Abstract
To provide a systematic review of scientific literature on functional magnetic resonance imaging (fMRI) studies on sustained attention in psychosis. We searched PubMed to identify fMRI studies pertaining sustained attention in both affective and non-affective psychosis. Only studies conducted on adult patients using a sustained attention task during fMRI scanning were included in the final review. The search was conducted on September 10th, 2013. 15 fMRI studies met our inclusion criteria: 12 studies were focused on Schizophrenia and 3 on Bipolar Disorder Type I (BDI). Only half of the Schizophrenia studies and two of the BDI studies reported behavioral abnormalities, but all of them evidenced significant functional differences in brain regions related to the sustained attention system. Altered functioning of the insula was found in both Schizophrenia and BDI, and therefore proposed as a candidate trait marker for psychosis in general. On the other hand, other brain regions were differently impaired in affective and non-affective psychosis: alterations of cingulate cortex and thalamus seemed to be more common in Schizophrenia and amygdala dysfunctions in BDI. Neural correlates of sustained attention seem to be of great interest in the study of psychosis, highlighting differences and similarities between Schizophrenia and BDI.
Keywords: Sustained attention, Affective psychosis, Non-affective psychosis, Schizophrenia, Bipolar disorder, Functional magnetic resonance imaging, Insula
Core tip: In the present paper, we systematically reviewed functional magnetic resonance imaging studies investigating sustained attention in affective and non-affective psychosis. We found that differences between cases (patients, unaffected relatives of psychotic probands) and controls in terms of functional activation of sustained attention system structures were detectable even when the groups performed comparably. In particular, the insular cortex seems to be a trait marker for psychosis in general, whereas other regions (thalamus, cingulate cortex, amygdala) seem to be differently impaired in affective and non-affective psychosis.
INTRODUCTION
Sustained attention can be defined as the ability to maintain a high vigilance level for prolonged periods of time, allowing the subjects to respond in an appropriate way to infrequent and unpredictable stimuli[1].
Abnormalities in sustained attention have been reported in both schizophrenic[2-4] and Bipolar Disorder (BD) patients[5-8] and several studies suggested a correlation with a worse prognosis and a poorer quality of life[9-12]. Sustained attention deficits seem to be independent from medications[13,14] and illness states[15]. Studies comparing directly schizophrenic and BD patients found that the two groups were qualitatively similar in sustained attention deficits[16], even though schizophrenic patients were usually quantitatively more impaired[17-20]. A reduced attentional performance has also been higlighted in non-affected relatives of schizophrenic[21,22] and bipolar patients[23]: it has been therefore proposed as a candidate endophenotype for both affective[24-26] and non-affective psychosis[27-30]. Candidate endophenotypes must be associated with illness, state independent, heritable, and found in unaffected relatives of probands at a higher rate than in general population[31]. By contrast, some behavioral studies failed to find any significant performance deficit in schizophrenic patients[32], in bipolar patients[33] or in unaffected relatives of bipolar probands[34,35], so the role of sustained attention as a trait-market of psychosis is still controversial. The discordant results reported in scientific literature may be due to the differences in experimental paradigms and inclusion/exclusion criteria.
The most commonly used tasks to assess sustained attention are the “oddball paradigms”, where subjects are required to identify rare and unpredictable target stimuli presented among a stream of frequent non-target stimuli[36,37] or among both frequent and rare non-target stimuli, usually called “standards” and “novels” respectively[38,39]. A particular kind of oddball paradigm is the Continuous Performance Test (CPT), initially developed by Beck et al[40] and nowadays considered a well validated instrument to measure sustained attention in both research and clinical settings[41]. There are numerous versions of CPT, differing from one another for the sensorial modalities (visual or auditory)[42,43] the perceptual complexity of the stimuli (CPT with degraded stimuli: DS-CPT)[44] and the response required: only on targets, on both targets and non-targets and only on non-targets (Conners’ CPT II)[45]. Other CPT versions increase the number of stimuli presented per minute to intensify the attentional load (e.g., the Rapid Visual Information Processing task, RVIP)[46]. Some CPTs are designed to assess both sustained attention and working memory resources, e.g., the CPT-AX (a character or number preceded by another character or number as a target)[47] or the CPI-IP (identical pairs of stimuli as a target)[48].Several scores are used to measure the behavioral performance in oddball paradigms: the rate of correct targets (“hits”, “H”) and incorrect targets (“omission errors”); the rate of correct non-target (“correct rejections”) and incorrect non-target (“false alarms”, “FA”) “commission”) and the mean reaction times (RT) to the stimuli. Subjects who respond accurately and rapidly to both target and non-target are considered good performers, whereas a high number of omissions indicate a reduced attention and a high number of commissions indicate augmented impulsivity. Using the signal detection theory[49] other measures of accuracy may be calculated, such as the sensitivity index (d’, d-prime), its nonparametric analog (A’, A-prime) and the response criterion (B’’, beta, ln b). d’ is the standardized difference between hit rate and false alarm rate [d’ = z(Hits) - z (False alarms)] and it is considered a good measure of discriminability. B’’ instead represents an index of response bias, the subject’s tendency to under respond or over respond [B’’ = (1-H) - FA (1-FA)/H(1-H)+FA (1-FA)].
Functional neuroimaging studies increase the possibility to detect subtle differences in brain functioning even in behaviorally intact subjects. In healthy individuals, sustained attention tasks usually elicit a widespread cortical and subcortical network, including dorsal and medial prefrontal cortex, parietal, temporal and occipital areas, cingulate gyrus, insula, cerebellum, and basal ganglia[50-53]. Different components of sustained attention have their anatomical and functional correlates in different brain regions: subcortical structures have been associated with arousal control, dorsal frontal and temporoparietal cortex with attention maintenance over time, and anterior ventromedial regions, such as anterior cingulate cortex (ACC) and anterior insula, with conflict monitoring, target detection and error signaling[54]. Moreover, ACC and insula are reported to play a crucial role in emotional regulation, linking emotion to cognition[55,56].
The aim of the present paper is to review fMRI correlates of sustained attention in affective and non-affective psychosis, discussing the literature findings and the role of sustained attention as a candidate endophenotype for psychotic disorders.
SEARCH
We searched PubMed to identify functional magnetic resonance (fMRI) studies investigating sustained attention in affective and non-affective psychosis. The following search words were used, both alone and in combination: sustained attention, fMRI, affective psychosis, non-affective psychosis, Schizophrenia, Bipolar Disorder. The search was conducted on September 10th, 2013 and yielded 42 records. Moreover, we manually checked the reference lists of the identified articles and we found 9 further potential studies, for a total number of 51 records. Inclusion criteria were the following: articles written in English, patients’ age ≥ 18 years, psychotic patients and/or subjects at augmented risk for psychosis, studies providing both behavioral and fMRI results during a sustained attention task. Structural MRI studies and fMRI studies reporting data acquired during paradigms other than sustained attention tasks or during resting state were excluded.
By reading titles and abstracts, we excluded 18 records. By reading the full texts of the 33 remaining articles, we identified15 papers meeting our inclusion criteria and therefore included in the qualitative synthesis (Figure 1).
Figure 1.
