Abstract
The vertebrate ectoderm gives rise to organs that produce mineralized or keratinized substances, including teeth, hair, and claws. Most of these ectodermal derivatives grow continuously throughout the animal’s life and have active pools of adult stem cells that generate all the necessary cell types. These organs provide powerful systems for understanding the mechanisms that enable stem cells to regenerate or renew ectodermally derived tissues, and remarkable progress in our understanding of these systems has been made in recent years using mouse models. We briefly compare what is known about stem cells and their niches in incisors, hair follicles, and claws, and we examine expression of Gli1 as a potential example of a shared stem cell marker. We summarize some of the features, structures, and functions of the stem cell niches in these ectodermal derivatives; definition of the basic elements of the stem cell niches in these organs will provide guiding principles for identification and characterization of the niche in similar systems.
Keywords: stem cells, niche, tooth, hair, nail, claw, ectodermal derivatives, Gli1
Several ectodermally derived follicular structures, such as teeth, hair and nails, produce mineralized or keratinized substances that are secreted throughout the animal’s life. The functions of these appendages include physical protection, camouflage, thermal insulation, feeding, and sensory perception. Comparing these three organs is useful for several reasons. First, a number of human genetic diseases, notably the ectodermal dysplasias, affect teeth, hair and nails in patients. Second, abnormalities of these organs can be readily detected because of their accessible exterior location, and this accessibility also can enable experimental procedures. Third, their relatively simple physiology makes these organs convenient models for deciphering the molecular underpinnings of postnatal renewal and regeneration. This is further facilitated by being able to capture all cell types, at successive stages of differentiation, in a single histological section.
Ectodermal appendages originate from an ectoderm-derived epithelium and a neural crest or mesoderm-derived mesenchyme [1–3]. Sequential epithelial-mesenchymal interactions drive development of these organs, which occurs in three phases: initiation, morphogenesis, and differentiation [4]. An epithelial thickening appears prior to an invagination into the mesenchyme. The mesenchyme then condenses into a papilla, followed by differentiation of specific epithelial and mesenchymal cell types. The morphogenesis of tooth, hair and claw utilize many of the same signaling pathways, as reviewed elsewhere [5–9], whereas differentiation of the mineralized or keratinized components is governed by organ-specific events.
The regenerative ability of tooth, hair and claw, as well as many other adult tissues, is dependent on tissue-specific stem cell (SC) populations that self-renew to maintain stable numbers and that possess the capacity to differentiate into distinct cell lineages. Adult SCs usually reside in a niche, which is a physiologically limited microenvironment whose nature and location vary, depending on the tissue [10]. Deciphering the signaling pathways that control the delicate balance between self-renewal and differentiation is fundamental to understanding how SCs are regulated in their niches.
Here, we provide an overview and comparison of the structures and signaling pathways involved in renewal and regeneration of mouse incisors, hair follicles and claws. Additionally, to illustrate potential similarities between these systems, we have compared the expression of Gli1, a known dental SC marker, in tooth, hair and claw.
Incisors
Teeth are used to catch and chew food and, in some species, for defense. The dentition of mammals encompasses great diversity. In contrast to humans, who replace their deciduous teeth, mice have only one set of teeth in their lifespan, consisting of four incisors and twelve molars. In some rodents, all teeth grow continuously, but in mice only the incisors grow continuously throughout the life of the animal.
The ability of the incisor to grow depends on the presence of epithelial and mesenchymal SCs that have the capacity to self-renew and differentiate into all cell types of the adult tooth (Fig. 1A). SCs located in the labial cervical loop (laCL) contribute to a population of transit-amplifying (T-A) cells that undergo several rounds of cell division before they move distally and differentiate into ameloblasts or stratum intermedium cells [11]. The ameloblasts secrete enamel matrix that mineralizes. The pre-odontoblasts, which are derived from SCs in the mesenchyme, are located adjacent to the inner dental epithelium and give rise to dentin-secreting odontoblasts that maintain contact with the basement membrane. Rodent incisors do not have a typical crown or root but rather possess a crown-like labial (near the lip) surface covered by enamel and a root-like lingual (near the tongue) surface where enamel is absent. Periodontal ligament and alveolar bone are derived from the mesenchyme and anchor the teeth to the bones in the jaw.
