Abstract
Background
The aim was to investigate the association between sleep disturbances and cognitive function in younger and older individuals from an ageing population.
Methods
3,968 male and 4,821 female white participants, aged 50 years and over, from the English Longitudinal Study of Ageing (ELSA) were studied. Information on sleep quality and quantity as well as both amnestic (memory, ACF) and non-amnestic (non-memory, nACF) function was available at Wave 4 (2008). Analysis of covariance was used to evaluate the relationship between sleep and cognitive function.
Results
After adjustment for multiple confounders in the younger group (50–64 years) duration of sleep explained 15.2% of the variance in ACF (p = 0.003) and 20.6% of nACF (p = 0.010). In the older group (65+ years) the estimates were 21.3% (p<0.001) and 25.6% (p<0.001), respectively. For sleep quality, there was a statistically significant association between sleep quality and both ACF (p<0.001) and nACF (p<0.001) in the older age group, but not in the younger age group (p = 0.586 and p = 0.373, respectively; interaction between age and sleep quality in the study sample including both age groups: p<0.001 for ACF and p = 0.018 for nACF). Sleep quality explained between 15.1% and 25.5% of the variance in cognition. The interaction with age was independent of duration of sleep. At any level of sleep duration there was a steeper association between sleep quality and ACF in the older than the younger group.
Conclusions
The associations between sleep disturbances and cognitive function vary between younger and older adults. Prospective studies will determine the temporal relationships between sleep disturbances and changes in cognition in different age groups.
Introduction
Developments in the fields of sleep neurobiology and cognitive neuroscience have produced converging evidence of a fundamental role for sleep in cognition. As we age the amount of time spent in good quality, continuous sleep decreases [1] and there are links between sleep disturbances and cognitive impairment in vulnerable populations, such as those at risk of various dementing illnesses [2], [3]. Sleep disturbances, including specific reduction in fast sleep spindles, may be one of the earliest signs of neurodegenerative disorders, including early Alzheimer’s disease (AD) [4].
The term ‘cognition’ refers to a range of mental processes including memory, problem solving, language, forward planning and attention, which can all be differentially affected by inadequate sleep. Specific cognitive processes can be grouped into two broader categories of amnestic function (ACF, referring to memory) and non-amnestic function (nACF, or non-memory). This dichotomy is of particular importance in relation to the progression from normal cognitive ageing to mild cognitive impairment (MCI), since MCI is typically diagnosed as amnestic (aMCI) or non-amnestic (naMCI) subtype [5]. These two subtypes of MCI may possibly have different trajectories, where aMCI may potentially develop into AD, and naMCI may develop into other forms of dementia (e.g. vascular dementia, Dementia with Lewy bodies (DLB), fronto-temporal dementia) [6], [7], although the validity of this prediction has previously been questioned [8], [9].
In cross-sectional studies both short and long sleep durations are associated with diminished global cognition [10] and memory function [11] in adult populations. However, whilst there may be different associations between sleep disturbances and subtypes of MCI [12], most studies have reported the effects of sleep on global cognition, using generalised tests such as the Mini-Mental State Exam (MMSE) [13].
A recent study [14] attempted to distinguish between amnestic and non-amnestic cognitive impairments with relation to sleep patterns. The specific amnestic cognitive impairments were identified however, simply by using the scores on the delayed recall task from the MMSE.
Amongst women, long sleep (>9 hr per night) predicted cognitive impairment over one year, whilst in men, it was short sleep (<5 hr per night) that predicted cognitive decline. In a separate study, disturbed sleep was strongly associated with a decline in executive (non-amnestic) function but less so for global cognition [15].
The current study explores the associations between measures of sleep and amnestic and non-amnestic cognitive function in younger and older adults in a representative population of English men and women over the age of 50 years.
Methods
Study Population
The English Longitudinal Study of Ageing (ELSA) is a representative sample of the English population aged 50 years and over (N = 11,050) [16], [17]. Data were selected from Wave 4 (2008), at which point sleep data was included for the first time, alongside routine measures of health, disease, cognition, finances, lifestyle and anthropometrics. The full methodology, sampling procedures and details on previous waves of screening have been reported elsewhere [18]. Non-whites (3.5%) and participants under the age of 50 (n = 301) or aged 90 years or above (n = 137) were excluded, as were those with inaccurate or incomplete essential data (n = 1432). The remaining subjects with full data on sleep quantity and quality and cognitive function were included (n = 8,789; 3,968 males and 4,821 females) [18]. Since research has shown pre- and post-retirement changes in health conditions [19] and sleep behaviour [20], the main objective of our analysis was to explore the patterns of associations between sleep and cognition at different stages of ageing. As significant interactions were detected between sleep disturbances and age (quantity: p<0.001 for ACF and p = 0.06 for nACF; quality: p<0.001 for ACF and p = 0.018 for nACF), the respondents were separated into younger (50–64 years; n = 4,660) and older (65+ years; n = 4,129) age groups and analyses carried out separately.
Ethics statement
All participants of ELSA provided signed consent, and ethical approval was granted by the London Multi- Centre Research Ethics Committee. Anonymised unlinked data for this secondary data analysis initiative study was provided by the UK Data Service (previously known as Economic and Social Data Service (ESDS)).
Measures of Exposure
Sleep Quantity
Respondents reported the number of hours they slept, on average, per weeknight. Open-ended responses ranged between 3 and 14 hours, and were categorised as <6 hrs, 6–8 hrs, and >8 hrs for comparability with previous publications [21], [22], whilst maintaining adequate numbers in each group. Throughout the description of results, we will refer to the <6 hrs group as ‘short sleepers’, the 6–8 hrs group as ‘optimal sleepers’ [23], and the >8 hrs group as ‘long sleepers’.
Sleep Quality
Respondents reported the frequency with which they experienced the following over the previous month: delay in falling asleep, inability to stay asleep, and waking up feeling tired. The response categories were: ‘no difficulties’, ‘less than once a week’, ‘once or twice a week’, and ‘three times or more a week’, and were assigned a numerical score from 1 to 4. An overall rating of sleep quality over the previous month, ranging from ‘very good’, ‘good’, ‘fairly bad’, to ‘very bad’, was assigned a numerical score of 1 to 4. All scores were summed and then categorised into tertiles, with the first tertile representing those reporting the least sleep disturbance (lowest scores), and the third tertile those reporting the most sleep disturbance (highest scores).
Measures of Outcome
Amnestic tasks
Tests assessing amnestic function in ELSA consist of a prospective memory task (remembering to carry out a task at a specified time during the test; range 0–3), orientation questions (reporting the day, date, month and year; range 0–4), and immediate and delayed recall tasks (memory for a list of 10 everyday words; each range 0–10). Details of each amnestic test have been described in detail elsewhere [24].
Non-amnestic tasks
The non-amnestic tests in ELSA consist of verbal fluency (number of animals named in one minute; range 1–55), speed of processing (total number of letters searched in one minute range 34–780), visual search accuracy (percentage of target letters detected in one minute; range 0–100) [24], and numeracy (mental arithmetic questions of increasing difficulty; range 0–6) [25].
Raw scores were standardised to allow meaningful comparisons between scores and to allow combined scores to be calculated, with standardisation being performed separately for each age group. Within each age group, each cognitive test score was converted to a z score using the standard formula (where x = raw score; x– = group mean; s = standard deviation):
For ease of interpretation and comparison with previous studies [26] z scores were converted to T scores using the standard formula:
The same procedure of converting raw scores into z scores, and then z scores into T scores, was repeated for each cognitive test. To calculate an average T score across amnestic tests for each respondent, T scores for prospective memory, date questions, immediate recall and delayed recall were summed and divided by 4. Likewise, to calculate an average T score across non-amnestic tests for each respondent, T scores for verbal fluency, speed of processing, search accuracy and numeracy were summed and divided by 4.
