Abstract
Introduction
The liver donor risk index (LDRI), originally developed in 2006 by Feng et. al and since modified, is a method of evaluating liver grafts from deceased donors by determining the relative risk of graft failure post transplantation.
Methods
Online and paper surveys sent to liver transplant physicians asking about their attitudes and practices regarding decision-making in liver transplantation and the role of LDRI.
Results
147 of 401 (37%) eligible respondents returned partial or complete surveys. The majority of the respondents were male (116/134 or 87%) and practiced in academic medical centers (128/138 or 93%). Transplant coordinators initially contacted the candidate with an offer in 81% of programs. Eighty-eight of 143 (62%) respondents reported that they were very familiar with LDRI, but the vast majority (114/137 or 83%) rarely or never discuss the concept of LDRI with their patients. A majority of respondents (96/132 or 73%) believe that LDRI does not adequately describe a liver’s relative risk of graft failure and that there are factors that make LDRI potentially misleading (122/138 or 88%). Nevertheless, 60 of 130 (46%) believe that LDRI would increase/improve shared decision making.
Discussion
The LDRI has not been widely adopted because of concerns that 1) it does not accurately reflect post-transplant survival; 2) it excludes relevant donor and recipient factors, and 3) it is too complicated for candidates to grasp. There is a need to improve it or to develop other decision making tools to help promote shared decision making. There is also great diversity in how liver offers are made to ambulatory candidates, and how transplant programs address a candidate’s refusal. Research is needed to determine evidence-based best practice.
Keywords: deceased donor liver transplantation, liver donor risk index, shared decision-making, ethics, autonomy
Introduction
Due to the increasing shortage of livers available for transplantation, the use of marginal (e.g., expanded criteria donor grafts--those liver grafts with a higher likelihood of graft failure) and high risk organs (as defined by the Centers for Disease Control and Prevention [CDC]) has increased. The liver donor risk index (LDRI) was initially developed in 2006 by Feng et. al as a method of evaluating liver grafts from deceased donors by determining the relative risk of graft failure post transplantation. Initially, seven donor characteristics were used to calculate the LDRI: age, race, size (height), cause of death (cerebrovascular accident [CVA]), cause of death (other), donation after circulatory death (DCD), and whether the graft is partial/split or whole.[1] The LDRI was later modified to include cold ischemia time (CIT) and national/regional sharing.
One potential benefit of a risk index is to standardize the evaluation of deceased donor liver quality, rather than relying on individual physician intuition. LDRI, however, has its critics. First, the current LDRI model was derived using data from the pre-MELD (Model for End Stage Liver Disease) allocation era, and some argue that it should be re-evaluated using a more recent, independent data set.[2,3] Second, the inclusion of some of the donor characteristics (such as race) into the LDRI was based on statistical significance, but this may not correlate with an actual biological effect in increasing the risk of graft failure.[4,5] Third, some argue that there are other donor factors, which are not included in the LDRI, that are more important predictors of graft failure (e.g. steatosis).[6-9] Fourth, the LDRI does not include any information about the recipient, although the health status of the recipient greatly affects the likelihood of graft failure.[7-8,10-14]
In this study, we surveyed transplant hepatologists and surgeons to ascertain their opinions on the LDRI and whether or not they incorporate it into their discussion with liver transplant candidates. We also surveyed their practice regarding how offers are made to ambulatory candidates, how they respond to refusals by ambulatory candidates, and what impact LDRI might have on candidate acceptance of these offers. Finally, we also examined whether transplant professionals use LDRI explicitly, and if not, whether and how frequently they discuss certain individual characteristics that factor into the LDRI (e.g., age, DCD, split/partial graft) with their patients.
Methods
We surveyed 482 liver transplant physicians (hepatologists and transplant surgeons) identified by transplant center websites and the American Society of Transplant (AST) and the American Society of Transplant Surgeons (ASTS) websites. Physicians were excluded if 1) they did not have an email address, 2) they did not reside in the United States, 3) they had previously recused themselves on surveymonkey from receiving survey requests, and/or 4) they were not an appropriate respondent for the survey.