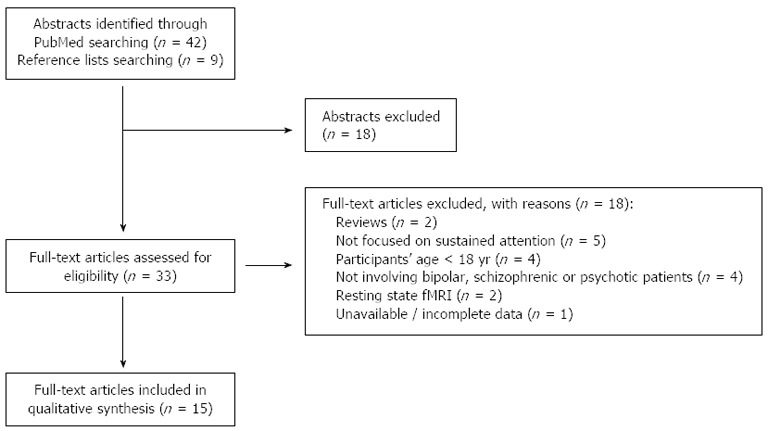
Flow chart of the systematic review. fMRI: Functional magnetic resonance imaging.
RESEARCH
A total number of 578 subjects was tested by the 15 studies included in the qualitative synthesis: 272 normal comparisons (NC), 173 schizophrenic patients (SCZ), 17 unaffected relatives of schizophrenics (REL-SCZ), 10 subjects at ultra high risk for Schizophrenia (UHR-SCZ), 84 Bipolar Disorder type I patients (BDI) and 22 unaffected relatives of BDI patients (REL-BDI). The majority of SCZ were male (68.8%), conversely to what observed in BDI, where males represented only 30.9% of the total.
Sustained attention in schizophrenia
A total number of 12 studies was selected[57-68]. The characteristic of the groups and the results of the studies are depicted in Table 1.
Table 1.
Functional magnetic resonance imaging studies of sustained attention in Schizophrenia
| Ref. | Participants | Task and behavioral results | fMRI methods and results |
| Volz et al[57], 1999 | SCZ (n = 14), age 34.1 ± 12.3, males 78.6%, medicated 100% | CPT-IP. Type of stimuli: lettersTNS = 720, Target = 25%, SET = 600 ms, ISI = 1200 ms, TET = 30 min | 1.5 T, block design (4 blocks). baseline: finger tapping Whole brain analysis. Imaging package: SPM96 |
| NC (n = 20), age 28.2 ± 5.7, males 60% | Required response: on targets.Behavioral measures: hit rate, mean RT, d’, ln b Results: no between group differences | Results: NC > SCZ in the R mesial PFC, ACC and L TH | |
| Eyler et al[58], 2004 | SCZ (n = 8)/SCA (n = 1) age 58.9 ± 9.9, males 55.6%, illness duration: 33 yr, medicated 100% | CPT-X. Type of stimuli: letters. TNS = 72Target = 33.3% ISI = 500 ms, TET = 6 min 3 s | 1.5 T, block design. baseline: digit fixation 8 task blocks and 9 baseline blocks Whole brain analysis. Imaging package: AFNI |
| NC (n = 10), age 59.8 ± 5.7, 12 males 60% | Required response: on targets Behavioral measures: mean RT, d’ Results: no between group differences | Results: NC > SCZ in R IFG/insula (BA 47/45)SCZ > NC in R postcentral gyrus (BA 3) and L cerebellum | |
| Salgado-Pineda et al[59], 2004 | SCZ (n = 14), age 25.5 ± 4.1, males 50%, medicated 100%, illness duration: 1.9 yr | CPT-IP. Type of stimuli: numbers. TNS = 900Target = 15% ISI = 1100 ms, TET = 14min | 1.5 T, block design. baseline: digit response |
| 2 task blocks and 2 baseline blocks. Whole brain analysis. Imaging package: SPM2 | |||
| NC (n = 14), age 25.1 ± 3.3, males 50% | Required response: on targets Behavioral measures: omission errors, commission errors, mean RT, d’, ln bResults: Between group differences-omissions, commission and d’: NC > SCZ-mean RT: SCZ > NC | Results:NC > SCZ in R IFG (BA 44), R angular gyrus (BA 39), R STG (BA 37), R MTG (BA 21), R TH | |
| Honey et al[60], 2005 | N-SCZ: SCZ with both negative and positive symptoms: (n = 11), age 42.6 ± 9.2, males 90.9%, age of onset: 22.2 yr, medicated 100% | CPT-X with 2 levels of difficulty: undegraded and degraded stimuli (0% and 40% pixel inverted). Type of stimuli: digits TNS = 280 Target = 25% SET = 42 ms, ISI = 958 ms TET = 6 min | 3T, block design, baseline: screen fixation 10 task blocks and 10 baseline blocks. Imaging package: SPM2 Masked brain analysis (ROIs involved in attention processing, differentiating the groups and showing a task related activity associated to attentional load). Connectivity analysis (seed ROIs: ACC and cerebellar vermis) |
| P-SCZ: SCZ with predominant positive symptoms: (n = 11) age 41.1 ± 9.2, males 81.8%, age of onset: 24.7 yr, medicated 100% | Required response: on targets Behavioral measures: mean RT, d’Results: N-SCZ were less accurate than NC in target discrimination (d’) | Results Task vs baseline:NC > (P-SCZ = N-SCZ) in R and L angular gyrus, MFG, L putamen(P-SCZ = N-SCZ) > NC in R and L SFG, R and L IPL, R SPL, R post central gyrus, L precentral gyrus, R and L TH, ACC, PCC, R MiFG, R IFG, cerebellum; P-SCZ > N-SCZ in R STG, R MiFG and L SPL | |
| NC (n = 12), age 33.3 ± 11.8, 12 males 83.3% | Connectivity with ACC:NC > (P-SCZ = N-SCZ) in R and L MSFG, R and L IFG; (P-SCZ = N-SCZ) > NC in R and L precentral gyrus, R postcentral gyrus, cerebellum; P-SCZ > N-SCZ in ACC; N-SCZ > P-SCZ in SMA | ||
| Connectivity with cerebellum:NC > (P-SCZ = N-SCZ) in R and L MSFG, L MFG; (P-SCZ = N-SCZ) > NC in L MiFG; P-SCZ>N-SCZ in R and L IFG, ACC, L SPL, R precentral gyrus, L postcentral gyrus | |||
| Morey et al[61], 2005 | UHR (n = 10), age 22.6 ± 4.4, male 50%, medicated 20%, | Visual oddball taskType of stimuli: circles (“targets”), squares (frequent non targets-“standards”), objects (rare non targets-“novels”) | 1.5T, 7 runs. Event-related design. Imaging package: SPM99ROI analysis: ACC, MiFG, IFG, BG, and TH. Conditions: -Targets-Novels-Standards (baseline) |
| Early SCZ (n = 15) age 24.1 ± 6.5, male 67%, age of onset 22.3 yr, illness duration 1.7 yr, medicated 86.7% | TNS = 1400 Target = 3% SET = 500 ms, ISI = 1500 ms, TET = 36 min 24 s Required response: on both targets and non-targets | ResultsTargets vs novels activations-in ACC, MiFG and IFC: NC > UHR, Early SCZ and Chronic SCZ-in IFG: (1) NC> Early SCZ and Chronic SCZ; (2) only NC and UHR showed R > L activations, whereas Early SCZ and Chronic SCZ showed a reduced laterality | |
| Chronic SCZ (n = 11) age 38.1 ± 7.7, male 82%, age of onset 22.9 yr, illness duration 15.3 yr, medicated 100% | Behavioral measures: hit rate, d’, B’’ | Target vs baseline activations: -in ACC, MiFG and IFC: NC > Early SCZ and Chronic SCZ -in BG and TH: NC > Early SCZ and Chronic SCZ (results confirmed comparing Chronic SCZ with Older NC) | |
| NC (n = 16) age 28.0 ± 11.6, male 59% Older NC (n = 10) age 34.0 ± 12.1, male 67% | Results: Between group differences: -Hit rate: NC > Early SCZ and chronic SCZ -d’: NC > UHR, Early SCZ and Chronic SCZ | ||
| Liddle et al[62], 2006 | SCZ (n = 24)/SCA (n = 4) age 31.6 ± 10.1, males 67.9%, illness duration 7 yr, medicated 96.4% | Auditory oddball task Type of stimuli: 1500 Hz tones (“targets”), 1000 Hz tones (frequent non targets-“standards”), noises (rare non targets-“novels”) | 1.5 T, event related design, whole brain analysis Imaging package: SPM99. Conditions: -correct targets-correct novels-correct standards (baseline)-missed targets-standard false alarms |
| NC (n = 28), age 28.2 ± 8.9, males 75 % | TNS = 488 Target = 10% SET = 200 ms, ISI = 2000 ms, TET = 16 min Required response: on targets Behavioral measures: RT, omissions, commissions | ResultsTargets vs baseline activations: NC > SCZ: in L and R amygdala, R hippocampus, R and L STS, L and R insula, R and L orbitofrontal cortex (BA 47), ACC, PCC, L and R SPL, R and L IPL, L and R middle IFG, L and R superior MFG, L and R TH, L and R striatum, L and R cerebellum | |