Measures of Covariates
Standard adjustments were made for age and sex, where age was measured in full years and entered into the models as a continuous variable. The effects of further covariates known to influence sleep and/or cognition were assessed at each stage of the analysis, although not all were included in the final models. These covariates were all derived from the questionnaire administered in ELSA Wave 4, and were as follows: highest educational qualification (none, intermediate, or degree/higher; [27]); employment grade (managerial/professional, intermediate, or routine/manual; [26]); marital status (single, married, separated/divorced, or widowed; [27]); depression (categorised as either ‘depressed’, where CES-D score ≥4 [28], [29], or there had been a previous diagnosis of depression; or ‘not depressed’, where CES-D score <4 and there was no reported diagnosis of depression); quality of life (total CASP-19 score; [30]); physical activity (sedentary, low, moderate, or high; composite measure derived from four questions in the main ELSA interview, approximating closely to the classification used in the Allied Dunbar Survey of Fitness [31]); smoking (never, current smoker, or previous smoker; [32]); alcohol consumption (units per week); chronic disease (reported diagnosis of one or more of the following cardiovascular, chronic or respiratory complaints; high blood pressure, angina, myocardial infarction, congestive heart failure, heart murmur, arrhythmia, diabetes or high blood sugar, stroke, high cholesterol, arthritis, osteoporosis, cancer, Parkinson’s Disease, psychiatric disease, Alzheimer’s Disease, dementia or memory impairment, lung disease i.e. chronic bronchitis or emphysema, or asthma); limiting longstanding illness (any self-reported illness, disability or infirmity which has limited activity over a period of time); troubled by pain; and finally, self-reported general health (excellent, very good, good, fair or poor).
Statistical Analysis
Age and basic characteristics were derived from the ELSA questionnaire at Wave 4, where chi-square and one-way analysis of variance (ANOVA) were used to determine differences in the distribution of variables. Analysis of covariance (ANCOVA) was performed to test the association between sleep quantity and sleep quality, and scores of amnestic and non-amnestic cognition separately, whilst controlling for the effects of confounding/contributing factors. ANCOVAs were carried out separately for sleep quantity and sleep quality, for amnestic and non-amnestic scores, and for each age group, although the final models for each analysis were the same. We tested for interactions between each of the sleep factors and each covariate separately, with the intention to include any interactions in the final models. There were interactions between the sleep variables and age at this level for both the ACF and the nACF (p<0.001 and p = 0.06 with sleep quantity and p<0.001 and p = 0.018 for sleep quality, respectively). The final models included either average amnestic or non-amnestic T scores as the dependant variable (measure of outcome), either sleep quantity categories or sleep quality tertiles as the independent variables (measure of exposure), and all of the following covariates: age, sex, education, employment grade, depression, physical activity, smoking, general health, plus a sleep*age interaction term. A two-sided p value <0.05 is considered statistically significant. All analyses were performed in IBM SPSS Statistics version 21 (IBM Corp, Armonk, NY).
Results
Population characteristics
The basic characteristics and demographics of the study sample are presented by sleep quantity categories (Table 1) and sleep quality tertiles (Table 2), in younger (50–64 years; n = 4,660) and older (65+ years; n = 4,129) age groups.
Table 1. Characteristics of study population by sleep QUANTITY categories.
| YOUNGER (50–64 years) | OLDER (65+ years) | |||||||
| Variable1 | <6 hr | 6–8 hr | >8 hr | p 2 | <6 hr | 6–8 hr | >8 hr | p 2 |
| N [%] unless otherwise stated | 646[13.9] | 3778[81.1] | 236[5.1] | 607[14.7] | 3136[76.0] | 386[9.3] | ||
| Sex (% male) | 37.3 | 46.2 | 38.6 | <0.001 | 37.4 | 47.5 | 45.3 | <0.001 |
| Age (years) | 57.9 (3.8) | 57.9 (3.8) | 58.3 (4.2) | = 0.252 | 73.9 (6.1) | 73.2 (6.1) | 74.5 (6.5) | <0.001 |
| Amnestic function (T score) | 48.4 (6.3) | 50.4 (6.2) | 48.6 (6.9) | <0.001 | 49.4 (6.6) | 50.4 (6.7) | 47.5 (8.4) | <0.001 |
| Non-amnestic function (T score) | 48.8 (5.5) | 50.3 (5.4) | 48.4 (5.8) | <0.001 | 49.2 (5.7) | 50.4 (6.0) | 48.3 (6.2) | <0.001 |
| Educational Qualification: | [ N = 610] | [ N = 3577] | [ N = 226] | [ N = 605] | [ N = 3122] | [ N = 385] | ||
| None | 26.1 | 15.5 | 28.8 | 44.6 | 34.7 | 37.7 | ||
| Intermediate | 45.6 | 41.8 | 42.5 | 35.0 | 36.7 | 37.1 | ||
| Higher/degree | 28.4 | 42.6 | 28.8 | <0.001 | 20.3 | 28.6 | 25.2 | <0.001 |
| Employment Grade: | [ N = 611] | [ N = 3594] | [ N = 227] | [ N = 597] | [ N = 3077] | [ N = 376] | ||
| Managerial/Professional | 29.0 | 39.8 | 31.7 | 22.6 | 32.9 | 25.3 | ||
| Intermediate | 24.5 | 25.8 | 23.8 | 25.5 | 26.3 | 26.1 | ||
| Routine/Manual | 46.5 | 34.4 | 44.5 | <0.001 | 51.9 | 40.9 | 48.7 | <0.001 |
| Marital Status: | ||||||||
| Single | 6.3 | 6.9 | 5.1 | 5.3 | 4.4 | 4.7 | ||
| Married | 66.1 | 76.0 | 78.8 | 54.7 | 63.7 | 58.3 | ||
| Divorced/Separated | 20.7 | 13.2 | 13.1 | 9.4 | 8.6 | 4.4 | ||
| Widowed | 6.8 | 3.9 | 3.0 | <0.001 | 30.6 | 23.2 | 32.6 | <0.001 |
| Depression 3 : | [ N = 645] | [ N = 3775] | [ N = 236] | [ N = 607] | [ N = 3130] | [ N = 385] | ||
| Yes | 53.0 | 22.6 | 33.9 | <0.001 | 51.1 | 26.9 | 30.1 | <0.001 |
| Quality of Life: | [ N = 565] | [ N = 3345] | [ N = 202] | [ N = 494] | [ N = 2668] | [ N = 304] | ||
| CASP19 score | 37.4 (10.1) | 42.4 (8.4) | 40.7 (9.2) | <0.001 | 37.1 (9.0) | 41.4 (7.9) | 40.0 (8.7) | <0.001 |
| Physical Activity level: | [ N = 645] | [ N = 3777] | [ N = 236] | [ N = 607] | [ N = 3134] | [ N = 386] | ||
| Sedentary | 5.6 | 2.0 | 5.1 | 12.0 | 7.7 | 10.4 | ||
| Low | 25.0 | 16.5 | 22.9 | 35.6 | 26.3 | 31.1 | ||
| Moderate | 48.4 | 53.8 | 51.3 | 40.9 | 49.5 | 46.1 | ||
| High | 21.1 | 27.6 | 20.8 | <0.001 | 11.5 | 16.5 | 12.4 | <0.001 |
| Smoking status: | ||||||||
| Never smoked | 62.1 | 70.5 | 67.4 | 78.4 | 81.1 | 78.0 | ||
| Current smoker | 19.8 | 17.3 | 17.8 | 12.2 | 10.7 | 10.4 | ||
| Previous smoker | 18.1 | 12.2 | 14.8 | <0.001 | 9.4 | 8.3 | 11.7 | = 0.152 |
| Alcohol consumption: | [ N = 325] | [ N = 2548] | [ N = 145] | [ N = 269] | [ N = 1797] | [ N = 177] | ||
| Units per week | 16.8 (17.2) | 17.8 (16.3) | 17.8 (16.7) | = 0.436 | 14.2 (15.9) | 16.0 (15.6) | 13.0 (11.8) | = 0.013 |
| Diagnosed disease 3 | 82.2 | 69.2 | 78.8 | <0.001 | 89.1 | 87.7 | 86.8 | = 0.491 |
| Limiting longstanding illness 3 | 46.4 | 24.1 | 38.6 | <0.001 | 49.9 | 35.5 | 42.7 | <0.001 |
| Troubled by pain 3 | 57.9 | 34.3 | 41.1 | <0.001 | 56.8 | 38.5 | 36.3 | <0.001 |
| Self-reported general health: | ||||||||
| Excellent | 10.5 | 18.0 | 11.4 | 6.1 | 10.0 | 7.0 | ||
| Very good | 20.5 | 34.5 | 28.0 | 18.8 | 27.8 | 27.5 | ||
| Good | 28.4 | 30.4 | 33.5 | 30.6 | 35.2 | 33.7 | ||
| Fair | 25.9 | 13.4 | 17.8 | 28.3 | 20.6 | 23.1 | ||
| Poor | 14.7 | 3.8 | 9.3 | <0.001 | 16.1 | 6.3 | 8.8 | <0.001 |
Results expressed as mean (sd) or %.