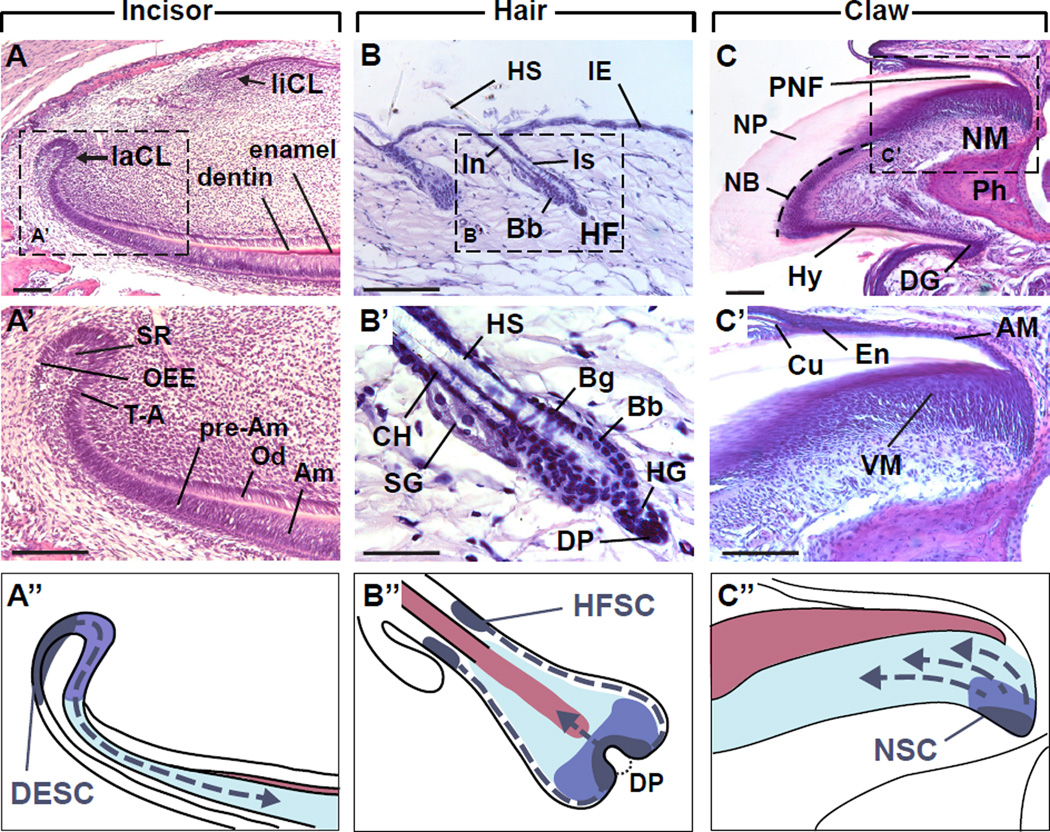
Figure 1. Stem cell location and direction of progeny movement in adult mouse incisor, hair and claw.
(A) In the proximal part of the incisor, the labial cervical loop (laCL) gives rise to enamel-producing ameloblasts, whereas on the lingual cervical loop (liCL) side, dentin is the only mineralized tissue. (A’) Stem cells in the laCL produce T-A cells, which differentiate into pre-ameloblasts (pre-Am). Subsequently, functional ameloblasts (Am) produce enamel, while the mesenchymal odontoblasts (Od) produce dentin. The SCs are housed in the stellate reticulum (SR) and outer enamel epithelium (OEE). (A”) The dental epithelial stem cell (DESC) niche is located in the proximal part of the laCL, and the progeny cells differentiate as they move distally. (B) The hair shaft, which protrudes from the interfollicular epidermis (IE), is produced by the hair follicle (HF) which consists of several portions: infundibulum (In), isthmus (Is), and bulb. (B’) Telogen hair follicle (HF) with sebaceous gland (SG) and club hair (CH). The bulb (Bb), hair germ (HG) and mesenchymal dermal papillae (DP) are located below the hair follicle bulge (Bg). (B”) The hair follicle stem cells (HFSC) are located in the outer root sheath in the bulge region. During anagen, the T-A matrix cells generate a new HS and surrounding cell layers. (C) The nail unit consists of a proximal nail fold (PNF), nail matrix (NM), nail bed (NB, dashed line), and hyponychium (Hn), and produces the nail plate (NP). The nail epithelium ends at the distal groove (DG). The terminal phalanx bone (Ph) supports the nail unit. (C’) The nail matrix consists of a ventral matrix (VM) and an apical matrix (AM). The eponychium (En) covers the distal part of the NP. The cuticle (Cu) is made of dead cells. (C”) The nail stem cells (NSC) appear to be located in the proximal part of the VM and provide differentiating cells that migrate distally to produce keratinized layers of the nail plate. A’ B’ and C’ depict hematoxylin and eosin stained tissue sections from 6 week old wild-type mice. In A”, B", and C", the SCs (dark blue), transit-amplifying cells (T-A, purple), differentiating/differentiated cells (light blue), and the mineralized or keratinized secretion (pink) are diagrammed. Scale bars: (A, A’, C, C’) 100 µm; (B, B’) 30 µm. Tissue processing and staining was performed as previously reported [12].