The survey inquired about the attitudes and practices of physicians regarding decision making in liver transplantation and the role of LDRI. Respondents were asked about the utility and accuracy of the LDRI, how it influences their discussion with a potential recipient about an organ offer, and what impact LDRI has on shared decision making between health care providers and candidates. Respondents were also asked about the general process of how a liver offer is made to an ambulatory candidate at their institution when a deceased donor liver becomes available and how they handled a candidate’s refusal of an organ. Demographic questions included age, gender, type of physician, type of practice, responsibilities, estimated number of deceased donor liver transplants done at institution yearly (<10, 10-19; 20-29; 30-39, etc.), estimated percentage of candidates who are ambulatory at time of transplant (<10%; 10-25%; >25-50%; >50-75%; >75-90%, >90%), and estimated average MELD range (<15, 15-20; 21-25; 26-30; 31-35; 36-40) for ambulatory patients with blood type O at time of transplant. Several questions provided space for respondents to elaborate. Qualitative responses were coded by two researchers (LAM and LFR) for themes. Complete consensus was reached on all codes and themes.
Each physician was contacted a maximum of four times. The first, second, and fourth rounds were sent electronically through surveymonkey (www.surveymonkey.com). For the third round, a paper survey was sent through the US mail, which could be returned via US mail or fax.
For comparative purposes we divided transplant programs approximately equally for 2 factors: 1) programs that performed ≥ 70 deceased donor transplants per year (48%) and < 70 deceased donor transplants annually (52%); and 2) programs in which the average ambulatory patient of blood type O had a MELD score > 30 (59%) or a MELD score ≤ 30 (41%) at the time of transplant. Statistical analysis was performed with SPSS v21.0 (SPSS Inc., Chicago, IL). Results were compared through the use of χ2 tests of independence.
Approval from the University of Chicago Institutional Review Board was obtained and waived the requirement for written consent.
Results
Four hundred and eighty-two surveys were distributed (see Appendix A). Eighty-one respondents were excluded because they did not have a valid email address (n=12), they were not appropriate respondents for the survey (n=33), or they had previously recused themselves from receiving surveymonkey requests (n=36). Of the remaining 401 potential respondents, 284 (71%) were transplant surgeons and 117 (29%) were hepatologists; 147 (37%) returned partial or complete surveys and three (1%) declined to participate.
Respondent demographics and center information are provided in Table 1. Ninety-one of 138 (66%) respondents were transplant surgeons and 47 of 138 (34%) were hepatologists. The majority of the respondents were male (116/134 or 87%) with surgeons more likely to be male than hepatologists (81/87 (93%) vs. 35/47 (74%), respectively, p<0.005). Most of the respondents (56%) were ≤50 years of age, and practiced in an academic medical center (93%), and spent >50% of their time in direct patient care (83%). Seventy-one of 136 respondents (52%) came from programs performing <70 deceased donor liver transplants (DDLT) per year. The average MELD range for ambulatory patients with blood type O at time of transplant was 26-30.
Table 1.