ANOVA for continuous data, chi-square for categorical data (where p value represents differences between all categories).
See Methods section for description of variables.
Table 2. Characteristics of study population by sleep QUALITY1 tertiles.
| YOUNGER (50–64 years) | OLDER (65+ years) | |||||||
| Variable2 | 1st | 2nd | 3rd | p 3 | 1st | 2nd | 3rd | P 3 |
| N [%] unless otherwise stated | 1714[36.8] | 1487[31.9] | 1459[31.3] | 1573[38.1] | 1424[34.5] | 1132[27.4] | ||
| Sex (% male) | 53.9 | 45.6 | 32.6 | <0.001 | 52.4 | 47.8 | 34.1 | <0.001 |
| Age (years) | 57.8 (3.8) | 58.2 (3.8) | 57.8 (3.8) | = 0.001 | 73.3 (6.3) | 73.4 (6.0) | 73.5 (6.1) | = 0.610 |
| Amnestic function (T score) | 50.3 (6.3) | 50.2 (6.1) | 49.4 (6.4) | <0.001 | 49.7 (7.3) | 50.5 (6.7) | 49.8 (6.7) | = 0.002 |
| Non-amnestic function (T score) | 50.5 (5.6) | 50.3 (5.3) | 49.1 (5.5) | <0.001 | 50.1 (6.2) | 50.4 (5.8) | 49.4 (5.9) | <0.001 |
| Educational Qualification: | [ N = 1610] | [ N = 1418] | [ N = 1385] | [ N = 1567] | [ N = 1421] | [ N = 1124] | ||
| None | 14.7 | 15.2 | 23.6 | 33.8 | 33.2 | 44.1 | ||
| Intermediate | 39.7 | 44.2 | 43.6 | 36.1 | 38.4 | 34.8 | ||
| Higher/degree | 45.6 | 40.6 | 32.8 | <0.001 | 30.1 | 28.4 | 21.1 | <0.001 |
| Employment Grade: | [ N = 1630] | [ N = 1419] | [ N = 1383] | [ N = 1547] | [ N = 1398] | [ N = 1105] | ||
| Managerial/Professional | 42.9 | 37.8 | 32.0 | 32.1 | 32.5 | 26.2 | ||
| Intermediate | 24.8 | 25.4 | 26.5 | 27.0 | 26.0 | 25.1 | ||
| Routine/Manual | 32.3 | 36.9 | 41.4 | <0.001 | 40.9 | 41.5 | 48.8 | <0.001 |
| Marital Status: | ||||||||
| Single | 6.9 | 6.0 | 7.4 | 4.5 | 4.6 | 4.5 | ||
| Married | 77.6 | 75.9 | 70.3 | 64.5 | 62.2 | 58.0 | ||
| Divorced/Separated | 12.3 | 13.7 | 17.1 | 7.2 | 9.1 | 8.9 | ||
| Widowed | 3.3 | 4.4 | 5.2 | <0.001 | 23.8 | 24.0 | 28.6 | = 0.016 |
| Depression 4 : | [ N = 1711] | [ N = 1487] | [ N = 1458] | [ N = 1570] | [ N = 1421] | [ N = 1131] | ||
| Yes | 9.6 | 21.5 | 54.1 | <0.001 | 15.7 | 26.9 | 56.5 | <0.001 |
| Quality of Life: | [ N = 1526] | [ N = 1319] | [ N = 1267] | [ N = 1339] | [ N = 1197] | [ N = 930] | ||
| CASP19 score | 44.6 (7.7) | 42.3 (7.9) | 37.3 (9.6) | <0.001 | 43.3 (7.5) | 40.6 (7.5) | 36.7 (8.8) | <0.001 |
| Physical Activity level: | [ N = 1713] | [ N = 1487] | [ N = 1458] | [ N = 1573] | [ N = 1424] | [ N = 1130] | ||
| Sedentary | 2.3 | 1.5 | 4.3 | 6.7 | 7.0 | 13.1 | ||
| Low | 12.7 | 16.0 | 26.3 | 23.5 | 27.2 | 35.8 | ||
| Moderate | 55.0 | 53.4 | 50.0 | 51.9 | 49.5 | 40.4 | ||
| High | 29.9 | 29.1 | 19.4 | <0.001 | 17.9 | 16.3 | 10.7 | <0.001 |
| Smoking status: | ||||||||
| Never smoked | 70.5 | 71.9 | 64.8 | 79.8 | 82.3 | 78.7 | ||
| Current smoker | 17.5 | 15.8 | 19.9 | 11.4 | 10.0 | 11.2 | ||
| Previous smoker | 12.0 | 12.3 | 15.4 | <0.001 | 8.8 | 7.7 | 10.1 | = 0.157 |
| Alcohol consumption: | [ N = 1197] | [ N = 1000] | [ N = 821] | [ N = 910] | [ N = 798] | [ N = 535] | ||
| Units per week | 18.6 (17.2) | 18.4 (17.3) | 15.8 (15.2) | = 0.001 | 16.4 (16.1) | 15.8 (15.4) | 13.6 (14.2) | = 0.003 |
| Diagnosed disease 4 | 62.3 | 71.8 | 82.0 | <0.001 | 83.9 | 88.4 | 92.4 | <0.001 |
| Limiting longstanding illness 4 | 16.1 | 24.4 | 45.4 | <0.001 | 26.3 | 37.5 | 56.1 | <0.001 |
| Troubled by pain 4 | 23.0 | 36.5 | 56.7 | <0.001 | 26.3 | 40.7 | 61.6 | <0.001 |
| Self-reported general health: | ||||||||
| Excellent | 24.8 | 15.7 | 8.0 | 13.7 | 8.3 | 4.1 | ||
| Very good | 38.0 | 34.7 | 22.8 | 35.7 | 25.0 | 15.3 | ||
| Good | 27.2 | 32.3 | 31.7 | 34.1 | 37.3 | 31.2 | ||
| Fair | 8.6 | 13.7 | 24.9 | 13.2 | 23.3 | 32.6 | ||
| Poor | 1.3 | 3.6 | 12.7 | <0.001 | 3.4 | 6.1 | 16.9 | <0.001 |
Total sleep quality score divided into tertiles. 1st tertile = least disturbance; 3rd tertile = most disturbance. See Methods for further details.
