Abstract
In the non-transplant setting, aberrant serum adipokine levels associate with cardiovascular (CV) disease. The effects of liver transplantation (LT) on serum adipokine levels, and their association with post-LT CV disease have not been studied.
Methods
A nested case-control study of 77 patients with major CV events beyond 4 months post-LT had serum adiponectin, resistin, leptin, C-reactive protein and apolipoprotein levels measured prior to transplant and at 4, 12 and 24 months post-LT.
Results
Adiponectin and resistin levels decrease dramatically after LT in all patients. Recipients with CV disease achieved lower levels of adiponectin and higher levels of resistin, leptin, C-reactive protein and apolipoprotein B100 than controls. Pre-LT adiponectin level was associated with a 16% increase risk of CV event for every 1μg/mL decrement in adiponectin (HR 0.84, p=0.046). Pre-LT C-reactive protein (HR 1.03, p=0.047) and 12 month C-reactive protein (HR1.03, p=.029) were associated with CV events post-LT. Diabetes (HR 2.14, p=0.09), resistin (HR1.07, p=0.07) and Apolipoprotein B (HR 1.07, p=0.08) were associated with a non-significant increased risk of CV events in this small sample size.
Conclusion
Pre and post-LT changes in serum adipokine and inflammatory markers may be a signal for increased risk of CV events after liver transplantation, but further study is needed.
MESH terms: Adiponectin, Leptin, Resistin, Apolipoprotein, morbidity, outcomes
Introduction
Cardiovascular disease is a common cause of long term post-transplant morbidity and a leading cause of non-graft related mortality among liver transplant recipients (1, 2). Obesity, hypertension, diabetes and dyslipidemia have an increased prevalence after liver transplantation (2, 3). Reasons for the increased prevalence of metabolic syndrome and cardiovascular disease in liver transplant recipients are poorly understood.
Adipose (white fat) tissue is the production site of a wide number of inflammatory molecules and hormones (4). These hormones (adipokines) are associated with energy metabolism, glucose and lipid metabolism, cardiovascular function, immunity, and are closely linked to obesity. Abnormal levels of these markers, either individually or in combination have been associated with varying degrees of increased risk for metabolic syndrome and cardiovascular disease in the general population (5). These same hormones and inflammatory markers affect and are affected by liver function and fibrosis (6, 7). In addition, after a liver transplant, factors including the transplanted allograft itself and immunosuppressant medications may impact adipokine level and function. Corticosteroids are known to stimulate leptin release (8) and tacrolimus, thought to be diabetogenic, may decrease serum adiponectin levels (9). Many immunosuppressant medications including cyclosporine, tacrolimus and sirolimus result in elevated serum lipid levels (10, 11). Little is known about lipoprotein metabolism after liver transplant in adult recipients except that in the early phase (first weeks) after transplant apolipoprotein A-1 levels decrease and apolipoprotein B levels increase (12). We do not know if these levels increase or remains disturbed long term after transplant. Renal insufficiency is a common complication after liver transplantation, and both adiponectin and leptin are metabolized and excreted, at least in part, by the kidney. Whether these markers impact the prevalence of metabolic syndrome or cardiovascular disease post liver transplant is unclear.
The purpose of this pilot study is to measure adipokine levels and inflammatory markers including adiponectin, leptin, resistin, C-reactive protein and lipoprotein levels prior to and after liver transplantation. We aim to determine the effect of end-stage liver disease and liver transplantation on levels of these adipokines and inflammatory markers. In addition, we attempt to identify potential a clinical impact of these changes on metabolic syndrome and cardiovascular events after liver transplantation.
Methods
This study was a nested case-control study using data and serum samples of the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) liver transplant followup database. This database consists of 798 adult (≥18 years) patients receiving a liver transplant during the years April 1990 to June 1994 at three centers (Mayo Clinic, Rochester, University of Nebraska Medical Center, and University of California San Francisco Medical Center) with follow-up data obtained by January 2003 (median follow-up of 10 years, no followup is available beyond 2003). These patients had fasting serum samples frozen and stored prior to transplant and at all followup visits after transplantation.
The database was used to determine pre and posttransplant recipient metabolic variables (BMI, diabetes, hypertension, hyperlipidemia) and major cardiovascular outcomes (limited to myocardial infarction, cardiac arrest, cerebrovascular event, and/or heart failure). Arrhythmias not leading to heart failure or cardiac arrest were not included as major CV events in this study as we focused on potential atherosclerotic disease. Peripheral vascular disease was infrequently documented and thus not included. These events were diagnosed based on standard clinical criteria by the transplant physicians involved in the database of each of the 3 centers. Patients with reported cardiovascular (CV) events beyond 4 months of followup were identified and matched for age, transplant date and gender with control transplant patients whom were CV event-free at the time of the case’s event. The restriction to CV events beyond 4 months after transplant was chosen to ensure data from at least 2 serum analyses were available and events were less likely to be perioperative arrhythmia or fluid overload based events.
