Abstract
AIM: To evaluate the feasibility of coronary artery calcium score (CACS) on low-dose non-gated chest CT (ngCCT).
METHODS: Sixty consecutive individuals (30 males; 73 ± 7 years) scheduled for risk stratification by means of unenhanced ECG-triggered cardiac computed tomography (gCCT) underwent additional unenhanced ngCCT. All CT scans were performed on a 64-slice CT scanner (Somatom Sensation 64 Cardiac, Siemens, Germany). CACS was calculated using conventional methods/scores (Volume, Mass, Agatston) as previously described in literature. The CACS value obtained were compared. The Mayo Clinic classification was used to stratify cardiovascular risk based on Agatston CACS. Differences and correlations between the two methods were compared. A P-value < 0.05 was considered significant.
RESULTS: Mean CACS values were significantly higher for gCCT as compared to ngCCT (Volume: 418 ± 747 vs 332 ± 597; Mass: 89 ± 151 vs 78 ± 141; Agatston: 481 ± 854 vs 428 ± 776; P < 0.05). The correlation between the two values was always very high (Volume: r = 0.95; Mass: r = 0.97; Agatston: r = 0.98). Of the 6 patients with 0 Agatston score on gCCT, 2 (33%) showed an Agatston score > 0 in the ngCCT. Of the 3 patients with 1-10 Agatston score on gCCT, 1 (33%) showed an Agatston score of 0 in the ngCCT. Overall, 23 (38%) patients were reclassified in a different cardiovascular risk category, mostly (18/23; 78%) shifting to a lower risk in the ngCCT. The estimated radiation dose was significantly higher for gCCT (DLP 115.8 ± 50.7 vs 83.8 ± 16.3; Effective dose 1.6 ± 0.7 mSv vs 1.2 ± 0.2 mSv; P < 0.01).
CONCLUSION: CACS assessment is feasible on ngCCT; the variability of CACS values and the associated re-stratification of patients in cardiovascular risk groups should be taken into account.
Keywords: Coronary artery calcium score, Lung cancer screening, High-resolution computed tomography, Unenhanced chest computed tomography, Cardiovascular risk stratification
Core tip: Low dose chest computed tomography (CT)/high-resolution CT (HRCT) is entering the clinical practice for the screening of individuals at high risk of lung cancer. This study provides evidence that a surrogate stratification of cardiovascular risk can be performed on low-dose chest CT performed in the settings of lung cancer screening. This finding has some relevant consequences since lung cancer and atherosclerosis share some similarities concerning risk factors (smoking), patients’ population (age decade and gender prevalence).
INTRODUCTION
Coronary artery calcium score (CACS) has been regarded as an independent predictor for cardiovascular risk stratification[1,2]. CACS has been performed in asymptomatic individuals for at least two decades using Electron-Beam Computed Tomography (EBCT) and more recently using Multi-Slice Computed Tomography (MSCT), however it has not entered guidelines for cardiovascular risk stratification until recently. In several countries it is applied as a part of primary prevention mostly based on self-referral.
With the introduction of MSCT the potential for anatomical screening has become a clinical reality[3,4]. Besides Coronary Artery Disease (i.e., CACS), also colon cancer (i.e., Virtual Colonoscopy) and lung cancer (i.e., low-dose chest CT) have been proposed as topics for screening[5]. While CACS has already showed a potential for screening asymptomatic individuals, colon cancer screening and lung cancer screening are undergoing large multicenter studies to test whether CT is viable tool for this purpose[6-8].
A very basic observation relies on the fact that in the context of lung cancer screening, the data collected (i.e., low-dose chest CT) are somehow similar to the ones collected for CACS assessment. The main difference relies on the fact that low-dose chest CT is not performed with ECG synchronization protocols (i.e., retrospective ECG gating or prospective ECG triggering), however the increasing speed of MSCT equipment may reduce the discrepancy between the two protocols. The hypothesis is that in principle it is possible to assess CACS on low-dose chest CT (ngCCT: non-gated low-dose chest CT). Therefore, we wanted to test the differences between the CACS values obtained with ngCCT and conventional ECG-gated CT protocol (gCCT: gated Cardiac CT) in the same population of asymptomatic individuals. The aim of the study was compare the CACS values obtained with gCCT (as the reference standard) to ngCCT, and to verify the feasibility and variability of cardiovascular risk stratification using the two protocols.