Results expressed as mean (sd) or %.
ANOVA for continuous data, chi-square for categorical data (where p value represents differences between all categories).
See Methods section for description of variables.
Many of the measured variables showed significant differences across categories of sleep quantity or sleep quality in both younger and older age groups. As in previous studies, the proportion of individuals who were long sleepers increased in the older age group; in both age groups there was a strong association between short sleep and depression, reduced quality of life, limiting long standing illness, troubled by pain and poorer self-reported general health. Coexisting diagnosed disease was associated with short sleep in the younger, but not in the older, age group, probably due to the high prevalence of diagnosed disease in the latter (Table 1). For sleep quality, in both age groups, individuals were more likely to have good quality sleep; there were however strong associations between poor quality sleep and depression, reduced quality of life, reduced physical activity, coexisting diagnosed disease, limiting longstanding illness, troubled by pain and poorer self-reported general health (Table 2).
Sleep quantity, sleep quality and cognitive function
Unadjusted
The association between sleep domains (quantity and quality) are shown in Figure S1 (Supplementary Information). In both the younger and older age groups, short sleepers reported the more sleep disturbance. There was a significant variation (inverted U shape) in both amnestic and non-amnestic cognition by sleep quantity in both the younger and older age groups (all p<0.001), whereby cognition scores were lower in both short and long sleepers (Table 1). Likewise for sleep quality, there was a significant variation in amnestic function for younger (p<0.001) and older (p = 0.002) groups, as well as in non-amnestic cognition (both p<0.001), whereby lower cognition scores were associated with poorer quality (Table 2).
Adjusted
After adjustment for multiple confounders, there was a statistically significant association between sleep quantity (Table 3 and Figure 1) and both amnestic and non-amnestic cognitive function (mean T score) in both age groups. In a fully adjusted model (Model 3), in the younger group duration of sleep explained 15.2% of the variance in the amnestic domain (p = 0.003) and 20.6% of non-amnestic cognitive function (p = 0.010). In the older group the estimates were 21.3% (p<0.001) and 25.6% (p<0.001), respectively. Applying the same adjustments to sleep quality, there was a statistically significant association between sleep quality and both amnestic (p<0.001) and non-amnestic (p<0.001) cognition in the older age group, but no longer in the younger age group (p = 0.586 and p = 0.373, respectively; Table 4). Again, sleep quality explained between 15.1% and 25.5% of the variance in the cognition domains (Table 4 and Figure 2). When these analyses were carried out after the exclusion of participants with previous stroke, Parkinson’s, Alzheimer’s and dementia or memory impairment (n = 419) the results did not change substantially (data not shown).
Table 3. Mean amnestic and non-amnestic T scores by sleep quantity categories for the younger and older age groups.
| AMNESTIC | YOUNGER GROUP (50–64 yrs) | OLDER GROUP (65+yrs) | |||
| Model | Sleep Quantity | Mean T Score(SE) | p and R2 values | Mean T Score(SE) | p and R2 values |
| 0 | <6 hr | 48.4 (0.2) | 49.4 (0.3) | ||
| (unadjusted) | 6–8 hr | 50.4 (0.1) | 50.4 (0.1) | ||
| >8 hr | 48.6 (0.4) | p<0.001; R2 = 0.014 | 47.5 (0.4) | p<0.001; R2 = 0.016 | |
| 1 | <6 hr | 48.3 (0.2) | 49.4 (0.3) | ||
| (age+sex, sleep*age) | 6–8 hr | 50.3 (0.1) | 50.4 (0.1) | ||
| >8 hr | 48.6 (0.4) | p<0.001; R2 = 0.028 | 48.1 (0.3) | p<0.001; R2 = 0.125 | |
| 2 | <6 hr | 48.5 (0.2) | 50.0 (0.3) | ||
| (Model 1+ education, | 6–8 hr | 49.8 (0.1) | 50.5 (0.1) | ||
| employment grade) | >8 hr | 48.7 (0.4) | p<0.001; R2 = 0.123 | 48.4 (0.3) | p<0.001; R2 = 0.189 |
| 3 | <6 hr | 47.8 (0.3) | 49.7 (0.3) | ||
| (Model 2+ depression, physical, | 6–8 hr | 48.5 (0.2) | 49.7 (0.2) | ||
| activity, smoking, general health) | >8 hr | 47.6 (0.4) | p = 0.003; R2 = 0.152 | 47.7 (0.4) | p<0.001; R2 = 0.213 |
| NON-AMNESTIC | |||||
| 0 | <6 hr | 48.8 (0.2) | 49.2 (0.2) | ||
| (unadjusted) | 6–8 hr | 50.3 (0.1) | 50.4 (0.1) | ||
| >8 hr | 48.4 (0.4) | p<0.001; R2 = 0.014 | 48.3 (0.3) | p<0.001; R2 = 0.013 | |
| 1 | <6 hr | 48.8 (0.2) | 49.4 (0.2) | ||
| (age+sex, sleep*age) | 6–8 hr | 50.3 (0.1) | 50.3 (0.1) | ||
| >8 hr | 48.5 (0.4) | p<0.001; R2 = 0.023 | 48.7 (0.3) | p<0.001; R2 = 0.110 | |
| 2 | <6 hr | 49.0 (0.2) | 50.1 (0.2) | ||
| (Model 1+ education, | 6–8 hr | 49.7 (0.1) | 50.5 (0.1) | ||
| employment grade) | >8 hr | 48.5 (0.3) | p<0.001; R2 = 0.182 | 49.1 (0.3) | p<0.001; R2 = 0.225 |
| 3 | <6 hr | 48.7 (0.2) | 49.8 (0.2) | ||
| (Model 2+ depression, physical, | 6–8 hr | 48.9 (0.2) | 49.7 (0.2) | ||
| activity, smoking, general health) | >8 hr | 47.9 (0.4) | p = 0.010; R2 = 0.206 | 48.4 (0.3) | p<0.001; R2 = 0.256 |
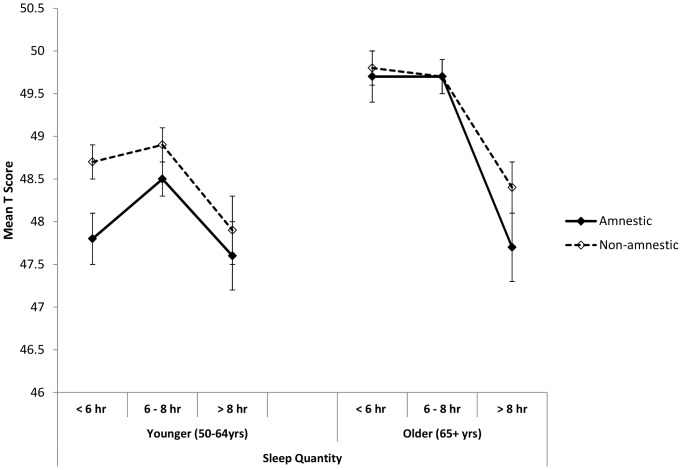
Figure 1. Cognitive function scores by sleep quantity.
Fully adjusted mean T scores for amnestic and non-amnestic cognition scores for each sleep quantity category, in younger and older age groups. Adjusted for age, sex, sleep*age, education, employment grade, depression, physical activity, smoking, general health (Model 3).