Respondent Demographics and Center Information
| All participants [n (%), N=138] |
Transplant surgeons N=91 [n (%)] |
Hepatologists N=47 [n (%)] |
|
|---|---|---|---|
| Age (N=133*) | |||
| ≤50 years | 75 (56) | 47 (53) | 28 (62) |
| >50 years | 58 (44) | 41 (47) | 17 (38) |
| Gender (N=134*)# | |||
| Female | 18 (13) | 6 (7) | 12 (26) |
| Male | 116 (87) | 81 (93) | 35(74) |
| Practice setting^ | |||
| Academic medical center | 128 (93) | 84 (92) | 44 (94) |
| Private practice | 7(5) | 5(5) | 2 (4) |
| Other | 3(2) | 2(2) | 1 (2) |
| % time spent in direct patient care## | |||
| ≤ 50% | 23 (17) | 11(12) | 12 (26) |
| > 50% | 115 (83) | 80 (88) | 35(74) |
| Center Size (N=136*) | |||
| Smaller (< 70 DDLTs/year) | 71(52) | 48 (54) | 23 (49) |
| Larger (≥ 79 DDLTs /year) | 65 (48) | 41 (46) | 24 (51) |
| Center Average MELD Score (for ambulatory patient with blood type O (N =135*) | |||
| Lower MELD (0-30) | 80 (59) | 55 (62) | 25 (54) |
| Higher MELD (31-40) | 55 (41) | 34 (38) | 21 (46) |
N varies because not all respondents answered all questions
Percentages do not add up to 100% due to rounding
Difference between surgeons and hepatologists significant at P<0.005
Difference between surgeons and hepatologists significant at P<0.05
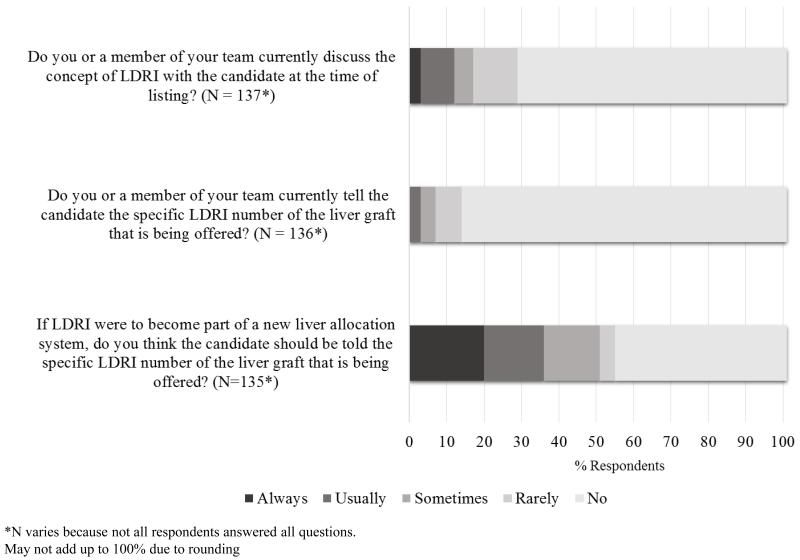
Eighty-eight of 143 (62%) respondents reported that they were very familiar with LDRI, and there was no significant difference in familiarity between specialties (27/47 or 57% of hepatologists vs. 58/91 or 64% of transplant surgeons, p = 0.47). Figure 1 describes how transplant surgeons and hepatologists use LDRI in practice. A significant majority (114/137 or 83%) rarely or never discuss the concept of LDRI with their patients, however this varies depending on the respondents’ specialty. Transplant hepatologists were more likely to discuss the concept of LDRI than surgeons (13/42 or 31% vs. 9/91 or 10%, respectively, p < 0.01). An even greater majority (127/136 or 93%) of respondents rarely or never currently tell the candidate the specific LDRI number of the graft being offered, and this did not vary between specialties (39/42 or 93% of hepatologists vs. 85/91 or 93% of transplant surgeons, p = 0.91). If LDRI were to become part of a new liver allocation system, 67 of 135 respondents (50%) believed that candidates should never or rarely be told the specific LDRI number. Again, this did not vary significantly between specialties (16/42 or 38% of hepatologists vs. 50/91 or 55% of transplant surgeons, p = 0.07).
Figure 1.