A novelty of our paper is the comparison of gCCT CACS values with ngCCT ones obtained analyzing dataset reconstructed with 5 mm slice thickness and 5.0mm increment unlike other previously published studies based on dataset with 3 mm, 2.5mm, 1.5 mm or 1mm slice thickness[3,4,9,10]. The feasibility of cardiovascular risk stratification using non gated CACS values obtained with the same standard dataset routinely reconstructed (5mm slice thickness) would allow a wide and fast use in daily clinical practice. The description of the degree of cardiovascular risk reclassification using ngCCT vs gCCT values is another innovative aspect of our study.
MATERIALS AND METHODS
Patients
During a period of 6 mo we prospectively enrolled 60 consecutive asymptomatic individuals (30 males; 72 ± 10 years) who were referred for the assessment of CACS (Table 1). The individuals were excluded based on conventional contra-indications to Radiation Exposure (i.e., potential or actual pregnancy, age < 50 years). All patients underwent two CT scans: the first (gCCT) for CACS purposes and the second for lung parenchyma (ngCCT) assessment, using conventional parameters.
Table 1.
Demographics n (%)
| Clinical characteristics | Population (n = 60) |
| Age (yr, mean ± SD) | 73.4 ± 7.1 |
| Male gender | 30 (50) |
| BMI (kg/m², mean ± SD) | 25.9 ± 4.5 |
| BSA (m², mean ± SD) | 1.8 ± 0.2 |
| Mean heart rate (bpm, mean ± SD) | 73.0 ± 15.7 |
| Additional negative chronotropic drugs | None |
Baseline characteristics of study population. BMI: Body mass index; BSA: Body surface area.
We collected demographics (age, gender, height, weight), heart rate during the scans, and radiation dose for both scans in all patients.
Informed consent was obtained from all patients and the local Medical Ethical Committee approved the study.
CT scan
All individuals underwent two un-enhanced CT scans (Table 2). For gCCT, scanning was performed by using 64-slice CT system (Sensation 64 Cardiac, Siemens Medical Solutions, Forchheim, Germany) and spiral retrospective ECG gating technique. All CT scans of the heart (from the carina to the apex of the heart) were acquired during one inspiratory breath-hold without the use of the contrast medium and without the additional administration of chronotropic drugs. Scan parameters were: detector collimation: 32 mm × 0.6 mm, Z-axis focal spot alternation resulting in simultaneous acquisition of 64 slices; gantry rotation time: 330 ms; effective temporal resolution 165 ms; table feed per rotation: 3.84 mm; pitch 0.2; tube voltage 120 kV; tube power 150 mAs; direction in which data acquisition proceeded: cranio-caudal.
Table 2.
scan and reconstruction parameters
| Parameters | gCCT | ngCCT |
| Scan parameters | ||
| Technique | Spiral | Spiral |
| kV | 120 | 120 |
| mAs | 150 | 30 |
| Gantry rotation time (ms) | 330 | 330 |
| Individual detector width (mm) | 1.2 | 0.6 |
| Number of detectors | 20 × 2 | 32 × 2 |
| ECG gating | Yes | No |
| Prospective ECG modulation of tube current | Yes | No |
| Table feed/s (mm) | 14.4 | 86.4 |
| Pitch | 0.2 | 1.5 |
| Scan range | Carina-Apex | Chest |
| DLP (mGy; mean ± SD) | 116 ± 51 | 84 ± 16a |
| Effective dose (mSv; factor = 0.014; mean ± SD) | 2.0 ± 0.9 | 1.2 ± 0.5a |
| Effective dose (mSv; factor = 0.014; median, IQR) | 1.61; 1.15-2.0 | 1.13; 1.05-1.20a |
| Reconstruction parameters | ||
| Effective slice width (mm) | 3.0 | 5.0 |
| Reconstruction increment (mm) | 1.5 | 5.0 |
| FOV (mm) | 140-160 | 250-300 |
| Kernel filtering | B35f1 | B30f(medium-smooth) |
The Table shows the scan parameters for gCCT and ngCCT. gCCT vs ngCCT,
P < 0.05;
Dedicated filter for calcium scoring. gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography; FOV: Field of view; ECG: Electrocardiogram; DLP: Dose length product.