Table 4. Mean amnestic and non-amnestic T scores by sleep quality categories for the younger and older age groups.
| AMNESTIC | YOUNGER GROUP (50–64yrs) | OLDER GROUP (65+yrs) | |||
| Model | Sleep Quality | Mean TScore (SE) | p and R2 values | Mean TScore (SE) | p and R2 values |
| 0 | 1st tertile (least disturbance) | 50.3 (0.2) | 49.7 (0.2) | ||
| (unadjusted) | 2nd tertile | 50.2 (0.2) | 50.5 (0.2) | ||
| 3rd tertile (most disturbance) | 49.4 (0.2) | p<0.001; R2 = 0.005 | 49.8 (0.2) | p = 0.002; R2 = 0.003 | |
| 1 | 1st tertile (least disturbance) | 50.4 (0.2) | 49.6 (0.2) | ||
| (age+sex, sleep*age) | 2nd tertile | 50.2 (0.2) | 50.5 (0.2) | ||
| 3rd tertile (most disturbance) | 49.1 (0.2) | p<0.001; R2 = 0.020 | 49.7 (0.2) | p<0.001; R2 = 0.114 | |
| 2 | 1st tertile (least disturbance) | 49.8 (0.2) | 49.8 (0.2) | ||
| (Model 1+ education, | 2nd tertile | 49.7 (0.2) | 50.7 (0.2) | ||
| employment grade) | 3rd tertile (most disturbance) | 49.1 (0.2) | p = 0.005; R2 = 0.119 | 50.2 (0.2) | p = 0.002; R2 = 0.181 |
| 3 | 1st tertile (least disturbance) | 48.1 (0.2) | 48.6 (0.2) | ||
| (Model 2+ depression, physical | 2nd tertile | 48.3 (0.2) | 49.9 (0.2) | ||
| activity, smoking, general health) | 3rd tertile (most disturbance) | 48.4 (0.2) | p = 0.586; R2 = 0.151 | 50.2 (0.2) | p<0.001; R2 = 0.213 |
| NON-AMNESTIC | |||||
| 0 | 1st tertile (least disturbance) | 50.5 (0.1) | 50.1 (0.2) | ||
| (unadjusted) | 2nd tertile | 50.3 (0.1) | 50.4 (0.2) | ||
| 3rd tertile (most disturbance) | 49.1 (0.1) | p<0.001; R2 = 0.011 | 49.4 (0.2) | p<0.001; R2 = 0.005 | |
| 1 | 1st tertile (least disturbance) | 50.4 (0.1) | 50.1 (0.1) | ||
| (age+sex, sleep*age) | 2nd tertile | 50.4 (0.1) | 50.4 (0.2) | ||
| 3rd tertile (most disturbance) | 49.2 (0.1) | p<0.001; R2 = 0.020 | 49.5 (0.2) | p<0.001; R2 = 0.105 | |
| 2 | 1st tertile (least disturbance) | 49.7 (0.1) | 50.3 (0.1) | ||
| (Model 1+ education, | 2nd tertile | 49.8 (0.1) | 50.6 (0.1) | ||
| employment grade) | 3rd tertile (most disturbance) | 49.1 (0.1) | p = 0.001; R2 = 0.181 | 50.1 (0.2) | p = 0.067; R2 = 0.221 |
| 3 | 1st tertile (least disturbance) | 48.7 (0.2) | 49.1 (0.2) | ||
| (Model 2+ depression, physical | 2nd tertile | 48.9 (0.2) | 49.8 (0.2) | ||
| activity, smoking, general health) | 3rd tertile (most disturbance) | 48.8 (0.2) | p = 0.373; R2 = 0.204 | 49.9 (0.2) | p<0.001; R2 = 0.255 |
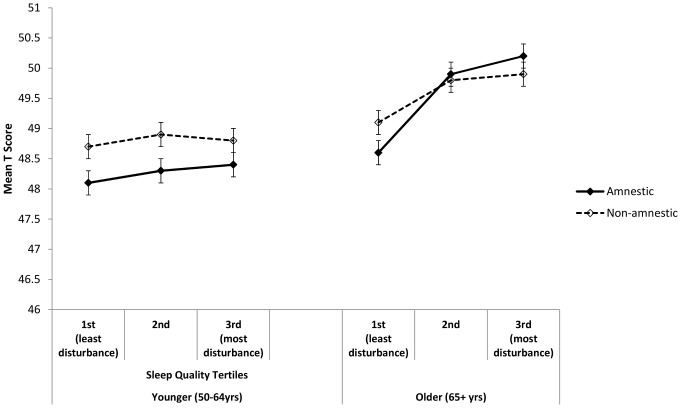
Figure 2. Cognitive function scores by sleep quality.
Fully adjusted mean T scores for amnestic and non-amnestic cognition scores for each sleep quality tertile, in younger and older age groups. Adjusted for age, sex, sleep*age, education, employment grade, depression, physical activity, smoking, general health (Model 3).
Sleep quantity and amnestic function in younger group (Figure 1)
After Bonferroni correction short sleepers had significantly lower mean amnestic T scores than optimal sleepers (p = 0.012). There was no significant difference in amnestic scores between short sleepers and long sleepers (p>0.999), or between optimal sleepers and long sleepers (p = 0.088).
Sleep quantity and amnestic function in older group (Figure 1)
Long sleepers had significantly lower amnestic scores than both short sleepers (p<0.001) and optimal sleepers (p<0.001), whereas there was no significant difference between short and optimal sleepers (p>0.999).
Sleep quantity and non-amnestic function in younger group (Figure 1)
Long sleepers had significantly lower mean non-amnestic T scores than optimal sleepers (p = 0.010), but there was no significant difference in non-amnestic T scores between short sleepers and optimal sleepers (p = 0.789), or short sleepers and long sleepers (p = 0.161).
Sleep quantity and non-amnestic function in older group (Figure 1)
Long sleepers had significantly lower non-amnestic scores than both short sleepers (p<0.001) and optimal sleepers (p<0.001), but there was no significant difference in non-amnestic scores between short and optimal sleepers (p>0.999).
Sleep quality and amnestic function in younger group (Figure 2)
There were no significant differences in amnestic scores between any of the sleep quality tertiles (all p>0.917).
Sleep quality and amnestic function in older group (Figure 2)
Those reporting the least amount of sleep disturbance had the lowest cognitive function scores. Mean T scores were significantly lower in the 1st tertile (least disturbance) than in the 2nd and 3rd tertiles (both p<0.001), but the difference between 2nd and 3rd tertile was not significant (p = 0.461).
Sleep quality and non-amnestic function in younger group (Figure 2)
There were no significant differences in non-amnestic scores between any of the sleep quality tertiles for the younger age group (all p>0.484).
Sleep quality and non-amnestic function in older group (Figure 2)
Those in the 1st tertile (least disturbance) had lower T scores than those in the 2nd (p = 0.003) or 3rd (p = 0.001) tertile, but there was no significant difference in T scores between the 2nd and 3rd tertile (p>0.999).