LDRI is not currently utilized
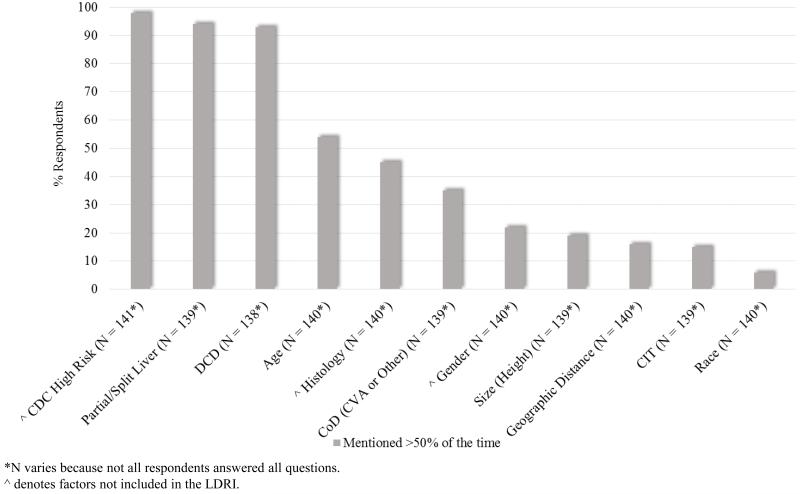
Figure 2 illustrates the practices of our respondents when discussing deceased donor organs with their candidates. Of all the organ factors surveyed, the ones mentioned with the highest frequency were CDC high-risk, partial/split liver, and DCD, each with over 90% of the respondents mentioning them >50% of the time. Only one other factor, age, had a majority of respondents mentioning it >50% of the time. Interestingly, providers from smaller centers more frequently disclosed donor age than providers from larger centers (44/69 or 64% vs. 26/63 or 41% respectively, p < 0.05). Respondents from centers where ambulatory candidates were transplanted with higher average MELDs were also more likely to disclose donor age compared to respondents from centers with lower average MELDs (36/53 or 68% vs. 36/79 or 46%, p < 0.05). Histology (31/53 or 58% vs. 27/79 or 34%, p < 0.01) and donor gender (18/53 or 34% vs. 11/79 or 14%, p < 0.01) were also mentioned more often in centers where the ambulatory transplant candidates had higher average MELDs.
Figure 2.
Deceased donor liver factors mentioned to candidates
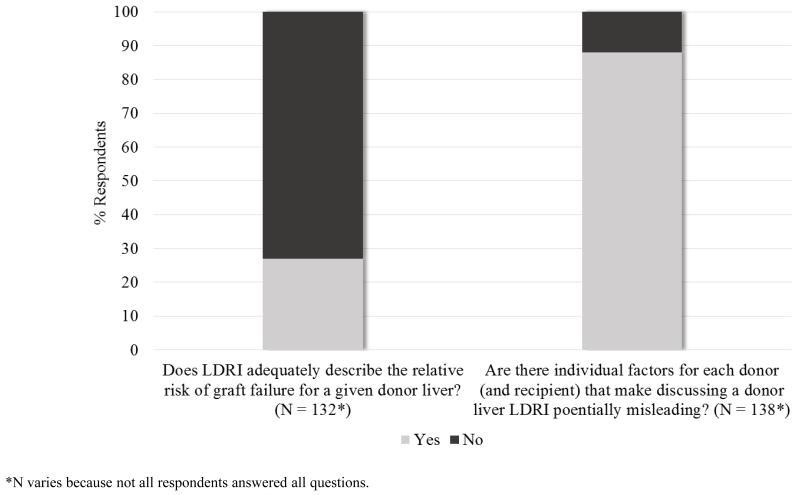
A majority of respondents (96/132 or 73%) believe that LDRI does not adequately describe a liver’s relative risk of graft failure (see Figure 3). An even greater majority believe that there are factors that make LDRI potentially misleading (122/138 or 88%). When asked to elaborate on these questions, 45 respondents submitted 50 free responses. The most common theme (n = 17) focused on the poor predictive ability of LDRI—some respondents expressing the need for further validation and others objecting that it is not reflective of graft and patient survival or that it cannot replace the judgment and technical skill of the transplant team. The next most common theme (n = 14) was that LDRI includes some donor factors that are irrelevant (e.g. race) and does not include other factors that are relevant (recipient factors [e.g. portal vein thrombosis, MELD, retransplant, sodium, hepatic encephalopathy] and other donor factors [e.g. biopsy, steatosis, pH, pressor support]). Another theme (n = 11) focused on the concern of mortality risk for the recipient if he or she declines what the respondent thinks is an appropriate offer and instead remains on the waitlist. Concern about patients’ lack of understanding of LDRI was mentioned by eight respondents.