| Results: SCZ were significantly slower and less accurate than NC | |||
| ResultsTargets vs novels activations: NC > SCZ in: L amygdala, L orbitofrontal cortex (BA 47), L anterior insula, rostral ACC and L striatum | |||
| Gur et al[63], 2007 | SCZ (n = 22), age 30.5 ± 9.1, males 59.1%, age of onset 22.5 yr, illness duration 12.4 yr, medicated 95.5% | Visual oddball task. Stimuli: colored shapes Type of stimuli: red circles (“targets”), green circles (frequent non targets-“standards”), fractal images (rare non targets-“novels”) | 4T, event related design, whole brain analysis. Imaging package: FEAT/FMRIB. Conditions: -targets-novels-standards (baseline) |
| NC (n = 28), age 31.6 ± 8.5, males 57.1 % | TNS = 200 Target = 15% SET = 1000 ms, ISI = 2000 ms, TET = 7 minRequired response: on targetsBehavioral measures: hit rate, RT | ResultsTargets vs baseline activations: NC > SCZ in R and L STG, L insula, R and L putamen, ACC, PCC, L SFG, L THSCZ > NC in R insula, R MiFG, L IPL | |
| Results: no between group differences | Novels vs baseline activations: NC > SCZ in L IOG and L IPL SCZ > NC in L MOG, L fusiform, L precuneus, L IFG, R angular gyrus, SOG, SPL, MiFG | ||
| Harrison et al[64], 2007 | SCZ (n = 12), age 32.2 ± 8.0, males 100%, medicated 100% | Multi-Source Interference Task (MSIT). Type of stimuli: digits SET = 2000 ms, ISI = 500 ms TNS = 160, TET = 11 min Required response: on all stimuli Behavioral measures: correct responses, RT | 3T, block design. Imaging package: SPM5 Conditions: -low difficulty Task (“baseline”)-high difficulty Task (“Task”)-fixation (“Rest”) |
| Whole brain analysis and ROI analysis of deactivation (“Rest”-“Task”) in medial PCC/rostral ACC and PCC/Precuneus | |||
| NC (n = 14), age 31.7 ± 8.0, males 100% | Results: no between group differences | ResultsSCZ > NC in deactivation of medial PCC/rostral ACC and PCC/Precuneus. In SCZ the magnitude of deactivation correlates with response speed and level of emotional awareness | |
| Wolf et al[65], 2008 | SCZ (n = 16)/SCA (n = 1) age 31.9 ± 7.1, males 53 %, illness duration 9.9 yr, medicated 94.1% | Auditory oddball taskType of stimuli: 2000 Hz tones (“targets”), 1000 Hz tones (frequent non targets-“standards”), sounds (rare non targets-“novels”) | 4T, event-related whole brain analysis. Imaging package: FEAT/FSL. Conditions:-targets -novels -standards (baseline) |
| NC (n = 21), age 28.6 ± 7.1, males 52% | TNS = 200 Target = 15% SET = 150 ms, ISI = 1850 ms, TET = 6 min and 40 s Required response: on targets Behavioral measures: hit rate, RTResults: SCZ were significantly slower than NC | ResultsTargets vs baseline activations: SCZ > NC in: L precentral gyrus, ACC/SMA, L and R insula, L hippocampus, L and R STG/MTG, L superior MOGNovels vs baseline activations: SCZ > NC in: L IFG | |
| Filbey et al[66], 2008 | POC-SCZ (n = 6) age 53, males 33.3% medicated 0% (drug naïve) | Sustained attention taskType of stimuli: colored circlesRequired response: on targets Behavioral measures: RT | 1.5T. Block design. Imaging package: FSL Whole brain analysis baseline condition: circles fixation |
| NC (n = 8) age 41, males 62.5% | Results: no between group differences | ResultsNC > POC-SCZ in: R IPL (BA 7), R SPL (BA 7), R MTG (BA 21), R MOG (BA 18), R PCC (BA 31), R SFG (BA 10), R lingual gyrus (BA 18/19), R precentral gyrus (BA 9/43), R parahippocampal gyrus (BA 28), L cuneus (BA 18), L striatum; POC-SCZ > NC in: L STG (BA 21), L SFG (BA9) and R MTG (BA 19) | |
| Carter et al[67], 2010 | SCZ (n = 9), age 29.8 ± 12.0, males 100% | Visual selective attention task Type of stimuli: colored circles | 4T. Block/event-related mixed design. 10 runs. Imaging package: SPM. Conditions: -target events (3 s before-13.5 s after the event) -transient activation (3 s before-7.5 s after the onset of task block) -sustained activation (15 s before-70.5 s after the onset of task block) |
| NC (n = 12), age 25.5 ± 4.6, males 100% | TNS = 1960 Target = 5 %SET = 500 ms, ISI = 1000 ms TET = 49 minRequired response: on both targets and non-targets Behavioral measures: correct targets, RT | Masked brain analysis and ROI analysis in ACC, IFG, MiFG, IPS, BG, caudate and TH | |
| Results-correct target percentage: NC > SCZ-RT: SCZ > NC | Results -During transient activation NC > SCZ in: MiFG, IPS, caudate and TH-During target events NC > SCZ in THIn NC: positive correlation between accuracy and TH activation during sustained activation condition; In SCZ: negative correlation between RT and BG activation during target events | ||
| Sepede et al[68], 2010 | REL-SCZ (n = 11), age 34.4 ± 8.8, males 45.5% medicated 0% (drug naïve), smokers 36.4% | CPT-X with 3 levels of difficulty: undegraded and degraded stimuli (0%, 25% and 40% pixel inverted). Type of stimuli: digits | 1.5T, event-related design, 3 runs (0%, 25% and 40% degraded) Whole brain analysis. Imaging package: BrainVoyager QX 1.9 |
| NC (n = 11), age 32.0 ± 5.2, males 45.5%, smokers 36.4% | TNT = 210, Target = 16% SET = 200 ms, ISI = 2000 ms, TET = 42 min | Task conditions: -correct responses on target -incorrect responses on target -correct responses on non-targets (baseline) | |
| Required response: on targets and non-targets Behavioral measures: correct targets, correct non-targets, RT Results: no between group differences | Results Correct targets vs baseline: NC > REL-SCZ in R precentral gyrus (BA 6/9), R and L insula (BA 13), MFG/dorsal ACC (BA 9/32)REL-SCZ > NC in deactivating PCC/retrosplenial cortex (BA 23/31) Incorrect target vs baseline: REL-SCZ > NC in L insula/IFG (BA 13/47) and R TH |
SCZ: Schizophrenic patients; NC: Normal comparisons; SCA: Schizoaffective patients; REL-SCZ: Unaffected first degree relatives of schizophrenic patients; POC-SCZ: Presumed Obligate carriers of schizophrenic patients; UHR: Ultra high risk subjects; SET: Stimulus exposure time; ISI: Interstimulus interval; TNS: Total number of stimuli; TNT: Total number of targets; TET: Total experiment time; RT: Response time; R: Right; L: Left; PFC: Prefrontal cortex; ACC: Anterior cingulate cortex; PCC: Posterior cingulate cortex; MFG: Medial frontal gyrus; MSFG: Medial superior frontal gyrus; IFG: Inferior frontal gyrus; SFG: Superior frontal gyrus; SMA: Supplementary motor area; IPL: Inferior parietal lobule; SPL: Superior parietal lobule; IPS: Intraparietal sulcus; MTG: Middle temporal gyrus; STG: Superior temporal gyrus; STS: Superior temporal sulcus; MOG: Middle occipital gyrus; IOG: Inferior occipital gyrus; TH: Thalamus; BG: Basal ganglia.
