Abstract
Background
Rheumatoid arthritis (RA) treatment regimens are not adjusted in response to active disease as frequently as indicated. The objective of this study was to examine how disease activity and patients’ illness beliefs combine to predict future treatment changes in patients who are currently under the care of a rheumatologist.
Methods
We interviewed RA patients at baseline, 2, 4 and 6 months. We examined the association of disease activity (RAPID-4) and five specific illness beliefs (consequences, treatment control, symptom burden, concern, emotional impact) with future escalation of treatment using logistic regression. Analyses were adjusted for age, current biologic use, and disease duration. Disease activity and illness beliefs were dichotomized at the median to create four dummy variables (e.g. Low disease activity + High illness belief) in order to examine the combined impact of disease activity and illness beliefs on escalation.
Results
Twenty-nine percent of the participants (N=142) had an escalation of treatment during the follow-up period. When examined separately, disease activity and four of the illness beliefs (consequences, symptom burden, concern, and emotional impact) were associated with future escalation. High disease activity was predictive of future escalation only when combined with high levels of consequences, concern, and emotional impact. The combinations of high disease activity and high consequences, concern, and emotional impact were much stronger predictors or future escalation than either factor in isolation.
Conclusions
The findings suggest that patients’ illness beliefs are an important determinant of treatment decisions which are not fully captured by disease activity measures alone.
The current standard of care for rheumatoid arthritis (RA) includes aggressive management of disease activity in order to minimize inflammation and prevent future morbidity and disability (1–3). Despite the proven benefits of this approach, studies suggest that treatment regimens are not adjusted in response to active disease as frequently as indicated (4–8). The reasons for this gap in care are not well understood.
Access to specialists and inadequate insurance coverage are frequent barriers to implementation of best practices. This is particularly true for recommendations involving the use of expensive medications (9–11). Recent data suggest, however, that lower than expected rates of escalation occur in RA patients with adequate access to care. Specifically, Harrold et al (4), found significant underuse of disease modifying antirheumatic drugs (DMARDs) among insured patients under the care of a rheumatologist. Less than 60% of patients cared for by physicians participating in the Consortium of Rheumatology Researchers of North America registry had their treatment adjusted according to ACR guidelines in this study (4). Moreover, the guidelines for management of RA are based on high quality studies and there appears to be widespread endorsement of the principles underlying aggressive management of RA as indicated by a recent large survey of practicing rheumatologists (12). Thus, physician disagreement with published recommendations is also not a likely a barrier to implementation of guidelines (13).
Escalation of care according to prespecified targets requires routine monitoring of disease activity. While this practice has not yet been uniformly adapted, data suggest that escalation does not occur as frequently as expected even among patients found to have high disease activity using standardized assessments (14). Therefore, underuse of routine monitoring does not fully account for the lower than expected escalation rates in clinical practice.
Recommendations regarding the need for additional treatment are based primarily on composite measures which include at a minimum the patient’s assessment of their pain and function. While these factors are undoubtedly important, they may not adequately capture how patients conceptualize their illness. Specifically, patients’ judgments regarding the need for additional treatment are also likely influenced by the physical and emotional impact that symptoms have on their lives and by the beliefs they have regarding the potential efficacy of proposed new treatment(s) (15). If available disease activity tools do not adequately capture these factors, their scores may not correlate with patients’ perceived need for escalation of treatment in clinical practice.
One of the most extensive and prolific research programs dedicated to understanding how patients conceptualize their illness comes under the rubric of the “Common Sense Model” (CSM), developed by Leventhal and associates (16, 17). The CSM conceptualizes the patient as an active problem-solver, who seeks information and tests hypotheses about symptoms, evaluates whether they pose a health risk, and determines the need for treatment (15, 16). It proposes that these actions are guided by people’s representations of their illness. These representations, which are frequently referred to as illness beliefs, can be described across distinct domains, several of which might influence escalation rates including: 1) consequences: the patient’s perceived current impact of the illness on their life; 2) treatment: the patient’s perceived potential effectiveness of medical treatment; 3) identity: the burden of symptoms the patient identifies as being due to the illness; 4) concern: the patient’s concern about the illness; and 5) the emotional impact of the illness (18).