Figure 3.
LDRI does not adequately describe relative risk and is potentially misleading
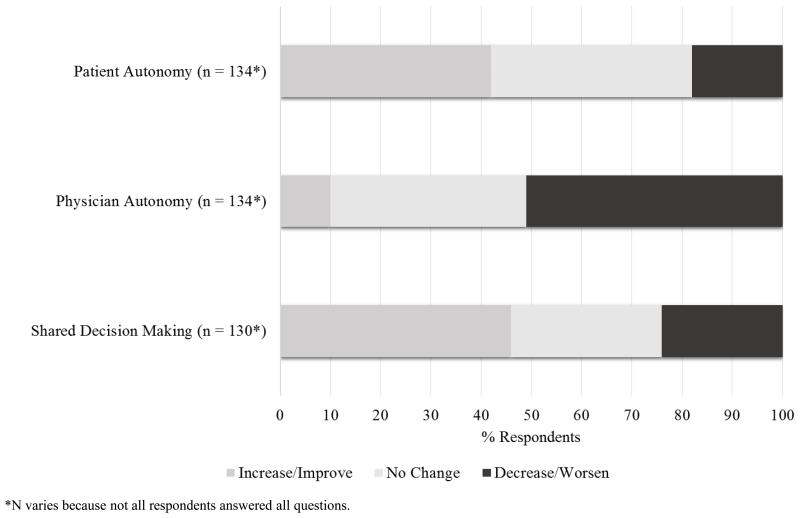
Figure 4 depicts the opinions of respondents about the impact of LDRI on decision making. There was no clear consensus among the respondents as to whether or not LDRI would increase/improve (56/134 or 42%) or not change (54/134 or 40%) patient autonomy, however transplant hepatologists believed that LDRI would increase/improve patient autonomy more than surgeons (26/43 or 60% vs. 30/88 or 34% respectively, p < 0.05). Over half of the respondents (68/134 or 51%) believed that LDRI would decrease physician autonomy, and no significant difference between specialties was found (21/43 or 49% of hepatologists vs. 45/88 or 51% of transplant surgeons, p = 0.34). Sixty of 130 (46%) believed that LDRI would increase/improve shared decision making, and transplant hepatologists were more likely to believe this than surgeons (26/42 or 62% vs. 33/85 or 39%, respectively, p < 0.05).
Figure 4.
Impact of LDRI on Decision Making
One hundred seventeen of 145 (81%) identified the transplant coordinator alone as the person who initially contacts the candidate about the organ. In addition, 59 of 144 (41%) identified the transplant coordinator alone as the person who makes the offer and discusses the suitability of the donor with the candidate, while 57 of 144 (40%) identified the transplant surgeon, and 28 of 144 (19%) identified a combination of surgeon/hepatologist/coordinator.
The organ offerer knew the transplant candidate >50% of the time in 50% (73/147) of responses. However, this was more often the case in smaller centers than in larger ones (47/71 or 66% vs. 17/65 or 26%, respectively, p < 0.001). The hepatologist was usually not involved in deciding the suitability of the organ for the candidate, with over half of respondents (79 of 145 or 54%) reporting hepatologist involvement ≤ 25% of the time. This again varied by center size, with smaller centers including hepatologists more frequently (39/70 or 56% vs. 22/65 or 34%, respectively, p < 0.05). The hepatologist was even less frequently involved in discussing the organ offer with the candidate, with 82 of 144 respondents (57%) reporting this occurring <10% of the time. This did not vary by center size (30/71 or 42% vs. 27/64 or 42%, p = 0.99).