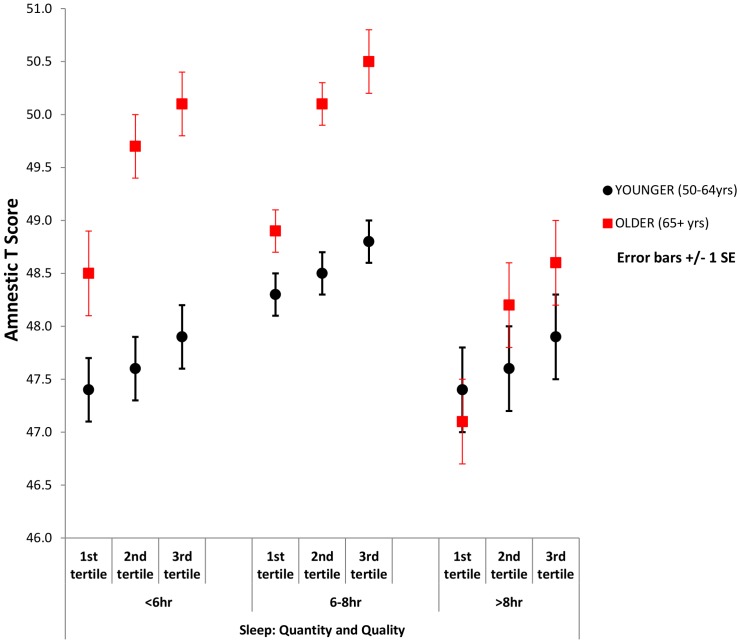
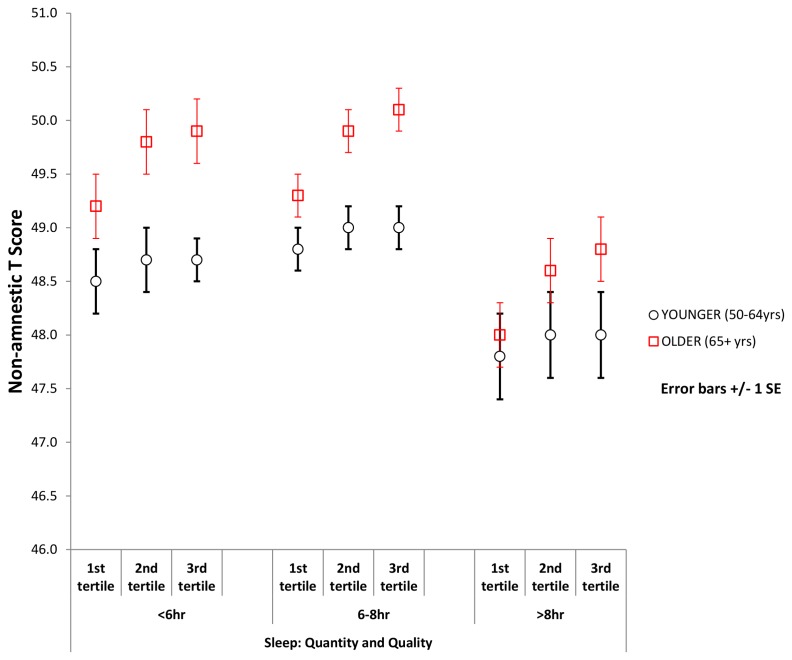
Interaction of sleep quality with age on cognitive function
We detected a significant interaction between sleep quality and age independent from sleep quantity, more evident for the association with amnestic cognitive function (see above). These interactions are shown in Figure 3 for amnestic function and Figure 4 for non-amnestic function when stratified by sleep duration. At any level of sleep duration there was a steeper association between sleep quality and amnestic cognition in the older than the younger group.
Figure 3. Mean amnestic T scores by sleep categories adjusted for sleep quality.
Adjusted mean amnestic T Score by sleep quantity categories, per sleep quality tertile, in younger and older age groups.
Figure 4. Mean non-amnestic T scores by sleep categories adjusted for sleep quality.
Adjusted mean non-amnestic T Score by sleep quantity categories, per sleep quality tertile, in younger and older age groups.
Discussion
Main Findings
The results from this study suggest that sleep quantity and quality are associated with both amnestic and non-amnestic cognition, and that these associations differ with age. Following adjustment for potential confounders, in the younger age group (50–64 years), both short (<6 hrs per night) and long (>8 hrs per night) sleep were associated with lower amnestic and non-amnestic scores. Whereas in the older age group (65+ years), associations were only significant with long sleep. However, whilst sleep quality was associated with both amnestic and non-amnestic scores in the older age group, it was not in the younger age group. These effects were regardless of duration of sleep. Finally, the older individuals with higher cognitive function reported more often sleep disturbances, regardless of sleep quantity.
External validity and comparison with other studies
Previous cross-sectional studies have demonstrated that long sleep durations are associated with diminished global cognition [33]. Furthermore, in older populations there may be an inverse U-shaped relationship between sleep and cognition, with both short and long sleep being associated with poorer memory function [11].
In our analysis we separated the individuals into younger and older groups, to examine the potential differences in the associations between sleep and cognition in these two age groups. In agreement with Faubel et al. [33] who showed an association between long sleep and diminished global cognition, we found a significant decrease in amnestic and non-amnestic cognitive function in long sleepers, but this only reached significance in the older group. In our younger group, amnestic scores were significantly lower in short sleepers, whereas non-amnestic scores were lower in long sleepers. Our results also support those of Xu et al. [11], who suggested that memory function is impaired in short and long sleepers. Again however, we have explored the effect of sleep on memory in further detail by examining this in different pre- and post-retirement age groups. Our results show that an inverted U-shaped relationship exists in younger adults, where the amnestic scores for short sleepers were significantly lower than those for optimal sleepers, and whilst the amnestic scores for the long sleepers were reduced, this difference was not statistically significant. These findings could be interpreted in the context of recent findings in mice, which suggest that sleep deprivation causes irreversible damage to the brain which could impair cognitive function, particularly alertness [34]. However, if this is the case, it is not clear why the effect of short sleep is not evident in the older group. That is, in the older adults, there was no observed effect in short sleepers but the amnestic scores in long sleepers were significantly lower than those for optimal sleepers. These findings are consistent with the results described in a recent cross-sectional analysis of an elderly population [35]. Using the MMSE long, but not short, sleep was associated with significantly lower cognitive function scores. Furthermore, whilst another cross-sectional study reported that both short and long sleep durations were associated with diminished global cognition, the individuals in that study were aged 30 years or older and hence included both ‘much younger’ and ‘older’ adults [10].
Our results indicate that the age of the population under investigation may be an important determinant of study outcome. In ELSA, those over the age of 89 years are coded with an age of 99 years to promote anonymity within the sample, and as such, the coded age is not accurate for individuals over the age of 89 years. Therefore, the age range in our sample was 50–89 years. It may be pertinent for future studies to examine the association between sleep and cognition and the influence of extraneous health and lifestyle factors, in the very old (i.e. those over the age of 90 years) to give a more comprehensive and inclusive overview of the cognitive ageing process.
In this study, we did not find any significant interaction with gender (interactions between sleep and sex factors in Model 3 for younger and older groups, all p≥0.085) and hence the data was not analysed in men and women separately. However, it is of interest to note that it has been suggested [36] that whereas women have higher baseline cognition scores than men, decline may be faster in women, and so longitudinal trajectories of male and female cognitive decline converge in later life. This could explain the lack of sex interactions in our results, but it is important to note for future studies of younger adult populations.
In a study in men only, Blackwell et al. [15] suggested that disturbed sleep is strongly associated with decline in executive function (or non-amnestic function), and less so for global cognition, whereas we found the opposite to be true in older adults. Indeed in our older group, the highest cognitive function scores (both amnestic and non-amnestic) were seen in those individuals with the greatest reported disturbances in sleep. In younger individuals however, there was no significant association between cognition and sleep quality, indicating that until we reach the age of around 65 years, there may be no association between sleep quality and cognitive function. The reason for these differences is unclear and prospective analyses of the effects of sleep quality on the decline in cognition could help rule out possible influences of reverse causality due to pre-existing ill-health or other confounders.