CSM studies of RA patients have found that beliefs about duration and treatment control have a greater influence on treatment adherence than does their knowledge of the disease (19, 20) and that beliefs about the identity and consequences of the illness influence disability and quality of life assessments (21). Patients’ illness perceptions are also associated with treatment preferences. In a recent study, the illness perception “consequences”, reflecting impact of disease, better predicted patients’ willingness to accept a highly effective/high risk treatment for pain versus a mildly effective/no risk treatment for pain compared to widely used disease activity measures (11-point numeric rating scale of pain intensity and SF-12 physical function component) (22). These findings suggest that RA patients’ illness perceptions may also influence the rates of escalation of treatment in clinical practice.
In this prospective study, we explore how disease activity and patients’ illness perceptions combine to predict future treatment changes in patients with RA who are currently under the care of a rheumatologist and have agreed to be treated with DMARDs. Specifically, we examine the association of 1) disease activity, 2) five illness beliefs, and 3) the combined impact of disease activity and patient beliefs on future escalation of treatment.
METHODS
Subjects and Interview Schedule
Patients with RA were recruited from four community-based rheumatology practices to participate in interviews at two-month intervals conducted over a six month period. Patients with RA on DMARDs were identified by the treating physician. The research assistant mailed all patients a letter briefly describing the study and notifying them that they would be telephoned by a research assistant. Potential subjects were offered the opportunity to refuse this contact by calling an answering machine and leaving a message. The research assistant contacted all patients who did not “opt out” by telephone to describe the study and confirmed additional inclusion criteria: 18 years of age or older; having seen their rheumatologist at least twice over the past 12 months; having pain rated at least “3” on an 11-point numeric rating scale; and currently prescribed at least one DMARD. Patients reporting a contraindication to biologics (diagnosis of cancer within the past five years, congestive heart failure, chronic lung disease, hepatitis C or B, open skin ulcers or a current infection) were excluded. An in-person interview was scheduled for patients who expressed interest in participating. The interview began by obtaining written consent and then proceeded to the baseline interview. Participants were given $25.00 after the baseline and final (6 month). The study protocol was approved by the Human Subjects Committee at our institution.
Measures
All data were collected in face-to-face interviews using paper and pencil questionnaires. Participant sociodemographic characteristics, duration of RA, and current medications were assessed at baseline. Disease activity was measured using the RAPID-4, one of the ACR-recommended measures for assessing and monitoring disease activity (23), which includes four components of the Multi-Dimensional Health Assessment Questionnaire: physical function assessment, arthritis-related pain numeric rating scale, patient global assessment, and Rheumatoid Arthritis Disease Activity Index (RADAI) self-reported joint count (24–26). While there are many disease activity measures available for RA, the RAPID-4 was chosen for this study because it can be administered without a physician’s assessment and does not require laboratory results. It has acceptable reliability, good validity and individual components are responsive to change (23). Patients’ illness beliefs were measured by five items from the 8-item Brief Illness Perception Questionnaire (BIPQ) (27). The BIPQ is based on the 57-item Revised Illness Perception Questionnaire and has been validated for acute and chronic pain patients (18). Each item is scored on a 10-point scale, with higher scores indicating greater severity or influence. Because each BIPQ item represents a specific belief, individual item scores are analyzed separately. We examined the five beliefs (all reflecting patients’ perceptions) having an expected relationship with patients’ treatment preference:
The Consequences scale measures how extensively the patient believes that the illness affects his or her life. Scores range from 0, no affect at all, to 10, severely affects my life.
The Treatment Control scale measures patients’ beliefs about the effectiveness of medical treatment. Scores range from 0, not at all, to 10, extremely helpful.
The Experience of Symptoms scale measures the extent that patients believe they experience symptoms of the illness. Scores range from 0, no symptoms at all, to 10, many severe symptoms.
The Concern scale measures the extent of patients’ concern about the illness. Scores range from 0, not at all concerned, to 10, extremely concerned.
The Emotion scale measures how much the illness affects patients emotionally. Scores range from 0, not at all affected emotionally, to 10, extremely affected emotionally.
Treatment escalation, the dependent variable, was defined as adding or increasing the dose of corticosteroids, and/or adding or switching DMARDs since the previous interview. Escalation was coded as an interview-level binomial variable (1 for an adjustment since the previous interview, 0 no adjustment since the previous interview). For the two patients who had two adjustments during the follow-up period, only the first adjustment was coded. The addition of an anti-inflammatory drugs and medication changes due to adverse events were not classified as treatment escalations.