Slow-cycling SC populations have been identified using label retention experiments, either through injection with BrdU or with transgenic mice harboring a tetracycline-sensitive, histone 2B(H2B)-GFP cassette under the control of a tissue specific trans-activator [11, 12]. Label-retaining cells (LRCs) in the mouse incisor are found in the most proximal region of the mesenchyme and in the proximal halves of both the labial and lingual cervical loop. A number of genetic markers of dental SCs have been identified in recent years using in vivo lineage tracing. SCs expressing Gli1 (Fig. 2A) or Bmi1 reside in the LRC-containing regions of both epithelium and mesenchyme, whereas Sox2 marks SCs exclusively in the laCL [12–14]. How much the epithelial SC populations marked by these three factors overlap, or whether there exists a hierarchical relationship between them, remains to be determined. Expression of Lgr5 is found in a number of cells in the stratum intermedium of the laCl [15, 16]. In culture, these cells act like dental epithelial SCs [16]. As Lgr5 expression does not colocalize with the slow-cycling SCs, it has been suggested that this gene marks a subpopulation of active epithelial SCs. Recently, based on in vitro assays and in vivo expression analysis, integrin α6 (CD49f) was also suggested to be a marker of epithelial SCs [14, 17, 18].
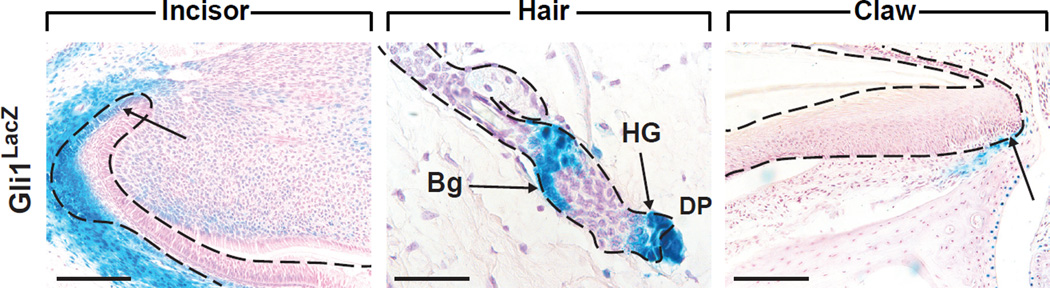
Figure 2. Gli1 expression in mouse incisor, hair and claw.
(A) In the incisor, Gli1 is expressed in the labial cervical loop epithelium proximal to the T-A area (arrow), as well as in the mesenchyme. (B) In the telogen phase hair follicle, cells in the bulge (Bg), hair germ (HG) and mesenchymal dermal papilla (DP) express Gli1. (C) In the nail unit, a small number of cells near the basal layer of the proximal ventral matrix are stained. Scale bars: (A, C) 100 µm; (B) 30 µm. X-Gal staining on Gli1lacZ/+ mice was performed as previously described [12].
Several signaling pathways regulate SCs in the incisor, including the FGF, BMP/TGF-β, Notch, and Hedgehog (HH) cascades; these have been reviewed in detail elsewhere [5] and are only briefly described here. Mesenchymal FGF3 and FGF10 and epithelial FGFR1b and FGFR2b levels regulate the size and shape of the epithelial SC niche [19, 20]. Follistatin (Fst) regulates Fgf3 expression [19], and deletion of Tgfbr1 (Alk5) in the mesenchyme led to down-regulation of Fgf3, Fgf9, Fgf10, and a reduced laCL size [21]. Sprouty genes, which are FGF inhibitors, prevent the generation of ectopic ameloblasts by the lingual CL SCs [22]. Furthermore, FGF signaling regulates E-cadherin expression, cell proliferation and migration in the incisor [23] and is essential for self-renewal of the epithelial SCs [16].
Notch signaling is also an important regulator of SCs. Notch1 and Notch2 are expressed in the incisor epithelium and mesenchyme, whereas Notch3 is restricted to the mesenchyme [11]. Inhibition of Notch signaling led to a reduction in the size of the laCL in explant experiments [24]. The SHH signaling pathway regulates SC progeny formation, such that the differentiating progeny of epithelial SCs express Shh, which signals back in a positive feedback fashion to the SCs [12]. Inhibition of HH signaling disrupts the generation of ameloblasts but not of another cell type, the stratum intermedium, from Gli1-positive SCs. Interestingly, the SCs do not appear to require HH signaling for survival. Finally, miRNAs play a role in the renewal and differentiation of adult SCs during SC-fueled incisor growth [13, 25], and how miRNAs regulate SCs in the tooth is an exciting new topic that is beginning to be explored.