Right handedness was an inclusion criteria in 6 studies[57,59,60,63,66,68]. Nine studies enrolled only SCZ, one had both a SCZ group and an additional group of UHR-SCZ[61] and two had only REL-SCZ[66,68]. In the study by Morey et al[61], patients were divided into early SCZ (mean illness duration 1.7 years) and chronic SCZ (mean illness duration 15.3 years). In the study by Honey et al[60], patients were divided into SCZ with both negative and positive symptoms (n = 11) and SCZ with predominantly positive symptoms (n = 11). The SCZ (n = 173) enrolled in the studies were clinically stable and in the majority of cases were medicated (range: 87.5%-100%). Only 2 of the 10 UHR-SCZ received medications at the moment of the scanning, whereas all the REL-SCZ (n = 17) and the NC (n = 204) were drug naïve. In seven of the 10 studies including a SCZ group, the mean illness duration was also reported[58-63,65] and it ranged from 1.7 to 33 years. The UHR-SCZ group in the study by Morey et al[61] met at least one of the following criteria: (1) reporting brief intermittent psychotic states; (2) reporting attenuated positive symptom states; and (3) being first-degree relatives of schizophrenic/schizotypal probands plus reporting a significant recent loss of social/work functioning. The 11 REL-SCZ enrolled by Sepede et al[68] were all unaffected siblings of schizophrenic patients, whereas the 6 REL-SCZ enrolled by Filbey et al[66] were presumed obligate carriers of schizophrenia (POCs): unaffected subjects having a first-degree relative (sibling or parent) plus a child affected by schizophrenia. Ten of the 12 studies used visual stimuli, whereas the other two[62,65] used auditory stimuli. The tasks administered were: oddball tasks (n = 4), CPT-X (n = 1), DS-CPT-X (n = 2), CPT-IP (n = 2), RVIP (n = 1), and other attention tasks (n = 2), with a total duration of the experiment ranging from 6 to 49 min.
FMRI images were acquired using a 1.5 T scanner in seven studies, a 3 T scanner in 2 studies and a 4 T scanner in three studies. A block design was used to present the tasks in six studies, whereas an event-related design was used in other five studies. Only one study[67] used a block/event-related mixed design. A whole brain approach was used in eight studies to analyze the BOLD FMRI signal whereas three studies[60,61,67] used a region of interest (ROI) approach and/or a masked brain analysis, limiting the analysis to areas known to be involved in sustained attention processing and/or to areas showing significant between-group or within-condition differences. Only one study[64] used the ROI analysis after the whole brain analysis. In the study by Honey et al[60], connectivity analyses were also performed.
Behavioral results
In four of the ten studies involving SCZ (n = 57), no significant behavioral differences were found with respect to NC[57,58,63,64]. In the other six studies, SCZ (n = 116) performed worse than NC: a reduced accuracy was evidenced by Honey et al[60] and Morey et al[61], whereas Wolf et al[65] reported an increased mean reaction time and Salgado et al[59], Liddle et al[62] and Carter et al[67] reported both reduced accuracy and increased mean reaction times with respect to NC. In their 10 UHR subjects, Morey et al[61] reported a reduced accuracy. On the contrary, the 17 REL-SCZ enrolled by Filbey et al[66] and Sepede et al[68] performed similarly to controls.
FMRI results
Significant between-group differences in several brain regions were found in all the selected studies, even when the groups performed comparably. The most reported differences were observed in cingulate gyrus, thalamus (TH), inferior parietal lobule (IPL), inferior frontal gyrus (IFG) and insula. The anterior part of the cingulate cortex (ACC) significantly differentiated the groups in seven studies: SCZ showed a reduced activation with respect to NC in 4 SCZ groups[57,61-63], and the same pattern was observed in the UHR subjects enrolled by Morey et al[61] and in the REL-SCZ enrolled by Sepede et al[68] By contrast, two studies[60,65] reported an augmented activation in SCZ with respect to NC. Also the posterior part of the cingulate cortex (PCC) significantly differentiated the groups in six studies. Honey et al[60] reported an increased activation in the SCZ with respect to NC, whereas a decreased activation was found by Gur et al[63] and Liddle et al[62] Interestingly, in three studies, the PCC was reported to be deactivated during attention task, with respect to the baseline/control task, and the amount of the deactivation was larger in SCZ[64] and REL-SCZ[66,68] with respect to NC. A significant hypoactivation of the IFG was found in five studies[58-62]. The medial regions of the prefrontal cortex, located dorso-rostrally with respect to the cingulate cortex, appeared to be hyperactivated in SCZ[57,60] or REL-SCZ[68].
The insular cortex significantly differentiated the groups in five studies. A reduced activation in SCZ was reported bilaterally by Liddle et al[62] and limited to the right emisphere by Eyler et al[58] By contrast, Gur et al[63] found a reduced activation in the R insula, counterbalanced by an augmented activation in the L insula, and Wolf et al[65] reported a bilateral augmented activation. In the event-related study by Sepede et al[68], REL-SCZ hyperactivated the bilateral insula during correct target responses and hyperactivated the L insula during wrong target responses. An altered functioning of the inferior parietal lobule (IPL) was detected in 4 studies, three showing a reduced activation in SCZ with respect to NC[60,62,66], one an increased activation[63]. Other parietal regions were also reported to differentiate the groups. In the angular gyrus Salgado et al[59] and Honey et al[60] reported a reduced activation, Gur et al[63] an increased activation; in the superior parietal lobule (SPL) Liddle et al[62] and Filbey et al[66] found a reduced activation, Honey et al[60] an increased activation in SCZ with respect to NC.
When considering the subcortical regions, SCZ significantly hyperactivated the TH in six studies[57,59,61,62,63,67], whereas Sepede et al[68] found an increased activation during wrong target responses in REL-SCZ. Other subcortical structures, such as the basal ganglia, appeared to be less activated in SCZ with respect to NC in 4 studies[60,61,63].
SUSTAINED ATTENTION IN BIPOLAR DISORDER
Three studies enrolled BDI patients[69-71] and one of these studies had also a group of unaffected, drug-naïve, BDI-REL[71]. The characteristic of the groups and the results of the studies are depicted in Table 2. Right handedness was an inclusion criteria in two studies[69,71]. The BDI (n = 84) enrolled in the three studies were euthymic (n = 34) or affected by a manic/mixed episode (n = 50). About 80% of the 74 patients in the studies by Fleck et al[70] and Sepede et al[71] were under medication at the moment of the fMRI scanning, whereas the 10 BDI in the study by Strakowsky et al[69] were drug free. All the NC (n = 68) and the BDI-REL (n = 22) were drug naïve. In two of the studies[69,71], the mean illness duration was also reported and it was 2.2 and 4.7 years respectively. The presence of psychotic features during the acute phases of the illness (95.8%) was reported only by Sepede et al[71] All the three selected studies used a visual CPT to assess sustained attention (CPT-IP: n = 2; DS-CPT-X: n = 1), with a total duration of the experiment ranging from 6 to 15 min.