Data Analysis Procedure
Analyses were conducted using SAS version 9.2. Escalations in treatment were predicted from disease activity and illness belief measures obtained from the preceding interview. Thus, if a treatment escalation was reported at the 6-month follow-up, the disease activity and illness belief scores were obtained from the 4-month follow-up, or from the closest earlier interview if the 4-month interview was not conducted. For patients who had no escalation in their treatment, the disease activity and illness belief scores were obtained from the data collected during the second to last interview.
We describe subject characteristics by escalation status and report correlations between each of the illness beliefs and the RAPID-4. We examined the association of disease activity and each illness belief using logistic regression. Analyses were adjusted for variables which might influence escalation of care: age, current biologic, and disease duration. We then created dummy variables in order to examine the combined impact of disease activity and illness beliefs on escalation. Each independent variable was dichotomized at the median to create four groups: Low disease activity + Low illness belief, Low disease activity + High illness belief, High disease activity + Low illness belief, High disease activity + High illness belief. These groups were subsequently analyzed using logistic regression, with the Low disease activity + Low levels of each illness belief group as the reference category.
RESULTS
Of 205 eligible subjects, 156 agreed to participate in the study. Of these, 15 dropped out after the baseline interview. Of the remaining 142, 94 patients completed all four interviews, 28 completed three of the four, and 21 completed two of the four. The 49 patients who completed fewer than four interviews were asked at follow-up why they did not participate. Reasons included unavailability due to work or vacation schedules, personal emergencies, or family obligations.
The majority of the participants were female, White, and had at least some college education. Additional subject characteristics are presented in Table 1. Sixty-four subjects were classified as having high disease activity. Twenty-nine percent of the participants (n=41) had an escalation of treatment during the follow-up period. Five were dosage increases of prednisone and 36 were a new disease-modifying agent.
Table 1.
Subject Characteristics at Baseline
| Variable | No Escalation N = 101 |
Escalation N = 41 |
Overall N = 142 |
|---|---|---|---|
| Mean Age (SD) | 58.39 (12.19) | 60.46 (12.93) | 58.77 (12.93) |
| Female (%) | 86 (85) | 36 (89) | 122 (86) |
| White (%) | 86 (85) | 29 (71) | 115 (81) |
| Married/partner (%) | 70 (69) | 18 (44) | 88 (62) |
| College graduates (%) | 39 (39) | 10 (24) | 49 (34) |
| Current medications (%) | |||
| Prednisone | 38 (38) | 15 (37) | 53 (37) |
| Methotrexate | 49 (49) | 26 (63) | 75 (53) |
| Other non-biologic DMARD | 15 (15) | 7 (17) | 22 (15) |
| Biologic | 48 (48) | 23 (56) | 71 (50) |
| Mean disease duration (SD) | 13 (12) | 11 (12) | 13 (12) |
| Mean disease activity* (SD) | 3.15 (1.41) | 4.04 (1.98) | 3.41 (1.64) |
| Illness beliefs* | |||
| Mean consequences (SD) | 6.06 (2.34) | 7.41 (2.44) | 6.45 (2.44) |
| Mean treatment control (SD) | 7.45 (2.19) | 7.10 (2.29) | 7.35 (2.21) |
| Mean experience of symptoms (SD) | 6.27 (2.34) | 7.29 (2.36) | 6.56 (2.38) |
| Mean concern (SD) | 7.53 (2.61) | 8.41 (2.41) | 7.79 (2.57) |
| Mean emotion (SD) | 5.17 (3.04) | 6.59 (2.85) | 5.58 (3.05) |
Possible range: 0–10.
Each illness belief was correlated with disease activity as measured by the RAPID-4 [consequence (r = 0.51, p<0.001), treatment control (r = −0.48, p<0.001), experience of symptoms (r = 0.60, p<0.001), concern (r = 0.33, p<0.001), and emotion (r = 0.49, p<0.001)].
Table 2 describes the relationship between disease activity, as measured by the RAPID-4, and the five illness beliefs with future escalation of treatment. Disease activity and all illness beliefs were significantly associated with escalation of treatment except for “Treatment Control” (i.e., participants’ perceived efficacy of treatment). The associations for the combinations of disease activity and the four significant illness beliefs on future escalation of treatment (using the Low disease activity/Low level of each illness belief as the reference group) are reported in Table 3. High disease activity was not associated with future escalation in patients reporting low levels of perceived consequences, concern and emotional impact. Subjects with high disease activity and low symptom burden were more likely to escalate treatment (Adjusted odds ratio = 2.39); however, this association did not reach statistical significance. Only subjects with both high disease activity and high levels of consequences, concern, and emotional impact were more likely to escalate treatment compared to those with low disease activity and low levels of each respective illness belief. Moreover, the combination of disease activity and illness belief better predicted future escalation than either factor in isolation.