Hair
A defining characteristic that distinguishes mammals from other vertebrates is that their skin is covered with hair, at least in part and at some point during their lifetime. Hair serves various functions, including protection of the body and sensory organs against environmental stresses, regulation of body temperature, sensory perception, facilitation of social interactions, and camouflage.
Each hair has two main portions. The visible part is the hair shaft that projects outside the skin and consists of dead keratinized cells. The part embedded into the skin, from which the hair shaft emerges, is the hair follicle; this is the living portion of the hair. As with developing teeth, both the development and the regeneration of hair during adulthood are tightly regulated by signal interactions between ectodermal epithelium and the underlying mesenchyme. The initial development of the hair follicle occurs in successive, morphologically distinguishable stages [7, 8, 26, 27]. In the mouse, epithelial cells start to form a placode that can be observed at day 14 of embryonic development (E14). Mesenchymal cells start to condense around the epithelial placode, and cell proliferation leads to formation of the dermal papilla in the mesenchyme and down-growth of the epithelial hair germ (E15.5). Following further down-growth (E16.5), the dermal papilla is almost entirely surrounded by keratinocytes. The subsequent bulbous peg stage (E18.5) is characterized by differentiation of epithelial keratinocytes to consecutively form the concentric epithelial layers that make up the mature hair follicle. The outermost of these layers, the outer root sheath (ORS), maintains contact with the basal layer of the epidermis and, in the bulge region, houses the hair follicle stem cells (HFSC). The layers of the inner root sheath (IRS) function as a channel for the emerging hair. The first hairs of the murine coat become visible around day 4 of postnatal development.
Throughout life, hair follicles undergo degeneration and regeneration in a cyclic manner. The hair follicle bulge, the distally adjacent, narrower region of the isthmus, and the infundibulum, the portion of the hair follicle located above the opening of the sebaceous duct, comprise the permanent portion of the hair follicle (Fig. 1B). During anagen, the growth phase, HFSCs give rise to highly proliferative T-A matrix cells, which in turn produce the differentiated cells that make up the new hair shaft and circumjacent IRS layers. Epithelial cells encompass the dermal papilla, and the entire hair follicle is dramatically elongated. During the following catagen phase, the proximally extended epithelium undergoes apoptosis and regresses, and the dermal papilla becomes re-positioned close to the permanent part of the hair follicle. Before re-entering anagen, the permanent portion of the hair follicle rests in relative quiescence in telogen stage.
HFSCs were initially discovered as LRCs in the bulge [28–30], and subsequent studies showed that these cells self-renew, are multipotent, and are able to reconstitute hair follicles when grafted together with dermis cells [31, 32]. Based on their expression within the bulge, integrin α6 and CD34 were proposed as SC markers, and these have been widely used for sorting HFSCs by flow cytometry [33]. Lineage tracing studies using tamoxifen-inducible Cre lines identified Krt14 [34], Krt15 [35], Lgr5 [36], and Gli1 [37] as markers expressed in HFSCs. Furthermore, hair follicle cells are derived from cells that express Shh or Sox9 during embryonic development [38, 39]. Cells within the bulge show non-overlapping patterns of expression of the proposed SC markers, and Lgr5 marks cycling cells rather than LRCs, which points to the existence of sub-populations of cells that may be heterogeneous with regard to their regeneration potential [36, 40]. HFSCs are located in the mid-region of the permanent portion of the hair follicle during active phases, and in closer proximity to the dermal papilla during the quiescent telogen phase.
Cyclic regeneration of hair follicles in the adult skin is tightly regulated by the interplay of numerous signaling pathways. This process involves extensive interactions between the SCs in the bulge and the surrounding mesenchymal compartment. Most of these signaling interactions are very similar to the ones that guide hair follicle morphogenesis [32]. Wnt signaling plays a dominant role during induction, patterning and morphogenesis of the hair follicle and is thought to constitute the first inductive signal for hair follicle formation. Numerous gain and loss of function studies have identified Wnt signaling as a driving force for HFSC activation and anagen entry [41]. Both overexpression of Wnt signaling and upregulation of HH signaling cause early anagen entry, hyperproliferation and formation of hair follicle tumors ([41, 42] and references therein). In the normal hair follicle, HH activity is dramatically increased during anagen and is required for regulating hair follicle re-growth [43, 44]. In contrast, BMP signals from the dermis and underlying adipocytes repress HFSC activation [45, 46]. During the hair cycle, BMP2 and BMP4 expression occurs periodically but out-of-phase with peaks of Wnt/β-catenin activation. The balance between both signaling pathways is critical and divides telogen into a refractory phase (BMP high, Wnt low) and a phase competent for hair regeneration (BMP low, Wnt high) [46, 47]. Furthermore, transient TGF-beta activation during late telogen through signals from the dermal papilla antagonizes BMP signaling in the HFSCs [48]. FGF7 and FGF10 also promote the shift to hair follicle regeneration that has been proposed to occur in two phases, involving activation of hair germ cells during the initial stage [49]. FGF18, in contrast, is crucial for maintaining the telogen phase and has repressive effects on the SCs in the bulge [32, 50].