Table 2.
Functional magnetic resonance imaging studies of sustained attention in bipolar disorder
| Ref. | Participants | Task and behavioral results | FMRI methods and results |
| Strakowski et al[69], 2004 | BDI (n = 10), age 25.5 ± 8.1, males 40%, euthymic, age of onset 23 yr, illness duration 2.2 yr, medicated 0% (drug free) | CPT-IP. Type of stimuli: digits TNS = 400 SET = 700 ms, ISI = 750, TET = 6 min Required response: on targets. Behavioral measures: d’, percent correct, percent false positive | 3T, block design, 5 task blocks and 5 baseline blocks Baseline: digits fixation Whole brain analysis. Imaging package: CHIPS |
| NC (n = 10), age25.3 ± 7.3, males 40% | Results: no between-group differences | Results:BDI > NC in: R IFG/insula (BA 13/47), R and L ventral PFC (BA 10/47), parahippocampus/amygdala (BA 34), MOG/MTG (BA 18/19/39), R IPL (BA 40), R SPL (BA 7/40), L postcentral gyrus (BA 43), hypothalamus; NC > BDI in: L fusyform gyrus (BA 20) and L MFG (BA 11) | |
| Fleck et al[70], 2012 | BDI (n = 50), age 30 ± 10, males 30%, manic, medicated 80% | CPT-IP. Type of stimuli: digits TNS = 900, Target = 15%, SET = 750 ms, ISI = 1000 ms, TET = 15 min | 4T, Event-related design, 3 runs (periods) Whole brain analysis. Imaging package: AFNI ROi based analysis: anterior-limbic network (IFG, BG, TH, amygdala, cerebellar vermis) + SFGBaseline: visual count down condition Task conditions: -hits, misses and false alarms -correct rejections on non-targets |
| NC (n = 34), age 31 ± 9, males 41% | Required response: on targets Behavioral measures: A’, B’’, RT, correct rejectionResults: patients performed worse in terms of correct rejections and showed a trend vs a reduced A’ | Results: In period 1: NC > BD in cerebellum; BD > NC in TH; NC > BD in deactivation of L PCC and R angular gyrusIn period 2: NC > BD in bilateral IFG and L THIn period 3: NC > BD in activation of R IFGOver time: BD activated and NC deactivated L striatum and bilateral amygdala Unmedicated BD > medicated BD in activation of R IFG and cerebellum | |
| Sepede et al[71], 2012 | BDI (n = 24), age 34.8 ± 8.0, males 41.7%, euthymic, age of onset 29.9, illness duration 4.7 yr, psychotic features during acute phases 95.8%, medicated 83.3% | CPT-X with 2 levels of difficulty: undegraded and degraded stimuli (0% and 40% pixel inverted)Type of stimuli: digits TNT = 80, Target = 20%, TNS = 408 ± 30 SET = 200 ms, ISI = 2000 ms, TET = 14 min | 1.5T, event-related design, 2 runs (0%, and 40% degraded). Whole brain analysis. Imaging package: BrainVoyager QX 1.9.Task conditions: -correct responses on target -incorrect responses on target -correct responses on non-targets (baseline) |
| REL-BDI (n = 22), age 31.5 ± 7.3, males 31.8% medicated 0% (drug naïve) | Required response: on targets and non-targets Behavioral measures: correct target, correct non-targets, incorrect target, incorrect non-target, mean RT | ResultsCorrect target vs baseline: (NC = REL-BDI) > BDI in R insula (BA13) REL-BDI > (NC = BDI) in deactivating PCC/retrosplenial cortex (BA 23/29) During the 40% degraded run, correct target condition: REL-BDI > (NC = BDI) in R and L IPL (BA 40), L insula/IFG (BA 13/45) | |
| NC (n = 24), age 32.5 ± 6.2, males 33.3% | Results: both BDI and REL-BDI were less accurate than NC in target recognition (percent correct target ) | Incorrect target vs baseline:(BDI = REL-BDI) > NC in middle PCC (BA 31) and R insula/IFG (BA 13/45)BDI > REL-BDI > NC in L insula (BA 13) |
BDI: Bipolar disorder type I patients; REL-BDI: Unaffected relatives of bipolar disorder type I patients; NC: Normal comparisons; PFC: Prefrontal cortex; ACC: Anterior cingulate cortex; PCC: Posterior cingulate cortex; MFG: Medial frontal gyrus; SFG: Superior frontal gyrus; IFG: Inferior frontal gyrus; IPL: Inferior parietal lobule; SPL: Superior parietal lobule; MTG: Middle temporal gyrus; MOG: Middle occipital gyrus; TH: Thalamus; BG: Basal ganglia; R: Right; L: Left.
FMRI images were acquired using a 1.5 T (n = 1), a 3 T (n = 1) or a 4 T (n = 1) scanner, presenting the tasks with an event-related design (n = 2) or a block design (n = 1). A whole brain approach was used in two studies to analyze the BOLD FMRI, whereas Fleck et al[70] performed a whole brain analysis followed by a ROI analysis.
Behavioral results
In their group of 10 euthymic and unmedicated BDI, Strakowski et al[69] did not find any behavioral deficit with respect to NC. On the contrary, both Fleck et al[70] and Sepede et al[71] reported a reduced target accuracy in their manic/mixed (n = 50) or euthymic (n = 24) BDI patients. An impaired performance was also found in the group of 22 unaffected and unmedicated BDI-REL enrolled by Sepede et al[71].
FMRI results
Significant between-group differences in several brain regions were found in all the three selected studies, even when the groups performed comparably. The regions more reported to differentiate the groups were: IFG, insula, amygdala and IPL.
The IFG/insula showed an altered pattern of activation in all the three selected studies: an augmented activation in BDI with respect to NC was found by Strakowski et al[69], whereas Fleck et al[70] reported a reduced activation. In the study by Sepede et al[71], BDI showed a reduced activation during correct target responses and an augmented activation during wrong target responses, with REL-BDI showing an intermediate pattern of functioning between BDI and NC. The amygdala was found to be more activated with respect to NC in two studies, involving euthymic[69] or manic[70] BDI. The IPL seemed to be hyperactivated in the euthymic BDI enrolled by Strakowski et al[69] and in the REL-BDI by Sepede et al[71].
DISCUSSION
In this paper we systematically reviewed fMRI studies on sustained attention in affective and non-affective psychosis.
We found several studies on Schizophrenia that met our inclusion criteria, whereas the publications on BDI were very few. This result is quite surprising, considering the large amount of behavioral data that reported sustained attention deficits in both acute and euthymic phases of BDI.
Summarizing the literature findings on affective and non-affective psychosis, we highlighted that patients and at-risk subjects significantly differed from healthy comparisons in the functioning of several brain regions belonging to the sustained attention system, even when they were behaviorally intact. There were regions that seemed more impaired in Schizophrenia, other more impaired in Bipolar Disorder and other that appeared altered in both conditions.
In the studies on schizophrenic patients and subjects at augmented risk for schizophrenia, the most frequent dysfunctions were located in the cingulate gyrus and in the thalamus. The anterior part of the cingulate gyrus is a key region in sustained attention, cognitive control and error processing[72-74]. An altered function of ACC has been consistently reported in both schizophrenic patients and unaffected relatives[75,76] during attentional control[77] conflict/error monitoring[78-81], working memory[82-84] and semantic[85] tasks.