The suggestion that cognitive function increases with increasing sleep disturbance in older individuals appears to be counterintuitive. There are a number of possible reasons for this that need to be explored. Higher cognitive scores in older individuals could be due to practice effects – i.e. the older group may have more experience with the cognitive function tests in ELSA [37]. It may reflect the fact that those individuals who are more cognitively able are better at recording sleep disturbance data. Alternatively, it may indicate that in an elderly population, individuals who are more cognitively active may process the day’s events and/or experience more worry or anxiety than those who are less cognitively active, and hence this may lead to an associated increase in self-reported frequency of sleep disturbance. Further, previous research has indicated that mild anxiety symptoms in older adults are associated with better cognitive function [38], which would support this suggestion. Confounding effects of medications may also be more important in an older group. Likewise, in those participants with memory problems, we cannot exclude the possibility that their responses might have been erroneous to some extent due to their memory impairment. The number of such individuals was small and their exclusion did not substantially change the results. Some antihypertensives and corticosteroids acts as stimulants and, the night-time use of diuretics can promote repeated awakening to go to the bathroom. These ideas and the temporal sequence of events, however, need to be addressed in longitudinal studies.
A small number of prospective studies have investigated whether poor sleep can predict cognitive impairment in later life, but these have produced inconsistent findings [39]–[43]. The heterogeneity of results between these studies could be due to a number of methodological differences, including age [14], [26] and sex [3], [44] of participants, duration of follow-up [41]–[43], population culture or ethnicity [45], [46], cognitive assessments or sleep measures [41], [47], and statistical adjustments made for various potential confounders. There are two recent prospective studies, which are most pertinent to our line of enquiry [14], [26]. The Whitehall II study shows that adverse changes in sleep quantity over time (either a decrease from 6, 7 or 8 hours, or an increase from 7 or 8 hours, over a mean period of 5.4 years) are associated with lower scores on a variety of tests of cognitive function, with the exception of memory tasks [26]. Thus, detrimental changes in sleep quantity over time, particularly a shift to longer sleep durations, may have a domain-specific effect on non-amnestic cognitive performance, whilst memory function may remain relatively preserved. This study was carried out in a middle-aged cohort. Whilst it remains to be seen whether changes in sleep duration would have similar effects on different cognitive domains in older adults one report suggests a detectable effect on verbal memory in participants >70 years of age [48].
It is of interest that alcohol and quality of life scores were associated with an attenuation of the association between sleep and cognition. This may be due to protective effects of alcohol [49] and quality of life [50] on cognitive function.
Strengths and limitations
Whilst in a recent study it was demonstrated that the MMSE can be used as a tool to extract scores pertaining to amnestic and non-amnestic function specifically [14], the findings should be interpreted with caution. That is, reliance on scores from one item of a test of global cognition is not a robust method of diagnosing or even suggesting the existence of a memory impairment - not merely because there are so many more tests which comprise the non-amnestic score on the MMSE. A major strength of our study is that the cognitive function assessments are robust and have been used extensively in previous population studies, such as the Medical Research Council Cognitive Function and Aging Study (MRC CFAS) [51], Health and Retirement Study (HRS) [52], and the MRC National Survey of Health and Development (also known as the British 1946 birth cohort) [53]. Furthermore, the amnestic and non-amnestic composite scores used in our analyses were derived from multiple tests, thus minimising ceiling and floor effects.
Limitations of this study include the exclusion of non-white individuals due to the limited sample size (3.5%) and hence lack of statistical power for the analysis, the assessment of sleep domains based exclusively on self-reporting and the cross-sectional design. The latter precludes investigation of the temporal sequence of sleep disturbances in relation to cognitive function. This will be addressed in future analyses when further waves of ELSA follow-up data are available. Repeated measures in longitudinal studies can however introduce a possible practice effect which masks cognitive decline over time [37]. Finally, data on the use of hypnotics was not collected in ELSA and hence we were unable to adjust for a potential important confounder.
Some studies have categorised short sleep as <5 hrs and long sleep as >9 hrs [14], [26], but this was not possible for our analyses, owing to the very small numbers in these groups and subsequent loss of statistical power. Nevertheless, it is widely accepted that around 7 hrs of sleep per night is considered to be optimal [23], and so our sleep quantity categories are generally consistent with, and comparable to, the majority of studies in this field.
Implications
The results from this study suggest that sleep quantity and sleep quality, if causally related to cognition, may have different effects in younger and older adults, on both amnestic and non-amnestic cognitive function. Furthermore, there are clear interactions between sleep quantity and sleep quality with age, which would indicate that future studies need to be designed to investigate both the independent effects, and interplay between, these different sleep measures throughout the life-course.
Conclusions
Ideally, the diagnosis of mild cognitive impairment needs to be made at a very early stage, where intervention might delay or even prevent the disease process and potential progression to various forms of dementia. Further research is needed, however, to investigate the temporal sequence of events underlying the association between sleep quantity and quality and cognitive function and decline over time. Furthermore, it is important that studies develop validated and standardised tests, which are specifically designed to detect amnestic and non-amnestic cognitive impairments in the normal ageing population. This would allow direct comparison between studies with different populations, and in in particular, those with differing age groups.
Supporting Information
Sleep quantity and sleep quality. Mean sleep quality score for each sleep quantity category (upper panel, S1A-S1B), and mean sleep quantity (hours) for each sleep quality tertile (lower panel, S1C-S1D), in younger and older age groups. All unadjusted ANOVAs p<0.001; see figures for p values for multiple comparisons (Bonferroni-corrected).
(TIF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are available from the UK Data Service for researchers who meet the criteria for access to confidential data. Data are from WAVE 4 of the English Longitudinal Study of Ageing (ELSA). Data and contact details may be obtained via the website http://www.adls.ac.uk/find-administrative-data/linked-administrative-data/english-longitudinal-study-of-ageing/
Funding Statement
MAM and FPC received funding from the The Economic and Social Research Council (ESRC) grant number ES/K002910/1(www.esrc.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The study is part of the Sleep, Health and Society Research Programme of The University of Warwick.
References
- 1.Bliwise DL (2005) Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: WB Saunders Company. 24–38.
- 2. Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, et al. (1998) REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology 51: 363–370. [DOI] [PubMed] [Google Scholar]
- 3. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, et al. (2011) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA: The Journal of the American Medical Association 306: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, et al. (2008) Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport 19: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: 183–194. [DOI] [PubMed] [Google Scholar]
- 6. Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, et al. (2012) Risk profiles of subtypes of mild cognitive impairment: the sydney memory and ageing study. J Am Geriatr Soc 60: 24–33 10.1111/j.1532-5415.2011.03774.x [doi] [DOI] [PubMed] [Google Scholar]
- 7. Palmer K, Backman L, Winblad B, Fratiglioni L (2008) Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry 16: 603–611 10.1097/JGP.0b013e3181753a64 [doi];16/7/603 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC (2003) Mild cognitive impairment: prevalence and predictive validity according to current approaches. Acta Neurol Scand 108: 71–81. 118a [pii]. [DOI] [PubMed]
- 9. Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, et al. (2007) Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 68: 288–291 68/4/288 [pii];10.1212/01.wnl.0000252358.03285.9d [doi] [DOI] [PubMed] [Google Scholar]
- 10. Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, et al. (2009) Self-reported sleep duration and cognitive functioning in the general population. 18: 436–446 10.1111/j.1365–2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 11. Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, et al. (2011) Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep 34: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes TL, Riley T, Mattek N, Pavel M, Kaye JA (2013) Sleep Habits in Mild Cognitive Impairment. Alzheimer Dis Assoc Disord. 10.1097/WAD.0000000000000010 [doi]. [DOI] [PMC free article] [PubMed]
- 13.Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. 0022-3956(75)90026-6 [pii]. [DOI] [PubMed]
- 14. Potvin O, Lorrain D, Forget H, Dubé M, Grenier S, et al. (2012) Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 35: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, et al. (2011) Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 34: 1347–1356 10.5665/SLEEP.1276 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussey D, Lessof C, Ward K, Wood N (2010) Methodology. In: Banks J, Lessof C, Nazroo J, Rogers N, Stafford M et al., editors. Financial circumstances, health and well-being in the older population in England: the 2008 English Longitudinal Study of Ageing (Wave 4). London: Institute for Fiscal Studies. 386–409.