Claws
Nails and claws provide protection to fingertips and toes and are used for climbing, self-defense, and manipulating food. Despite being easily accessible, these small organs have not been the focus of much study to date. However, as evidence has emerged pointing to a requirement for partial retention of the nail organ for mammalian digit regeneration [51, 52], the mechanisms underlying nail renewal have attracted more attention [53].
The nail unit [54], including the proximal nail fold, nail matrix, nail bed, and hyponychium, produces the nail plate (Fig. 1C). In the adult mouse, the ventral matrix consists of multiple layers of proliferating keratinocytes. These express epithelial keratins and eventually undergo apoptosis, migrating distally to produce keratinized layers of the nail plate [55]. The nail bed, which is more distal, consists of only 2 to 3 cell layers. A granular epithelium (hyponychium) starts after the nail plate detachment and ends at the distal groove. On the opposite dorsal side, the ventral matrix reflects outward to cover the proximal nail plate. The apical matrix becomes a granular ventral epithelium (eponychium), which joins the digit epidermis and produces the cuticle.
The nail stem cell (NSC) niche has not yet been well-defined. In BrdU pulse-chase experiments in mice, LRCs were described in the basal cell layer of the distal nail matrix adjacent to the nail bed [56]. In human fetal nails, which have a tissue architecture that is similar to the mouse claw [57], NSCs were suggested to be located in the ventral proximal nail fold [58] based on expression of follicular SC markers (KRT15, KRT19 and PHLDA1). Recentlyin vivo lineage tracing of Krt14-expressing cells provided further evidence that self-renewing SCs that sustain tissue growth are located in the proximal matrix [53]. Surprisingly, these cells are highly proliferative and express Ki67 [53], similar to Lgr5-expressing SCs in the intestine [59]. The Krt14-expressing cells in the proximal nail matrix are also positive for KRT17, a marker not expressed in the distal matrix, and double positive cells showed the highest regeneration potential based on their ability to form colonies in vitro [53].
Whether progenitors that normally facilitate nail growth also play a role in the regeneration of mammalian digits after amputation is a question of major interest. Conditional inactivation of β-catenin or Wntless in Krt14-expressing cells revealed an essential role for Wnt signaling in nail differentiation [53]. These mutants not only failed to produce a nail structure, but also were unable to regenerate bone after amputation of the digit tip distal to the SC niche. High levels of Wnt pathway activation can be observed in the distal nail matrix, but not in the SC-containing proximal part. Wnt pathway activation has an important role in the distal nail matrix in attracting the nerves required for promoting mesenchymal blastema growth.
Discussion
Almost 200 years ago, the naturalist de Blainville established his theory of the vertebrate “phanere” (phaneros in ancient Greek meaning “apparent”) as a “follicular organ…wherein the produced solid portion is excreted… and constantly remains on the surface of the animal” [60]. He was the first to consider teeth as components of the integumentary system, similar to hair, nail, claw, hoof, feather, horn and antlers, all of which were believed to be inorganic secretions [61, 62]. Today we know that, in addition to their externally visible, keratinized or mineralized acellular portion, teeth, hair, and nails are in fact very much alive, and they undergo stem-cell fuelled, directional growth as part of their normal homeostasis. This growth is a continuous renewal, in the case of the murine incisors and claws, whereas hair follicles show cyclic regeneration. By comparing the SCs and their niches in the mouse incisor, hair follicle, and claw, some common features, structures, and functions can be identified.
Location of the stem cells
Incisors, hair follicles and nails share a common tissue origin as ectodermal derivatives, and they undergo similar morphogenetic programs [5, 8, 26, 63]. These developmental parallels likely account for some of the similarities in their SC niches. In both tooth and nail, which continuously renew, SCs are located at the most proximal end, with the forming progeny progressing towards the distal aspect (Fig. 1A, C). In contrast, in the active hair follicle, the slowly cycling, label-retaining bulge stem cells are concentrated mostly in the mid-region of the permanent portion of the hair follicle (Fig. 1B). During the quiescent phase, these HFSCs are located in closer proximity to the mesenchymal dermal papilla. When activated, the organ first becomes extended in a proximal direction, from where a pool of T-A cells gives rise to differentiating cells that start moving distally to form a new hair shaft.