The posterior part of the cingulate gyrus is usually deactivated during active tasks with respect to rest conditions, and it is therefore considered a part of the Default Mode Network (DMN) of the brain[86]. It has a crucial role not only in internally focused tasks, but also in active regulation of the arousal state and in balancing between internally and externally oriented attention[87]. A lower volume of PCC/retrosplenial cortex has been associated to a poorer outcome in Schizophrenia[88], and an altered function of this region has been evidenced during semantic[89], self-evaluation[90,91] and fear-conditioning[92] tasks.
The thalamus is a subcortical structure whose integrity is needed to the correct functioning of cognitive processes. It is not a simple passive relay station, but a nodal link actively connecting top-down to bottom-up components of the attention/arousal system[1,93] and different cortical regions via cortico-thalamo-cortical pathways[94]. Both structural and functional MRI studies on Schizophrenia frequently reported significant abnormalities in schizophrenic patients, so a disruption of thalamocortical connections was suggested as one of the possible neural basis of cognitive and sensorial symptoms of Schizophrenia[95-98].
With regard to Bipolar Disorder, amygdala was found to be altered in two of the three reviewed. In humans, the amygdala plays a key role in detecting dangers and other emotionally salient stimuli in the environment, in order to make the subject ready to react in an appropriate way[99]. During emotional tasks, an altered functioning of the amygdala in BD has been extensively reported, especially in manic patients[100-103], but also during depressive[104] and euthymic states[105,106] of the illness. An important finding highlighted by the current review is that an augmented activation of the amygdala was observed also during attention tasks without any emotional components, this results suggesting that emotional limbic areas may interfere with cognition in BD[107].
In our systematic review we reported that an altered functioning of the insula during sustained attention task was frequently found in both Schizophrenia and Bipolar Disorder.
The insular cortex, due to its location at the interface of frontal, parietal and temporal lobes, is involved in cognitive, emotional and somato-sensorial processes[56,108], providing a hub that integrates salient stimuli with autonomic and sensorial data[109]. Many studies reported an insular dysfunction in Schizophrenia, Bipolar Disorder, and “at-risk subjects”, during both tasks[110-115] and resting state[116-119], thus suggesting a key role of this region in vulnerability for psychosis, regardless of the affective or non-affective diagnostic distinction.
CONCLUSION
In the present paper, we systematically reviewed fMRI studies pertaining sustained attention in affective and non-affective psychosis.
We found that differences between cases (patients, unaffected relatives of psychotic probands) and controls in terms of functional activation in brain regions belonging to the sustained attention system were detectable even when the groups performed comparably. In particular, the insular cortex seems to be a trait marker for psychosis in general, whereas other regions seem to be differently impaired in affective and non-affective psychosis: alterations of the cingulate cortex and thalamus appear to be more common in Schizophrenia whereas amygdalar dysfunctions may be more frequently observed in Bipolar Disorder. Therefore, investigating neural correlates of sustained attention seem to be of great interest in the study of affective and non-affective psychosis as it may clarify differences and similarities between these two disabling psychiatric conditions.
Limits of the study
An important limitation of the present paper is that we included in the qualitative synthesis only those studies conducted on selected versions of CPTs that were focused on sustained attention, excluding papers with CPT versions designed to measure other cognitive functions, such as working memory or emotional processing. Moreover, it’s possible that our search strategy did not succeed in finding all the available literature on the topic and that adding other search words (i.e., Continuous performance Test, oddball task) or other data bases would have improved the results. Due to the small number of published studies on Bipolar Disorder, our results should be interpreted with caution and further research are needed to clarify the role of sustained attention in affective psychosis.
Footnotes
P- Reviewers: Serafini G, J Aguilar E S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
References
- 1.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 2.Suwa H, Matsushima E, Ohta K, Mori K. Attention disorders in schizophrenia. Psychiatry Clin Neurosci. 2004;58:249–256. doi: 10.1111/j.1440-1819.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- 3.Hahn B, Robinson BM, Kaiser ST, Matveeva TM, Harvey AN, Luck SJ, Gold JM. Kraepelin and Bleuler had it right: people with schizophrenia have deficits sustaining attention over time. J Abnorm Psychol. 2012;121:641–648. doi: 10.1037/a0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Üçok A, Direk N, Koyuncu A, Keskin-Ergen Y, Yüksel Ç, Güler J, Karadayı G, Akturan E, Devrim-Üçok M. Cognitive deficits in clinical and familial high risk groups for psychosis are common as in first episode schizophrenia. Schizophr Res. 2013;151:265–269. doi: 10.1016/j.schres.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Harmer CJ, Clark L, Grayson L, Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia. 2002;40:1586–1590. doi: 10.1016/s0028-3932(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 6.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 8.Robinson LJ, Thompson JM, Gallagher P, Gray JM, Young AH, Ferrier IN. Performance monitoring and executive control of attention in euthymic bipolar disorder: employing the CPT-AX paradigm. Psychiatry Res. 2013;210:457–464. doi: 10.1016/j.psychres.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Hofer A, Baumgartner S, Bodner T, Edlinger M, Hummer M, Kemmler G, Rettenbacher MA, Fleischhacker WW. Patient outcomes in schizophrenia II: the impact of cognition. Eur Psychiatry. 2005;20:395–402. doi: 10.1016/j.eurpsy.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.González-Blanch C, Perez-Iglesias R, Pardo-García G, Rodríguez-Sánchez JM, Martínez-García O, Vázquez-Barquero JL, Crespo-Facorro B. Prognostic value of cognitive functioning for global functional recovery in first-episode schizophrenia. Psychol Med. 2010;40:935–944. doi: 10.1017/S0033291709991267. [DOI] [PubMed] [Google Scholar]
- 11.Martino DJ, Marengo E, Igoa A, Scápola M, Ais ED, Perinot L, Strejilevich SA. Neurocognitive and symptomatic predictors of functional outcome in bipolar disorders: a prospective 1 year follow-up study. J Affect Disord. 2009;116:37–42. doi: 10.1016/j.jad.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Pattanayak RD, Sagar R, Mehta M. Neuropsychological performance in euthymic Indian patients with bipolar disorder type I: correlation between quality of life and global functioning. Psychiatry Clin Neurosci. 2012;66:553–563. doi: 10.1111/j.1440-1819.2012.02400.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu SK, Chen WJ, Chang CJ, Lin HN. Effects of atypical neuroleptics on sustained attention deficits in schizophrenia: a trial of risperidone versus haloperidol. Neuropsychopharmacology. 2000;22:311–319. doi: 10.1016/S0893-133X(99)00137-2. [DOI] [PubMed] [Google Scholar]
- 14.Goswami U, Sharma A, Varma A, Gulrajani C, Ferrier IN, Young AH, Gallagher P, Thompson JM, Moore PB. The neurocognitive performance of drug-free and medicated euthymic bipolar patients do not differ. Acta Psychiatr Scand. 2009;120:456–463. doi: 10.1111/j.1600-0447.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Morla EM, Barabash A, Martínez-Vizcaíno V, Tabarés-Seisdedos R, Balanzá-Martínez V, Cabranes-Díaz JA, Baca-Baldomero E, Gómez JL. Comparative study of neurocognitive function in euthymic bipolar patients and stabilized schizophrenic patients. Psychiatry Res. 2009;169:220–228. doi: 10.1016/j.psychres.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Liu SK, Chiu CH, Chang CJ, Hwang TJ, Hwu HG, Chen WJ. Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry. 2002;159:975–982. doi: 10.1176/appi.ajp.159.6.975. [DOI] [PubMed] [Google Scholar]