Image were reconstructed with the following parameters: slice thickness 3 mm, slice increment 1.5 mm, field of view (FOV) 150-180 mm, convolution kernel filtering for CACS (b35f). Temporal windows were set at -350ms prior to the next R wave.
For ngCCT, scanning was performed by using 64-slice CT system (Sensation 64 Cardiac, Siemens Medical Solutions, Forchheim, Germany) and spiral non ECG gated technique. All CT scans of the whole lung were acquired during one deep inspiratory breath-hold without the use of IV contrast medium. Neither electrocardiographic triggering, nor any dose-modulation system were used. The scanner was regularly calibrated to allow reliable measurements and comparison between examinations. Standard low dose chest CT parameters were: detector collimation 0.6 mm, slices per rotation 32 × 2, gantry rotation time 330 ms, pitch 1.5, tube voltage 120 kV, tube power 30 mAs.
Image were reconstructed with the following parameters: slice thickness 5 mm, slice increment 5mm, field of view (FOV) 250-300 mm, convolution kernel filtering medium-smooth (b30f).
CT data analysis
The images of the study population were transferred to a dedicated CT workstation (MMWP, Siemens Medical Solutions, Forchheim, Germany) and analyzed by one operator with more than five years of experience in cardiac imaging, who was blinded to participants’ data and scan protocol. CACS assessment was performed using a dedicated software (CaScore, Siemens, Forchheim, Germany). For the purpose of intra- and inter-observer variability assessment the main observer re-red all scans and a second operator with three years of experience in cardiac imaging red the entire CT dataset. There was a time of 2 months between the two additional readings (one of the main operator for intra-observer variability and one for the additional reader for the inter-observer variability).
Statistical analysis
Continuous variables are expressed as mean ± SD and/or median, where appropriate. Differences between groups were compared using the Student’s t and Wilcoxon tests, as appropriate. Correlation was assessed using the Pearson’s r test while the bias was assessed using the Bland-Altman method. Intra- and inter-observer variability were assessed for all CACS measurements and compared using the Bland-Altman method.
Radiation dose (Dose Length Product: DLP) was estimated using the CTDIvol multiplied for the scan length. For the estimation of the effective dose a factor of 0.014 was applied[11].
Statistical analysis was performed with SPSS (version 12.0, SPSS Inc., Chicago, IL, United States) and MedCalc (version 9.3.0.0., MedCalc Software, Mariakerke, Belgium) software. Significance was set at P < 0.05.
RESULTS
The scans were successful in all patients. Mean heart rate during the scans was 73 ± 16 bpm.
CACS assessment
The time required to perform CACS was 3 ± 1 min.
Mean CACS values were significantly higher for gCCT as compared to ngCCT (Volume: 418 ± 747 vs 332 ± 597; Mass: 89 ± 151 vs 78 ± 141; Agatston: 481 ± 854 vs 428 ± 776; P < 0.05). The mean difference (Delta) between gCCT and ngCCT was 86 ± 267 for Volume, 11 ± 36 for Mass and 53 ± 188 for Agatston score, showing a sharp tendency for global underestimation of CACS in ngCCT (Table 3, Figures 1-3).
Table 3.
coronary artery calcium score values
| Parameters (mean ± SD) | gCCT | ngCCT | Delta (absolute) | Delta | P | r | R2 |
| Volume | 418.1 ± 746.5 | 332.0 ± 596.9 | -86.1 ± 263.8 | -21% | < 0.05 | 0.947 | 0.896 |
| Mass | 88.9 ± 151.2 | 77.9 ± 140.5 | -11.1 ± 35.5 | -12% | < 0.05 | 0.973 | 0.946 |
| Agatston | 480.8 ± 853.7 | 427.7 ± 776.3 | -53.1 ± 188.4 | -11% | < 0.05 | 0.978 | 0.956 |
The Table shows the mean values of CACS Volume, Mass, and Agatston score for the study population. On average gCCT shows CACS values significantly higher with a high correlation to ngCCT. The percentage underestimation (delta) of ngCCT of Agatston score is -11%. CACS: Coronary artery calcium score; gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography.