Table 2.
Relationship between Disease Activity and Illness Beliefs with Future Escalation of Treatment
| Variable | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Disease activity | 1.40 (1.11 –1.77) | 1.43 (1.12 – 1.82) |
| Illness beliefs | ||
| Consequences | 1.29 (1.09 – 1.53) | 1.34 (1.13 – 1.60) |
| Treatment control | 0.93 (0.79 – 1.10) | 0.91 (0.77 – 1.08) |
| Experience of symptoms | 1.21 (1.03 – 1.43) | 1.25 (1.05 –1.48) |
| Concern | 1.16 (0.99 – 1.36) | 1.21 (1.02 – 1.44) |
| Emotion | 1.18 (1.04 –1.34) | 1.19 (1.04 – 1.35) |
Table 3.
Relationship between Subgroups of Disease Activity Combined with Illness Beliefs and Future Escalation of Treatment
| Subgroup defined by disease activity and illness belief levels: | Unadjusted Odds Ratio (95% CI)* | Adjusted Odds Ratio (95% CI)* |
|---|---|---|
| Low disease activity/High consequences (n= 28) | 2.10 (0.69 – 6.41) | 2.44 (0.78 – 7.69) |
| High disease activity/Low consequences (n= 16) | 1.21 (0.28 – 5.23) | 1.09 (0.25 – 4.79) |
| High disease activity/High consequences (n= 48) | 4.44 (1.73 – 11.44) | 5.06 (1.91 – 13.44) |
| Low disease activity/High symptom burden (n= 26) | 1.26 (0.40 – 3.95) | 1.34 (0.44 – 4.49) |
| High disease activity/Low symptom burden (n= 17) | 2.29 (0.68 – 7.69) | 2.39 (0.70 – 8.25) |
| High disease activity/High symptom burden (n= 47) | 2.85 (1.16 – 7.03) | 2.95 (1.18 – 7.38) |
| Low disease activity/High concern (n= 32) | 1.15 (0.38 – 3.49) | 1.30 (0.41 – 4.13) |
| High disease activity/Low concern (n=22) | 1.21 (0.35 – 4.16) | 0.95 (0.26 – 3.44) |
| High disease activity/High concern (n= 42) | 3.74 (1.45 – 9.64) | 4.57 (1.65 – 12.66) |
| Low disease activity/High emotional impact (n= 29) | 1.02 (0.33 – 3.17) | 0.92 (0.29 – 2.89) |
| High disease activity/Low emotional impact (n= 18) | 1.11 (0.30 – 4.13) | 0.95 (0.25 – 3.60) |
| High disease activity/High emotional impact (n= 46) | 3.28 (1.33 – 8.10) | 3.39 (1.33 – 8.64) |
Reference groups are patients with low disease activity and low levels of the specified illness belief.