The database definition of diabetes mellitus (DM) required treatment with insulin or oral hypoglycemics. Clinicians would have determined this treatment based on standard clinical diagnostic criteria at that time. Post transplant diabetes was defined similarly at 1 year, which would exclude the transient peri-operative diagnosis. Renal insufficiency/failure (RI) was defined as serum creatinine ≥ 2mg/dL. The definition of hypertension (HTN) was a sustained blood pressure >150/95 or the use of antihypertensive medication. Smoking pre transplant was defined as currently smoking. Tacrolimus based immunosuppression was used in the minority of this cohort, (N=34).
Frozen serum samples obtained from fasting pre-transplant and post-transplant serum at 4 months, 12 months and 24 months were thawed and serum withdrawn for analysis using the following assays: high sensitivity c-reactive protein (ELISA ALPCO Immunoassays Cat #30-9710s, range of this assay is 1.9–150 ng/mL), Leptin (Millipore Cat #EZHL-80SK, range of this assay is 0.78 to 100 ng/mL human leptin), Adiponectin (ELISA Kit Millipore Cat # EZHADP-61K, standard range of this assay is 1.56 ng/mL to 100 ng/mL), Resistin (Millipore Cat #EZHR-95K, standard range of this assay is 0.02 to 100 ng/mL and corresponds to approximately 2 times the value in comparison to other commercially available Resistin Elisa kits, www.millipore.com\userguides), Apolipoprotein A1 (MABTECH Cat #3710-1HP-2, standard range of this assay is 0.316–31.6 ng/mL) and Apolipoprotein B (MABTECH Cat #3715-1HP-2, standard range of this assay is 10–1000 ng/mL). Ghrelin was determined unstable in stored serum and therefore not suitable for this analysis.
Statistical Analysis
Since subjects are matched on follow-up time this is a nested case-control study and the primary risk analysis is based on a Cox model, stratified by case/control group, resulting in hazard ratios (HR) and 95% confidence intervals for the association of each predictor with cardiac outcome. A P value of <.05 was considered significant. Baseline data is summarized using mean and standard deviation for the continuous variables, and counts and percentages for categorical variables. The statistical analysis was computed using SAS version 9.2 software (Copyright © 2002–2008 by SAS Institute Inc., Cary, NC, USA).
Results
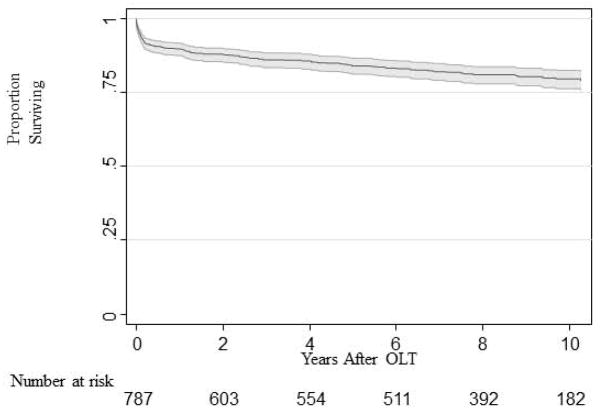
Over a mean of 10 years followup, 147/798 (18.4%) patients had a documented first major CV event. Overall, 72 first events were myocardial infarction/cardiac arrest, 49 congestive heart failure and 26 cerebrovascular events. Seventy-seven of the 147 patients were documented to have experienced this cardiovascular event more than 4 months after transplant. In this group, 43 myocardial infarction/cardiac arrests, 20 congestive heart failure episodes, and 14 cerebrovascular accidents were documented. Figure 1 demonstrates the major CV event-free survival of the entire 798 patients in this database.
Figure 1.
Cardiac Event free survival for the whole database cohort (N=798, Kaplan-Meier survival estimate).
Demographics of 77 cases and 77 controls are shown in Table 1. Diabetes was documented in 27 (18.8%) of all study patients prior to transplant and 58 (39.6%) of patients by 12 months after liver transplant. In the study cohort, mean BMI increased after transplant with a change from 25.6 (±5.5) pre transplant (corrected for ascites) to 25.4 (±4.9) by 4 months, 27.3 (± 5.7) by 12 months and 28.0(±5.9) by 24 months after transplant. Notably, only 8 patients in this cohort had a mean BMI above 35 kg/m2 before liver transplantation.
Table 1.