Figure 1.
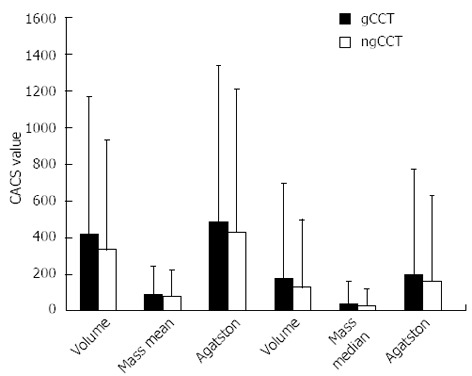
Coronary artery calcium score values. The Figure shows the mean and median values of Volume, Mass, and Agatston Score for gCCT and ngCCT in the total population. There is a sharp tendency to underestimate of ngCCT for all types of CACS score. gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography; CACS: Coronary artery calcium score.
Figure 3.
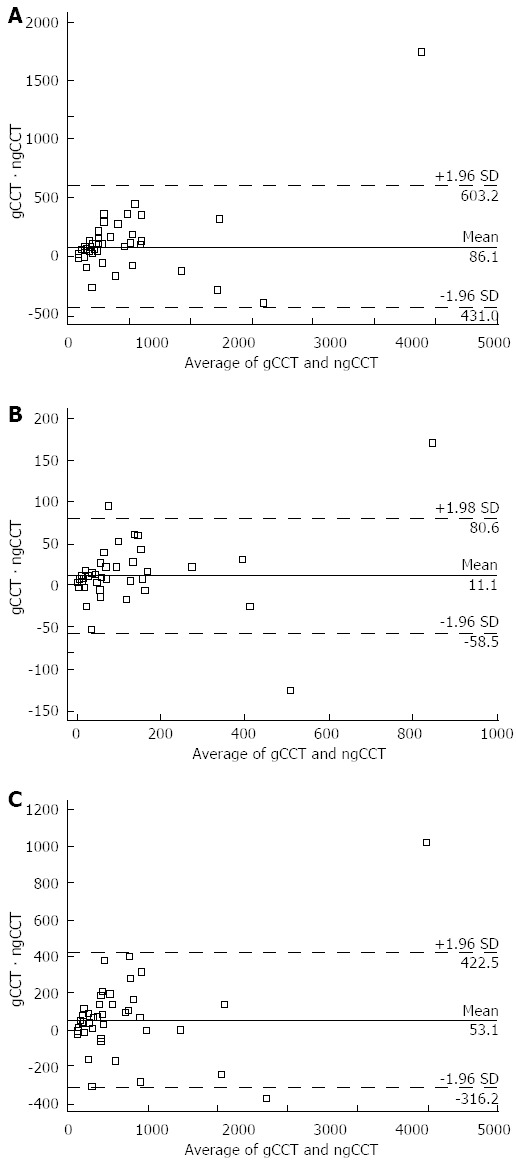
Bland-Altman plots of coronary artery calcium score values. The Bland-Altman plots show the slight but significant tendency to overestimate Volume (A), Mass (B), and Agatston (C) scores of ngCCT as compared to gCCT. There are 2 patients with values far off in ngCCT vs gCCT. However, they are both with very high values and this does not affect significantly further reclassification. gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography.
Figure 2.