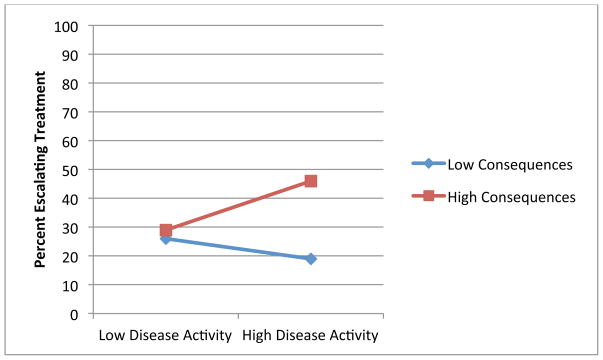
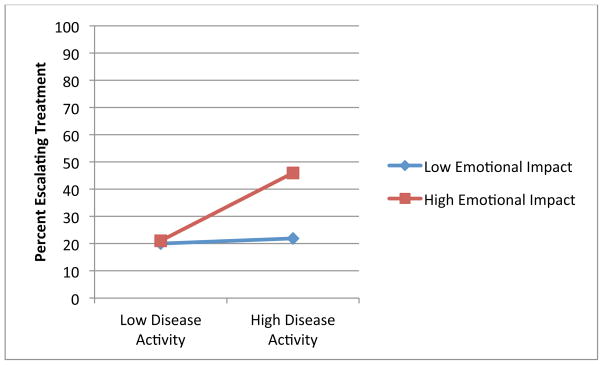
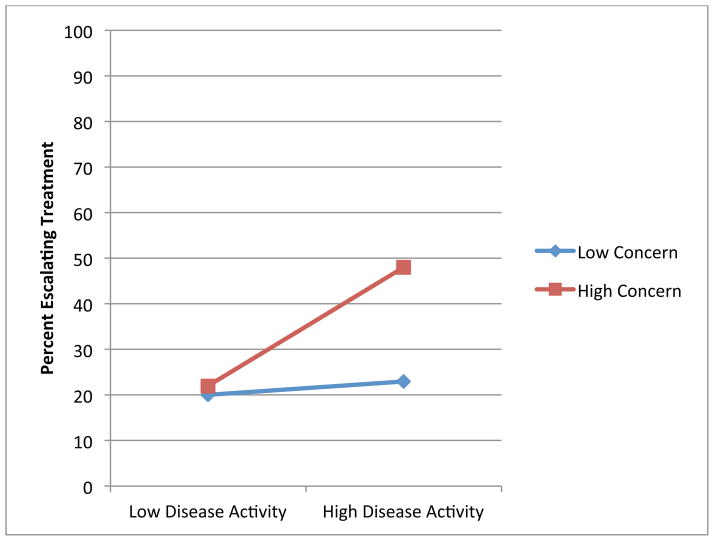
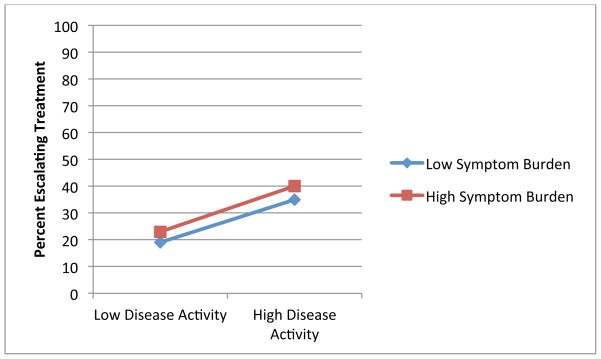
Figures 1 through 4 illustrate the clinical significance of the interaction between disease activity and illness beliefs. The percent of participants escalating care is uniformly low except for subjects with high disease activity who are also significantly impacted by, and concerned about, their RA as illustrated by Figures 1, 3 and 4. We did not find an interaction with symptom burden and disease activity.
Figure 1.
Association of Disease Activity and Treatment Escalation by Consequences of RA
Blue: Low Levels of Illness Belief
Red: High Levels of Illness Belief
Figure 4.
Association of Disease Activity and Treatment Escalation by Level of Emotional Impact
Blue: Low Levels of Illness Belief
Red: High Levels of Illness Belief
Figure 3.
Association of Disease Activity and Treatment Escalation by Level of Concern
Blue: Low Levels of Illness Belief
Red: High Levels of Illness Belief
DISCUSSION
While randomized controlled trials have demonstrated the efficacy of adjusting treatment in response to disease activity, recent studies have shown that many RA patients are not treated according to widely endorsed recommendations. Recommendations to escalate treatment are based on well-validated disease activity measures; however, these measures may not fully capture the patients’ perspective. In this study, we found that the combination of both disease activity and specific patient illness beliefs were much stronger predictors of future escalation of treatment than either factor alone. Our results suggest that patients’ illness beliefs likely play a role in the treatment planning process and may be one of the factors accounting for the observed gap between guideline recommendations and DMARD use.
One explanation is that the illness belief measures, while highly correlated with disease activity, capture information at a level that is more meaningful to patients. For example, patients may not be willing to add a DMARD because certain joints are swollen, if the swelling in those joints is not having a significant impact on their lives. Moreover, because many factors (e.g., use of assistive devices, employment adaptations, joint protection techniques, and ergonomic modifications) can moderate the effects of disease activity, measures which directly assess the physical and emotional consequences of disease are likely more immediate indicators of patients’ perceived need for treatment escalation.