Some similarities in the components of the niches of these three organs have been identified. Epithelial (E)-cadherin is a cell-cell junctional protein essential in promoting morphogenesis and maintaining skin and its appendages [64, 65]. E-cadherin has recently been described as an important factor for the maintenance of incisor SCs as well [23]. Similarly, integrin alpha-6 marks incisor SCs [17, 18], and this protein is also expressed around the hair bulge [32] and in the basal layer of both nail matrix and epidermis [66].
The relationship between nerves and SCs in these organs is intriguing, as nerves are known to regulate the niche of certain SCs in mammals [67]. It has recently been shown that digit tip regeneration depends on nerves and mesenchymal SCs beneath the nail bed [53]. In the hair, the arrector pili muscle, inserted on the bulge, is stimulated by the sympathetic nervous system. The upper domain of the hair follicle bulge is Gli1 positive (Fig. 2), and this expression has been proposed to be due to SHH produced by nerves [37]. In the case of the tooth, the role of the nerves as potential sources of SHH is an area of active investigation.
Cycling status
SC pools with different cycling properties may be present in all three organs. In the incisor, so far only a group of slow cycling SCs in the proximal cervical loop has been identified, although as discussed above, hints that subpopulations of SCs may exist are beginning to emerge. In contrast, the SCs in the claw appear to turn over rapidly [53], and the role of the LRCs identified further distally in the nail bed remains unclear at present. In the case of hair, both slow and fast cycling SCs have been identified [28, 35, 36, 68]. At the beginning of anagen, hair germ cells are the first to be activated, and these start proliferating prior to activation of their progenitors in the bulge to regenerate the hair follicle [48, 49]. Early stem cell descendants cycle slower than further committed progeny, and both were shown to re-enter the bulge, as stem and niche cells respectively, at the end of the cycle [69]. A recent study using intravital microscopy for in vivo lineage tracing elegantly demonstrated the influence of spatial location within the niche on stem cell fate [70]. Furthermore, ablation experiments revealed that stem cells in the HF bulge are dispensable for HF regeneration and demonstrated a remarkable plasticity of epithelial HF cells, which are able to regenerate the missing SC population. Similar dynamics may exist in the incisor and nail systems but have not been studied to date.
Signaling and markers
The major signaling pathways that are responsible for orchestrating development and regeneration throughout the body, including FGF, HH, Wnt, BMP/TGF-β, and Notch, are either known or presumed to be active in the organs under discussion here. Gli1 is an important marker that has been demonstrated to label SCs in both the incisor and the hair follicle. Interestingly, in both systems there are multiple, physically or compartmentally separated pools of cells that express Gli1. In the incisor, expression is found in the LRC-containing regions of both labial and lingual epithelial SC niches as well as in the proximal mesenchyme. In the telogen hair follicle, cells in the upper bulge, the hair germ and the mesenchymal dermal papilla are Gli1-positive. This co-expression in (1) slowly cycling SCs, (2) cells with a capacity to quickly respond to anagen-inducing cues [49], and (3) cells that constitute a signaling center crucial for regulating cyclic growth is intriguing and points to a role for HH signaling in coordinating SC-regulating signals across the entire organ.
To investigate Gli1 as a potential marker for SCs in the nail system, we analyzed expression in the adult murine claw (Fig. 2). Interestingly, Gli1 marks a small group of cells near the proximal nail matrix where the fast cycling, Krt14-expressing, KRT17-positive SCs are housed [53], pointing to potential conservation of Gli1 as a marker for SCs in all three systems. Genetic lineage tracing studies in the nail will be required in the future to definitively determine if Gli1 marks SCs in this organ as well. While Gli1 itself is dispensable [71], HH signalling is required for tooth and hair follicle morphogenesis [72–74], as well as for generation of ameloblasts during adult renewal of the incisor and for anagen entry during the hair cycle and maintenance of molecular identity of the hair follicle stem cells [12, 37]. Expression of Gli1, which marks cells that respond to HH signalling [75], in the stem cell-containing region of the nail unit suggests a role for the HH pathway also during renewal of the nail. The precise role of HH signaling in this organ, and whether HH regulation directs a conserved function of the stem cells in all three organs, remains to be elucidated. In contrast to HH signaling, which appears to be active in all three organs, Wnt signaling is active in hair and nail, but at least based on the existing data, does not seem to be active in the incisor epithelium. It is interesting to note that the Wnt target gene Lgr5, which marks cycling SCs in the hair follicle, has been reported to be expressed in the laCL epithelium [15, 16]. However, lineage tracing from Lgr5-positive cells has not yet been performed in either the incisor or the claw. Comparison of the role of Wnt and HH signaling, as well as of other signaling pathways such as FGF and BMP, will likely reveal additional similarities between tooth, nail and hair epithelial SCs in the future.