Pre-transplant Demographics
| Case N=77 |
Control n=77 |
P value | |
|---|---|---|---|
| Age, Mean (SD) | 55 (9.6) | 55 (8.8) | 0.647 |
| Male gender, N (%) | 50 (65%) | 50 (65%) | 1.000 |
| MELD score (SD) | 19.8 (8.5) | 18.8 (10.5) | 0.685 |
| Underlying disease % | |||
| Hepatitis C | 11.4% | 17.6% | 0.297 |
| Hepatitis B | 4.3% | 2.7% | 0.604 |
| Alcohol | 22.9% | 10.8% | 0.053 |
| Cholestatic | 27.1% | 33.8% | 0.387 |
| Cryptogenic | 15.7% | 8.1% | 0.157 |
| Other | 18.6% | 27% | 0.228 |
| BMI (corrected)* | 25.9 | 25.3 | 0.650 |
| Diabetes pre LT | 19 (25%) | 11 (14%) | 0.103 |
| Smoker | 38 (49%) | 39 (51%) | 0.795 |
| Hypertension | 19 (25%) | 15 (20%) | 0.452 |
| Renal failure** | 18 (24%) | 10 (13%) | 0.088 |
MELD= model end stage liver disease, BMI= body mass index corrected for ascites removed at transplant, LT = liver transplant
creatinine >2 mg/dL
Adiponectin (Figure 2A)
Figure 2.
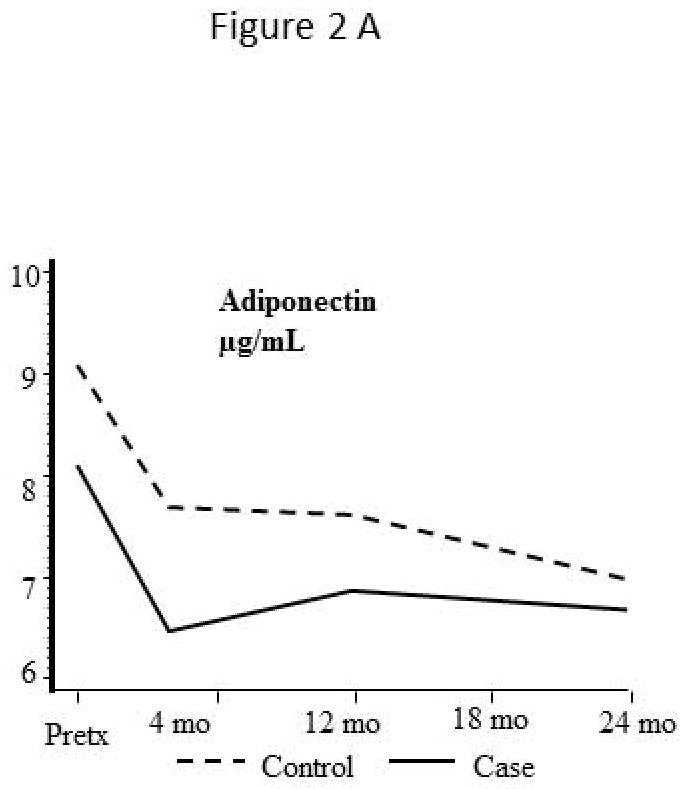
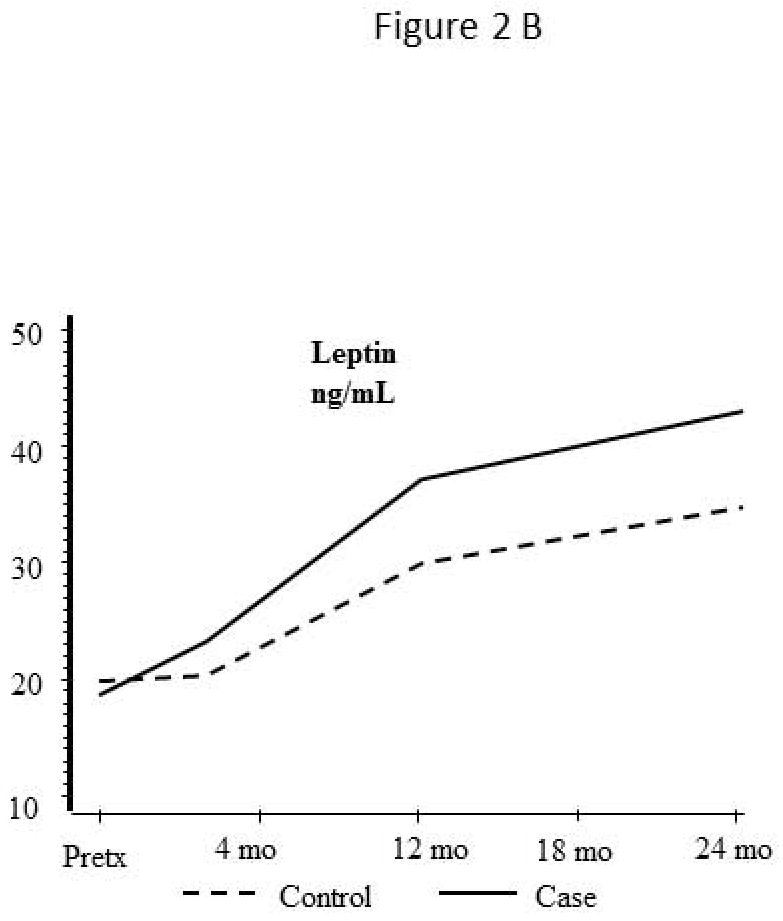
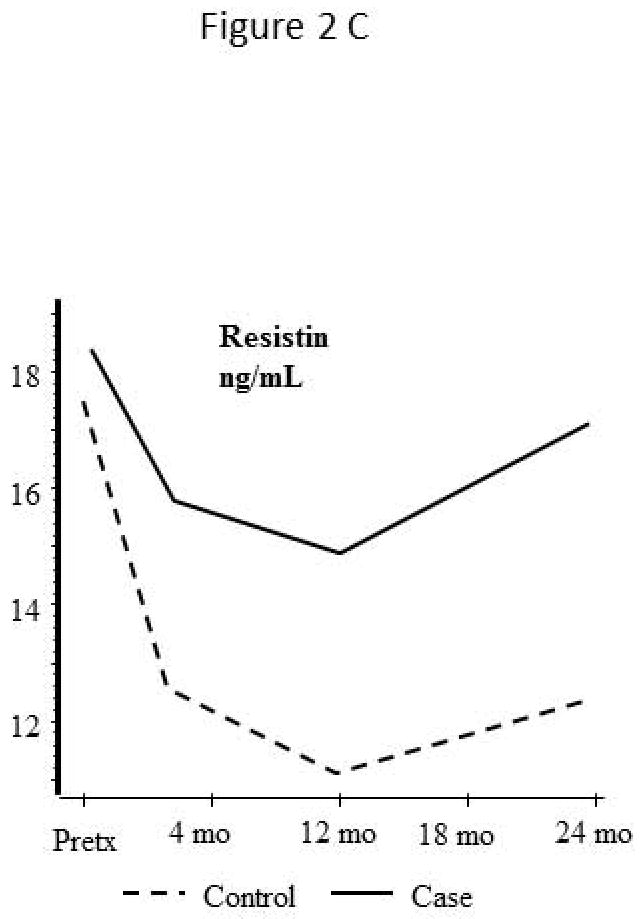

Levels of adipokine and inflammatory markers before and after liver transplantation
Serum levels of adiponectin are elevated in cirrhotic patients prior to transplant and decrease immediately after liver transplant. Adiponectin levels at the time of transplant were lower in major CVD cases (8.1 ±3.5 μg/mL) than controls (9.1 ±3.2 μg/mL), decreased by a mean of 1.9 μg/mL and 1.6 μg/mL respectively by 4 months post transplant and remain stable thereafter. Tacrolimus immunosuppression (used in 34 patients) was associated with reduced adiponectin levels after transplant, compared to cyclosporine based immunosuppression with median adiponectin levels at 24 months of 6.0±3.4 μg/mL compared to 7.6±3.5 μg/mL (p>0.05) respectively.
Leptin (Figure 2B)
Serum leptin levels were similar at the time of transplant in both groups and continue to rise over the 24 months after transplant. The increased level in CVD cases did not reach statistical significance (p =.19).
Resistin (Figure 2C)
Serum resistin levels are elevated in cirrhotic patients prior to transplant and decrease substantially after transplant. Patients with CV events in long term followup had a non-significant trend to higher serum resistin levels at 12 months post transplant (HR 1.07, per ng/mL increase, 0.99–1.15, p=0.072) than control patients.