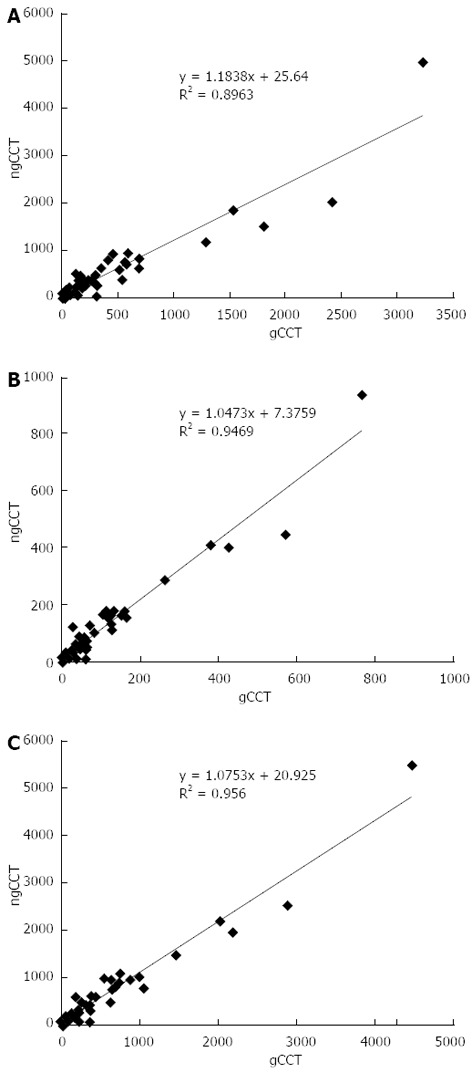
Scatter plots coronary artery calcium score values. The scatter plots show the high correlation of Volume (A), Mass (B), and Agatston (C) scores obtained with gCCT and ngCCT. gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography.
The correlation between values was always high (r = Volume: 0.95; Mass: 0.97; Agatston: 0.98) between the two scans. The correlation between heart rate and the difference between the two scans was very low (r = -0.09), suggesting that the variability was not related to heart rate. Instead there was a moderate correlation (r = 0.50) between the differences (delta) between the two scans and the Agatston score in gCCT, suggesting that the higher the CACS value, the higher the variability.
Intra- and Inter-observer variability was very limited for both gCCT and ngCCT (P > 0.05). Intra-observer variability of Agatston score showed a bias of -0.148 for ngCCT and 0.057 for gCCT. Inter-observer of Agatston score showed a bias of -0.259 for ngCCT and 0.178 for gCCT.
Reclassification
With the tendency of ngCCT to underestimate CACS values we observed a relevant degree of reclassification (Table 4, Figure 4).
Table 4.
Cardio vascular risk stratification based on absolute coronary artery calcium score classes n (%)
| CACS class | gCCT | ngCCT |
| 0 | 6 (10) | 10 (16.7) |
| 1-10 | 6 (10) | 7 (11.7) |
| 11-100 | 14 (23.3) | 10 (16.7) |
| 101-400 | 14 (23.3) | 17 (28.3) |
| 400-1000 | 14 (23.3) | 10 (16.7) |
| > 1000 | 6 (10) | 6 (10) |
The Table shows how the total Agatston score distributed using the Mayo Clinic Classification (Rumberger et al[17]) with gCCT and ngCCT within the same population. In total 23 (38.3%) patients shifted to a different cardiovascular risk group. gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography.
Figure 4.
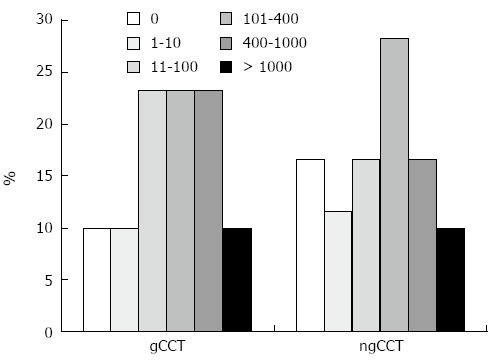
Restratification. The Figure shows the pattern of reclassification of cardiovascular risk categories using the Mayo Clinic Classification (Rumberger et al) with gCCT and ngCCT within the same population (CACS score according to Agatston). ngCCT determines a shift in the ranges between 1 and 400 Agatston score. Some of these patients shift to a lower category (in particular the ones with Agatston score between 1 and 10), while some of the patients shifts towards higher risk categories (in particular the ones with Agatston score between 11 and 100). In total 23 patients shifted to another cardiovascular risk category in ngCCT. gCCT: ECG gated cardiac computed tomography; ngCCT: Non-gated chest computed tomography; CACS: Coronary artery calcium score.
Overall, 23 (38%) patients were reclassified in a different cardiovascular risk category, mostly (18/23; 78%) shifting to a lower risk in the ngCCT; in 5/23 (22%) cases the CACS score shifted to a higher cardiovascular risk category.