In summary, the three organs discussed here have many similarities in terms of SC organization and function, as well as some important differences. By comparing SCs and their niches in these related yet distinct organs, we will obtain further insight into the myriad ways that renewal and regeneration are regulated.
Highlights.
-
-
Mouse incisor, hair and claw utilize stem cell-based continuous growth and have similar embryonic origins and architectures
-
-
Comparing the stem cell niches of these organs will improve our understanding of the mechanisms that enable renewal or regeneration in mammals
-
-
Gli1 is potentially a common stem cell marker for mouse tooth, hair and nail
Acknowledgements
We would like to thank R. Hesse for helpful comments and discussion. This work was supported by R01-DE021420 to O.D.K.; A.N is supported by Université Paris Descartes – Sorbonne Paris Cité, Fondation Bettencourt-Schueller, Institut Servier, Fondation des Gueules-Cassées, Fondation Philippe, Assistance Publique-Hôpitaux de Paris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement
The authors declare no competing financial interests.
REFERENCES
- 1.Narytnyk A, Gillinder K, Verdon B, Clewes O, Sieber-Blum M. Neural Crest Stem Cell-specific Deletion of the Pygopus2 Gene Modulates Hair Follicle Development. Stem Cell Rev. 2013 doi: 10.1007/s12015-013-9466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohazama A, Haworth KE, Ota MS, Khonsari RH, Sharpe PT. Ectoderm, endoderm, and the evolution of heterodont dentitions. Genesis. 2010;48:382–389. doi: 10.1002/dvg.20634. [DOI] [PubMed] [Google Scholar]
- 3.Soukup V, Epperlein HH, Horacek I, Cerny R. Dual epithelial origin of vertebrate oral teeth. Nature. 2008;455:795–798. doi: 10.1038/nature07304. [DOI] [PubMed] [Google Scholar]
- 4.Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39:35–50. [PubMed] [Google Scholar]
- 5.Jheon AH, Seidel K, Biehs B, Klein OD. From molecule to mastication: the development and evolution of teeth. Wiley Interdiscip Rev Dev Biol. 2013:165–182. doi: 10.1002/wdev.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernvall J, Thesleff I. Tooth shape formation and tooth renewal: evolving with the same signals. Development. 2012;139:3487–3497. doi: 10.1242/dev.085084. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 9.Hamrick MW. Development and evolution of the mammalian limb: adaptive diversification of nails, hooves, and claws. Evol Dev. 2001;3:355–363. doi: 10.1046/j.1525-142x.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 10.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 11.Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, Schober M, Fuchs E, Joyner A, Martin GR, de Sauvage FJ, Klein OD. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juuri E, Saito K, Ahtiainen L, Seidel K, Tummers M, Hochedlinger K, Klein OD, Thesleff I, Michon F. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev Cell. 2012;23:317–328. doi: 10.1016/j.devcel.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biehs B, Hu JK, Strauli NB, Sangiorgi E, Jung H, Heber RP, Ho S, Goodwin AF, Dasen JS, Capecchi MR, Klein OD. BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat Cell Biol. 2013;15:846–852. doi: 10.1038/ncb2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suomalainen M, Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 2010;239:364–372. doi: 10.1002/dvdy.22106. [DOI] [PubMed] [Google Scholar]
- 16.Chang JY, Wang C, Liu J, Huang Y, Jin C, Yang C, Hai B, Liu F, D'Souza RN, McKeehan WL, Wang F. Fibroblast Growth Factor Signaling Is Essential for Selfrenewal of Dental Epithelial Stem Cells. J Biol Chem. 2013;288:28952–28961. doi: 10.1074/jbc.M113.506873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JY, Wang C, Jin C, Yang C, Huang Y, Liu J, McKeehan WL, D'Souza RN, Wang F. Self-renewal and multilineage differentiation of mouse dental epithelial stem cells. Stem Cell Res. 2013;11:990–1002. doi: 10.1016/j.scr.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez MG, Yu W, Biehs B, Harada H, Snead ML, Lee JS, Desai TA, Klein OD. Characterization of dental epithelial stem cells from the mouse incisor with twodimensional and three-dimensional platforms. Tissue Eng Part C Methods. 2013;19:15–24. doi: 10.1089/ten.tec.2012.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsa S, Kuremoto K, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, Klein OD, Bellusci S. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development. 2010;137:3743–3752. doi: 10.1242/dev.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavanagh KD, Evans AR, Jernvall J. Predicting evolutionary patterns of mammalian teeth from development. Nature. 2007;449:427–432. doi: 10.1038/nature06153. [DOI] [PubMed] [Google Scholar]