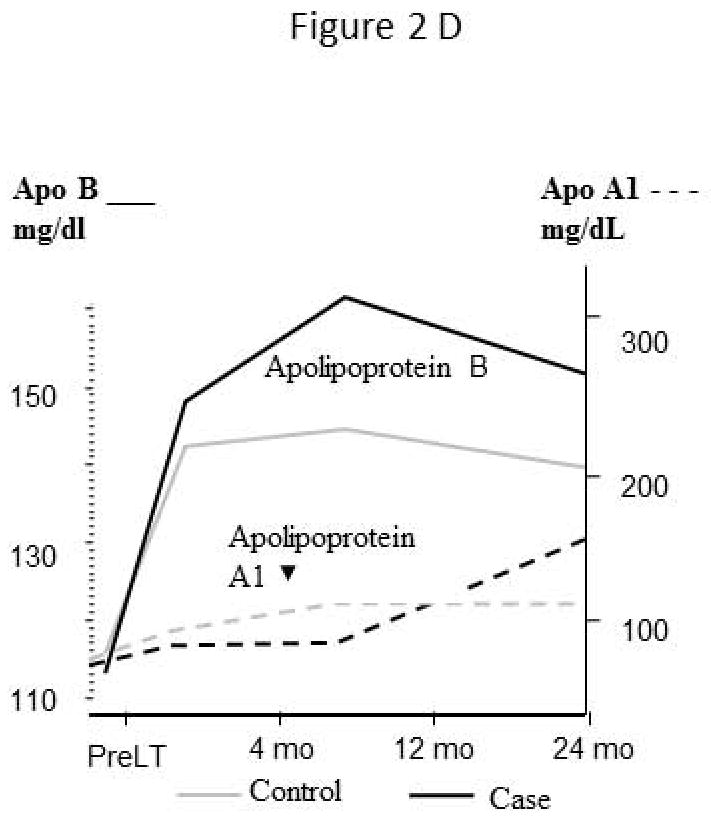
Apolipoproteins (Figure 2D)
Serum apolipoprotein A1 levels are similar in both groups and do not change substantially after liver transplantation to 12 months. Whereas, apolipoprotein B levels increased by 4 months post transplant in both groups. After 4 months however, the level of apolipoprotein B in patients with CV events continues to rise relative to the control group, but did not reach statistical significance (HR per ng/dL 1.08, 0.99–1.17, p=0.080).
High sensitivity C-Reactive Protein (hs-CRP) (Figure 2E)
Serum hs-CRP levels, which are elevated in cirrhotic patients, decrease after liver transplant but remained significantly higher in patients with future CV events than controls (HR per ng/mL1.03, 1.00–1.06, p=0.029).
Risk Analysis
Only a limited risk analysis could be performed given the small sample size. Univariate analysis for risk factors predictive of CV events is shown in Table 2a&b. Pre transplant serum adiponectin levels and hs-CRP were associated with increased risk of CV events within the case-control subset. For every 1 μg/mL decrease in pre transplant serum adiponectin levels, the risk of late (>4 months) CV events increased 16%. Pre transplant diabetes (HR 2.14, CI 0.87–5.25, p=0.09) and renal failure (HR 1.71, CI 0.67–4.53, p=0.26) also show clinically important effect sizes but with lack of statistical significance due to the small sample. When tested in the cohort as a whole (778 subjects) the overall hazard ratios for diabetes and renal failure do not differ significantly from the hazard ratio value found in the case-control subset, but p value becomes p=0.02. Only diabetes and renal failure at 12 months post-transplant were associated with CV events after transplant.
Table 2a.
Univariate analysis
Pretransplant variables associated with cardiovascular events post transplant.
| PreLT Variable | Hazard Ratio | CI | P value |
|---|---|---|---|
| Adiponectin* | 0.84 | 0.72–0.99 | 0.04 |
| Leptin | 0.99 | 0.97–1.01 | 0.50 |
| Resistin | 1.01 | 0.98–1.04 | 0.61 |
| Apo B:A1 | 0.94 | 0.74–1.19 | 0.59 |
| Apo B | 1.02 | 1.00–1.00 | 0.67 |
| Hs-CRP | 1.03 | 1.00–1.05 | 0.05 |
| Smoking | 1.27 | 0.58–2.80 | 0.55 |
| Diabetes | 2.14 | 0.87–5.25 | 0.09 |
| Hypertension | 1.20 | 0.52–2.78 | 0.67 |
| Renal Failure | 1.71 | 0.67–4.35 | 0.26 |
| BMI | 0.99 | 0.92–1.07 | 0.81 |
per 1 μg/mL
Apo= apolipoprotein, Hs-CRP= high sensitivity-C-reactive protein, BMI = body mass index
Table 2b.
Univariate analysis
Posttransplant variables associated with cardiovascular events after transplant
| 12 month Variable | Hazard Ratio | CI | P value |
|---|---|---|---|
| Adiponectin | 0.94 | 0.81–1.09 | 0.40 |
| Leptin | 1.01 | 0.99–1.03 | 0.20 |
| Resistin | 1.07 | 0.99–1.15 | 0.07 |
| Apo B/A1 | 0.88 | 0.59–1.28 | 0.41 |
| Apo B | 1.08 | 0.99–1.17 | 0.08 |
| Hs-CRP | 1.03 | 1.01–1.06 | 0.03 |
| Diabetes | 1.80 | 0.83–3.90 | 0.14 |
| Hypertension | 1.23 | 0.59–2.56 | 0.58 |
| Renal Failure* | 3.43 | 1.48–7.96 | 0.004 |
| BMI | 2.00 | 0.81–4.96 | 0.13 |
Apo = apolipoprotein, Hs-CRP= high sensitivity c-reactive protein, BMI = body mass index
defined as creatinine > 2mg/dL
In multivariate analysis, the effect of adiponectin was adjusted for each of the potential confounders. Due to the small sample size a full multivariate model is not feasible, and the adjustment was done for each variable 1 at a time. As shown in Table 3, the adjusted HR values range from 0.76 to 0.85. The attenuation is small and none are significantly different than the overall estimate of 0.85.