Illness beliefs may also be an important additional factor to consider over and above disease activity, because they reflect patients’ level of adaptation (or lack of) to RA. In this context, adaptation refers to the gradual diminished impact of RA on a patient’s quality of life over time. When a disease or condition initially develops, it is experienced as a loss from a previous health state. However, over time, most people adapt to their symptoms and/or functional limitations and establish a new reference point. The process of adaptation explains why paraplegics’ reported happiness is similar to that of non-disabled age-matched controls (28, 29). A patient who has adapted to their health state, as reflected by low levels of consequences, concerns, and emotional impact related to RA, might not perceive a need for additional treatment even if they have a high disease activity score. This hypothesis may also explain why we did not find an interaction between disease activity and symptom burden. This illness belief may measure the same constructs as those included in the RAPID-4, and while it quantifies the degree or extent of symptoms, it may not reflect the current impact of symptoms on patients’ lives.
There is a recognized reluctance to escalate care in response to a specified criterion when physicians perceive a disconnect between disease activity scores that are driven by subjective pain and not substantiated by objective findings (30, 31). Our results suggest that a second disconnect, i.e., high disease activity scores that are not accompanied by high physical and/or emotional impact of disease as perceived by patients, may also limit escalation rates. Patients who do not perceive that their condition is having a significant negative impact on their lives (e.g., patients with the RA robustus phenotype or those or those that have learned to cope with their illness and have adapted to a new steady state) are unlikely to be willing to accept the additional risks, inconveniences and costs associated with additional treatment. Future efforts should examine whether identifying this subset of patients and tailoring education efforts to 1) better explain the rational underling the ACR and EULAR guidelines, and 2) ensure accurate perceptions regarding the risks of toxicity, might improve escalation rates among patients with evidence of active RA who meet the criteria for treatment escalation.
The strengths of this study include the prospective design. In addition, we were able to measure actual adjustment of care (in contrast to behavioral intention). Eligibility criteria specified that patients had to be treated with at least one DMARD demonstrating that the lower than expected escalation rates were not solely due to patients’ reluctance to consider prescription medications. There are several important limitations to this study. The sample was not drawn from a registry of all RA patients, but relied on compiled lists from treating physicians. While we interviewed patients every two months, we did miss flares in RA, which explains the escalations occurring in subjects with low disease activity. In addition, the majority of subjects were White and female thereby limiting generalizability. Although our results improve our understanding of the thresholds used to adjust treatment in clinical practice, the proportion of patients escalating care was low, indicating the significant impact of other barriers.
This study advances the literature focused on improving our understanding of the barriers of adhering to guideline recommendations in RA. The findings suggest that patients’ illness beliefs are an important determinant of treatment decisions which are not fully captured by disease activity measures. To realize the benefits observed in clinical trials and to improve patient outcomes, a better understanding of the metrics used by patients and their physicians in clinical practice to adjust treatment is needed.
Figure 2.
Association of Disease Activity and Treatment Escalation by Burden of Symptoms
Blue: Low Levels of Illness Belief
Red: High Levels of Illness Belief
Significance and Innovations.
Treatment regimens in patients with active rheumatoid arthritis (RA) are not adjusted as frequently as indicated.
In this prospective study, we found that high disease activity was predictive of future escalation of treatment only when combined with high levels of RA-related consequences, concern, and emotional impact.
Illness beliefs in combination with disease activity scores may capture information that is more proximal to patients’ perceived need for additional treatment than disease activity scores alone.
Acknowledgments
This work was made possible by a grant from the Arthritis Foundation. Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR060231-01.
