic factor); and (C): HGF (hepatocyte growth factor). Each data point represents the mean value (n = 6) ± standard error of the mean. Lines correspond to the best fit regression line at 24 (solid line) and 96 h (dashed line) that were statistically significant.
MSC Secretion of Anti-Inflammatory Molecules
For anti-inflammatory molecule IL-10, no significant secretion could be detected at either the 24 or 96 h time-points for untreated cells or cells treated with fluidMAG-D. For HGF also, little could be detected at the 24 h time-point. However, significant concentrations were detectable in culture media at the 96 h time-point suggesting a statistically significant suppression of HGF with increasing fluidMAG-D dosage (R2 = 0.49; p = 0.0001) (Fig. 1(c)). These results are in contrast with results observed in vivo where significant levels of both IL-10 and HGF were detected in retinal tissue containing fluidMAG-D treated cells [3].
B. Secretion of Retinoschisin From Genetically Modified MSCs
Increasing fluidMAG-D concentration had no statistically significant effect on retinoschisin secretion into culture media (at 24 h timepoint R2 = 0.1; p = 0.43 and at 96 h timepoint R2 = 0.03; p = 0.68 [Fig. 2(a)]. However, it was noted that increasing fluidMAG-D concentration was associated with increasing retinoschisin concentration in cell lysate (at the 96 h timepoint R2 = 0.83; p = 0.01) [Fig. 2(b)]. This former result at first might appear unsurprising since the retinoschisin construct is driven by a CMV promoter and is independent of normal cell mechanisms. The latter results, however, suggest that this might be an oversimplification. This may be explained as fluidMAG-D increasing retinoschisin production but with a corresponding inhibitory effect on retinoschisin secretion from the MSCs. An alternative explanation might be that the transport and secretory mechanisms employed by MSCs to secrete retinoschisin are easily saturated and are unable to deliver ever increasing amounts of retinoschisin production.
Fig. 2.
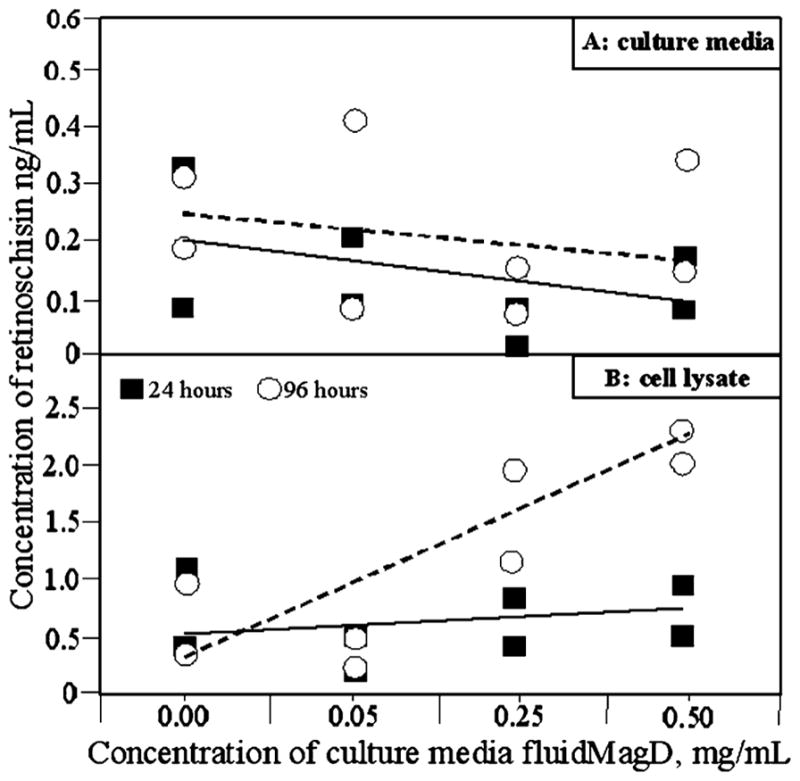
ELISA assays of—(A): culture media and (B): cell lysate, of mouse MSCs genetically modified to secrete retinoschisin. Untreated cells were compared with cells cultured with: 0.05 mg/mL; 0.25 mg/mL; or 0.5 mg/mL fluidMAG-D. Measurements were undertaken after 24 h and 96 h. Each point represents a separate biological repeat sample. Lines correspond to the best fit regression line at 24 (solid line) and 96 h (dashed line).