Table 3.
Bivariate analyses
| Pre-LT Variable | Hazard Ratio | CI | P value |
|---|---|---|---|
| Adiponectin | 0.850 | 0.70–1.02 | 0.084 |
| BMI | 0.991 | 0.87–1.13 | 0.889 |
| Adiponectin | 0.794 | 0.65–0.98 | 0.029 |
| Diabetes | 3.738 | 0.63–22.3 | 0.148 |
| Adiponectin | 0.838 | 0.69–1.01 | 0.068 |
| Smoking history | 1.546 | 0.49–4.88 | 0.458 |
| Adiponectin | 0.762 | 0.61–0.96 | 0.019 |
| Hypertension | 2.769 | 0.71–10.8 | 0.142 |
LT = Liver Transplant CI = confidence interval, BMI = body mass index, 4 m = 4 months
Discussion
This is first study to document the evolution of multiple serum adipokine and inflammatory markers before and after liver transplantation and reveals important insights into the metabolic changes associated not only with underlying liver failure (pre transplant) but also with the subsequent change over time after a liver transplantation. These changes are studied in association with important metabolic or cardiovascular clinical outcomes in the long term post-transplant setting. The ability to reach statistical significance in analysis of these changes is limited by the size and design of this pilot study, but the trends noted in these adipokine and inflammatory markers warrant further investigation.
High adiponectin levels (which normally protect against diabetes and obesity) prior to liver transplant has been presumed to be related to impaired liver clearance but does not appear associated with BMI, lipid or glucose metabolism in cirrhotic patients (13, 14). These levels decrease after successful liver transplantation. High adiponectin levels in kidney transplant patients were also shown to decrease after successful kidney transplant (15), suggesting a possible role of renal clearance of the hormone as well. Low adiponectin levels have been associated with atherosclerotic CV disease in the general population (16). Our study suggests lower adiponectin levels in the pre transplant setting are associated with increased CV events in the long term followup of liver transplant recipients. Determining appropriate risk levels in prospective studies would be of value and would require a much larger study.
The association of hs-CRP to CV disease is not novel, but this marker has not been proven to be a useful screening tool in end-stage liver disease patients or immunosuppressed liver transplant patients. However, hs-CRP in patients receiving kidney transplantation was strongly predictive of overall mortality and cardiovascular mortality over 3–5 year followup after kidney transplantation (17). Both hs-CRP and insulin resistance have been associated with increased cardiac transplant graft vasculopathy and worse outcomes related to this (18). In contrast to a previous study unable to link hs-CRP levels prior to liver transplant to post transplant CV disease (19), this study demonstrated both elevated pre and post transplant hs-CRP levels were associated with increased CV events over long term followup. The difference in findings may be related to the case-control study design. Hs-CRP serum levels are easily obtained, but unfortunately are non-specific markers of inflammation and can be increased in a great number of clinical circumstances. The combination of this marker with other predictors of increased CV disease risk may be a more appropriate application.
Serum leptin levels have been shown to be elevated in heart, liver and kidney transplant recipients measured at varying times post transplant compared to a non transplant control population. These elevated levels correlated with increased BMI, but the clinical significance of this finding was unknown (20). Importantly, our study shows the increase level is not static, but a progressive increase over time after liver transplantation. In addition, although not statistically significant in this pilot study, this increase in serum leptin levels was greater in the first 12 months in patients with CV events on long term followup. One cannot glean causality in this study, but it has been shown previously that leptin has direct effects on hypertension as well as renal and cardiovascular disease (21), thus warranting further prospective evaluation.
A high level of resistin is associated with obesity, pro-inflammatory states, insulin resistance and cardiovascular disease in the general population (22). It is upregulated in cirrhosis and further amplified in end stage liver disease (23, 24). Resistin levels were predictive of poor outcomes in patients with cirrhosis in one study (24). This study is the first to assess resistin levels in liver transplant recipients. We confirm the elevated levels pre transplant and show resistin levels decrease within the first year of transplant (less so, however, in patients with future cardiac events), but increase thereafter. Patients that experienced cardiac events had a more rapid rise in serum resistin levels within the following year than those that remain event free. Further studies following resistin post transplant may be helpful to elucidate its full effect.