- 22.Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CY, Cha W, Luder HU, Charles RP, McMahon M, Mitsiadis TA, Klein OD. Ecadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor. Dev Biol. 2012;366:357–366. doi: 10.1016/j.ydbio.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felszeghy S, Suomalainen M, Thesleff I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing mouse incisor. Differentiation. 2010;80:241–248. doi: 10.1016/j.diff.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Jheon AH, Li CY, Wen T, Michon F, Klein OD. Expression of microRNAs in the stem cell niche of the adult mouse incisor. PLoS One. 2011;6:e24536. doi: 10.1371/journal.pone.0024536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 28.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 29.Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of labelretaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 30.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 32.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 34.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 36.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 37.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim X, Nusse R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang LC, Liu ZY, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, Williams KP, Taylor FR, Barrandon Y, Ling L, Burkly LC. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901–908. doi: 10.1046/j.1523-1747.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- 44.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 45.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc Natl Acad Sci U S A. 2013;110:1351–1356. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oshimori N, Fuchs E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura-Ueki M, Oda Y, Oki J, Komi-Kuramochi A, Honda E, Asada M, Suzuki M, Imamura T. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J Invest Dermatol. 2012;132:1338–1345. doi: 10.1038/jid.2011.490. [DOI] [PubMed] [Google Scholar]
- 51.Borgens RB. Mice regrow the tips of their foretoes. Science. 1982;217:747–750. doi: 10.1126/science.7100922. [DOI] [PubMed] [Google Scholar]
- 52.Zhao W, Neufeld DA. Bone regrowth in young mice stimulated by nail organ. J Exp Zool. 1995;271:155–159. doi: 10.1002/jez.1402710212. [DOI] [PubMed] [Google Scholar]
- 53.Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, Taketo MM, Ito M. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499:228–232. doi: 10.1038/nature12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaias N. The nail in health and disease. Norwalk, CT: Appleton & Lange; 1990. [Google Scholar]
- 55.Norton LA. Incorporation of thymidine-methyl-H3 and glycine-2-H3 in the nail matrix and bed of humans. J Invest Dermatol. 1971;56:61–68. doi: 10.1111/1523-1747.ep12291905. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura M, Ishikawa O. The localization of label-retaining cells in mouse nails. J Invest Dermatol. 2008;128:728–730. doi: 10.1038/sj.jid.5701062. [DOI] [PubMed] [Google Scholar]
- 57.Fleckman P, Jaeger K, Silva KA, Sundberg JP. Comparative anatomy of mouse and human nail units. Anat Rec (Hoboken) 2013;296:521–532. doi: 10.1002/ar.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sellheyer K. Nail stem cells. J Dtsch Dermatol Ges. 2013;11:235–239. doi: 10.1111/ddg.12030. [DOI] [PubMed] [Google Scholar]
- 59.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 60.Ducrotay de Blainville MHM. De l'organisation des animaux ou principes d'anatomie compareée. Paris: 1822. [Google Scholar]
- 61.Hunter J. The natural history of the human teeth: Explaining their structure, use, formation, growth and deseases. London: Johnson, J.; 1771. [Google Scholar]
- 62.Cuvier FG. Des dents des mammifères, considérées comme caractères zoologiques. Strasbourg: Levrault, F.G.; 1825. [Google Scholar]
- 63.Chapman RE. Hair, wool, quill, nail, claw, hoof and horn. In: Bereither-Hahn J, Matoltsy GA, Sylvia-Richards K, editors. Biology of the integument. Vol. 2. Berlin, Heidelberg, New York: Vertebrates, Springer-Verlag; 1986. pp. 293–312. [Google Scholar]
- 64.Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis. Development. 1989;105:271–277. doi: 10.1242/dev.105.2.271. [DOI] [PubMed] [Google Scholar]
- 65.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cameli N, Picardo M, Tosti A, Perrin C, Pisani A, Ortonne JP. Expression of integrins in human nail matrix. Br J Dermatol. 1994;130:583–588. doi: 10.1111/j.1365-2133.1994.tb13103.x. [DOI] [PubMed] [Google Scholar]
- 67.Kumar A, Brockes JP. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012;35:691–699. doi: 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greco V. The death and growth connection. Nat Rev Mol Cell Biol. 2013;14:6. doi: 10.1038/nrm3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 72.Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 73.Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129:5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- 74.St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 75.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]