Refusal of an organ offer by ambulatory patients was rare overall, with the majority (97/147 or 66%) reporting its occurrence <10% of the time. However, refusal was more likely to occur if a physician (surgeon or hepatologist) was involved in making the organ offer compared to the transplant coordinator alone making the offer (37/85 or 46% vs. 13/59 or 22%, p < 0.01). If a refusal did occur, a majority (78/141 or 55%) answered that the refusal was accepted without further discussion >50% of the time. Only 36% (49/138) attempted to convince the candidate to accept the organ >50% of the time. A second colleague was rarely involved in the event of a refusal, with only 48 of 136 respondents (35%) reporting its occurrence >25% of the time. A majority (80/141 or 57%) stated that candidates were recontacted after refusing an organ >75% of the time for further education about transplant, however this was more likely to occur in smaller transplant centers compared to larger ones (47/70 or 67% vs. 29/62 or 47%, respectively, p < 0.05). If a refusal occurred, the overwhelming majority of respondents (125/139 or 90%) reported that candidates were rarely or infrequently removed from the waitlist (≤ 25% of the time).
Discussion
While the majority of our respondents (62%) state that they are very familiar with LDRI, only a small minority (17%) discuss the concept of LDRI with their candidates, and even less (7%) disclose a specific LDRI number. This may be because they had several concerns regarding LDRI, the most common being that it is unable to accurately predict graft and patient survival, which is consistent with the literature.[15-17] Our respondents were also critical of the inclusion of donor race, the exclusion of donor histology, and the exclusion of all recipient factors.[4-8,10-14] They also expressed concern that LDRI is too complex for patients to fully understand.
Although our respondents are not discussing LDRI with their candidates, they are discussing certain individual factors that are included in the LDRI. Over 90% discuss whether the liver is split/partial and whether it is a DCD organ. DCD and split/partial livers are both significantly associated with graft failure.[1] In the original study by Feng et al., donor age was the more important donor demographic factor associated with risk of graft failure, and it is striking that it is only discussed by slightly more than half of our respondents. All of the other LDRI factors are discussed less than half of the time. Although not included in LDRI, over 90% of respondents do discuss CDC high risk which is not surprising since CDC high risk organs need specific candidate consent.
Our respondents rarely discuss the concept of LDRI, let alone provide a specific number, and over half of all respondents believe that reporting LDRI will decrease physician autonomy, forcing them to rely on an algorithm rather than their own expertise and experience. There is also no clear consensus among them whether it would affect patient autonomy. However, transplant hepatologists who answered our survey believe that LDRI will increase patient autonomy and shared decision making more so than surgeons.
In the process of making organ offers, a great majority (81%) of respondents report that their center has the transplant coordinator initially contact the candidate. Fifty-nine of 144 respondents (41%) report that the transplant coordinator alone makes the actual offer and discusses the suitability of the organ with the candidate. This suggests that a physician does not discuss the donor organ with the patient until after a patient has already decided to accept or refuse it in almost half of transplant centers. The method of organ offer correlated with center size. In smaller centers, it is more likely that 1) the offering provider has personally evaluated the candidate; and 2) that the hepatologist is involved in deciding the suitability of the organ for the candidate. Hepatologists are rarely involved in discussing the offer with the candidate, and this did not vary among center size.
Although refusals are rare, even in ambulatory candidates, they are more likely to occur if a physician was involved in making the offer. Further research is needed to determine if this is due to greater involvement of physicians in more marginal (e.g. expanded criteria donor grafts) or high-risk offers, if this is due to a more robust consent process, and what impact this has on outcomes.
When an ambulatory candidate refuses an organ offer, the majority of centers accept the refusal without further discussion. Transplant hepatologists, however, are more likely to attempt to convince the candidate to accept the organ. Although there may be an attempt to convince, second colleagues are rarely involved in the event of a refusal and candidates are even less frequently removed from the waitlist. Neither of these responses varied by center size or respondent specialty. Almost always, the candidate is recontacted for further education about transplant, however this is more likely to happen in smaller centers. Further research is needed to understand the actual process of organ offers to determine if one practice style leads to more frequent refusals at a later stage of the process, patient satisfaction with the consent process, and other quality measures.