Lipid and lipoprotein dysregulation with insulin resistance is associated with increased atherosclerosis and cardiovascular disease (25). It has been suggested that Apolipoprotein B is a better predictor of CV events than cholesterol levels (26). Apolipoprotein A and B levels in patients with end stage liver disease have been previously noted to be approximately 25% lower than the general population (27). In the first 2 weeks following transplant, apolipoprotein A-1 levels decrease and apolipoprotein B levels increase relative to hepatic allograft synthetic function (12). This study extends these findings with a relatively low and stable apolipoprotein A-1 level and an increasing apolipoprotein B level in the long term following liver transplantation. There is a well-documented association between high apolipoprotein B: apolipoprotein A-1 ratio and cardiovascular disease (28) and thus the increase in apolipoprotein B in conjunction with the relatively stable level of apolipoprotein A-1 would result in increased ratio in the liver transplant recipient, potentially increasing their risk for CV disease in the future. We were unable to confirm this in this study, but despite the small samples size, a trend to increased CV events was associated with elevated apolipoprotein B levels after the first year of followup was noted.
An unfortunate weakness of this study is the unclear effect of prolonged frozen serum on our assay results but if anything it would be expected to increase the risk of mis-measurement and a null result. In addition, many other factors that we could not account for, including medications, can influence serum adipokine and apolipoprotein levels. Detailed information on lipid abnormalities or medications were not available and certainly impacts the interpretation of apolipoprotein data. In addition, the transplant era represented in this database is different than the current era of recipients. The prevalence of CV events in this database cohort may not correspond to the current era of transplant recipients. However, the goal was not to determine prevalence but to measure adipokine trends and potential risk associations. By identifying those that had an actual cardiac event post transplant, we hoped to minimize the era effect, as we were using an outcome measure as the inclusion criteria for the study cases (ie it not a cohort study of a that era of recipients). Despite these issues and deficient power to identify anything less than a striking association, we have identified potential markers for increased risk of major CV events after transplant that warrant further investigation.
In summary, documenting the evolution of adipokines and inflammatory markers over time after liver transplantation helps to develop future studies of their potential clinical impact and possible therapeutic interventions. We have demonstrated lower serum adiponectin levels and hs-CRP levels before and after transplant may be associated with CV events in the long term followup of liver transplant recipients. Further research into the multiple effects of altered adipokines in our transplant population is needed.
Acknowledgments
This study is supported by the National Institutes of Diabetes and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with the investigators of this study and does not reflect the opinions or views of the NIDDK.
This project was supported by Grant Number 1 UL1 RR024150* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. It is awarded as the “CReFF award” by the Mayo Clinic Center for Translational Science Activities (CTSA), a multi-disciplinary research and education center.
Footnotes
No conflicts of interest for any of the authors
References
- 1.Johnston S, Morris J, Cramb R, Gunson B, Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73(6):901–6. doi: 10.1097/00007890-200203270-00012. [DOI] [PubMed] [Google Scholar]
- 2.Watt K, Pedersen R, Kremers W, Heimbach J, Charlton M. Evolution of Causes and Risk Factors for Mortality Post Liver Transplant: Results of the NIDDK Long Term Follow-up Study. Am J Transplant. 2010;10:1–8. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, et al. Metabolic Syndrome in Liver Transplant Recipients:Prevalence and Association with Cardiovascular Events. Liver Transpl. 2007;13(8):1109–14. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 4.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 5.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. American journal of physiology. 2005 May;288(5):H2031–41. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 6.Tiftikci A, Atug O, Yilmaz Y, Eren F, Ozdemir FT, Yapali S, et al. Serum levels of adipokines in patients with chronic HCV infection: relationship with steatosis and fibrosis. Arch Med Res. 2009 May;40(4):294–8. doi: 10.1016/j.arcmed.2009.04.008. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 7.Anty R, Lemoine M. Liver fibrogenesis and metabolic factors. Clin Res Hepatol Gastroenterol. 2011 Jun;35(Suppl 1):S10–20. doi: 10.1016/S2210-7401(11)70003-1. [DOI] [PubMed] [Google Scholar]