C. Iron Load
The amount of iron carried by the cells after 24 h of incubation was measured. It was found that increasing fluidMAG-D concentration in culture media was associated with increasing concentration within cultured MSCs. Emission spectroscopy results were averaged (n = 3 for each datapoint) and showed that no fluidMAG-D in culture media resulted in nondetectable iron oxide load. Doses of 0.05, 0.25 and 0.5 mg/mL fluidMAG-D resulted in corresponding iron oxide loads in MSCs of 7, 120 or 274 pg iron oxide per cell. These figures correlate with iron oxide levels reported in other studies [3], [4], [8].
Although our previous studies [3] had suggested that SPION treatment had little impact upon MSC viability in vitro, this present study does suggest some effect on MSC secretion in vitro. An initial stimulatory effect on GDNF secretion was seen along with suppression in the longer term (96 h). It is possible that increasing fluidMAG-D dosage initially induces a ‘stress response’ in cells, increasing the secretion of protective molecules but that this is eventually overtaken by a toxic effect inhibiting secretion [12]. It is unclear, however, why fluidMAG-D did not have the same effect on CNTF secretion. This suggests that fluidMAG-D has selective effects on cells rather than a general toxic influence affecting many biochemical pathways. This might be explained in two ways. Firstly that fluidMAG-D has an effect on the intracellular manufacture of only some molecules or inhibits their intracellular transport and secretion at the cytoplasmic membrane. Further studies are required to establish the exact mechanism of action; however, effects on retinoschisin secretion in genetically modified MSCs could suggest an inhibition of retinoschisin transport and/or secretion rather than an inhibitory effect on protein production (since lysate concentrations actually increased with fluidMAG-D concentration without increase in culture supernatant). The secretion of retinoschisin from cells is partially controlled by actin polymerization [13]. This may be affected by SPION treatment since at a concentration of 0.5 mg/mL, SPIONs can cause formation of actin stress fibers in endothelial cells [14]. It is, therefore, possible to hypothesize that SPIONs such as fluidMAG-D have some inhibitory effects on cell protein secretion whilst also stimulating or having no effect on intracellular production of these proteins. Given that our in vitro results, showing little IL-10 and HGF secretion, are opposite to our observations on secretion of these molecules in vivo, this would suggests that extrapolation of in vitro results should be done with caution.
In a study similar to that reported here [9], adipose-derived rat MSCs were incubated in the presence of two different concentrations of iron-oxide nanoparticle (0.05 mg/mL and 0.1 mg/mL). Total amounts of VEGF, HGF and insulin like growth factor mRNA were reported as unaffected by nanoparticle incubation. Our study, however, differs from this in key areas. The SPION used in our study, fluidMAG-D, is starch coated whereas in this previous study the magnetic nanoparticle used was dextran coated (ferumoxides). A difference between cellular handling of the dextran and starch coating might result in different metabolic effects. Interestingly though, we previously compared cell viability after treatment with starch coated and dextran coated nanoparticles and found that, at comparable concentrations, dextran coated SPIONs appeared more toxic (67% viability compared to 95%). In addition, the range of concentrations used (0.05–0.1 mg/mL) was much more restricted than the range used in our study (0.05–0.5 mg/mL). Finally, the total mRNA concentrations measured in this previous study reflect total cellular concentrations rather than concentrations of these molecules when secreted into the culture media.
IV. Conclusion
MSCs have demonstrable value in the treatment of neurodegenerative diseases such as retinopathy—specific examples of which include age-related macular degeneration and retinitis pigmentosa, the leading causes of blindness in the western World [15]. In particular, secretion of neuroprotective and immunomodulatory molecules suggest significant therapeutic potential [1]. Most pertinent to the present study, previous reports have already demonstrated a retinal neuroprotective effect with MSCs delivered either by intravenous or intravitreal injection [16], [17]. In addition, ex vivo genetic modification of MSCs could extend the range of therapeutic benefits of MSC therapy [18].
A significant problem, however, is how to efficiently deliver such cells to target organs. Systemic delivery has significant advantages, in particular in situations where cell therapy needs to be repeated over many years. Magnetic targeting (after SPION treatment) has already been shown to be a feasible and practical adjunct to systemic cell delivery [3]–[5]. However, there are contradictory reports on the effects of SPION treatment on the metabolism of MSCs. A number of studies have reported that SPION treatment does not affect pluripotency of the MSCs [3], [8]. However, it has also been reported that nanoparticle labelling can affect iron metabolism, migration capacity and colony formation although this does not appear to correlate with poorer cell viability [19]. The same study also suggested that SPION treatment enhances the adipogenic differentiation of the MSCs [19]. SPIONs have been shown to accumulate in cell endosomes and lysosomes but with time these nanoparticles are released into the cytoplasm, ultimately increasing the total cellular iron pool. It has been speculated that this could result in a range of effects including stimulation of cell proliferation and accumulation of toxic reactive oxygen species [7], [20].