- 18.Dickerson F, Boronow JJ, Stallings C, Origoni AE, Cole SK, Yolken RH. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res. 2004;129:45–53. doi: 10.1016/j.psychres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pulver AE, Rivkin P, Rao VA, Diaz-Asper CM, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkett P, Sigmundsson T, Sharma T, Toulopoulou T, Griffiths TD, Reveley A, Murray R. Reaction time and sustained attention in schizophrenia and its genetic predisposition. Schizophr Res. 2007;95:76–85. doi: 10.1016/j.schres.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Hilti CC, Hilti LM, Heinemann D, Robbins T, Seifritz E, Cattapan-Ludewig K. Impaired performance on the Rapid Visual Information Processing task (RVIP) could be an endophenotype of schizophrenia. Psychiatry Res. 2010;177:60–64. doi: 10.1016/j.psychres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Trivedi JK, Goel D, Dhyani M, Sharma S, Singh AP, Sinha PK, Tandon R. Neurocognition in first-degree healthy relatives (siblings) of bipolar affective disorder patients. Psychiatry Clin Neurosci. 2008;62:190–196. doi: 10.1111/j.1440-1819.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- 24.Fridberg DJ, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, Malloy FW, O’Donnell BF. Relationships between auditory event-related potentials and mood state, medication, and comorbid psychiatric illness in patients with bipolar disorder. Bipolar Disord. 2009;11:857–866. doi: 10.1111/j.1399-5618.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arts B, Jabben N, Krabbendam L, van Os J. A 2-year naturalistic study on cognitive functioning in bipolar disorder. Acta Psychiatr Scand. 2011;123:190–205. doi: 10.1111/j.1600-0447.2010.01601.x. [DOI] [PubMed] [Google Scholar]
- 26.Ancín I, Santos JL, Teijeira C, Sánchez-Morla EM, Bescós MJ, Argudo I, Torrijos S, Vázquez-Alvarez B, De La Vega I, López-Ibor JJ, et al. Sustained attention as a potential endophenotype for bipolar disorder. Acta Psychiatr Scand. 2010;122:235–245. doi: 10.1111/j.1600-0447.2009.01532.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. Am J Med Genet. 2000;97:52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am J Med Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- 29.Giakoumaki SG, Roussos P, Pallis EG, Bitsios P. Sustained attention and working memory deficits follow a familial pattern in schizophrenia. Arch Clin Neuropsychol. 2011;26:687–695. doi: 10.1093/arclin/acr060. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Chan R, Sun J, Yao J, Deng W, Sun X, Liu X, Sham PC, Ma X, Meng H, et al. Reaction time of the Continuous Performance Test is an endophenotypic marker for schizophrenia: a study of first-episode neuroleptic-naive schizophrenia, their non-psychotic first-degree relatives and healthy population controls. Schizophr Res. 2007;89:293–298. doi: 10.1016/j.schres.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Jones LA, Cardno AG, Sanders RD, Owen MJ, Williams J. Sustained and selective attention as measures of genetic liability to schizophrenia. Schizophr Res. 2001;48:263–272. doi: 10.1016/s0920-9964(00)00136-5. [DOI] [PubMed] [Google Scholar]
- 33.Addington J, Addington D. Attentional vulnerability indicators in schizophrenia and bipolar disorder. Schizophr Res. 1997;23:197–204. doi: 10.1016/s0920-9964(96)00105-3. [DOI] [PubMed] [Google Scholar]
- 34.Clark L, Kempton MJ, Scarnà A, Grasby PM, Goodwin GM. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry. 2005;57:183–187. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Walshe M, Schulze KK, Stahl D, Hall MH, Chaddock C, Morris R, Marshall N, McDonald C, Murray RM, Bramon E, et al. Sustained attention in bipolar I disorder patients with familial psychosis and their first-degree relatives. Psychiatry Res. 2012;199:70–73. doi: 10.1016/j.psychres.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Huettel SA, McCarthy G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42:379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum Brain Mapp. 2014;35:2265–2284. doi: 10.1002/hbm.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Belliveau JW, Tengshe C, Ahveninen J. Brain networks of novelty-driven involuntary and cued voluntary auditory attention shifting. PLoS One. 2012;7:e44062. doi: 10.1371/journal.pone.0044062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaehle T, Bauch EM, Hinrichs H, Schmitt FC, Voges J, Heinze HJ, Bunzeck N. Nucleus accumbens activity dissociates different forms of salience: evidence from human intracranial recordings. J Neurosci. 2013;33:8764–8771. doi: 10.1523/JNEUROSCI.5276-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck LH, Bransome ED, Mirsky AF, Rosvold HE, Sarason I. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- 41.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol. 2002;17:235–272. [PubMed] [Google Scholar]
- 42.Baker DB, Taylor CJ, Leyva C. Continuous performance tests: a comparison of modalities. J Clin Psychol. 1995;51:548–551. doi: 10.1002/1097-4679(199507)51:4<548::aid-jclp2270510414>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Kasai K, Nakagome K, Hiramatsu K, Fukuda M, Honda M, Iwanami A. Psychophysiological index during auditory selective attention correlates with visual continuous performance test sensitivity in normal adults. Int J Psychophysiol. 2002;45:211–225. doi: 10.1016/s0167-8760(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 44.Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science. 1983;220:327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- 45.Conners CK, MHS Staff. Conners' Continuous Performance Test (CPT II) computer programs for Windows technical guide and software manual. North Tonawanda, NY: Multi-Health Systems: 2000. [Google Scholar]
- 46.Chen WJ, Hsiao CK, Hsiao LL, Hwu HG. Performance of the Continuous Performance Test among community samples. Schizophr Bull. 1998;24:163–174. doi: 10.1093/oxfordjournals.schbul.a033308. [DOI] [PubMed] [Google Scholar]
- 47.Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- 48.Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 49.Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley: 1966. [Google Scholar]
- 50.Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- 51.Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK. Neural correlates of a clinical continuous performance test. Magn Reson Imaging. 2008;26:504–512. doi: 10.1016/j.mri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44:265–274. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grahn JA, Manly T. Common neural recruitment across diverse sustained attention tasks. PLoS One. 2012;7:e49556. doi: 10.1371/journal.pone.0049556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- 55.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 56.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volz H, Gaser C, Häger F, Rzanny R, Pönisch J, Mentzel H, Kaiser WA, Sauer H. Decreased frontal activation in schizophrenics during stimulation with the continuous performance test--a functional magnetic resonance imaging study. Eur Psychiatry. 1999;14:17–24. doi: 10.1016/s0924-9338(99)80711-1. [DOI] [PubMed] [Google Scholar]
- 58.Eyler LT, Olsen RK, Jeste DV, Brown GG. Abnormal brain response of chronic schizophrenia patients despite normal performance during a visual vigilance task. Psychiatry Res. 2004;130:245–257. doi: 10.1016/j.pscychresns.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Salgado-Pineda P, Junqué C, Vendrell P, Baeza I, Bargalló N, Falcón C, Bernardo M. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage. 2004;21:840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liddle PF, Laurens KR, Kiehl KA, Ngan ET. Abnormal function of the brain system supporting motivated attention in medicated patients with schizophrenia: an fMRI study. Psychol Med. 2006;36:1097–1108. doi: 10.1017/S0033291706007677. [DOI] [PubMed] [Google Scholar]