Of the 6 patients with 0 Agatston score on gCCT, 2 (33%) showed an Agatston score > 0 in the ngCCT. Of the 3 patients with 1-10 Agatston score on gCCT, 1 (33%) showed an Agatston score of 0 in the ngCCT.
Two patients showed very different values of CACS between the two scans; they correspond both the very high values of Agatston score (> 1000); therefore, the difference is not relevant for stratification of risk. The main reason for such a difference (beside the lack of cardiac synchronization in ngCCT) was related to higher heart rates (> 80 bpm).
Radiation dose
The estimated radiation dose was significantly higher for gCCT (DLP 115.8 ± 50.7 vs 83.8 ± 16.3; Effective dose 1.6 ± 0.7 mSv vs 1.2 ± 0.2 mSv; P < 0.01).
DISCUSSION
A number of randomized lung cancer screening trials are currently undergoing with the aim of reducing mortality related to this life-threatening condition[12]. Although atherosclerotic vascular disease accounts for more death and disability than all types of cancer, the importance of detecting subclinical atherosclerosis and targeting prevention of future cardiovascular events is only now starting to be highlighted in the lung cancer screening setting[3,4,13-15]. Preliminary experiences shows a very high correlation between CACS assessed on low dose HRCT and cardiovascular events[4].
These findings are also suggesting that cardiovascular mortality as predicted by CACS is higher as compared to lung cancer mortality in a population of high risk smokers[4]. This finding can be expected from epidemiological data on all-cause mortality[8], however for the first time we have a tool that can stratify risk for both diseases. This is the first study that addressed the issue of CACS assessment in lung cancer screening context in a prospective cross-over fashion.
Our study is focused on showing the correlation between CACS obtained in conventional gCCT scans and in low-dose CT (ngCCT). The correlation we found is very high with a tendency of ngCCT to underestimate total calcium burden. The cardiovascular reclassification occurring in individuals undergoing HRCT, following the Mayo Clinic classification is important (about 38% of the individuals). Nevertheless, the predictive value on mortality has been shown to remain preserved[3,4].
The most important findings of our study are related to the classification of individuals with none or very low CACS values. In general one third of the individuals with CACS 0 (which means no evidence of CAD) are reclassified in higher category, while one third of the individuals with very low CACS (CACS 1-10) are reclassified as CACS 0. This is particularly important for the negative predictive value of CACS concerning obstructive CAD and cardiovascular events.
The technique applied to ngCCT in this study is the standard one (dataset reconstructed with 5 mm slice thickness and 5 mm slice increment) and does not require any additional reconstruction as suggested by other studies previously published (from 1 mm to 3 mm slice thickness)[3,4,9,10]. CACS assessment can be done using the same data routinely reconstructed for the purpose of lung cancer screening, resulting easily applicable in daily clinical practice.
Clinical implications
From our observation and the observation of Jacobs et al[3,4] we can suggest that individuals undergoing HRCT for lung cancer screening should be assessed for CACS. CACS assessment is a relatively simple procedure that requires a dedicated software application (currently available on all CT scanners able to perform Cardiac Imaging) and a basic training. It is not time consuming (it requires few minutes) but it has a very important prognostic value which is independent from conventional cardiovascular risk factors[1,2,15].
Limitations
Study has several limitations: The first is the relatively low number of patients. This also limits the sub-analysis of the CACS groups. However, this is the first study that prospectively tests in a cross-over design the hypothesis that ngCCT could be used for CACS purposes. Enrolling more patients would have exposed more individuals to un-necessary additional radiation.
The second limitation is the additional radiation dose delivered to the patients which is consistent with the design of the study and has been minimized by using available hardware and software solutions.
The third limitation is related to the absence of prospective ECG triggering protocol for gCCT. This feature was available but we decided not to use it due to the higher reproducibility of spiral CT scanning for CACS[16]. The use of prospective ECG triggering would have further reduced the radiation dose of gCCT. The fourth limitation is the lack of outcome data. However, this was beyond the aim of the study and the number of patients enrolled is not adequate for this purpose anyway. This aspect has been showed in other studies[4]. Recently another substudy of the MILD trial provided insight into this aspect and showed that individuals with > 400 modified Agatston score performed on non-gated chest CT scans have a worse prognosis in terms of mortality and cardiovascular events[8]. Actually, with this study we wanted to demonstrate the feasibility of CACS in ngCCT scans.