Our results demonstrate some inhibitory effects on secretion of innate and genetically modified molecules from adipose-derived rat MSCs when exposed to increasing concentrations of fluidMAG-D. This data adds to the accumulating literature suggesting complex effects of SPIONS on cell physiology which may limit their potential clinical effectiveness. However, recommending that lower dosages of fluidMAG-D be used in future studies (which would correspondingly reduce the magnetization of cells) may not always be necessary. For instance whereas increasing dosage does inhibit GDNF secretion, if an intended effect of MSC therapy would be through CNTF secretion then higher fluidMAG-D dosage might have no deleterious effect. Further studies are required to more fully assess the extent to which deleterious effects influence MSCs and whether these in vitro results correlate with in vivo effectiveness [1], [21].
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Team Grant No. 222728).
References
- 1.Joe AW, Gregory-Evans K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res. 2010 Nov;35:941–52. doi: 10.3109/02713683.2010.516466. [DOI] [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012 Jun;14:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Yanai A, et al. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012 Aug;21:1137–1148. doi: 10.3727/096368911X627435. [DOI] [PubMed] [Google Scholar]
- 4.Loebinger MR, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009 Dec;69:8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riegler J, et al. Magnetic cell delivery for peripheral arterial disease: A theoretical framework. Med Phys. 2011 Jul;38:3932–3943. doi: 10.1118/1.3593363. [DOI] [PubMed] [Google Scholar]
- 6.Yu MK, et al. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed Engl. 2008;47:5362–5365. doi: 10.1002/anie.200800857. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, et al. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and cell vision. PLoS One. 2012 Jan;7:e29997. doi: 10.1371/journal.pone.0029997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balakumaran A, et al. Superparamagnetic iron oxide nanoparticle labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”. PLoS One. 2010 Jul;5:e11462. doi: 10.1371/journal.pone.0011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, et al. Superparamagnetic iron oxide does not affect the viability and function of adipose-derived stem cells, superparamagnetic iron oxide-enhanced magnetic resonance imaging identifies viable cells. Magn Reson Imag. 2009 Jan;27:108–119. doi: 10.1016/j.mri.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Tang C, et al. Concise review: Nanoparticles and cellular carriers-allies in cancer imaging and cellular gene therapy? Stem Cells. 2010 Sep;28:1686–1702. doi: 10.1002/stem.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayasarathy C, et al. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Invest Ophthalmol Vis Sci. 2007 Mar;48:991–1000. doi: 10.1167/iovs.06-0915. [DOI] [PubMed] [Google Scholar]
- 12.Blanco-Gelaz MA, et al. Endoplasmic reticulum stress signals in defined human embryonic stem cell lines and culture conditions. Stem Cell Rev. 2010 Sep;6:462–472. doi: 10.1007/s12015-010-9135-4. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura E, et al. Regulation of retinoschisin secretion in Weri-Rb1 cells by the F-actin and microtubule cytoskeleton. PLoS One. 2011 Jun;6:e20707. doi: 10.1371/journal.pone.0020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buyukhatipoglu K, Clyne AM. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A. 2011 Jan;96:186–195. doi: 10.1002/jbm.a.32972. [DOI] [PubMed] [Google Scholar]
- 15.Evans JR, et al. Causes of visual impairment in people aged 75 years and older in Britain: An add-on study to the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2004 Mar;88:365–770. doi: 10.1136/bjo.2003.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, et al. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS One. 2010 Feb;5:e9200. doi: 10.1371/journal.pone.0009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, et al. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009 Apr;247:503–514. doi: 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 18.Gregory-Evans K, et al. Ex vivo gene therapy and vision. Curr Gene Ther. 2012 Apr;1:103–115. doi: 10.2174/156652312800099607. [DOI] [PubMed] [Google Scholar]
- 19.Schafer R, et al. Functional investigations on human mesenchymal stem cells exposed to magnetic fields and labeled with clinically approved iron nanoparticles. BMC Cell Biol. 2010 Apr;11:22. doi: 10.1186/1471-2121-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naqvi S, et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomed. 2010 Nov;5:983–989. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Chen L, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008 Apr;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]