- 8.Larsson H, Ahrén B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81(12):4428–32. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura K, Kishikawa H, Kato T, Kobayashi Y, Fujii N, Takahara S, et al. Tacrolimus and angiotensin receptor blockers associated with changes in serum adiponectin level in new-onset diabetes after renal transplantation: single-center cross-sectional analysis. Transpl Int. 2009 Jul;22(7):694–701. doi: 10.1111/j.1432-2277.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian S, Trence D. Immunosuppressive agents: effects on glucose and lipid metabolism. Endocrinol Metab Clin North Am. 2007;36(4):891–905. doi: 10.1016/j.ecl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Watt KD. Metabolic syndrome: Is immunosuppression to blame? Liver Transpl. 2011 Nov;17(Suppl 3):S38–42. doi: 10.1002/lt.22386. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong VW, Schutz E, Kaltefleiter M, Luy M, Helmhold M, Wieland E, et al. Relationship of apolipoproteins AI, B and lipoprotein Lp(a) to hepatic function of liver recipients during the early post-transplant period. European journal of clinical investigation. 1995 Jul;25(7):485–93. doi: 10.1111/j.1365-2362.1995.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 13.TietgeI U, Boker K, Manns M, Bahr M. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287(1):E82–9. doi: 10.1152/ajpendo.00494.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hui CK, Zhang HY, Lee NP, Chan W, Yueng YH, Leung KW, et al. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. Journal of hepatology. 2007 Aug;47(2):191–202. doi: 10.1016/j.jhep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Chudek J, Adamczak M, Karkoszka H, Budziński G, Ignacy W, Funahashi T, et al. Plasma adiponectin concentration before and after successful kidney transplantation. Transplant Proc. 2003;35(6):2186–9. doi: 10.1016/j.transproceed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clinical chemistry. 2003 Apr;49(4):650–2. doi: 10.1373/49.4.650. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 17.Varagunam M, Finney H, Trevitt R, Sharples E, McCloskey DJ, Sinnott PJ, et al. Pretransplantation levels of C-reactive protein predict all-cause and cardiovascular mortality, but not graft outcome, in kidney transplant recipients. Am J Kidney Dis. 2004 Mar;43(3):502–7. doi: 10.1053/j.ajkd.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Biadi O, Potena L, Fearon W, Luikart H, Yeung A, Ferrara R, et al. Interplay between systemic inflammation and markers of insulin resistance in cardiovascular prognosis after heart transplantation. J Heart Lung Transplant. 2007;26(4):324–30. doi: 10.1016/j.healun.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Coss E, Watt K, Pedersen R, Dierkhising R, Heimbach J, Charlton M. Predictors of Cardiovascular Events After Liver Transplantation: A Role for Pre-transplant Serum Troponin Levels. Liver Transplantation. 2011;17:23–31. doi: 10.1002/lt.22140. [DOI] [PubMed] [Google Scholar]
- 20.Kagan A, Haran N, Leschinsky L, Sarafian R, Aravot D, Dolberg J, et al. Serum concentrations of leptin in heart, liver and kidney transplant recipients. Isr Med Assoc J. 2002;4(3):213–7. [PubMed] [Google Scholar]
- 21.Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease. Current hypertension reports. 2008 Apr;10(2):131–7. doi: 10.1007/s11906-008-0025-y. [DOI] [PubMed] [Google Scholar]
- 22.Momiyama Y, Ohmori R, Uto-Kondo H, Tanaka N, Kato R, Taniguchi H, et al. Serum resistin levels and cardiovascular events in patients undergoing percutaneous coronary intervention. Journal of atherosclerosis and thrombosis. 18(2):108–14. doi: 10.5551/jat.6023. [DOI] [PubMed] [Google Scholar]
- 23.Bahr MJ, Ockenga J, Boker KH, Manns MP, Tietge UJ. Elevated resistin levels in cirrhosis are associated with the proinflammatory state and altered hepatic glucose metabolism but not with insulin resistance. Am J Physiol Endocrinol Metab. 2006 Aug;291(2):E199–206. doi: 10.1152/ajpendo.00291.2005. [DOI] [PubMed] [Google Scholar]
- 24.Yagmur E, Trautwein C, Gressner AM, Tacke F. Resistin serum levels are associated with insulin resistance, disease severity, clinical complications, and prognosis in patients with chronic liver diseases. The American journal of gastroenterology. 2006 Jun;101(6):1244–52. doi: 10.1111/j.1572-0241.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 25.Avramoglu R, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368(1–2):1–19. doi: 10.1016/j.cca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005 Nov 29;112(22):3375–83. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 27.Sposito AC, Vinagre CG, Pandullo FL, Mies S, Raia S, Ramires JA. Apolipoprotein and lipid abnormalities in chronic liver failure. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al. 1997 Nov;30(11):1287–90. doi: 10.1590/s0100-879x1997001100004. [DOI] [PubMed] [Google Scholar]
- 28.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. Journal of internal medicine. 2004 Feb;255(2):188–205. doi: 10.1046/j.1365-2796.2003.01276.x. [DOI] [PubMed] [Google Scholar]