The main limitation of our study is the low response rate of 37%, although this is typical of surveys of health care professionals.[18-19] In a positive vein, our respondents were similar to our non-respondents in terms of professional specialty (transplant surgeons comprised 71% of our mailing list and consisted of 66% of our respondents (data not shown, p = 0.28). However, we cannot be sure that there was not a bias in the responses we received.
A second limitation is that we asked our respondents to consider a potential organ for an “ambulatory candidate”. This could mean either a patient who is non-hospitalized, or it could refer to the subset of non-hospitalized patients who are functionally well enough to walk around. We did not clarify which was meant and so there may be some variation in responses depending on how the respondent understood the term.
A third limitation is that we focused on decision making regarding an organ already accepted by a liver transplant program. We did not address whether LDRI influenced a transplant program’s decision to accept an organ. We also did not address whether patients should be involved earlier in the process (i.e., at the time that an offer is made). Additional questions focused on these issues could help clarify 1) the value of LDRI for professionals; and 2) the boundaries of shared decision-making in organ allocation.
Conclusion
The liver donor risk index has not been widely adopted, due in part to concerns that 1) it does not accurately reflect post-transplant survival, 2) it excludes relevant donor and recipient factors, and 3) it is considered too complicated for candidates to grasp. There is a need to improve it or to develop other decision making tools to help promote shared decision making. There is also great diversity in how liver offers are made to ambulatory candidates, and how transplant programs address a candidate’s refusal. Research is needed to determine evidence-based best practice.
Supplementary Material
Acknowledgments
Funding: Leslie Mataya was funded by the Pritzker School of Medicine’s Summer Research Program and by the National Institute of Diabetes and Digestive and Kidney Diseases (grant number 2T35DK062719-26). No other funding.
Abbreviations
- AST
American Society of Transplantation
- ASTS
American Society of Transplant Surgeons
- CIT
cold ischemia time
- CVA
cerebrovascular accident
- DCD
donation after circulatory death
- DDLT
deceased donor liver transplant
- LDRI
living donor risk index
- MELD
Model for End Stage Liver Disease
Footnotes
Conflicts of Interest: None.
Contributor Information
Leslie Mataya, second year medical student at the University of Chicago Pritzker School of Medicine.
Andrew Aronsohn, section of Gastroenterology, University of Chicago.
Richard Thistlethwaite, Jr, Department of Surgery, Section of Transplantation, University of Chicago.
Lainie Friedman Ross, Carolyn and Matthew Bucksbaum Professor of Clinical Medical Ethics, Professor in the Departments of Medicine, Pediatrics and Surgery; and Associate Director of the MacLean Center for Clinical Medical Ethics, University of Chicago.