Footnotes
Both authors were involved in the design of the study and preparation of the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA. Updated ACR Rheumatoid Arthritis Guidelines American College of Rheumatology Annual Meeting; Chicago. 2011.2011. [Google Scholar]
- 3.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrold LR, Harrington JT, Curtis JR, et al. Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum. 2012;64:630–8. doi: 10.1002/art.33380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mease PJ. Improving the routine management of rheumatoid arthritis: The value of tight control. J Rheumatol. 2010;37:1570–8. doi: 10.3899/jrheum.091064. [DOI] [PubMed] [Google Scholar]
- 6.Schmajuk G, Schneeweiss S, Katz JN, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: Improved but not optimal. Arthritis Rheum. 2007;57:928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 7.Schmajuk G, Trivedi AN, Solomon DH, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–6. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hulst LTC, Hulscher MEJL, van Riel PLCM. Achieving tight control in rheumatoid arthritis. Rheumatology. 2011;50:1729–31. doi: 10.1093/rheumatology/ker325. [DOI] [PubMed] [Google Scholar]
- 9.Laires PA, Exposto F, Mesquita R, Martins AP, Cunha-Miranda L, Fonseca JE. Patients’ access to biologics in rheumatoid arthritis: A comparison between Portugal and other European countries. Eur J Health Econ. 2012;18:18. doi: 10.1007/s10198-012-0432-5. [DOI] [PubMed] [Google Scholar]
- 10.Orlewska E, Ancuta I, Anic B, et al. Access to biologic treatment for rheumatoid arthritis in Central and Eastern European (CEE) countries. Med Sci Monit. 2011;17:SR1–13. doi: 10.12659/MSM.881697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell AS, Olszynski WP, Davison KS, Koehn C, Haraoui B. Leveling the field in the treatment of rheumatoid arthritis with biologic therapies: Equal access for equal efficacy. Clin Rheumatol. 2010;29:233–9. doi: 10.1007/s10067-009-1338-1. [DOI] [PubMed] [Google Scholar]
- 12.Schoels M, Aletaha D, Smolen JS, et al. Follow-up standards and treatment targets in rheumatoid arthritis: Results of a questionnaire at the EULAR 2008. Annals Rheum Dis. 2010;69:575–8. doi: 10.1136/ard.2009.108472. [DOI] [PubMed] [Google Scholar]
- 13.Cabana Md, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines?: A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 14.van Hulst LTC, Creemers MCW, Fransen J, et al. How to improve DAS28 use in daily clinical practice? A pilot study of a nurse-led intervention. Rheumatology. 2010;49:741–8. doi: 10.1093/rheumatology/kep407. [DOI] [PubMed] [Google Scholar]
- 15.Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: A perceptual-cognitive approach. Psychol Health. 1998;13:717–33. [Google Scholar]
- 16.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Cogn Ther Res. 1992;16:143–63. [Google Scholar]
- 17.Cameron LD, Leventhal H. The self-regulation of health and illness behavior. New York: Routledge; 2003. [Google Scholar]
- 18.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- 19.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–67. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 20.Neame R, Hammond A. Beliefs about medications: A questionnaire survey of people with rheumatoid arthritis. Rheumatology. 2005;44(6):762–7. doi: 10.1093/rheumatology/keh587. [DOI] [PubMed] [Google Scholar]
- 21.Graves H, Scott DL, Lempp H, Weinman J. Illness beliefs predict disability in rheumatoid arthritis. J Psychosom Res. 2009;67:417–23. doi: 10.1016/j.jpsychores.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Fraenkel L, Falzer P, Fried T, et al. Measuring pain impact versus pain severity using a numeric rating scale. J Gen Intern Med. 2012;27:555–60. doi: 10.1007/s11606-011-1926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res. 2012;64:640–7. doi: 10.1002/acr.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pincus T. http://mdhaq.org/Content/Forms/RAPID/RAPIDScores.pdf [cited 2013]
- 25.Sullivan MB, Iannaccone C, Cui J, et al. Evaluation of selected rheumatoid arthritis activity scores for office-based assessment. J Rheumatol. 2010;37:2466–8. doi: 10.3899/jrheum.091349. [DOI] [PubMed] [Google Scholar]
- 26.Pincus T, Yazici Y, Bergman M, et al. A proposed continuous quality improvement approach to assessment and management of patients with rheumatoid arthritis without formal joint counts, based on quantitative routine assessment of patient index data (RAPID) scores on a multidimensional health assessment questionnaire (MDHAQ) Best Pract Res Clin Rheumatol. 2007;21:789–804. doi: 10.1016/j.berh.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception questionnaire. J Psychosom Res. 2006;60:631–7. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Schulz R, Decker S. Long-term adjustment to physical disability: The role of social support, perceived control, and self-blame. J Person Soc Psychol. 1985;48:1162–72. doi: 10.1037//0022-3514.48.5.1162. [DOI] [PubMed] [Google Scholar]
- 29.Wortman C, Silver R. Cataclysms, crises and catastrophies: Psychology in action. Washington D.C: 1987. Coping with irrevocable loss; pp. 189–23. Master lecture series. [Google Scholar]
- 30.Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum. 2012;64:2814–23. doi: 10.1002/art.34543. [DOI] [PubMed] [Google Scholar]
- 31.van Tuyl LH, Boers M. Patient’s global assessment of disease activity: What are we measuring? Arthritis Rheum. 2012;64:2811–3. doi: 10.1002/art.34540. [DOI] [PubMed] [Google Scholar]