CACS assessment is feasible on unenhanced chest CT, however the variability of CACS values and the associated re-stratification of patients in cardiovascular risk groups should be taken into account.
COMMENTS
Background
Lung cancer screening is a long standing topic. Low dose high resolution computed tomography (CT) is becoming the preferred mean for this purpose in high risk smoker population. Meanwhile, coronary artery calcium score by means of CT has become a strong and reliable method for cardiovascular risk stratification in asymptomatic individuals.
Research frontiers
This study demonstrates that coronary artery calcium score for cardiovascular risk stratification can be performed in the context of lung cancer screening by means of low dose CT.
Innovations and breakthroughs
There are no studies in literature performed as cross-over and prospective in such a population.
Applications
This study provides the clue on the feasibility of coronary calcium score in individuals undergoing low dose CT for lung cancer screening.
Terminology
Low dose computed tomography: it is a diagnostic method by which it is possible to detect small pulmonary nodules before they become larger tumors. Coronary artery calcium score: it is a test performed with CT that allows the quantification of coronary artery calcium as a surrogate and independent marker of cardiovascular risk.
Peer review
This is a well written manuscript that compares quantification of coronary artery calcium scoring by non-gated techniques with traditional thick slice reconstructions as used in lung screening exams with calcium scoring by traditional gated techniques with thin slice reconstructions.
Footnotes
P- Reviewers: Biondi-Zoccai G, Srichai-Parsia M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs PC, Gondrie MJ, van der Graaf Y, de Koning HJ, Isgum I, van Ginneken B, Mali WP. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198:505–511. doi: 10.2214/AJR.10.5577. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs PC, Prokop M, van der Graaf Y, Gondrie MJ, Janssen KJ, de Koning HJ, Isgum I, van Klaveren RJ, Oudkerk M, van Ginneken B, et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209:455–462. doi: 10.1016/j.atherosclerosis.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Ashar BH, Hughes MT, Marinopoulos SS, Prokopowicz GP, Berkenblit GV, Sisson SD, Simonson LA, Miller RG. Current evidence for the use of emerging radiologic technologies for disease screening. Am J Manag Care. 2005;11:385–392. [PubMed] [Google Scholar]
- 6.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Sverzellati N, Cademartiri F, Bravi F, Martini C, Gira FA, Maffei E, Marchianò A, La Vecchia C, De Filippo M, Kuhnigk JM, et al. Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology. 2012;262:460–467. doi: 10.1148/radiol.11110364. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, Nath H, Lopez-Garcia C, Black-Shinn J, Casaburi R. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5:113–118. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MT, Yang P, Huang YL, Chen JS, Chuo CC, Yeh C, Chang RS. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: concordance study with dedicated cardiac CT. AJR Am J Roentgenol. 2008;190:923–928. doi: 10.2214/AJR.07.2974. [DOI] [PubMed] [Google Scholar]
- 11.Bongartz G, Golding SJ. 2004 CT quality criteria. Luxembourg, Luxembourg. European Commission, 2004. Available from: http://www.msct.eu/CT_Quality_Criteria.htm.
- 12.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budoff MJ, Gul KM. Expert review on coronary calcium. Vasc Health Risk Manag. 2008;4:315–324. doi: 10.2147/vhrm.s1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shemesh J, Henschke CI, Farooqi A, Yip R, Yankelevitz DF, Shaham D, Miettinen OS. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30:181–185. doi: 10.1016/j.clinimag.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Shemesh J, Henschke CI, Shaham D, Yip R, Farooqi AO, Cham MD, McCauley DI, Chen M, Smith JP, Libby DM, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541–548. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 16.Ohnesorge B, Flohr T, Fischbach R, Kopp AF, Knez A, Schröder S, Schöpf UJ, Crispin A, Klotz E, Reiser MF, et al. Reproducibility of coronary calcium quantification in repeat examinations with retrospectively ECG-gated multisection spiral CT. Eur Radiol. 2002;12:1532–1540. doi: 10.1007/s00330-002-1394-2. [DOI] [PubMed] [Google Scholar]
- 17.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]