- 17.Marmot MG, Banks J, Blundell R, Lessof C, Nazroo J (2003) Health, wealth and lifestyles of the older population in England: the 2002 English Longitudinal Study of Ageing. London: Institute for Fiscal Studies.
- 18. Steptoe A, Breeze E, Banks J, Nazroo J (2013) Cohort profile: the English Longitudinal Study of Ageing. Int J Epidemiol 42: 1640–1648 dys168 [pii];10.1093/ije/dys168 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Behncke S (2012) Does retirement trigger ill health? Health Econ 21: 282–300 10.1002/hec.1712 [doi] [DOI] [PubMed] [Google Scholar]
- 20. Vahtera J, Westerlund H, Hall M, Sjosten N, Kivimaki M, et al. (2009) Effect of retirement on sleep disturbances: the GAZEL prospective cohort study. Sleep 32: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stranges S, Dorn JM, Shipley MJ, Kandala NB, Trevisan M, et al. (2008) Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States: the Whitehall II Study and the Western New York Health Study. Am J Epidemiol 168: 1353–1364 kwn337 [pii];10.1093/aje/kwn337 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lou P, Chen P, Zhang L, Zhang P, Chang G, et al. (2014) Interaction of sleep quality and sleep duration on impaired fasting glucose: a population-based cross-sectional survey in China. BMJ Open 4: e004436 bmjopen-2013–004436 [pii];10.1136/bmjopen-2013–004436 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappuccio FP, Miller MA, Lockley SW (2010) Sleep, Health and Society: From Aetiology to Public Health. Oxford University Press Inc.
- 24. Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, et al. (2008) Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J Am Geriatr Soc 56: 191–198 JGS1557 [pii];10.1111/j.1532-5415.2007.01557.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banks J, O’Dea C, Oldfield Z (2011) Cognitive function, numeracy and retirement saving trajectories. Econ J (London) 120: F381–F410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, et al. (2011) Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep 34: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huppert FA, Gardener E, McWilliams B (2006) Cognitive Function. In: Retirement, health and relationships of the older population in England: the 2004 English Longitudinal Study of Ageing (Wave 2). London: Institute for Fiscal Studies. 217–242.
- 28. Radloff LS (1977) The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1: 385–401. [Google Scholar]
- 29.Steffick DE (2000) Documentation of affective functioning measures in the Health and Retirement Study. HRS Documentation Report DR-005.
- 30. Hyde M, Wiggins RD, Higgs P, Blane DB (2003) A measure of quality of life in early old age: the theory, development and properties of a needs satisfaction model (CASP-19). Aging Ment Health 7: 186–194 10.1080/1360786031000101157 [doi];NRB8NHYDVLVJRN40 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Activity and Health Research (1992) Allied Dunbar National Fitness Survey: main findings. London: Sports Council and Health Education Authority.
- 32.Kumari M, Green R, Nazroo J (2010) Sleep duration and sleep disturbance. In: Banks J, Lessof C, Nazroo J, Rogers N, Stafford M et al., editors. Financial circumstances, health and well-being of the older population in England: the 2008 English Longitudinal Study of Ageing (Wave 4). London: Institute for Fiscal Studies. 178–226.
- 33. Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, et al. (2009) Usual sleep duration and cognitive function in older adults in Spain. 18: 427–435 10.1111/j.1365–2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Zhu Y, Zhan G, Fenik P, Panossian L, et al. (2014) Extended wakefulness: Compromised metabolics in and degeneration of locus ceruleus neurons. The Journal of Neuroscience 34: 4418–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramos AR, Dong C, Elkind MS, Boden-Albala B, Sacco RL, et al. (2013) Association between sleep duration and the mini-mental score: the Northern Manhattan study. J Clin Sleep Med 9: 669–673 10.5664/jcsm.2834 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, et al. (2009) Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol 170: 331–342 kwp154 [pii];10.1093/aje/kwp154 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rabbitt P, Diggle P, Smith D, Holland F, Mc Innes L (2001) Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia 39: 532–543. [DOI] [PubMed] [Google Scholar]
- 38. Bierman EJM, Comijs HC, Jonker C, Beekman ATF (2005) Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry 13: 686–693. [DOI] [PubMed] [Google Scholar]
- 39. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA (2013) Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36: 1027–1032 10.5665/sleep.2802 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, et al. (2012) What sleep characteristics predict cognitive decline in the elderly? Sleep Medicine 13: 886–892. [DOI] [PubMed] [Google Scholar]
- 41. Spiegel R, Herzog A, Köberle S (1999) Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biological Psychiatry 45: 435–442 10.1016/S0006-3223(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 42. Jelicic M, Bosma H, Ponds RWHM, Van Boxtel MPJ, Houx PJ, et al. (2002) Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS). International Journal of Geriatric Psychiatry 17: 73–77 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 43. Tworoger SS, Lee S, Schernhammer ES, Grodstein F (2006) The Association of Self-Reported Sleep Duration, Difficulty Sleeping, and Snoring With Cognitive Function in Older Women. Alzheimer Dis Assoc Disord 20: 41–48. [DOI] [PubMed] [Google Scholar]
- 44. Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, et al. (2011) Sleep disturbance and daytime sleepiness predict vascular dementia. Journal of Epidemiology and Community Health 65: 820–824 10.1136/jech.2009.100503. [DOI] [PubMed] [Google Scholar]
- 45. Cricco M, Simonsick EM, Foley DJ (2001) The impact of insomnia on cognitive functioning in older adults. Journal of the American Geriatrics Society 49: 1185–1189. [DOI] [PubMed] [Google Scholar]
- 46. Foley D, Monjan A, Masaki K, Ross W, Havlik R, et al. (2001) Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc 49: 1628–1632. [DOI] [PubMed] [Google Scholar]
- 47.Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S et al. (2014) Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. In press. [DOI] [PMC free article] [PubMed]
- 48. Loerbroks A, Debling D, Amelang M, Sturmer T (2010) Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry 25: 100–109 10.1002/gps.2305 [doi] [DOI] [PubMed] [Google Scholar]
- 49. Lang I, Wallace RB, Huppert FA, Melzer D (2007) Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing 36: 256–261 afm001 [pii];10.1093/ageing/afm001 [doi] [DOI] [PubMed] [Google Scholar]
- 50. Llewellyn DJ, Lang IA, Langa KM, Huppert FA (2008) Cognitive function and psychological well-being: findings from a population-based cohort. Age Ageing 37: 685–689 afn194 [pii];10.1093/ageing/afn194 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huppert FA, Johnson T, Nickson J (2000) High prevalence of prospective memory impairment in the elderly and in erly stage dementia. Applied Cognitive Psychology 14: S63–S81. [Google Scholar]
- 52.Ofstedal MB, Fisher GG, Herzog AR (2005) Documentation of cognitive function measures in the Health and Retirement Study. HRS Documentation Report DR-006.
- 53. Richards M, Kuh D, Hardy R, Wadsworth M (1999) Lifetime cognitive function and timing of the natural menopause. Neurology 53: 308–314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sleep quantity and sleep quality. Mean sleep quality score for each sleep quantity category (upper panel, S1A-S1B), and mean sleep quantity (hours) for each sleep quality tertile (lower panel, S1C-S1D), in younger and older age groups. All unadjusted ANOVAs p<0.001; see figures for p values for multiple comparisons (Bonferroni-corrected).
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are available from the UK Data Service for researchers who meet the criteria for access to confidential data. Data are from WAVE 4 of the English Longitudinal Study of Ageing (ELSA). Data and contact details may be obtained via the website http://www.adls.ac.uk/find-administrative-data/linked-administrative-data/english-longitudinal-study-of-ageing/