References
- 1.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 2.Akkina SK, Asrani SK, Peng Y, Stock P, Kim WR, Israni AK. Development of organ-specific donor risk indices. Liver Transpl. 2012;18:395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmiero HOM, Kajikawa P, Boin IFSF, Coria S, Pereira LA. Liver recipient survival rate before and after model for end-stage liver disease implementation and use of donor risk index. Transplant Proc. 2010;42:4113–5. doi: 10.1016/j.transproceed.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 4.Asrani SK, Lim Y-S, Therneau TM, Pedersen RA, Heimbach J, Kim WR. Donor race does not predict graft failure after liver transplantation. Gastroenterology. 2010;138:2341–7. doi: 10.1053/j.gastro.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckhoff DE, McGuire BM, Young CJ, Sellers MT, Frenette LR, Hudson SL, et al. Race: a critical factor in organ donation, patient referral and selection, and orthotopic liver transplantation? Liver Transpl Surg. 1998;4:499–505. doi: 10.1002/lt.500040606. [DOI] [PubMed] [Google Scholar]
- 6.De Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol. 2012;27:540–6. doi: 10.1111/j.1440-1746.2011.06844.x. [DOI] [PubMed] [Google Scholar]
- 7.Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745–753. doi: 10.1097/SLA.0b013e3182365081. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN. Development and validation of a model predicting graft survival after liver transplantation. Liver Transpl. 2006;12:1594–606. doi: 10.1002/lt.20764. [DOI] [PubMed] [Google Scholar]
- 9.Nafidi O, Marleau D, Roy A, Bilodeau M. Identification of new donor variables associated with graft survival in a single-center liver transplant cohort. Liver Transpl. 2010;16:1393–9. doi: 10.1002/lt.22176. [DOI] [PubMed] [Google Scholar]
- 10.Avolio AW, Siciliano M, Barbarino R, Nure E, Annicchiarico BE, Gasbarrini A, et al. Donor risk index and organ patient index as predictors of graft survival after liver transplantation. Transplant Proc. 2008;40:1899–902. doi: 10.1016/j.transproceed.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 11.Frühauf NR, Fischer-Fröhlich C-L, Kutschmann M, Schmidtmann I, Kirste G. Joint impact of donor and recipient parameters on the outcome of liver transplantation in Germany. Transplantation. 2011;92:1378–84. doi: 10.1097/TP.0b013e318236cd2f. [DOI] [PubMed] [Google Scholar]
- 12.Gambato M, Frigo AC, Rodríguez Castro KI, Senzolo M, Nadal E, D’Amico F, et al. Who fares worse after liver transplantation? Impact of donor and recipient variables on outcome: data from a prospective study. Transplantation. 2013;95:1528–34. doi: 10.1097/TP.0b013e318292827f. [DOI] [PubMed] [Google Scholar]
- 13.Northup PG, Pruett TL, Kashmer DM, Argo CK, Berg CL, Schmitt TM. Donor factors predicting recipient survival after liver retransplantation: the retransplant donor risk index. Am J Transplant. 2007;7:1984–8. doi: 10.1111/j.1600-6143.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 14.Salvalaggio PR, Dzebisashvili N, MacLeod KE, Lentine KL, Gheorghian A, Schnitzler MA, et al. The interaction among donor characteristics, severity of liver disease, and the cost of liver transplantation. Liver Transpl. 2011;17:233–42. doi: 10.1002/lt.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrem H, Reichert B, Frühauf N, Becker T, Lehner F, Kleine M, et al. The Donor-Risk-Index, ECD-Score and D-MELD-Score all fail to predict short-term outcome after liver transplantation with acceptable sensitivity and specificity. Ann Transplant. 2012;17:5–13. doi: 10.12659/aot.883452. [DOI] [PubMed] [Google Scholar]
- 16.Rauchfuss F, Zidan A, Scheuerlein H, Dittmar Y, Bauschke A, Settmacher U. Waiting time, not donor-risk-index, is a major determinant for beneficial outcome after liver transplantation in high-MELD patients. Ann Transplant. 2013;18:243–7. doi: 10.12659/AOT.883924. [DOI] [PubMed] [Google Scholar]
- 17.Vitale A, D’Amico F, Gringeri E, Valmasoni M, Pauletto A, Bonsignore P, et al. Prognostic evaluation of the donor risk index among a prospective cohort of Italian patients undergoing liver transplantation. Transplant Proc. 2009;41:1096–8. doi: 10.1016/j.transproceed.2009.03.097. [DOI] [PubMed] [Google Scholar]
- 18.Kellerman S, Herold J. Physician response to surveys: A review of the literature. Am J Prev Med. 2001;20:61–67. doi: 10.1016/s0749-3797(00)00258-0. [DOI] [PubMed] [Google Scholar]
- 19.Thorpe C, Ryan B, McLean SL, et al. How to obtain excellent response rates when surveying physicians. Fam Pract. 2009;26:65–68. doi: 10.1093/fampra/cmn097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.