- 63.Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, Pratiwadi R, Ragland JD, Bilker WB, Siegel SJ, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- 64.Harrison BJ, Yücel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007;91:82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 65.Wolf DH, Turetsky BI, Loughead J, Elliott MA, Pratiwadi R, Gur RE, Gur RC. Auditory Oddball fMRI in Schizophrenia: Association of Negative Symptoms with Regional Hypoactivation to Novel Distractors. Brain Imaging Behav. 2008;2:132–145. doi: 10.1007/s11682-008-9022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Filbey FM, Russell T, Morris RG, Murray RM, McDonald C. Functional magnetic resonance imaging (fMRI) of attention processes in presumed obligate carriers of schizophrenia: preliminary findings. Ann Gen Psychiatry. 2008;7:18. doi: 10.1186/1744-859X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter JD, Bizzell J, Kim C, Bellion C, Carpenter KL, Dichter G, Belger A. Attention deficits in schizophrenia--preliminary evidence of dissociable transient and sustained deficits. Schizophr Res. 2010;122:104–112. doi: 10.1016/j.schres.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sepede G, Ferretti A, Perrucci MG, Gambi F, Di Donato F, Nuccetelli F, Del Gratta C, Tartaro A, Salerno RM, Ferro FM, et al. Altered brain response without behavioral attention deficits in healthy siblings of schizophrenic patients: an event-related fMRI study. Neuroimage. 2010;49:1080–1090. doi: 10.1016/j.neuroimage.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 69.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 70.Fleck DE, Eliassen JC, Durling M, Lamy M, Adler CM, DelBello MP, Shear PK, Cerullo MA, Lee JH, Strakowski SM. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17:325–336. doi: 10.1038/mp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sepede G, De Berardis D, Campanella D, Perrucci MG, Ferretti A, Serroni N, Moschetta FS, Del Gratta C, Salerno RM, Ferro FM, et al. Impaired sustained attention in euthymic bipolar disorder patients and non-affected relatives: an fMRI study. Bipolar Disord. 2012;14:764–779. doi: 10.1111/bdi.12007. [DOI] [PubMed] [Google Scholar]
- 72.Badgaiyan RD, Posner MI. Mapping the cingulate cortex in response selection and monitoring. Neuroimage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- 73.Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- 74.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 75.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Curr Opin Genet Dev. 2011;21:340–348. doi: 10.1016/j.gde.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, Fazio L, Di Giorgio A, Latorre V, Sambataro F, et al. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex. 2010;20:837–845. doi: 10.1093/cercor/bhp146. [DOI] [PubMed] [Google Scholar]
- 78.Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- 79.Kerns JG, Cohen JD, MacDonald AW, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 80.Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 81.Sambataro F, Mattay VS, Thurin K, Safrin M, Rasetti R, Blasi G, Callicott JH, Weinberger DR. Altered cerebral response during cognitive control: a potential indicator of genetic liability for schizophrenia. Neuropsychopharmacology. 2013;38:846–853. doi: 10.1038/npp.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 83.Henseler I, Falkai P, Gruber O. A systematic fMRI investigation of the brain systems subserving different working memory components in schizophrenia. Eur J Neurosci. 2009;30:693–702. doi: 10.1111/j.1460-9568.2009.06850.x. [DOI] [PubMed] [Google Scholar]
- 84.Karch S, Leicht G, Giegling I, Lutz J, Kunz J, Buselmeier M, Hey P, Spörl A, Jäger L, Meindl T, et al. Inefficient neural activity in patients with schizophrenia and nonpsychotic relatives of schizophrenic patients: evidence from a working memory task. J Psychiatr Res. 2009;43:1185–1194. doi: 10.1016/j.jpsychires.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Sass K, Heim S, Sachs O, Straube B, Schneider F, Habel U, Kircher T. Neural correlates of semantic associations in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:143–154. doi: 10.1007/s00406-013-0425-0. [DOI] [PubMed] [Google Scholar]
- 86.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 87.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Tendolkar I, Weis S, Guddat O, Fernández G, Brockhaus-Dumke A, Specht K, Klosterkötter J, Reul J, Ruhrmann S. Evidence for a dysfunctional retrosplenial cortex in patients with schizophrenia: a functional magnetic resonance imaging study with a semantic-perceptual contrast. Neurosci Lett. 2004;369:4–8. doi: 10.1016/j.neulet.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 90.Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12:106. doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, David AS, Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull. 2013;39:1288–1295. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 94.Sherman SM, Guillery RW. Exploring the role of the thalamus and its role in cortical function. Cambridge: MIT Press; 2006. [Google Scholar]
- 95.Sim K, Cullen T, Ongur D, Heckers S. Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm. 2006;113:907–928. doi: 10.1007/s00702-005-0363-8. [DOI] [PubMed] [Google Scholar]
- 96.Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- 97.Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- 98.Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 99.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 100.Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 101.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bermpohl F, Dalanay U, Kahnt T, Sajonz B, Heimann H, Ricken R, Stoy M, Hägele C, Schlagenhauf F, Adli M, et al. A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disord. 2009;11:70–75. doi: 10.1111/j.1399-5618.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 103.Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee JH, Welge JA, DelBello MP, Fleck DE, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malhi GS, Lagopoulos J, Ward PB, Kumari V, Mitchell PB, Parker GB, Ivanovski B, Sachdev P. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- 105.Lagopoulos J, Malhi GS. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport. 2007;18:1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- 106.Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E, Phillips ML, Murray RM, McDonald C. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 107.Gruber O, Tost H, Henseler I, Schmael C, Scherk H, Ende G, Ruf M, Falkai P, Rietschel M. Pathological amygdala activation during working memory performance: Evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp. 2010;31:115–125. doi: 10.1002/hbm.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 109.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pujol N, Penadés R, Rametti G, Catalán R, Vidal-Piñeiro D, Palacios E, Bargallo N, Bernardo M, Junqué C. Inferior frontal and insular cortical thinning is related to dysfunctional brain activation/deactivation during working memory task in schizophrenic patients. Psychiatry Res. 2013;214:94–101. doi: 10.1016/j.pscychresns.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 111.Vu MA, Thermenos HW, Terry DP, Wolfe DJ, Voglmaier MM, Niznikiewicz MA, McCarley RW, Seidman LJ, Dickey CC. Working memory in schizotypal personality disorder: fMRI activation and deactivation differences. Schizophr Res. 2013;151:113–123. doi: 10.1016/j.schres.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 112.Linnman C, Coombs G, Goff DC, Holt DJ. Lack of insula reactivity to aversive stimuli in schizophrenia. Schizophr Res. 2013;143:150–157. doi: 10.1016/j.schres.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hummer TA, Hulvershorn LA, Karne HS, Gunn AD, Wang Y, Anand A. Emotional response inhibition in bipolar disorder: a functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol Psychiatry. 2013;73:136–143. doi: 10.1016/j.biopsych.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cooper D, Barker V, Radua J, Fusar-Poli P, Lawrie SM. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res. 2014;221:69–77. doi: 10.1016/j.pscychresns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 115.Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moran LV, Tagamets MA, Sampath H, O’Donnell A, Stein EA, Kochunov P, Hong LE. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. 2013;74:467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He Z, Deng W, Li M, Chen Z, Jiang L, Wang Q, Huang C, Collier DA, Gong Q, Ma X, et al. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med. 2013;43:769–780. doi: 10.1017/S0033291712001638. [DOI] [PubMed] [Google Scholar]
- 118.Vargas C, López-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 119.Whalley HC, Sussmann JE, Romaniuk L, Stewart T, Papmeyer M, Sprooten E, Hackett S, Hall J, Lawrie SM, McIntosh AM. Prediction of depression in individuals at high familial risk of mood disorders using functional magnetic resonance imaging. PLoS One. 2013;8:e57357. doi: 10.1371/journal.pone.0057357. [DOI] [PMC free article] [PubMed] [Google Scholar]


